Abstract
The epidemiological characteristics of Sjögren syndrome (SS) are significantly varied in different countries. We conducted the present study to survey the epidemiological characteristics of primary SS in China. We recruited 483 primary SS patients from 16 Chinese medical centers nationwide from January 2009 to November 2011 and assessed salivary and lacrimal gland dysfunction, organ involvement, and autoimmunity in these patients. The cohort included 456 women and 27 men (ratio, 17:1; mean age at onset, 42 ± 11 years; median age at diagnosis, 49 years; range, 41–56 years). Male patients showed a lower frequency of xerophthalmia (37.0% vs 60.7%) and a higher frequency of arthritis (40.7% vs 16.4%). Young-onset patients showed a higher frequency of low C3 levels (57.7% vs 36.3%) and pancytopenia (22.2% vs 8.8%). Patients with systemic involvement had a higher frequency of immunoglobulin A (IgA) (39.4% vs 22.5%) and immunoglobulin M (IgM) (12.4% vs 37.9%). Patients with pulmonary involvement had a higher parotid enlargement (21.4% vs 10.2%), purpura (12.1% vs 5.7%) and higher anti-La/SS-B (61.7% vs 41.8%), immunoglobulin G (IgG) (80.7% vs 64.6%) and IgA (48.9% vs 30.6%) levels. Patients with anti-Ro/SSA antibodies had more frequent exocrine gland symptoms and some extraglandular symptoms and immunological alterations. Compared with previous studies performed in other countries, SS patients in China showed particular clinical manifestation, systemic involvement, and immunological alterations.
INTRODUCTION
Primary Sjögren syndrome (SS) is an autoimmune disease that affects the exocrine glands and other parenchymal organs (ie, the kidney, lung, and liver), leading to dryness of the main mucosal surfaces and extraglandular manifestations.1,2 The disease overwhelmingly affects middle-aged women, and some patients (approximately 5%–10%) develop lymphoma.3 The prevalence of primary SS in China is approximately 0.33% to 0.77%, according to different criteria.4 Recent studies have reported that the prevalence ranges from 0.05% to 0.23% in other countries.5,6 Primary SS is associated with several immune abnormalities, of which antinuclear antibodies (ANAs) and increased immunoglobulin (Ig) levels are the most frequently detected; anti-Ro/SS-A is the most specific abnormality, and cryoglobulins and hypocomplementemia are the main prognostic markers. The histological hallmark is focal lymphocytic infiltration of the exocrine glands and other parenchymal organs.7
SS is a heterogeneous disease that has a wide spectrum.8,9 The variability of its presentation may significantly delay its diagnosis after the onset of symptoms.10,11 The presentation of SS may be significantly influenced by epidemiological characteristics, systemic involvement, or the immunological profile at diagnosis. Some researchers have analyzed such factors.12,13 These studies have yielded different results, likely because of the small number of patients included and the different classification criteria used. We conducted the present study to characterize the clinical presentation of primary SS in a large cohort of Chinese patients and to define epidemiologic, clinical, and immunologic subsets of patients to facilitate earlier diagnosis for Chinese SS patients.
METHODS
Patients
We registered 483 consecutive patients from 16 Chinese medical centers nationwide from January 2009 to November 2011 who fulfilled the 2002 classification criteria for primary SS.14 The following exclusion criteria were applied: chronic hepatitis C virus or human immunodeficiency virus infection and previous lymphoproliferative processes or associated systemic autoimmune diseases.
Heart involvement was indicated by persistently altered electrocardiographic examinations (with the exception of nodal tachycardia and bradycardia), and/or structural abnormalities detected by ultrasound. Pulmonary involvement was indicated by persistent cough and/or dyspnea with chronic diffuse interstitial infiltrates on X-rays, altered patterns on pulmonary function tests, and/or evidence of lung alveolitis or fibrosis in computed tomography (CT) scans. Nephropathy was defined as persistent proteinuria (>0.5 g/day), altered urine analysis (hematuria, pyuria, and red blood cell casts), a persistently elevated serum creatinine level (84 μmol/L), renal tubular acidosis, interstitial nephritis, or glomerulonephritis. Liver involvement was indicated by altered serum hepatic function test results (aminotransferase, alkaline phosphatase, gamma-glutamyltransferase, and bilirubin) and/or evidence of altered bile ducts in imaging-based examinations (ultrasound, CT, or magnetic resonance imaging).
Immunological tests were performed using commercial techniques standardized at Peking Union Medical College Hospital (indirect immunofluorescence for ANA, ELISA for anti-Ro/La antibodies and Ig, nephelometry for rheumatoid factor [RF], and immunoturbidimetry for C3/C4); anti-Ro/SSA antibodies were tested using commercial ELISA kits that detected IgG, IgA, and IgM antibodies to the 60-kDa and 52-kDa forms of Ro. This study was approved by the Ethics Committee of the Chinese Academy of Medical Sciences, Peking Union Medical College Hospital and, subsequently, by each participating center. The study design conformed to current Chinese ethical standards.
Statistical Analyses
Descriptive data are presented as means ± standard deviation for continuous variables when the data were normally distributed or as M (P25–P75) when the data were non-normally distributed; numbers (%) are indicated for the categorical variables. Continuous variables were analyzed with Student t test in large samples of similar variance, or with the nonparametric Mann–Whitney U test for small samples. Categorical data were compared using the χ2 or Fisher exact tests. A 2-tailed value of P < 0.05 indicated statistical significance. Multiple logistic regression was used in the univariate analysis, adjusted for the statistically significant variables (P < 0.05). Statistical analyses were performed with the 12.0 Stata/SE program (StataCorp LP, College Station, TX).
RESULTS
The patient cohort comprised 483 individuals, including 456 (94.4%) women and 27 (5.6%) men (female: male ratio, 17:1), with a mean age at onset of 41.7 ± 11.0 years (range, 14–77 years). The median age at diagnosis was 49 (41–56) years (range, 17–89 years). The median period of time from the first SS-related symptom to diagnosis was 12 (6–22) years (range, 0–65 years). There were 260 patients (85.8%) with positive salivary gland biopsies among 303 patients who were examined. The remaining patients with negative gland biopsies were diagnosed with SS due to positive anti-SSA and/or anti-SSB tests, as well as other SS-related symptoms (Table 1).
TABLE 1.
Demographic, Clinical, and Immunologic Features of 483 Chinese Patients With Primary SS
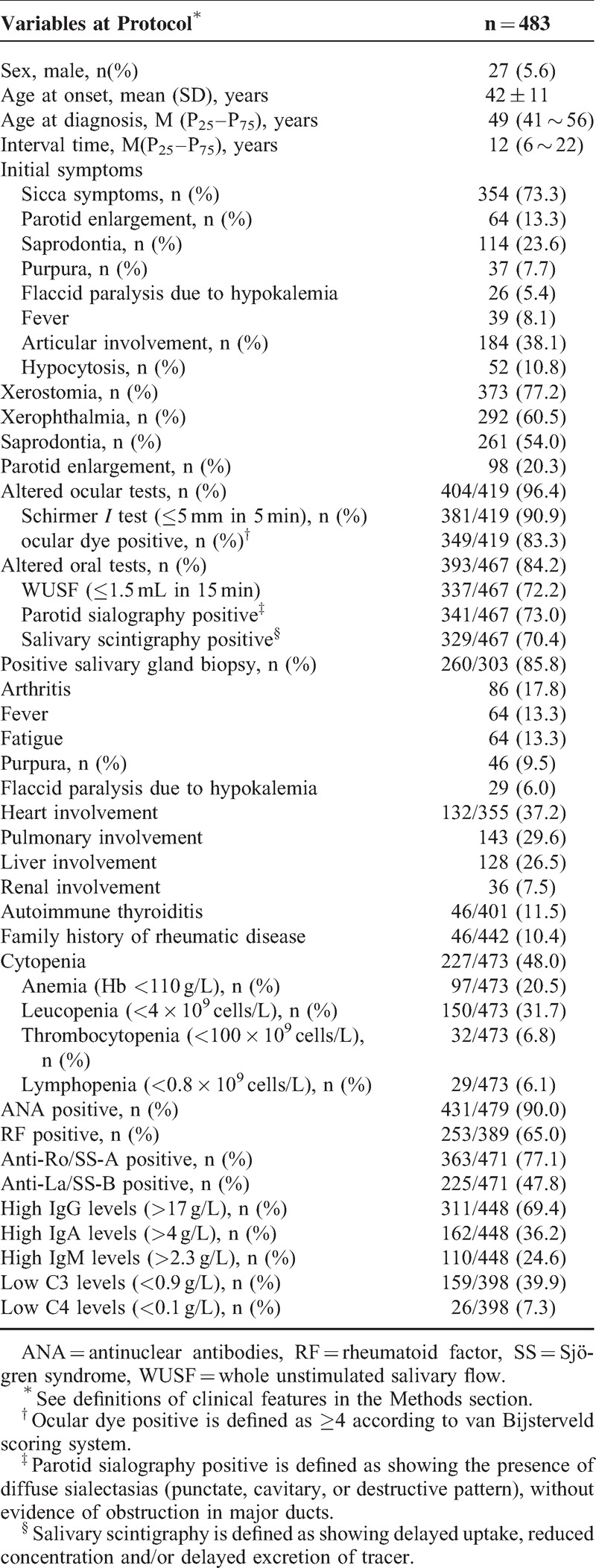
SS in Men
Among the 483 patients with primary SS, 27 (5.6%) were men. Men showed a lower frequency of xerophthalmia, leucopenia, erythrocyte sedimentation rate (ESR), RF positivity, and anti-La/SS-B positivity; in addition, arthritis was more prevalent in men compared with women by univariate analysis. Multivariate analysis identified xerophthalmia (P = 0.007) and arthritis (P = 0.006) as independent variables (Table 2).
TABLE 2.
Univariate and Multivariate Analyses of the Main Demographic, Clinical, and Immunologic Features in Patients with Primary SS, by to Sex
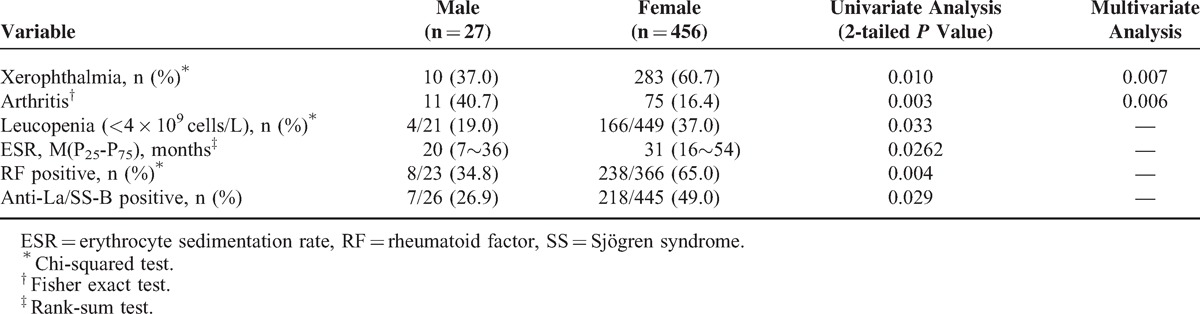
Young-onset SS (Age at Diagnosis 35 Years or Younger)
Primary SS was diagnosed before age 35 years in 75 of 455 (5.3%) patients. Among the initial symptoms, sicca, saprodontia, arthritis, and xerophthalmia were observed less frequently. In addition, there was a higher prevalence of purpura and flaccid paralysis resulting from hypokalemia and pancytopenia, and high IgG levels and low C3 levels were found at diagnosis in patients 35 years old and younger compared with patients older than 35 years, according to univariate analysis. Multivariate analysis identified pancytopenia (P = 0.04) among the initial symptoms and low C3 levels (P = 0.009) as independent variables (Table 3).
TABLE 3.
Univariate and Multivariate Analysis of the Main Demographic, Clinical, and Immunologic Features in Patients With Primary SS, According to Age at Diagnosis (35 Years’ Old or Younger or Older Than 35 Years of Age)
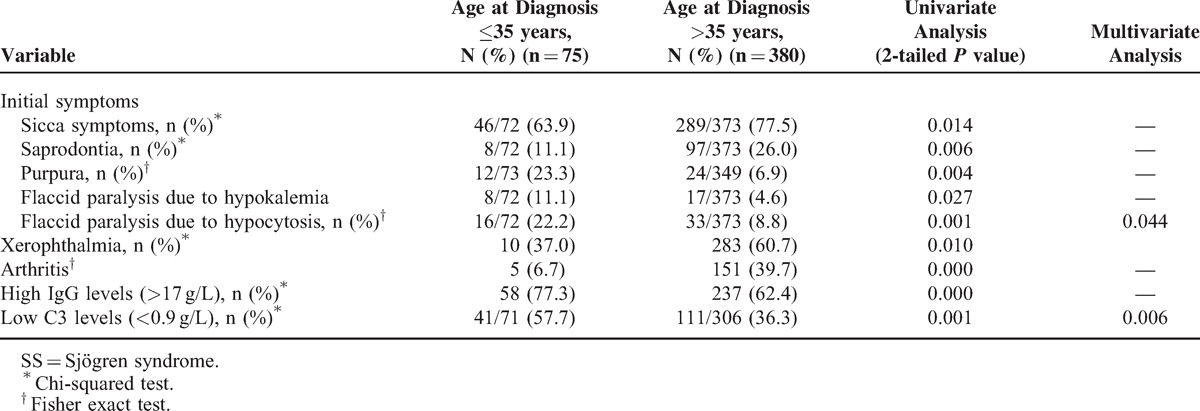
Systemic Disease Involvement
In total, 355 patients had their hearts tested, and 61 (17.2%) showed abnormalities, including heart package effusion (36/309, 11.8%) and atrioventricular blockage (27/355, 8.1%). Of the 384 patients who took the lung test, 143 (29.6%) showed pulmonary injury, including interstitial lung disease (59/317, 18.6%), abnormal pulmonary function (35/297, 11.8%), pulmonary hypertension (29/274, 10.6%), multiple lung bullae (30/317, 9.5%), and pleural effusion (20/317, 6.3%). Of the 384 patients who took the renal test, 36 (7.5%) showed renal injury, including urine protein positive (31/483, 6.4%), renal tubular acidosis (29/483, 6.0%), kidney stones and/or renal calcification (21/371, 5.7%), and renal insufficiency (26/483, 5.4%). In addition, there were 188 patients with abnormal liver tests, including abnormal liver function (107/483, 22.2%) and liver and spleen enlargement (71/483, 14.7%). Patients with systemic disease involvement had higher frequency of saprodontia, anti-Ro/SS-A, and anti-La/SS-B positivity and high levels of IgA and IgM, according to univariate analysis. Using multivariate analysis, we identified high levels of IgA (P = 0.03) and IgM (P = 0.02) as independent variables (Table 4).
TABLE 4.
Univariate and Multivariate Analysis of the Main Demographic, Clinical, and Immunologic Features in Patients with Primary SS, According to the Presence or Absence of Systemic Involvement
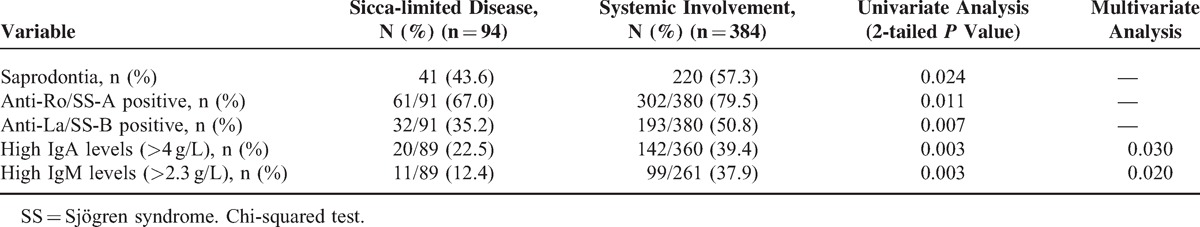
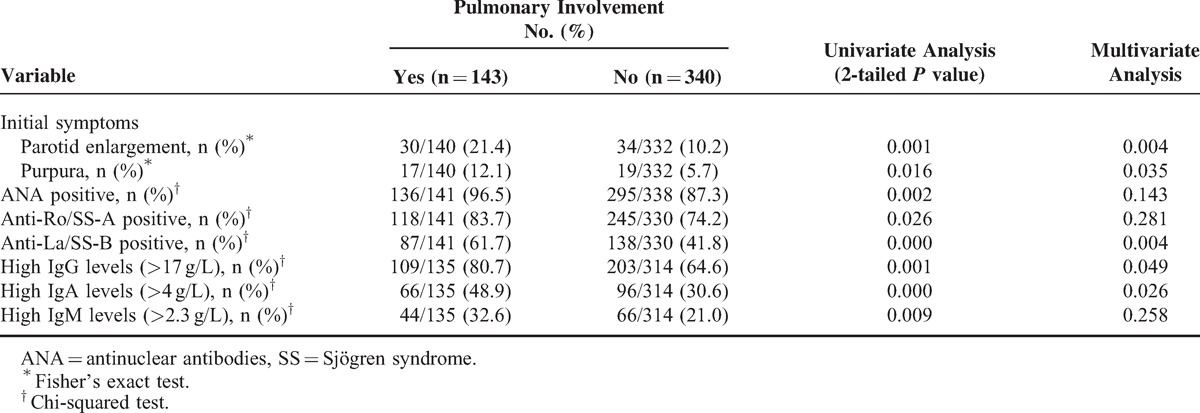
Patients With Pulmonary Involvement
Among the 483 patients, 143 (29.6%) had pulmonary injury. These patients had a higher prevalence of parotid enlargement and purpura among the initial symptoms and greater ANA, anti-Ro/SS-A, and anti-La/SS-B positivity, and higher levels IgG, IgA, and IgM by univariate analysis. Multivariate analysis identified parotid enlargement (P = 0.004) and purpura (P = 0.035) among the initial symptoms and anti-La/SS-B (P = 0.004) positivity, and higher levels of IgG (P = 0.049) and IgA (P = 0.026) as independent variables (Table 5).
TABLE 5.
Univariate and Multivariate Analysis of the Main Demographic, Clinical, and Immunological Features in Patients with Primary SS, According to Pulmonary Involvement (Years or N [%])
ANA-positive Patients
Compared with the ANA-negative patients, the ANA-positive patients had a higher frequency of systemic involvement (pulmonary involvement, heart involvement, and anemia) and altered immunological markers (RF, anti-Ro/SS-A, and anti-La/SS-B antibodies), according to univariate analysis. Multivariate analysis identified heart (P = 0.040) and anti-Ro/SS-A antibodies (P = 0.001) as independent variables (Table 6).
TABLE 6.
Analysis of the Main Demographic, Clinical, and Immunological Features in Patients with Primary SS, According to Presence or Absence of the Main Immunological Markers
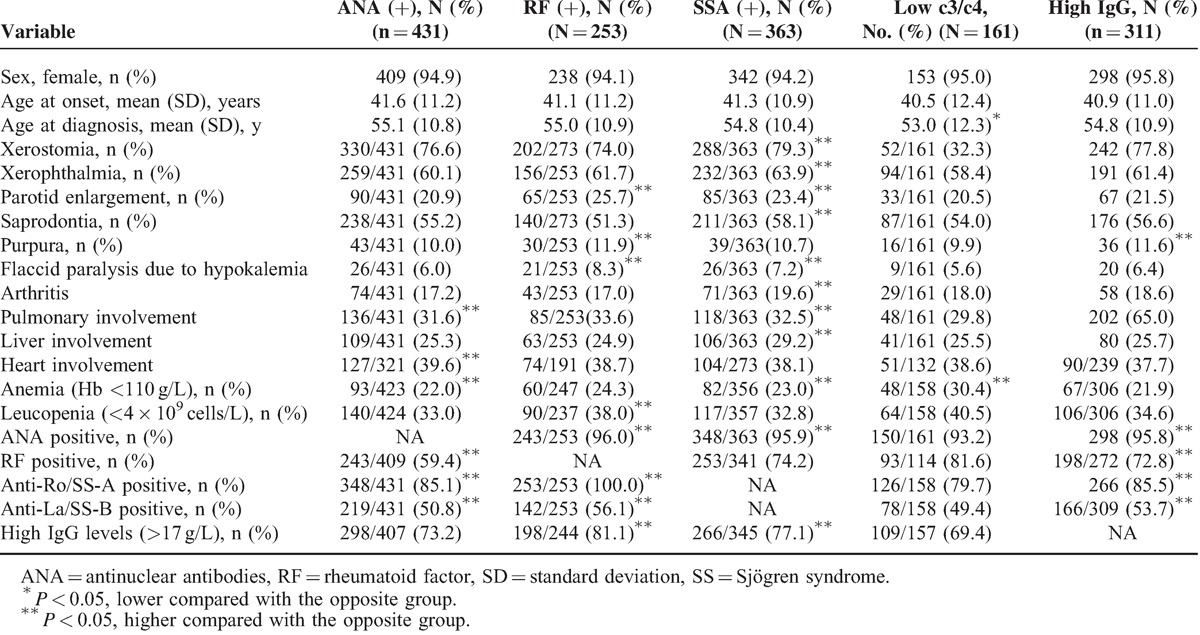
Patients with RF
Compared with the RF-negative patients, the RF-positive patients had a higher frequency of parotid enlargement, purpura, and flaccid paralysis because of hypokalemia, leucopenia, and positive immunological markers (ie, ANA, anti-Ro/SS-A and anti-La/SS-B antibodies, and higher IgG levels) by univariate analysis. Multivariate analysis identified leucopenia (P = 0.021), anti-La/SS-B (P = 0.035), and higher IgG levels (P = 0.003) as independent variables (Table 6).
Patients With Anti-Ro/La Antibodies
Compared with the Ro/La-negative patients, the Ro/La-positive patients had a higher frequency of glandular involvement (xerostomia, xerophthalmia, parotid enlargement, or saprodontia) and extraglandular symptoms (flaccid paralysis resulting from hypokalemia, arthritis, pulmonary involvement, liver involvement, or anemia), and positivity for immune markers (ANA and high IgG levels) by univariate analysis. Multivariate analysis identified saprodontia (P = 0.01), liver involvement (P = 0.009), ANA (P < 0.001), and high IgG (P < 0.001) levels as independent variables (Table 6).
Patients With Hypocomplementemia
Compared with the patients with normal C3 and C4 levels, the patients with hypocomplementemia had a lower mean age at diagnosis and a higher frequency of anemia in the univariate analysis. Multivariate analysis also identified the age at diagnosis (P = 0.013) and a higher frequency of anemia (P < 0.001) as independent variables (Table 6).
Patients With High IgG Levels
Compared with the patients with normal IgG levels, the patients with high IgG levels had a higher frequency of purpura and positivity for immune markers (ANA, anti-Ro/SS-A, and anti-La/SS-B) by univariate analysis. Multivariate analysis identified ANA (P = 0.01) and RF (P = 0.003) as independent variables (Table 6).
DISCUSSION
SS is a chronic autoimmune disease that typically affects middle-aged women, and a genome-wide association study has shown that genetic factors may play an important role in its pathogenesis.15 Although SS is classically considered to be an exocrine gland disease (mainly the salivary and lacrimal glands) that causes oral and ocular dryness, it is also characterized by diverse clinical manifestations. These manifestations can be related either to periepithelial infiltrates in parenchymal organs (kidney, lung, and liver) or to immune complex deposition because of B cell hyperactivity (purpura, peripheral neuropathy, or glomerulonephritis).1,16,17 Some researchers have studied the factors that affect diagnosis,12,13 and these analyses have yielded different results. In this study, we evaluated the clinical and immunological manifestations of primary SS in 483 consecutive Chinese patients, which allowed us to further confirm that xerostomia, xerophthalmia, ANA, anti-Ro/SS-A, RF, high IgG levels, and low C3 levels are the most frequently occurring features of SS. However, saprodontia manifestations were frequently observed in our study (54.0%), which are not included in the current classification criteria, and may be a strong suggestion for SS.
The expression of SS in males was characterized by a lower frequency of xerophthalmia, leucopenia, ESR, and RF or anti-La/SS-B positivity. These findings are consistent with a generally accepted idea in autoimmunity that women have higher levels of autoimmune processes (both clinical and serological) than men. Many previous studies have reported results18–24 that also support this notion. The lower frequency of autoimmunity in men may make it more difficult to diagnose this disease early.
Age is regarded as an important factor at SS diagnosis.2,13 Young-onset patients showed a low degree of sicca involvement and a high degree of immunological features in our study, indicating a specific pattern in the clinical expression of primary SS. The identification of this specific presentation pattern may allow earlier diagnoses in such patients, for whom the diagnosis may be complicated because of the less pronounced expression of sicca features. These findings confirm results in children and adolescents,25 and in other young-onset SS populations.12,14 Some studies have shown that the clinical presentation of elderly patients was diametrically opposite to young-onset SS patients, with a lower prevalence of some systemic and immunological features, which may reflect senescence of the immune system.13,26 However, our data did not support this pattern, although we caution that our sample of elderly patients was very small (n = 18).
The subset of patients with systemic disease involvement showed an increased prevalence of saprodontia, anti-Ro/SS-A, and anti-La/SS-B positivity, and higher levels of IgG and IgM, which may reflect an excessive immune process. The prevalence of saprodontia was highest among all physical signs (54.0%) on registry. The occurrence of saprodontia, resulting from reduced saliva production as a consequence of immune-mediated injury of the salivary glands, is a prominent sign in SS patients. Thus, typical saprodontia should be included in the diagnostic criteria, although it may be less common at the early disease stage.
Patients with pulmonary involvement had a greater prevalence of parotid enlargement and purpura among the initial symptoms, and a greater prevalence of anti-La/SS-B antibodies, as well as higher IgG and IgA levels by multivariate analysis, rather than differences in anti-Ro/SS-A antibodies. This finding was inconsistent with other studies,27–29 but a previous study reported that anti-La/SS-B levels had a higher specificity than anti-Ro/SS-A levels (92.6% vs 85.7%).29 Therefore, using anti-La/SS-B to predict pulmonary involvement in SS patients should be a cause for concern.
Positivity for autoantibodies is important in diagnosing SS and represents 1 of the 6 AECG criteria for SS. The 1993 European Criteria30 include the presence of ≥1 of 4 antibodies (ANA, RF, Ro/SS-A, and/or La/SS-B), whereas the 2002 Criteria includes only anti-Ro/SS-A and/or anti-La/SS-B antibodies.14 ANA is the most frequently detected antibody in primary SS (90% in the study presented here) and is closely associated with various extraglandular and immunological features; RF is also associated with the main extraglandular, histopathological, hematological, and immunological features of SS.19,26,31 Thus, a patient with sicca syndrome, positive ocular tests and parotid scintigraphy, and positive ANA and/or RF should be diagnosed with SS, even if the anti-Ro/La antibodies are negative. ANA and RF determinations may play a central role in differentiating SS from non-autoimmune causes of sicca syndrome, although they are not included in the AECG criteria. Considering the findings of our study and others13 we suggest an important diagnostic role for ANA/RF testing, as did the American College Of Rheumatology (ACR)32 in 2012.
Anti-Ro/SSA and anti-La/SSB antibodies can be detected in approximately 50% to 70% of primary SS patients33 and associated with some presentations. In the study presented herein, the subset of patients with anti-Ro/La antibodies not only had a higher prevalence of sicca symptoms but also showed extraglandular involvement, confirming previous reports that showed a close correlation between positivity for these autoantibodies and extraglandular manifestations,34–38 serological markers,38,39 and a higher focus score in salivary gland biopsies.31,40 These findings confirm that anti-Ro/La antibodies are considered to be a mandatory criterion for primary SS. However, the inclusion of positivity for anti-Ro/La antibodies as a mandatory criterion may limit the diagnosis of some cases of primary SS because some subsets, such as males and those with sicca-limited disease, had a lower prevalence of anti-Ro/La antibodies, thereby reducing their probability of fulfilling the 2002 criteria. The newly proposed ACR classification criteria for SS, in which anti-Ro/La antibodies are not mandatory, solved this problem.32
High IgG levels are a common feature of SS and might reflect greater B cell activation.41 In our study, patients with high IgG levels had a higher prevalence of purpura and immunological markers (ANA, RF, anti-Ro/SS-A, and anti-La/SS-B) that indicate hyperactive disease. Hypocomplementemia had been confirmed to be related to a lower mean age and a higher frequency of vasculitis, RF, and B cell lymphoma.13 We found a close association between hypocomplementemia and a lower mean age at diagnosis and a higher frequency of anemia. Ioannidis et al17 may have been the first to suggest a prognostic role for low C4 levels, which was confirmed, together with low C3 levels, by Theander et al.42 Some studies have also suggested a negative association between hypocomplementemia and survival.21,42,43 These data, all obtained from large prospective series of patients, confirmed that complement measurements should be considered as key immunological markers in the follow-up of patients with primary SS (as complement and anti-DNA levels are indicative of systemic lupus erythematosus).
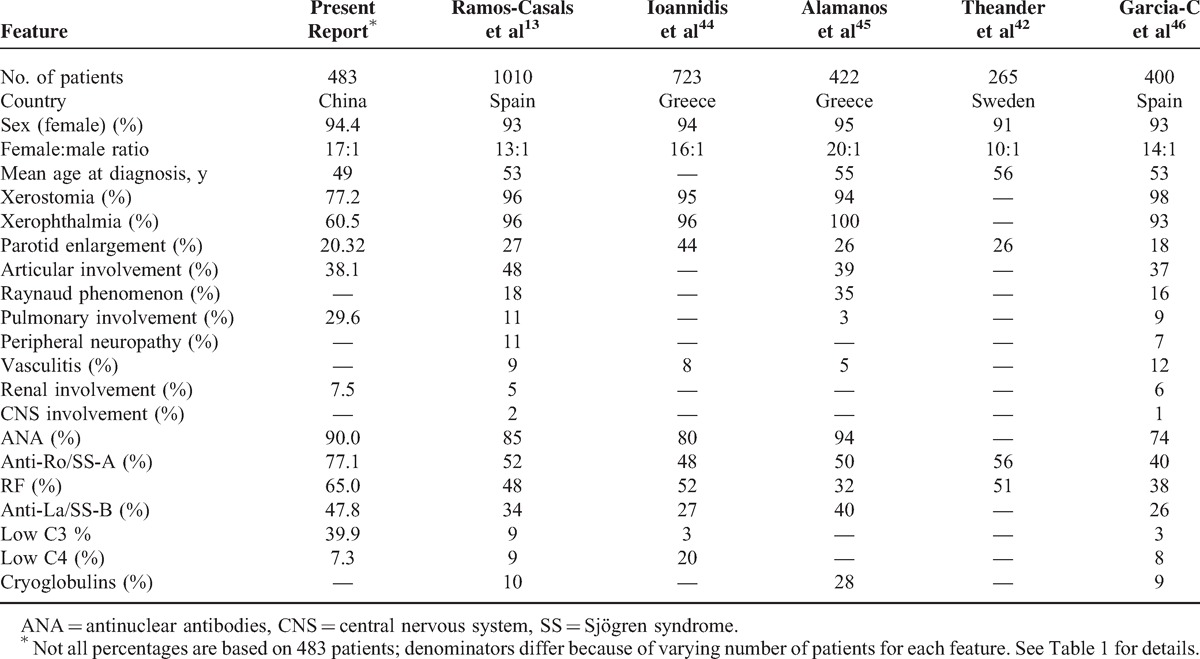
We found some marked differences in the main features of the current cohort of patients compared with those reported in previous studies13,42–46 (Table 7), including a lower prevalence of xerostomia and xerophthalmia, a higher prevalence of pulmonary involvement, a higher frequency of the main autoantibodies (ANA, RF, anti-Ro/La), and the prevalence of low C3 levels. These differences might be affected by patient ethnicity or clinical treatments. The lower frequencies of xerostomia and xerophthalmia in the present cohort compared with other cohorts could also be due to cultural differences and education levels. Indeed, the majority of Chinese people believe that dry mouth and dry eye are not significant problems, which were reflected in another study carried out in China.47 Pulmonary involvement in the present study was higher than in other studies; therefore, it should be included in routine screenings for SS patients in China.
TABLE 7.
Main Epidemiologic, Clinical and Immunological Features in a Large Series of Patients with Primary SS
Conclusively, some new associations were found in the present study. First, we found a high prevalence of saprodontia in Chinese SS patients and maybe a strong suggestion for SS. Second, we found a high prevalence of low C3 levels in Chinese SS patients and should be an important follow-up item. Third, we found that the prevalence of anti-La/SS-B was high in patients with pulmonary involvement, which may predict pulmonary involvement for Chinese SS patients. The broad heterogeneity in the clinical findings of patients with primary SS that we observed in this study shows that our understanding of this systemic autoimmune disease is still evolving and that the different criteria used to diagnose primary SS can lead to different appraisals of the disease. This study had some limitations. Although the study was multicentric, we were unable to accurately represent the entire spectrum of Chinese SS patients. Furthermore, as this was a cross-sectional study, we could not address dynamic changes in the patients’ condition; therefore, we plan on summarizing the clinical information for these patients in a period manner.
Acknowledgments
We would like to thank Guang-liang Shan for statistical consultations.
Footnotes
Abbreviations: ACR = American College of Rheumatology, ANA = antinuclear antibodies, CNS = central nervous system, CT = computed tomography, Ig = immunoglobulin, IgA = immunoglobulin A, IgM = immunoglobulin M, RF = rheumatoid factor, SS = Sjögren's syndrome.
YZ and YL contributed equally to this manuscript.
This work was supported by grants from the Research Special Fund for Public Welfare Industry of Health (201202004 to Feng-chun Zhang), the National Science Technology Pillar Program in the 11th Five-Year Plan (2008BAI59B03 to Feng-chun Zhang), the National Program on Key Research Project of New Drug Innovation (2012ZX09303006-002 to Feng-chun Zhang), the Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-EW-J-8 to J.W.), the CAS/SAFEA International Partnership Program for Creative Research Teams (Y2CX131003 to J.W.) and the National Natural Science Foundation of China (81072486 and 81172857 to Yong-zhe Li and 81101545 to K.Z.).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Malladi AS, Sack KE, Shiboski S, et al. Primary Sjögren's syndrome as a systemic disease: a study of participants enrolled in an international Sjogren's syndrome registry. Arthritis Care Res 2012; 64:911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, et al. Primary Sjögren syndrome. BMJ 2012; 344:e3821. [DOI] [PubMed] [Google Scholar]
- 3.Voulgarelis M, Ziakas PD, Papageorgiou A, et al. Prognosis and outcome of non-Hodgkin lymphoma in primary Sjögren's syndrome. Medicine 2012; 91:1–9. [DOI] [PubMed] [Google Scholar]
- 4.Zhang NZ, Shi CS, Yao QP, et al. Prevalence of primary Sjogren's syndrome in China. J Rheumatol 1995; 22:659–661. [PubMed] [Google Scholar]
- 5.Birlik M, Akar S, Gurler O, et al. Prevalence of primary Sjogren's syndrome in Turkey: a population-based epidemiological study. Int J Clin Pract 2009; 63:954–961. [DOI] [PubMed] [Google Scholar]
- 6.Anagnostopoulos I, Zinzaras E, Alexiou I, et al. The prevalence of rheumatic diseases in central Greece: a population survey. BMC Musculoskelet Disord 2010; 11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniels TE, Aufdemorte TB, Greenspan JS. Talal MH, Kassan SS. Histopathology of Sjogren's syndrome. Sjogren's Syndrome: Clinical and Immunological Aspects. Berlin: Springer; 1987. 266–286. [Google Scholar]
- 8.Brito-Zero’n P, Ramos-Casals M, Bove A, et al. Predicting adverse outcomes in primary Sjogren's syndrome: identification of prognostic factors. Rheumatology 2007; 46:1359–1362. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson R, Bolstad AI, Brokstad KA, et al. Sjogren's syndrome-a plethora of clinical and immunological phenotypes with a complex genetic background. Ann N Y Acad Sci 2007; 1108:433–447. [DOI] [PubMed] [Google Scholar]
- 10.Kassan SS, Moutsopoulos HM. Clinical manifestations and early diagnosis of Sjogren syndrome. Arch Intern Med 2004; 164:1275–1284. [DOI] [PubMed] [Google Scholar]
- 11.Fox R. Sjogren's syndrome. Lancet 2005; 366:321–331. [DOI] [PubMed] [Google Scholar]
- 12.Haga H, Jonsson R. The influence of age on disease manifestations and serological characteristics in primary Sjogren's syndrome. Scand J Rheumatol 1999; 28:227–232. [DOI] [PubMed] [Google Scholar]
- 13.Manuel RC, Roser S, Jose R, et al. Primary Sjogren Syndrome in Spain: Clinical and Immunologic Expression in 1010 Patients. Medicine 2008; 87:210–219. [DOI] [PubMed] [Google Scholar]
- 14.Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 2002; 61:554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yongzhe Li, Kunlin Zhang, Hua Chen, et al. A genome-wide association study in Han Chinese identifies a susceptibility locus for primary Sjögren's syndrome at 7q11.23. Nature Ge Netics 2013; 45:1361–1365. [DOI] [PubMed] [Google Scholar]
- 16.Skopouli FN, Dafni U, Ioannidis JP, et al. Clinical evolution, and morbidity and mortality of primary Sjogren's syndrome. Semin Arthritis Rheum 2000; 29:296–304. [DOI] [PubMed] [Google Scholar]
- 17.Mavragani CP, Moutsopoulos HM. The geoepidemiology of Sjogren's syndrome. Autoimmun Rev 2010; 9:A305–310. [DOI] [PubMed] [Google Scholar]
- 18.Anaya J, Liu G, D'Souza E, et al. Primary Sjogren's syndrome in men. Ann Rheum Dis 1995; 54:748–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Asmussen K, Andersen V, Bendixen G, et al. A new model for classification of disease manifestations in primary Sjogren's syndrome: evaluation in a retrospective long-term study. J Intern Med 1996; 239:475–482. [DOI] [PubMed] [Google Scholar]
- 20.Brennan M, Sankar V, Leakan R, et al. Risk factors for positive minor salivary gland biopsy findings in Sjogren's syndrome and dry mouth patients. Arthritis Rheum 2002; 47:189–195. [DOI] [PubMed] [Google Scholar]
- 21.Cervera R, Font J, Ramos-Casals M, et al. Primary Sjogren's syndrome in men: clinical and immunological characteristics. Lupus 2000; 9:61–64. [DOI] [PubMed] [Google Scholar]
- 22.Drosos A, Tsiakou E, Tsifetaki N, et al. Subgroups of primary Sjogren's syndrome: Sjogren's syndrome in male and paediatric Greek patients. Ann Rheum Dis 1997; 56:333–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Molina R, Provost T, Arnett F, et al. Primary Sjogren's syndrome in men. Clinical, serologic, and immunogenetic features. Am J Med 1986; 80:23–31. [DOI] [PubMed] [Google Scholar]
- 24.Saito T, Sato J, Kondo K, et al. Low prevalence of clinicopathologic and sialographic changes in salivary glands of men with Sjogren's syndrome. J Oral Pathol Med 1999; 28:312–316. [DOI] [PubMed] [Google Scholar]
- 25.Bartunkova J, Sediva A, Vencovsky J, et al. Primary Sjogren's syndrome in children and adolescents: proposal for diagnostic criteria. Clin Exp Rheumatol 1999; 17:381–386. [PubMed] [Google Scholar]
- 26.Farah S, Ronald PR, Frank CA, et al. Association of labial salivary gland histopathology with clinical and serologic features of connective tissue diseases. Arthritis Rheum 1990; 33:1682–1687. [DOI] [PubMed] [Google Scholar]
- 27.Isao I, Sonoko N, Masanori K, et al. Pulmonary Manifestations of Primary Sjogren's Syndrome: a clinical, radiologic, and pathologic study. Am J Respir Crit Care Med 2005; 171:632–638. [DOI] [PubMed] [Google Scholar]
- 28.Shi JH, Liu HR, Xu WB, et al. Pulmonary manifestations of Sjogren's syndrome. Respiration 2009; 78:377–386. [DOI] [PubMed] [Google Scholar]
- 29.Yazisiz V, Arslan G, Ozbudak IH, et al. Lung involvement in patients with primary Sjögren's syndrome: what are the predictors? Rheumatol Int 2010; 30:1317–1324. [DOI] [PubMed] [Google Scholar]
- 30.Vitali C, Bombardieri S, Moutsopoulos HM, et al. Preliminary criteria for the classification of Sjögren's syndrome. Results of a prospective concerted action supported by the European Community. Arthritis Rheum 1993; 36:340–347. [DOI] [PubMed] [Google Scholar]
- 31.Wise C, Woodruff R. Minor salivary gland biopsies in patients investigated for primary Sjogren's syndrome. A review of 187 patients. J Rheumatol 1993; 20:1515–1518. [PubMed] [Google Scholar]
- 32.Shiboski SC, Shiboski CH, Criswell L, et al. New classification criteria for Sjögren's Syndrome: a data-driven expert-clinician consensus approach within the SICCA Cohort. Arthritis Care Res 2012; 64:475–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Routsias JG, Tzioufas AG. Sjögren's syndrome-study of autoantigens and autoantibodies. Clin Rev Allergy Immunol 2007; 32:238–251. [DOI] [PubMed] [Google Scholar]
- 34.Alexander E, Arnett F, Provost T, et al. Sjogren's syndrome:association of anti-Ro(SS-A) antibodies with vasculitis, hematologic abnormalities, and serologic hyperreactivity. Ann Intern Med 1983; 98:155–159. [DOI] [PubMed] [Google Scholar]
- 35.Alexander E, Provost T, Stevens M, et al. Neurologic complications of primary Sjogren's syndrome. Medicine (Baltimore) 1982; 61:247–257. [DOI] [PubMed] [Google Scholar]
- 36.Davidson B, Kelly C, Griffiths I. Primary Sjogren's syndrome in the North East of England: a long-term follow-up study. Rheumatology (Oxford) 1999; 38:245–253. [DOI] [PubMed] [Google Scholar]
- 37.Harley J, Alexander E, Bias W, et al. Anti-Ro (SS-A) and anti-La (SS-B) in patients with Sjogren's syndrome. Arthritis Rheum 1986; 29:196–206. [DOI] [PubMed] [Google Scholar]
- 38.Moutsopoulos H, Zerva L. Anti-Ro (SSA)/La (SSB) antibodies and Sjogren's syndrome. Clin Rheumatol 1990; 9 (1 Suppl 1):123–130. [DOI] [PubMed] [Google Scholar]
- 39.Manoussakis M, Tzioufas A, Pange P, et al. Serological profiles in subgroups of patients with Sjogren's syndrome. Scand J Rheumatol 1986; Suppl61:89–92. [PubMed] [Google Scholar]
- 40.Markusse H, Veldhoven C, Swaak A, et al. The clinical significance of the detection of anti-Ro/SS-A and anti-La/SS-B autoantibodies using purified recombinant proteins in primary Sjogren's syndrome. Rheumatol Int 1993; 13:147–150. [DOI] [PubMed] [Google Scholar]
- 41.Kang K, Kim H, Kwok S, et al. Impact of interleukin-21 in the pathogenesis of primary Sjogren's syndrome: increased serum levels of interleukin-21 and its expression in the labial salivary glands. Arthritis Res Ther 2011; 13:R179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theander E, Manthorpe R, Jacobsson L. Mortality and causes of death in primary Sjogren's syndrome: a prospective cohort study. Arthritis Rheum 2004; 50:1262–1269. [DOI] [PubMed] [Google Scholar]
- 43.Ramos-Casals M, Brito-Zerón P, Yague J, et al. Hypocomplementaemia as an immunological marker of morbidity and mortality in patients with primary Sjogren's syndrome. Rheumatology 2005; 44:89–94. [DOI] [PubMed] [Google Scholar]
- 44.Ioannidis J, Vassiliou V, Moutsopoulos H. Long-term risk of mortality and lymphoproliferative disease and predictive classification of primary Sjogren's syndrome. Arthritis Rheum 2002; 46:741–747. [DOI] [PubMed] [Google Scholar]
- 45.Alamanos Y, Tsifetaki N, Voulgari PV, et al. Epidemiology of primary Sjogren's syndrome in north-west Greece, 1982–2003. Rheumatology (Oxford) 2006; 45:187–191. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Carrasco M, Ramos-Casals M, Rosas J, et al. Primary Sjogren syndrome: clinical and immunologic disease patterns in a cohort of 400 patients. Medicine (Baltimore) 2002; 81:270–280. [DOI] [PubMed] [Google Scholar]
- 47.Yan SM, Zhang W, LI MT, et al. The clinical characteristics of 573 cases of primary sjogren's syndrome. Chin J Rheumatol 2010; 14:223–227. [PubMed] [Google Scholar]


