Abstract
Computed tomography angiography (CTA) detects signs of large-vessel vasculitis (LVV) in about 67.5% of patients with giant-cell arteritis (GCA) at the time of diagnosis and early aortic dilatation in 15%. The outcome of CTA-findings of LVV upon glucocorticoid treatment has not been prospectively evaluated.
The aim of our study was to prospectively assess glucocorticoid-induced changes in CTA findings of LVV in patients with GCA.
Forty biopsy-proven GCA patients evaluated by CTA at diagnosis were prospectively followed and scheduled a new CTA approximately after 1 year of treatment. Vessel wall thickening, diameter, and contrast enhancement of the aorta and its tributaries were evaluated. Results were compared to those obtained at the time of diagnosis.
CTA was repeated to 35 patients after a median follow-up of 13.5 months (IQ25–75% 12.4–15.8). Arterial wall thickening was still present in 17 patients (68% of the patients who initially had LVV). The number of affected segments and wall thickness at various aortic segments significantly decreased and no patients developed new lesions, new aortic dilation or increase in previous dilation. Contrast enhancement disappeared in 15 (93.75%) of 16 patients in whom this finding could be assessed.
Signs of LVV improve with treatment. While contrast enhancement resolves in the majority of patients, vessel wall thickening persists in two thirds. However, the number of affected aortic segments as well as aortic wall thickness significantly decreases. Longer follow-up is necessary to determine the clinical significance of persisting wall thickening and its relationship with relapses or subsequent development of aortic dilatation or large-vessel stenoses.
INTRODUCTION
Giant-cell arteritis (GCA) is a systemic granulomatous vasculitis affecting large- and medium-sized vessels.1–3 Although GCA was initially considered as a vasculitis mainly involving the carotid and vertebral artery branches, prospective imaging studies have recently demonstrated that widespread large-vessel involvement is common.4–13
The prevalence of large-vessel inflammation in newly diagnosed patients has been evaluated in a few prospective studies only, using 18fluoro-deoxyglucose-positron emission tomography (FDG-PET), computed tomography angiography (CTA), or color duplex ultrasonography Frequency ranges from 29% to 74% of patients, depending on the vascular territory explored and the technique employed.4,6,8–12
Inflammatory involvement of the aorta has been evaluated in three sizable prospective imaging studies using FDG-PET or computed tomography, with or without angiography,4,6,7 and ranges from 45% to 65% of patients with GCA at the time of diagnosis. Moreover, retrospective studies have shown that, during follow-up, dilation of the aorta, particularly in the thoracic segment, is significantly more frequent in GCA patients than in the general population.14–17 Furthermore, a cross-sectional systematic screening of 54 patients with GCA demonstrated that aortic dilatation occurred in 12 (22.5%) after a median follow-up of 5.4 years.18 An extended follow-up of this cohort disclosed that 33.3% of patients develop aortic dilatation after a median of 10.3 years.19 To date, the specific mechanisms leading to aortic dilatation in GCA are unclear. It is conceivable and assumed that aortic dilatation is a consequence of previous inflammation but a direct link between initial inflammation and subsequent dilatation has not been established.
It has been hypothesized that aortic dilatation results from persistent remaining subclinical aortic inflammation.20,21 However, surgical or necropsy specimens from aortic aneurysms, usually obtained months or years after diagnosis, not always disclose persistent inflammatory infiltrates or these are residual.18–20 Consequently, early damage of the elastic fibers and muscular layer by inflammation, inefficient vascular repair or remodeling after injury, vascular ageing, and/or hemodynamic factors may also contribute to aneurysm development, regardless of whether or not active inflammation has resolved.3,4,16,22,23
In this study, we prospectively evaluated the outcome of CTA signs of large-vessel inflammation and remodeling in GCA patients after approximately 1 year of glucocorticoid treatment. Specific aims of our survey were (1) to investigate the persistence or resolution of CTA findings suggesting vascular inflammation (wall thickening and contrast enhancement) compared to the initial evaluation,4 (2) to assess the development of signs of structural damage or remodeling (dilation and/or stenosis), and (3) to identify clinical or blood test data associated with resolution or persistence of CTA signs of vascular inflammation.
PATIENTS AND METHODS
Patients
The initial cohort included 40 patients who had been evaluated by CTA at the time of GCA diagnosis in order to detect large-vessel involvement, as part of a prospective study.4 These patients were treated and followed by the investigators according to a defined protocol and tapering schedule18,24–26 and were appointed a new CTA examination between 12–18 months after the initiation of glucocorticoid treatment. Two patients were lost to follow-up (1 patient due to severe dementia and 1 patient because of unknown reasons) and 3 declined a new CTA. Follow-up CTA was completed in the remaining 35 patients.
Clinical and exploratory findings were prospectively recorded at the time of diagnosis, and at 1, 3, 6, 9, 12 month-follow-up and at the time of CTA imaging. The recorded items were: cranial symptoms (headache, scalp tenderness, and jaw claudication), polymyalgia rheumatica, systemic symptoms (weight loss, and fever), disease-related cranial ischemic complications, and presence of extremity claudication. Acute phase reactants including C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), and hemoglobin were also determined at every visit. A combination of clinical and blood test abnormalities was used to evaluate the intensity of the systemic inflammatory response (SIR) as previously reported.18 These included fever >37 °C, weight loss >3 kg, hemoglobin (Hb) <11 g/L, and erythrocyte sedimentation rate (ESR) ≥85 mm/h. Patients with 3 or 4 of these items were considered to have a strong SIR, whereas patients with ≤2 were considered to have a weak SIR.
CTA Protocol
CTA was performed using a multi-slice CT scanner (Somatom Definition Flash, Siemens Medical Solutions, Erlangen, Germany) with the following scanning conditions: collimation 0.6 mm, 120 kV, mAs determined by automatic modulation dose, and reconstruction slice thickness of 5.0 and 1 mm. One hundred milliliter of non-ionic contrast agent (370 mg I/ml) was injected through the ante-cubital vein using a power injector at the rate of 4 ml/s. Early arterial and late venous phases were acquired. We used the bolus-tracking method at the descending aorta level and set 100 Hounsfield units (HU) as the triggering threshold for the arterial phase. The venous phase scan was taken 60 seconds after the arterial phase was completed. Significant enhancement of the aortic wall was considered when an increase of 20 HU or more existed between the arterial and venous phases.
Vascular dilatation or stenosis, as well as vessel wall thickening, were assessed at 4 aortic segments (ascending thoracic aorta, aortic arch, descending thoracic aorta and abdominal aorta) in all patients. Moreover, in 16 patients, the presence of significant wall thickening and appropriate and timely imaging acquisition permitted evaluation of contrast enhancement of the aortic wall at both assessments. As in the first evaluation,4 aortitis was defined as circumferential aortic wall thicknening ≥2 mm observed in zones without adjacent atheroma. In addition, in the present study, contrast enhancement was evaluated, when feasible, in both CTA assessments, in order to distinguish between inflammatory activity (presence of contrast enhancement) and vascular remodeling (absence) (Figure 1).4,27–29 Aortic dilatation was defined by a diameter >4 cm in the ascending aorta, ≥4 cm in the rest of the thoracic aorta, and ≥3 cm in the abdominal aorta. Loss of the physiologically progressive diameter reduction of the aorta was also considered dilatation.18,30,31
FIGURE 1.

CTA of a newly diagnosed patient with GCA showing significant aortic wall thickening (panel A) with contrast enhancement in the late venous phase, sparing the intimal layer (panel B). Persistent aortic wall thickening after 1-year of treatment, devoid of contrast enhancement in the late venous phase (panels C and D).
The aortic tributaries, including brachiocephalic trunk, carotid, subclavian, axillary, splanchnic (celiac and mesenteric), renal, iliac, and femoral arteries, were also evaluated. Radiological findings of large-vessel inflammation were circumferential wall thickness >1 mm and contrast enhancement of the artery wall. Signs of abnormal large-vessel remodeling were arterial dilatation, and the presence of focal stenoses not related to atheroma.4
CTA evaluation was performed by the same radiologists (PA and JGC), blinded to the clinical and laboratory data.
Statistical Analysis
Mann–Whitney U test and Student's t test, for paired or independent data when applicable, were used for quantitative data. Fisher's exact test was applied to contingency tables for qualitative data. Bonferroni correction was applied to multiple comparisons. Calculations were performed with the IBM SPSS Statistics (Version 20.0, Armonk, NY).
RESULTS
Clinical and Laboratory Findings
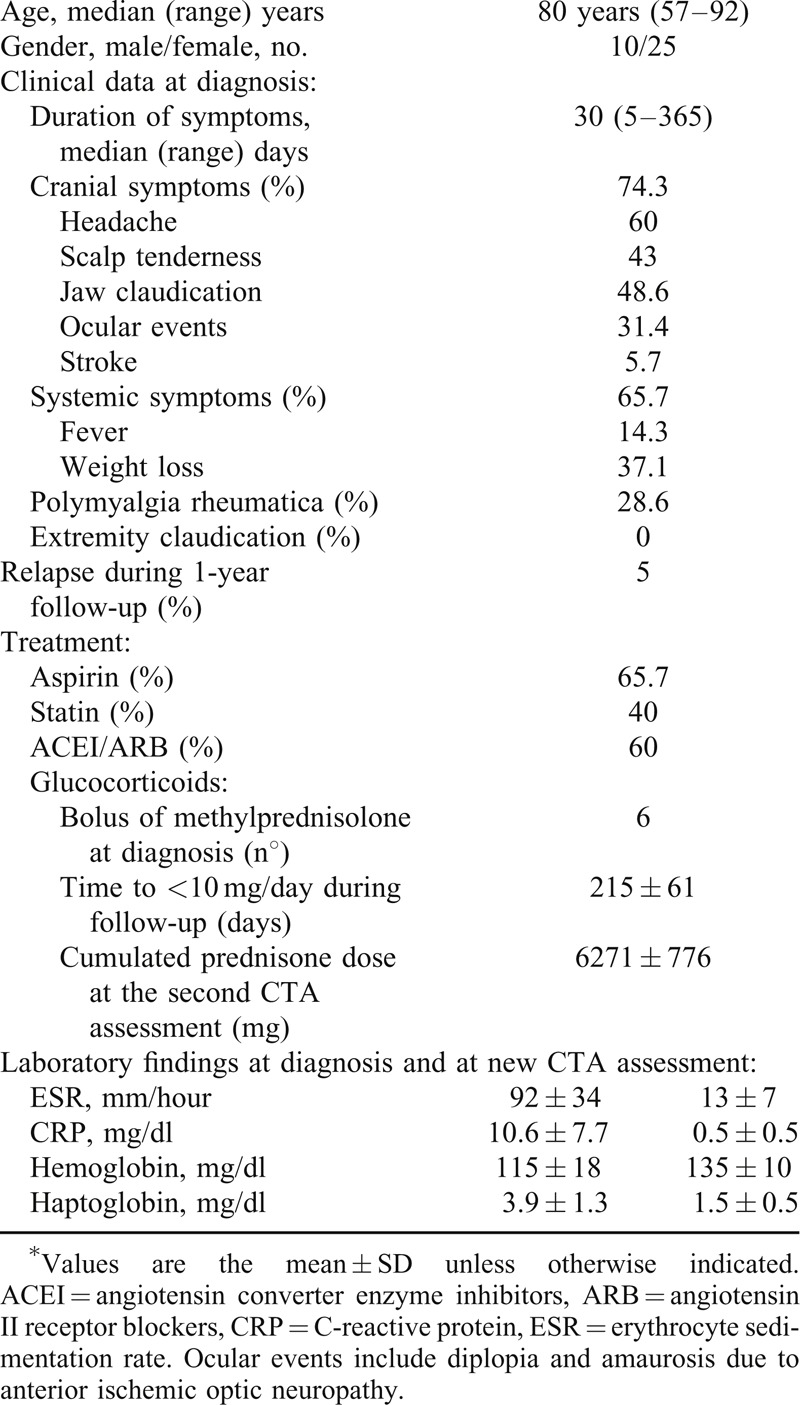
Of the 35 patients finally included, 25 were women and 10 men with a mean age of 79 ± 6 years (mean ± SD). Median follow-up time between both radiologic evaluations was 13.5 months (IQ25–75% 12.4–15.8).
Although two patients relapsed during the study period, at the time of the second CTA, all patients were in clinical remission and receiving glucocorticoid treatment, with a median prednisone dose of 5 mg/day (ranging from 10 mg/day, due to a recent relapse, to 2.5 mg/day). No specific symptoms or signs of large-vessel stenosis (bruits, asymmetry in arm blood pressure or extremity claudication) were referred or detected. The main clinical, laboratory and treatment data of the study cohort are summarized in Table 1.
TABLE 1.
Clinical and Biochemical Findings at Diagnosis and Treatment Data of the Investigated GCA Cohort of 35 Patients
Prevalence and Topography of Large-Vessel Involvement After 1-Year Follow-Up
Nineteen out of the 35 patients (54.3%) had abnormal CTA findings. Radiological signs suggesting LVV (wall thickening) were still seen in 17 patients (48.5%) and aortic dilation in 5 (14.3%). As in the first evaluation, the descending thoracic aorta and the aortic arch were the most affected segments: aortic thickening was observed in 13 (37.1%) of patients at the descending thoracic aorta and in 11 (31.4%) of patients at the aortic arch, followed by the abdominal aorta and the ascending aorta, that were involved in 9 (25.7%) and in 4 (11.4%) of patients, respectively. Furthermore, CTA-defined aortic branch inflammation was still detected in 12 (34.2%) patients. Detailed frequencies of radiological LVV at the different vascular segments evaluated and its distribution in the aorta at both evaluation times are summarized in Table 2 and Figure 2, respectively. Topography of the aortic segments with persistence, reduction or resolved radiological inflammation is depicted in Figure 2.
TABLE 2.
Topography of Inflammatory Large-Vessel Involvement in the Study Cohort at the Time of Diagnosis and at the Follow-Up Evaluation
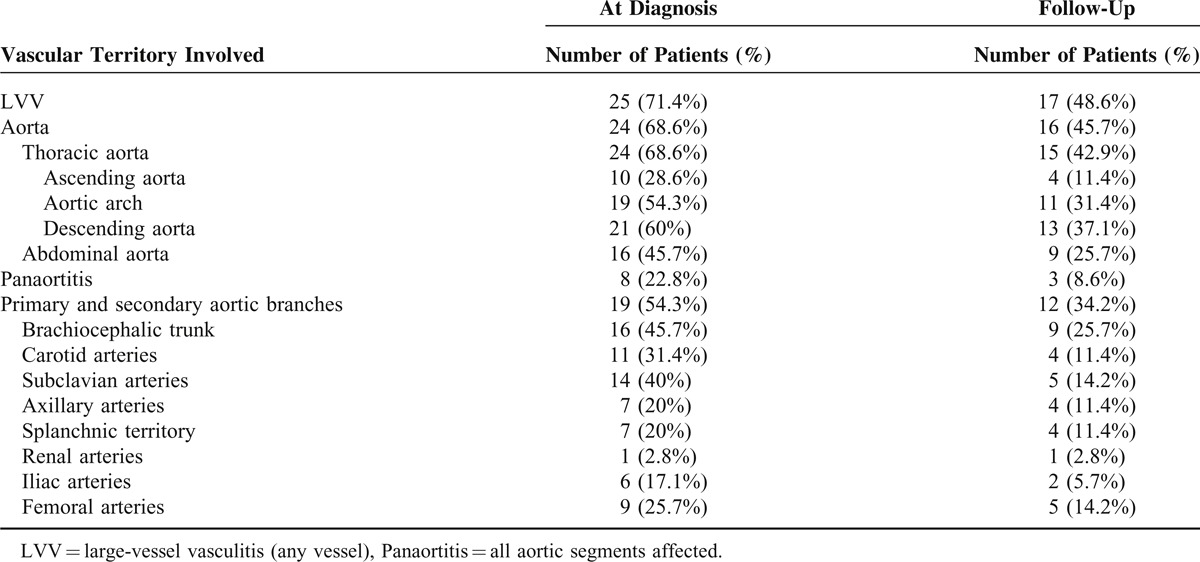
FIGURE 2.
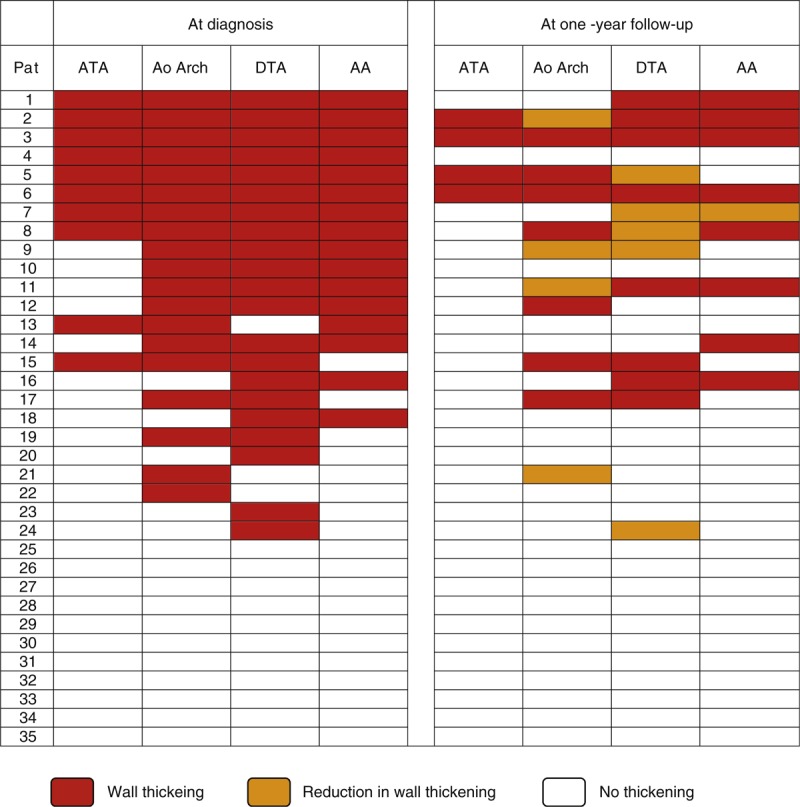
Distribution of the aortic wall thickening in the GCA cohort at both CTA assessments. ATA = ascending thoracic aorta; DTA = descending thoracic aorta; AA = abdominal aorta; Ao = aortic.
When comparing both evaluations, initial and present, vessel wall thickening was still present in 17 (68%) of the 25 patients who initially had this finding. A significant reduction in the mean aortic wall thickness was detected in all aortic segments (Table 3). Moreover, the number of vascular segments affected substantially decreased and none of the 35 patients developed new inflammatory lesions in previously unaffected areas (Figure 2). Post-hoc evaluation of the 16 patients in whom contrast enhancement assessment was feasible, disclosed that all patients but 1 (93.75%) had contrast enhancement of the aortic wall as a sign of active vasculitis27–29 in the first CTA evaluation performed at the time of GCA diagnosis. Conversely, at the second assessment, contrast enhancement was evident in the aortic wall in only one (6.25%) of the 16 evaluable patients (see Figure 3).
TABLE 3.
Aortic Wall Thickness at Diagnosis and at the follow-Up Assessment

FIGURE 3.
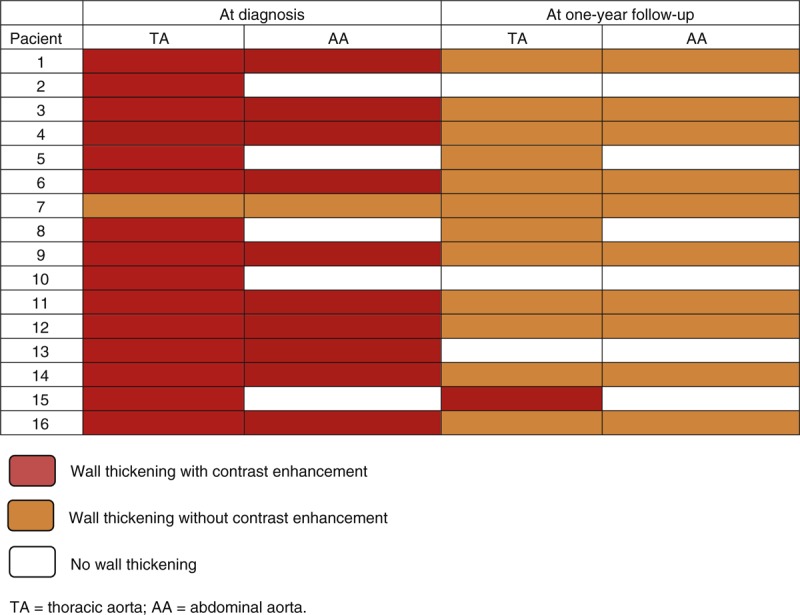
Distribution of the contrast enhancement in the followed GCA cohort at both radiologic assessments. TA = thoracic aorta; AA = abdominal aorta.
There were no significant differences in aortic diameters when comparing both evaluations. No new aortic dilatation was detected and, moreover, dilatation detected at the ascending thoracic aorta in 5 patients at the time of GCA diagnosis, remained stable. At the first assessment, only one patient showed a vascular stenosis, concomitant with wall thickening, located in the left renal artery. At the second assessment, these remained stable and another patient developed a new vascular stenosis in a segment located at the inferior mesenteric artery with no previously apparent CTA inflammatory signs.
Clinical, Laboratory and Treatment Features Associated With Persistence or Resolution of CTA Signs of Large-Vessel Inflammation
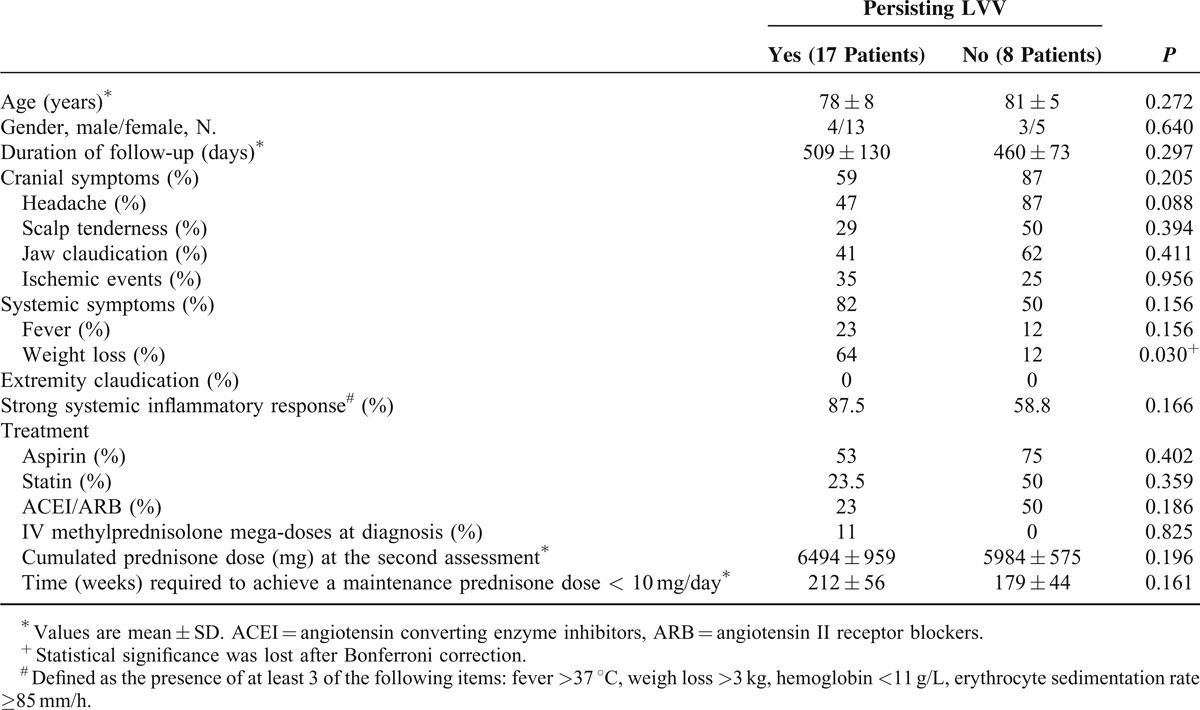
Twenty-five out of the 35 patients who completed both evaluations had signs of LVV at the time of diagnosis. Of those, 17 had persisting signs of LVV at follow-up whereas vessel wall thickening had resolved in the remaining 8. The time lapse between both CTA assessments was similar in patients with or without persisting LVV at follow-up. No significant differences in age, gender or clinical symptoms were found between both groups (Table 4). Only 2 patients suffered from relapse, 1 in each group.
TABLE 4.
Clinical Features at Baseline and Treatments Received by Patients With Persisting or Resolved Large-Vessel Thickening at the Second CTA Evaluation
Acute phase reactants at the specified time-points during follow-up in patients with or without persisting signs of LVV at the second CTA are shown in Figure 4. Only hemoglobin was significantly lower at the first follow-up visits in patients with persisting vessel wall thickening. When comparing acute phase reactants in patients in whom wall thickening persisted unchanged versus those who experienced improvement, only ESR was significantly higher in patients with unmodified wall thickening. No relationship was found between the presence of a strong or weak SIR at diagnosis and the outcome of the imaging abnormalities during follow-up (Table 4).
FIGURE 4.
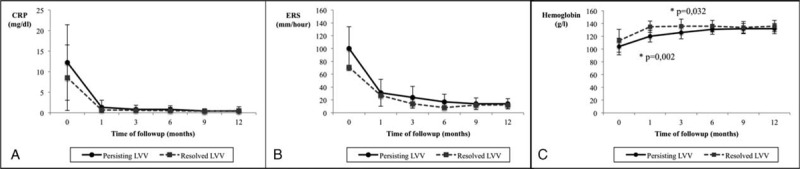
Acute phase reactants at scheduled time points during 1-year follow-up of patients with or without persisting LVV at the second assessment.
Data regarding patients’ therapy are summarized in Table 4 and Figure 5. There were no significant differences in the percentage of patients who had received IV methyl-prednisolone megadoses at diagnosis because of cranial ischemic manifestations, or other medications including aspirin, statins, or angiotensin-converting enzyme inhibitors /angiotensin II receptor blockers between both groups. There were no differences either in the time required to achieve a maintenance prednisone dose below 10 mg/day, in the cumulated prednisone dose at the second imaging between patients with persisting or resolved vessel wall thickening or in the prednisone doses at the different follow-up visits.
FIGURE 5.
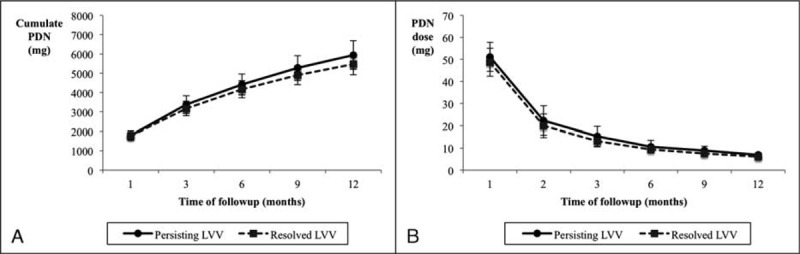
Cumulated prednisone dose (A) and daily prednisone dose (B) at scheduled time points during follow-up in patients with persisting or resolved LVV.
DISCUSSION
Recent prospective studies have revealed an elevated prevalence of inflammation of the aorta and its primary and secondary branches in GCA.4–13 However, the clinical impact of large-vessel inflammation detected at diagnosis on patient's outcome is unclear. Population-based studies have demonstrated an increased incidence of thoracic aortic aneurysm in GCA.14,15,17,32 However, a direct link between aortic inflammation and later development of aortic dilatation seems reasonable but it has not been clearly established. Male gender, smoking habit, and hypertension have been associated with higher risk of aortic aneurysm in patients with GCA in some studies.17,18,33 Blockmans et al demonstrated, indeed, larger aortic diameters detected by computed tomography (CT) in a heterogeneous cohort of GCA patients who had increased aortic FDG uptake in a PET performed 1–110 months before.34 Moreover, although there are reports of symptomatic stenoses of primary and secondary branches of the aorta leading to extremity claudication,35,36 mesenteric ischemia,37,38 myocardial infarction,39 or ischemic stroke,40,41 the percentage of patients who develop large-vessel stenosis is unknown and the relationship between stenosis and previous inflammatory involvement has not been demonstrated. It is estimated that between 5% and 15% of patients have signs or symptoms derived from large-vessel stenosis at diagnosis or during follow-up.32,35,42,43 However, since studies addressing this point are retrospective and not all patients were imaged, it is difficult to distinguish between signs and symptoms derived form atherosclerotic vascular disease, which is not unusual in the age group targeted by GCA, from those derived from vasculitic involvement.
To date, prospective longitudinal studies in unselected cohorts of GCA patients in order to define the outcome of large-vessel inflammation have not been reported. This is the first study prospectively evaluating the early outcome of CTA defined large-vessel vasculitis detected at the time of diagnosis in a cohort of patients with biopsy-proven GCA subjected to a standardized glucocorticoid treatment in accordance with international recommendations.44 We evaluated both the outcome of vessel wall thickening suggestive of vasculitis as well as changes in the vessel diameter indicating dilatation or stenosis after approximately 1 year of follow-up.
Unexpectedly, given the complete clinical response to treatment in the majority of patients, persistent vessel wall thickening was found in 48.5% of patients, two-third of the patients who had signs of LVV at the time of diagnosis. However, vessel wall thickening as well as the number of affected vessels significantly decreased and there was no new involvement of previously spared segments, indicating, to a certain extent, a substantial response of large-vessel inflammation to glucocorticoid therapy. Moreover, it has been repeatedly remarked that the capacity of imaging techniques to detect signs of large-vessel inflammation quickly decreases after gluococorticoid treatment.45–48 The significance of persistent vessel wall thickening during glucocorticoid treatment is unclear. It may represent persistent subclinical, pauci-symptomatic vascular inflammation, refractory to maintenance glucocorticoid therapy, which would strongly underline the need for more efficient therapies able to abrogate silent large-vessel inflammation.49 However, it is noteworthy that patients were in clinical remission with normal or close to normal acute phase reactants at the time of the second assessment. Moreover, no major differences in serum concentrations of acute phase reactants over time was observed between patients with resolved large-vessel involvement and those with persisting vascular thickening. Therefore, major persistence of inflammatory activity was unlikely.
Second temporal artery biopsies have been performed to some patients with GCA several months or years after the initiation of glucocorticosteroid treatment for a variety of reasons: including confirmation of a clinically based diagnosis, necropsy, or as part of an ancillary study in the context of an international multicentre clinical trial.50–53 In these specimens, scattered small foci of inflammatory cells persist but the more striking finding consists of prominent medial fibrosis and vascular wall remodeling. It is likely that persistent vascular wall thickening primarily represents fibrosis and vascular remodeling rather than major persistence of active inflammation. This is supported by the observation of contrast enhancement in only one out of 16 patients in whom this finding could be assessed. Concomitant PET scan may have been useful to differentiate active inflammation and scarring in patients with persisting vascular thickening as recently suggested for patients with cardiac involvement by eosinophilic granulomatosis with polyangiitis.12,54
No changes in aortic diameter indicative of dilatation were observed when comparing the initial and the follow-up assessments. This observation supports the concept that aortic dilatation is a delayed complication as suggested by retrospective, population-based studies14,15,17,32 and a cross-sectional study with its long-term follow-up.18,19
Regarding to vascular stenosis, only 1 patient developed reduction in the inferior mesenteric artery diameter and no patients had clinical signs or symptoms related to vascular stenosis in the second assessment indicating that the development of stenosis in the primary or secondary branches of the aorta is infrequent in the early outcome of unselected patients with GCA. Given the imaging modality used (CTA), conclusions about stenosis in more distal territories such as infrapopliteal arteries, which may be involved by GCA,3,9,55–58 cannot be drawn. These data underlines important differences with Takayasu disease. Based on the observation that GCA frequently targets large vessels, it has been recently proposed that GCA and Takayasu disease may be the edges of the spectrum of a single disorder and that clinico-pathological differences may be related to ageing of the immune and/or vascular system.59 Although in the absence of an identified etiology discussing whether or not Takayasu and GCA are different conditions or the same disease may be inconclusive, our observations support that there are important differences in vascular remodelling between both conditions. While patients with Takayasu disease frequently and typically develop vascular stenoses, sometimes in spite of immunosuppressive therapy, vascular lumen reduction is unusual in unselected patients with GCA.
Our study has the strengths of its prospective nature and careful imaging analysis of the vascular territories accessible to CTA. It has also some limitations including the relatively small size of the patient cohort and the short-term follow-up. Extended studies are necessary to delimitate the long-term outcome and clinical impact of large-vessel inflammation in GCA.
Footnotes
Abbreviations: AA = abdominal aorta, ACEI = angiotensin converting enzyme inhibitors, Ao = aortic, ARB = angiotensin II receptor blockers, ATA = ascending thoracic aorta, CRP = C-reactive protein, CTA = computed tomography angiography, DTA = descending thoracic aorta, ESR = erythrocyte sedimentation rate, FDG-PET = fluoro-deoxyglucose-possitron emission tomography, Hb = hemoglobin, HU = Hounsfield units, IQ = interquartile, LVV = large-vessel vasculitis, SD = standard deviation, SIR = systemic inflammatory response, TA = thoracic aorta.
Pedro Arguis and Maria C. Cid share senior authorship.
Preliminary results partially presented at the European Congress of Rheumatology in Berlin, June 2012, the Scientific Meeting of the American College of Rheumatology, Washington, November 2012, the Radiological Society of North America Annual Meeting, Chicago, December 2013, and at the European Congress of Rheumatology in Paris, June 2014.
Contributorship: Dr. Cid had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study design. Prieto-González, Arguis, García-Martínez, Cid.
Acquisition of data. Prieto-González, Arguis, Gutierrez-Chacoff, García-Martínez, Espígol-Frigolé, Tavera-Bahillo, Murgia, Alba, Hernández-Rodríguez, Cid.
Analysis and interpretation of data. Prieto-González, Arguis, Espígol-Frigolé, Gutiérrez-Chacoff, Hernández-Rodríguez, Cid.
Manuscript preparation. Prieto-González, García-Martínez, Hernández-Rodríguez, Cid.
Statistical analysis. Prieto-González, Alba, Cid.
Funding info: Supported by Ministerio de Economía y Competitividad (SAF 11/30073). Dr Itziar Tavera-Bahillo was supported by a research award from Hospital Clínic. Dr MA Alba was supported by Conacyt (Mexico) and AGAUR (Generalitat de Catalunya). Dr G Espígol-Frigolé was supported by Instituto de Salud Carlos III.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Cid MC, Font C, Coll-Vinent B, Grau JM. Large vessel vasculitides. Curr Opin Rheumatol 1998; 10:18–28. [DOI] [PubMed] [Google Scholar]
- 2.Salvarani C, Pipitone N, Versari A, Hunder GG. Clinical features of polymyalgia rheumatica and giant cell arteritis. Nat Rev Rheumatol 2012; 8:509–521. [DOI] [PubMed] [Google Scholar]
- 3.Cid MC, Prieto-Gonzalez S, Arguis P, et al. The spectrum of vascular involvement in giant-cell arteritis: clinical consequences of detrimental vascular remodelling at different sites. APMIS Suppl 2009; 10–20. [DOI] [PubMed] [Google Scholar]
- 4.Prieto-Gonzalez S, Arguis P, Garcia-Martinez A, et al. Large vessel involvement in biopsy-proven giant cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis 2012; 71:1170–1176. [DOI] [PubMed] [Google Scholar]
- 5.Prieto-Gonzalez S, Depetris M, Garcia-Martinez A, et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Ann Rheum Dis 2014; 73:1388–1392. [DOI] [PubMed] [Google Scholar]
- 6.Blockmans D, de Ceuninck L, Vanderschueren S, et al. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum 2006; 55:131–137. [DOI] [PubMed] [Google Scholar]
- 7.Agard C, Barrier JH, Dupas B, et al. Aortic involvement in recent-onset giant cell (temporal) arteritis: a case-control prospective study using helical aortic computed tomodensitometric scan. Arthritis Rheum 2008; 59:670–676. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt WA, Seifert A, Gromnica-Ihle E, et al. Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology (Oxford) 2008; 47 1:96–101. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt WA, Natusch A, Moller DE, et al. Involvement of peripheral arteries in giant cell arteritis: a color Doppler sonography study. Clin Exp Rheumatol 2002; 20:309–318. [PubMed] [Google Scholar]
- 10.Aschwanden M, Kesten F, Stern M, et al. Vascular involvement in patients with giant cell arteritis determined by duplex sonography of 2x11 arterial regions. Ann Rheum Dis 2010; 69:1356–1359. [DOI] [PubMed] [Google Scholar]
- 11.Ghinoi A, Pipitone N, Nicolini A, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology (Oxford) 2012; 51:730–734. [DOI] [PubMed] [Google Scholar]
- 12.Prieto-Gonzalez S, Arguis P, Cid MC. Imaging in vasculitis. Curr Opin Rheumatol 2015; 27:53–62. [DOI] [PubMed] [Google Scholar]
- 13.Espigol-Frigole G, Prieto-Gonzalez S, Alba MA, et al. Advances in the diagnosis of large vessel vasculitis. Rheum Dis Clin North Am 2015; 41:125–140. [DOI] [PubMed] [Google Scholar]
- 14.Evans JM, O’Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med 1995; 122:502–507. [DOI] [PubMed] [Google Scholar]
- 15.Kermani TA, Warrington KJ, Crowson CS, et al. Large-vessel involvement in giant cell arteritis: a population-based cohort study of the incidence-trends and prognosis. Ann Rheum Dis 2013; 72:1989–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackie SL, Hensor EM, Morgan AW, Pease CT. Should I send my patient with previous giant cell arteritis for imaging of the thoracic aorta? A systematic literature review and meta-analysis. Ann Rheum Dis 2014; 73:143–148. [DOI] [PubMed] [Google Scholar]
- 17.Robson JC, Kiran A, Maskell J, et al. The relative risk of aortic aneurysm in patients with giant cell arteritis compared with the general population of the UK. Ann Rheum Dis 2013; 10.1136/annrheumdis-2013-204113[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Martinez A, Hernandez-Rodriguez J, Arguis P, et al. Development of aortic aneurysm/dilatation during the followup of patients with giant cell arteritis: a cross-sectional screening of fifty-four prospectively followed patients. Arthritis Rheum 2008; 59:422–430. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Martinez A, Arguis P, Prieto-Gonzalez S, et al. Prospective long term follow-up of a cohort of patients with giant cell arteritis screened for aortic structural damage (aneurysm or dilatation). Ann Rheum Dis 2014; 73:1826–1832. [DOI] [PubMed] [Google Scholar]
- 20.Evans JM, Bowles CA, Bjornsson J, et al. Thoracic aortic aneurysm and rupture in giant cell arteritis. A descriptive study of 41 cases. Arthritis Rheum 1994; 37:1539–1547. [DOI] [PubMed] [Google Scholar]
- 21.Bongartz T, Matteson EL. Large-vessel involvement in giant cell arteritis. Curr Opin Rheumatol 2006; 18:10–17. [DOI] [PubMed] [Google Scholar]
- 22.Petursdottir V, Nordborg E, Nordborg C. Atrophy of the aortic media in giant cell arteritis. APMIS 1996; 104:191–198. [DOI] [PubMed] [Google Scholar]
- 23.Segarra M, Garcia-Martinez A, Sanchez M, et al. Gelatinase expression and proteolytic activity in giant-cell arteritis. Ann Rheum Dis 2007; 66:1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alba MA, Espígol-Frigolé G, Butjosa M, et al. Treatment of large-vessel vasculitis. Curr Immunol Rev 2011; 7:435–442. [Google Scholar]
- 25.Alba MA, Garcia-Martinez A, Prieto-Gonzalez S, et al. Treatment with angiotensin II receptor blockers is associated with prolonged relapse-free survival, lower relapse rate, and corticosteroid-sparing effect in patients with giant cell arteritis. Semin Arthritis Rheum 2014; 43:772–777. [DOI] [PubMed] [Google Scholar]
- 26.Alba MA, Garcia-Martinez A, Prieto-Gonzalez S, et al. Relapses in patients with giant cell arteritis: prevalence, characteristics, and associated clinical findings in a longitudinally followed cohort of 106 patients. Medicine (Baltimore) 2014; 93:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Restrepo CS, Ocazionez D, Suri R, Vargas D. Aortitis: imaging spectrum of the infectious and inflammatory conditions of the aorta. Radiographics 2011; 31:435–451. [DOI] [PubMed] [Google Scholar]
- 28.Zhu FP, Luo S, Wang ZJ, et al. Takayasu arteritis: imaging spectrum at multidetector CT angiography. Br J Radiol 2012; 85:e1282–e1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim SY, Park JH, Chung JW, et al. Follow-up CT evaluation of the mural changes in active Takayasu arteritis. Korean J Radiol 2007; 8:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aronberg DJ, Glazer HS, Madsen K, Sagel SS. Normal thoracic aortic diameters by computed tomography. J Comput Assist Tomogr 1984; 8:247–250. [PubMed] [Google Scholar]
- 31.Lu TL, Rizzo E, Marques-Vidal PM, et al. Variability of ascending aorta diameter measurements as assessed with electrocardiography-gated multidetector computerized tomography and computer assisted diagnosis software. Interact Cardiovasc Thorac Surg 2010; 10:217–221. [DOI] [PubMed] [Google Scholar]
- 32.Nuenninghoff DM, Hunder GG, Christianson TJ, et al. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum 2003; 48:3522–3531. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Gay MA, Garcia-Porrua C, Pineiro A, et al. Aortic aneurysm and dissection in patients with biopsy-proven giant cell arteritis from northwestern Spain: a population-based study. Medicine (Baltimore) 2004; 83:335–341. [DOI] [PubMed] [Google Scholar]
- 34.Blockmans D, Coudyzer W, Vanderschueren S, et al. Relationship between fluorodeoxyglucose uptake in the large vessels and late aortic diameter in giant cell arteritis. Rheumatology (Oxford) 2008; 47:1179–1184. [DOI] [PubMed] [Google Scholar]
- 35.Kermani TA, Matteson EL, Hunder GG, Warrington KJ. Symptomatic lower extremity vasculitis in giant cell arteritis: a case series. J Rheumatol 2009; 36:2277–2283. [DOI] [PubMed] [Google Scholar]
- 36.Czihal M, Piller A, Schroettle A, et al. Outcome of giant cell arteritis of the arm arteries managed with medical treatment alone: cross-sectional follow-up study. Rheumatology (Oxford) 2013; 52:282–286. [DOI] [PubMed] [Google Scholar]
- 37.Sujobert P, Fardet L, Marie I, et al. Mesenteric ischemia in giant cell arteritis: 6 cases and a systematic review. J Rheumatol 2007; 34:1727–1732. [PubMed] [Google Scholar]
- 38.Scola CJ, Li C, Upchurch KS. Mesenteric involvement in giant cell arteritis. An underrecognized complication? Analysis of a case series with clinicoanatomic correlation. Medicine (Baltimore) 2008; 87:45–51. [DOI] [PubMed] [Google Scholar]
- 39.Grant S. Giant cell arteritis, scalp necrosis and myocardial infarction. Intern Med J 2004; 34:138–139. [DOI] [PubMed] [Google Scholar]
- 40.Samson M, Jacquin A, Audia S, et al. Stroke associated with giant cell arteritis: a population-based study. J Neurol Neurosur Psychiatry 2014; 10.1136/jnnp-2014-307614[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 41.Zenone T, Puget M. Characteristics of cerebrovascular accidents at time of diagnosis in a series of 98 patients with giant cell arteritis. Rheumatol Int 2013; 33:3017–3023. [DOI] [PubMed] [Google Scholar]
- 42.Kermani TA, Warrington KJ. Lower extremity vasculitis in polymyalgia rheumatica and giant cell arteritis. Curr Opin Rheumatol 2011; 23:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein RG, Hunder GG, Stanson AW, Sheps SG. Large artery involvement in giant cell (temporal) arteritis. Ann Intern Med 1975; 83:806–812. [DOI] [PubMed] [Google Scholar]
- 44.Mukhtyar C, Guillevin L, Cid MC, et al. EULAR recommendations for the management of large vessel vasculitis. Ann Rheum Dis Mar 2009; 68:318–323. [DOI] [PubMed] [Google Scholar]
- 45.Hauenstein C, Reinhard M, Geiger J, et al. Effects of early corticosteroid treatment on magnetic resonance imaging and ultrasonography findings in giant cell arteritis. Rheumatology (Oxford) 2012; 51:1999–2003. [DOI] [PubMed] [Google Scholar]
- 46.Fuchs M, Briel M, Daikeler T, et al. The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis. Eur J Nucl Med Mol Imaging 2012; 39:344–353. [DOI] [PubMed] [Google Scholar]
- 47.Prieto-Gonzalez S, Garcia-Martinez A, Arguis P, Cid MC. Early improvement of radiological signs of large-vessel inflammation in giant cell arteritis upon glucocorticoid treatment. Rheumatology (Oxford) 2013; 52:1335–1336. [DOI] [PubMed] [Google Scholar]
- 48.Santoro L, D’Onofrio F, Bernardi S, et al. Temporal ultrasonography findings in temporal arteritis: early disappearance of halo sign after only 2 days of steroid treatment. Rheumatology (Oxford) 2013; 52:622. [DOI] [PubMed] [Google Scholar]
- 49.Schafer VS, Zwerina J. Biologic treatment of large-vessel vasculitides. Curr Opin Rheumatol 2012; 24:31–37. [DOI] [PubMed] [Google Scholar]
- 50.Fauchald P, Rygvold O, Oystese B. Temporal arteritis and polymyalgia rheumatica. Clinical and biopsy findings. Ann Intern Med 1972; 77:845–852. [DOI] [PubMed] [Google Scholar]
- 51.Achkar AA, Lie JT, Hunder GG, et al. How does previous corticosteroid treatment affect the biopsy findings in giant cell (temporal) arteritis? Ann Intern Med 1994; 120:987–992. [DOI] [PubMed] [Google Scholar]
- 52.Visvanathan S, Rahman MU, Hoffman GS, et al. Tissue and serum markers of inflammation during the follow-up of patients with giant-cell arteritis—a prospective longitudinal study. Rheumatology (Oxford) 2011; 50:2061–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffman GS, Cid MC, Rendt-Zagar KE, et al. Infliximab for maintenance of glucocorticosteroid-induced remission of giant cell arteritis: a randomized trial. Ann Intern Med 2007; 146:621–630. [DOI] [PubMed] [Google Scholar]
- 54.Marmursztejn J, Guillevin L, Trebossen R, et al. Churg-Strauss syndrome cardiac involvement evaluated by cardiac magnetic resonance imaging and positron-emission tomography: a prospective study on 20 patients. Rheumatology (Oxford) 2013; 52:642–650. [DOI] [PubMed] [Google Scholar]
- 55.Assie C, Janvresse A, Plissonnier D, et al. Long-term follow-up of upper and lower extremity vasculitis related to giant cell arteritis: a series of 36 patients. Medicine (Baltimore) 2011; 90:40–51. [DOI] [PubMed] [Google Scholar]
- 56.Tato F, Hoffmann U. Clinical presentation and vascular imaging in giant cell arteritis of the femoropopliteal and tibioperoneal arteries. Analysis of four cases. J Vasc Surg 2006; 44:176–182. [DOI] [PubMed] [Google Scholar]
- 57.McCain GA, Thompson JM. Isolated giant cell arteritis. J Rheumatol 1978; 5:474–476. [PubMed] [Google Scholar]
- 58.Cid MC, Cervera R, Font J, et al. Recurrent arterial thrombosis in a patient with giant-cell arteritis and raised anticardiolipin antibody levels. Br J Rheumatol 1988; 27:164–166. [DOI] [PubMed] [Google Scholar]
- 59.Maksimowicz-McKinnon K, Clark TM, Hoffman GS. Takayasu arteritis and giant cell arteritis: a spectrum within the same disease? Medicine (Baltimore) 2009; 88:221–226. [DOI] [PubMed] [Google Scholar]


