Abstract
Fibrosing cholestatic hepatitis (FCH) is an uncommon complication of renal transplantation and usually associated with hepatitis B and C viral infection. Without treatment, the prognosis is usually fatal within weeks of onset. There was rarely report with successful treatment intervention.
This case report describes a uremic patient with HCV infection who developed a fatal form of FCH after kidney transplantation. This is the first reported successful case with allogeneic hematopoietic stem cell transplantation (AHSCT) without ablative conditioning.
A dramatic virologic and clinical improvement was observed in this post-transplantation patient. But no adverse events related to AHSCT were observed. The patient returned to work full-time at 10 months of hospitalization and is still in good health by now. Serum HCV RNA gradually decreased from 2.5 × 106 Copies/mL at day 1 to 3.2 × 104 Copies/mL at day 98 and became negative (<400 Copies/mL) at day 126 of hospitalization and remains negative at the last available assessment.
Our report suggests that allogeneic HSCT may have a therapeutic role in FCH.
INTRODUCTION
Fibrosing cholestatic hepatitis (FCH) characterized by cholestasis leading to rapidly progressive hepatic failure is an aggressive and usually fatal form of viral hepatitis in immunocompromised patients, the characteristic histopathological features of which include: periportal fibrosis, ballooning degeneration of hepatocytes, cholestasis, with minimal inflammation.1 Liver injury in FCH is mediated via the direct viral cytopathic effects of virus antigen over-expression in hepatocytes.2 FCH has been reported almost exclusively in heavily immunosuppressed organ transplant recipients or subjects with human immunodeficiency virus (HIV) infection and to be usually associated with hepatitis B virus (HBV) and hepatitis C virus (HCV)3 and cytomegalovirus (CMV)4 infection. It has occurred mostly in patients after liver transplantation; some cases are also described as an uncommon complication of renal transplantation or bone marrow transplant.5–7 These findings may be a direct effect of immunosuppressive agents on the virus as well as a reduced immune response to virus. Without treatment, this condition is usually fatal within weeks of onset. The prognosis is very poor. There were a few reports with successful treatment intervention including lamivudine and adefovir dipivoxil.8,9 Allogeneic hematopoietic stem cell transplantation (AHSCT) without ablative conditioning may control host autoimmunity and induce transplantation tolerance in the host to the graft to prevent rejection of donor cells.10–13 Hematopoietic stem cells (HSCs) may potentially aid tissue repair and contribute to restoration of damaged hepatocytes.14–17 This case report describes a successful treatment of a uremic patient with previously stable chronic hepatitis C who developed FCH after kidney transplantation with AHSCT.
CASE REPORT
A 60-year-old male patient was admitted to the hospital with progressive deterioration, jaundice, and abdominal distension. He had undergone renal transplantation for renal failure 2 months earlier. He had suffered the chronic glomerulonephritis for 10 years and had to accept peritoneal dialysis for 1 year because of uremia prior to renal transplantation. During pre-transplant period and at the time of transplant his liver functions were normal and his serum was positive for anti-HCV antibody but negative for hepatitis B surface antigen (HBsAg), hepatitis B e antigen (HBeAg), HBV DNA, HIV RNA. Immunosuppressive therapy after transplantation included tacrolimus (6–8 mg/day), mycophenolate mofetil (1 g/day), and meprednisone (16 mg/day). In December 2007, he presented with yellowish discoloration of eyes and urine with decreased appetite for 3 weeks prior to admission. On examination he was afebrile, icteric, and had 2 cm nontender hepatomegaly with smooth surface and regular margins. There was no ascites clinically. At day 1 of hospitalization, his investigations revealed serum creatinine 105 μmol/L; total bilirubin 30 μmol/L with direct bilirubin of 12.8 μmol/L; aspartate aminotransferase (AST) 418 IU (N: up to 50 IU); alanine aminotransferase (ALT) 998 IU (N: up to 50 IU); gamma-glutamyl transferase (GGT) 698 U/L, albumin (ALB) 48 g/L; HBsAg negative; immunoglobulin M (IgM) hepatitis A (HAV) negative; IgM hepatitis E (HEV) negative; IgM hepatitis B core antigen (HBcAg) negative; HBeAg negative; CMV-IgM negative; anti-HCV positive, polymerase chain reaction (PCR) HBV negative. PCR HCV positive. The titer of HCV is 2.5 × 106 Copies/mL. abdomen ultrasound sonography test (USG) showed hepatomegaly.
Mycophenolate mofetil was stopped at admission and he was continued on 3 mg/kg of tacrolimus and 8 mg of meprednisone. Ursodeoxycholic acid (Losan Pharma GmbH), Compound Glycyrrhizin (Minophagen Pharmaceutical Co., Ltd) and Transmetil (Abbott Laboratories) were administrated to protect the live. At day 42, after stable disease condition for 6 weeks, the patient's condition suddenly deteriorated. He presents the clinical manifestation of acute liver failure such as further deepened jaundice, nausea, vomiting, extreme fatigue. His total bilirubin increased to 422 μmol/L. His other investigations revealed AST 314 IU/L; ALT 114 IU/L; alkaline phosphatase (ALP) 244 IU/L; prothrombin activity (PTA) <30%. He developed spontaneous bacterial peritonitis along with hypokalaemia. Lung infections including bacteria and fungi were also confirmed by sputum culture. Liver biopsy was performed and the result showed marked cholangiectasis, chronic inflammation and fibrosis with spotty necrosis. Hepatocytes showed focal ballooning along with intracellular cholestasis (Figure 1A and B). CK19 was highly expressed in portal areas and hepatic parenchyma, which showed significant fibroplasia of liver (Figure 1C). Immunohistochemistry for anti-HCV was positive (Figure 1D). All the immunosuppressive agents were stopped. He was started on IV injection of antibiotics and fresh frozen plasma for bacterial peritonitis. In view of the stop of immunosuppressive agents, which may give rise to renal rejection, the patient was advised to perform AHSCT to induce transplantation tolerance. Informed consents were obtained from the patients and his family. The experimental protocol was approved by our institutional committee on human research. Because renal allograft was from a dead body and the donors matching with the renal donor in human leukocyte antigen (HLA) were not found in Chinese hematopoietic stem cell donor databank, the stem cells had to be recruited from appropriate donor among his sons or brothers. Genotyping of the HLA in the renal donor were A203, 203; B 60, 55; DR9, 16 and the ABO blood group and RhD of renal donor are A and positive respectively. Fortunately, one of his sons was found to have the same blood group as the renal donor and have the haploidentical HLA typing . Genotyping of the HLA of his son were A 203, 24; B60, 61; DR9, 11. 3.4 × 108 granulocyte colony-stimulating factor–mobilized CD34+ peripheral blood cells were obtained by CD34+ positive selection (Isolex 300iMagnetic Cell Separator; Baxter, Deerfield, IL) from this son. The patient immediately received 5.8 × 106 CD34+ stem cells/kg by peripheral vein. At the same time, he continued to be treated with antibiotics and other adjunctive drug. Before day 70 he had made a remarkable recovery with resolution of all symptoms, and before day 160 liver biochemistry and prothrombin time returned to normal (Figure 2). A flow-cytometry analysis was used for detecting specific CD cell-surface epitopes on single fixed cells18 to assess chimerism after 4 months. The percentage of donor cells circulating in the recipient's peripheral blood, including leukocytes, CD3+ and CD34+ cells were 4.7%, 3.3%, 36.8%. A dramatic decrease of serum HCV RNA concentration was observed. Serum hepatitis C viral load gradually decreased from 2.5 × 106 Copies/mL at day 1 to 3.2 × 104 Copies/mL at day 98 and became negative (<400 copies/mL) at day 126 and remains negative at the last available assessment. At day 98 the patient has received continuous tacrolimus therapy (1 mg/d) to prevent renal rejection. At day 160 he was discharged, and at 10 months he was able to return to work. He was requested to visit monthly for the first year, and bimonthly from the second to the third year. Afterward, he visited every half a year. He was tested for liver function, kidney function, drug levels (tacrolimus, cyclosporin A, or mycophenolate), lipid profile, whole blood cell count, routine urine test, HCV RNA and anti-HCV at each visit. Renal graft and liver ultrasound were performed every 3 months or as ordered by doctors. The test results were normal and negative until last visit, respectively. He is currently working full-time. The last visit was in September, 2014. The test results included tacrolimus drug level of 4.10 ng/mL, white blood cell of 6.28 × 109/L, hemoglobin of 152 g/L, platelet 187 × 109/L, normal urine test, serum creatinine of 88 μmol/L, albumin of 40 g/L, ALT of 4 U/L, AST of 14 U/L, GGT of 15 U/L, TBIL of 11.3 μmol/L and normal ultrasound images of renal graft and liver.
FIGURE 1.
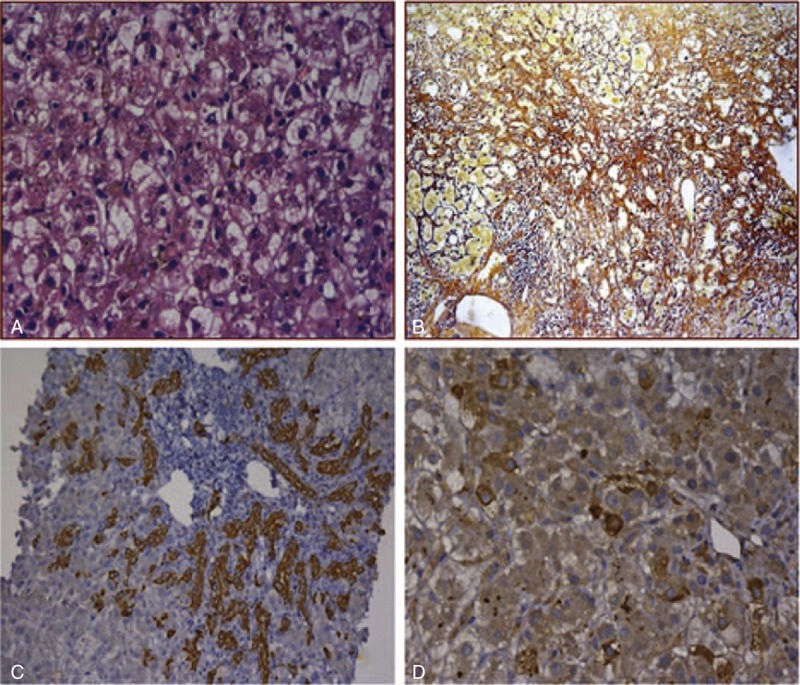
Liver histology. (A) Hepatocytes obviously displayed ballooning degeneration, necrosis with a ground glass aspect. Cholestasis and cholangiectasis also can be observed (HE 1×400). (B) There was marked periportal and septal fibrosis with moderate pericellular fibrosis (Silver staining). (C) CK19 was highly expressed in portal areas and hepatic parenchyma (1×200). (D) Immunohistochemical staining for HCV-Ag showed significantly positive.
FIGURE 2.
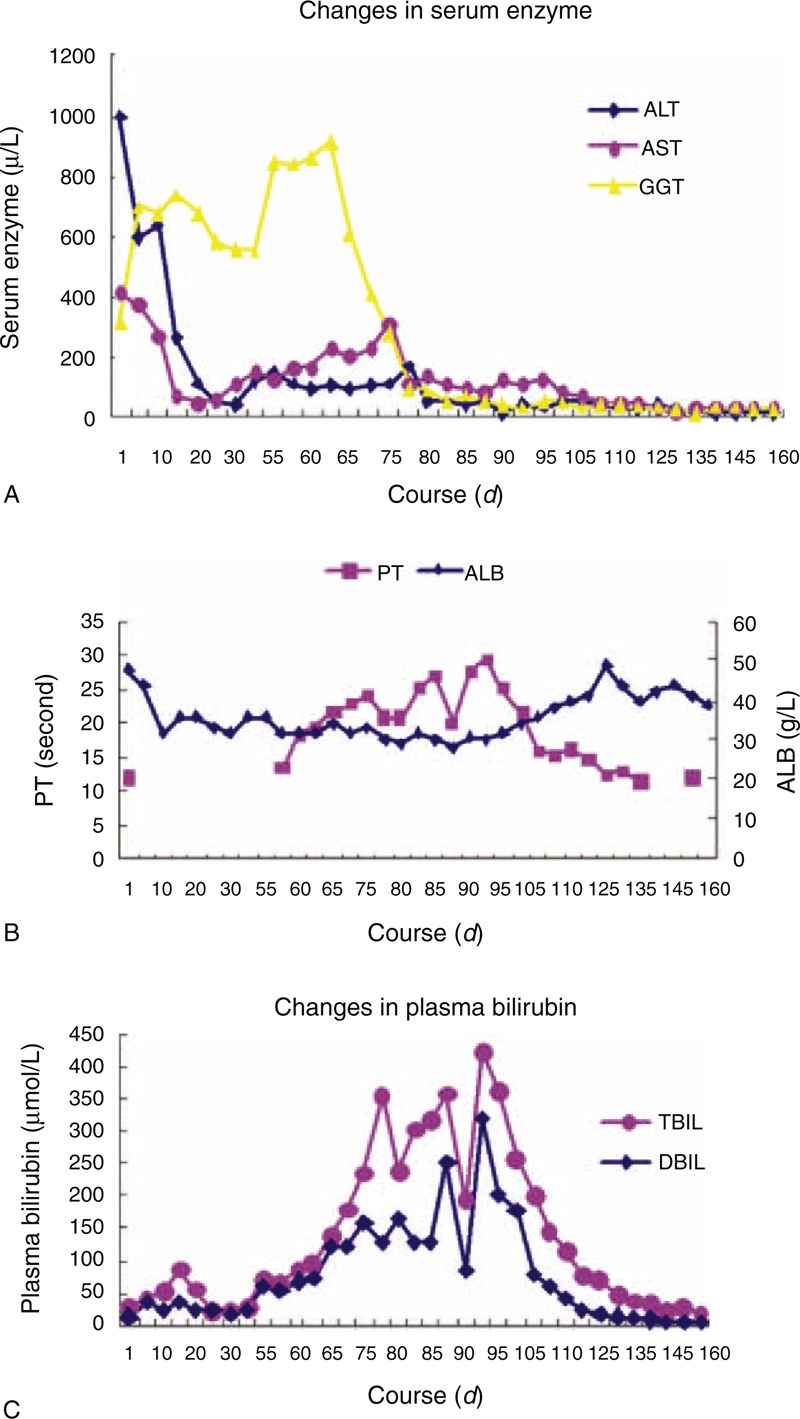
Changes in serum enzyme. The ALT and AST (reflects the hepatocyte injury) were high at first and decreased after treatment, while GGT (reflects cholestasis) were not high but gradually increased within 2 months. It is in accordance with the characteristics of FCH. Changes in prothrombin and plasma albumin. These two indicators reflect the liver synthetic function, their alterations were similar to those in figure C, which show the liver metabolic function. Changes in plasma bilirubin. From day 42 to 98, the patients showed severe cholestasis and the BIL curve ascended sharply. There were several drop-downs on the peak due to plasmapheresis. The curve descended remarkably after the liver function began to benefit from the treatment.
DISCUSSION
FCH is a rapidly progressive form of hepatitis that occurs in the organ transplant recipients or subjects with HIV infection in which there are the presence of significant immunosuppression. The clinical course is characterized by rapid progression to liver failure and death. The diagnosis of FCH was based on the typical histological picture with pericellular fibrosis, ductular proliferation, cholestasis, and intense positivity for HBs and HBc antigens in an immunocompromised setting.19 Different from the common virus hepatitis in which liver injury result from immune clearance of hepatotropic virus, FCH has been postulated to result from a direct cytopathic effect of HBV or HCV on hepatocytes associated with immunosuppression.20,21 FCH rapidly progresses to liver failure. Treatment by liver transplantation carries a high risk of mortality. In the last 40 years, Monaco's research laboratory has worked with donor bone marrow derived cells infusion to augment tolerance, and this has remained the central theme of his research work.22 Recently, different approaches have been successfully used in both experimental and clinical settings to increase the likelihood to induce chimerism and operational tolerance.23,24 Promotion of permanent chimerism resulting in exhaustive clonal deletion was considered to be the most probable mechanism for this effect. In this case, based on FCH, the patient developed spontaneous bacterial peritonitis and lung infection, a disease associated with high inpatient mortality. After renal transplantation, immunosuppressive therapy can result in an increased burden of HCV viremia. So all the immunosuppressive agents had to be stopped and antibiotics were administrated. In view of the possibility of renal rejection, we performed allogeneic HSC infusion to induce recipient hematopoietic chimerism and graft tolerance. In this successful case, there may be other mechanisms taking part in the liver repair such as infused HSC cell, transdifferentiation into functional hepatocytes and/or paracrine effects that promote liver regeneration and suppress local inflammation. At present, after renal transplantation, there is no current safe and efficient therapy for HCV infection. Alpha-interferon (alpha-IFN) does not give a sustained virological response, and is associated with a high rate of renal failure. Ribavirin and amantadine monotherapies are associated with a significant improvement in liver enzymes, but have no impact upon HCV viremia.25 In our patient described here, loss of serum HCV RNA from such a high baseline level may be result from recovery of anti-viral immune responses after stop of immuno suppressive agents.
In summary, this case suggests that HSCs from the donor who has haploidentical HLA typing with renal graft can induce the transplantation tolerance and may take part in the liver repair of the patient who suffers FCH after renal transplantation. It is the first report of renal transplantation patient who survived FCH with allogeneic HSC infusion. Clinical improvement was marked. But the therapeutic potential must be further verified by more cases.
Acknowledgments
This study was supported by the Key Project of Fujian Province Science and Technology (2011Y0043) and the Innovation of Medical Science and Technology Foundation of Nanjing Military (2007534).
Footnotes
Abbreviations: AHSCt = allogeneic hematopoietic stem cell transplantation, ALB = albumin, ALT = aminotransferase alanine aminotransferase, ALP = alkaline phosphatase, AST = aspartate, CMV = cytomegalovirus, FCH = fibrosing cholestatic hepatitis, GGT = gamma-glutamyl transferase, HAV = hepatitis A, HBV = hepatitis B virus, HBeAg = hepatitis B e antigen, HBsAg = hepatitis B surface antigen, HCV = hepatitis C virus, HIV = human immunodeficiency virus, HLA = human leukocyte antigen, IgM = immunoglobulin M, PCR = polymerase chain reaction, PTA = prothrombin activity, USG = ultrasound sonography test.
Key Project of Fujian Province Science and Technology (2011Y0043) and the Innovation of Medical Science and Technology Foundation of Nanjing Military (2007534).
The authors declare that they have no competing interests.
REFERENCES
- 1.Davies SE, Portmann BC, O’Grady JG, et al. Hepatic histological findings after transplantation for chronic hepatitis B virus infection, including a unique pattern of fibrosing cholestatic hepatitis. Hepatology 1991; 13:150–157. [PubMed] [Google Scholar]
- 2.Lau JY, Bain VG, Davies SE, et al. High-level expression of hepatitis B viral antigens in fibrosing cholestatic hepatitis. Gastroenterology 1992; 102:956–962. [DOI] [PubMed] [Google Scholar]
- 3.Schluger LK, Sheiner PA, Thung SN, et al. Severe recurrent cholestatic hepatitis C following orthotopic liver transplantation. Hepatology 1996; 23:971–976. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal SK, Kalra V, Dinda A, et al. Fibrosing cholestatic hepatitis in renal transplant recipient with CMV infection: a case report. Int Urol Nephrol 2004; 36:433–435. [DOI] [PubMed] [Google Scholar]
- 5.Lam PW, Wachs ME, Somberg KA, et al. Fibrosing cholestatic hepatitis in renal transplant recipients. Transplantation 1996; 61:378–381. [DOI] [PubMed] [Google Scholar]
- 6.Zylberberg H, Carnot F, Mamzer MF, et al. Hepatitis C virus-related fibrosing cholestatic hepatitis after renal transplantation. Transplantation 1997; 63:158–160. [DOI] [PubMed] [Google Scholar]
- 7.Toth CM, Pascual M, Chung RT, et al. Hepatitis C virus-associated fibrosing cholestatic hepatitis after renal transplantation: response to interferon-alpha therapy. Transplantation 1998; 66:1254–1258. [DOI] [PubMed] [Google Scholar]
- 8.Jung S, Lee HC, Han JM, et al. Four cases of hepatitis B virus-related fibrosing cholestatic hepatitis treated with lamivudine. J Gastroenterol Hepatol 2002; 17:345–350. [DOI] [PubMed] [Google Scholar]
- 9.Tillmann HL, Bock CT, Bleck JS, et al. Successful treatment of fibrosing cholestatic hepatitis using adefovir dipivoxil in a patient with cirrhosis and renal insufficiency. Liver Transplant 2003; 9:191–196. [DOI] [PubMed] [Google Scholar]
- 10.Trivedi H, Shah V, Shah P, et al. High dose DBMC associated tolerance in live-related renal allograft recipients. Transplant Proc 2000; 32:2001–2002. [DOI] [PubMed] [Google Scholar]
- 11.Trivedi HL, Shah VR, Shah PR, et al. Megadose approach to DBMC infusion-induced allograft hyporesponsiveness in living-related renal allograft recipients. Transplant Proc 2001; 33:71–76. [DOI] [PubMed] [Google Scholar]
- 12.Trivedi HL, Shah VR, Vanikar AV, et al. High-dose peripheral blood stem cell infusion: a strategy to induce donor-specific hyporesponsiveness to allografts in pediatric renal transplant recipients. Pediatr Transplant 2002; 6:63–68. [DOI] [PubMed] [Google Scholar]
- 13.Trivedi H, Vanikar A, Shah V, et al. Mega dose unfractionated donor bone marrow-derived cell infusion in thymus and periphery-an integrated clinical approach for tolerance in living related renal allografts. Transplant Proc 2003; 35:203–206. [DOI] [PubMed] [Google Scholar]
- 14.Petersen BE, Bowen WC, Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science 1999; 284:1168–1170. [DOI] [PubMed] [Google Scholar]
- 15.Theise ND, Nimmakayalu M, Gardner R, et al. Liver from bone marrow in humans. Hepatology 2000; 32:11–16. [DOI] [PubMed] [Google Scholar]
- 16.Alison MR, Poulsom R, Jeffery R, et al. Hepatocytes from non-hepatic adult stem cells. Nature 2000; 406:257. [DOI] [PubMed] [Google Scholar]
- 17.Lagasse E, Connors H, Al-Dhalimy M, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat Med 2000; 6:1229–1234. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Morales R, Esquenazi V, Zucker K, et al. An assessment of the effects of cadaver donor bone marrow on kidney allograft recipient blood cell chimerism by a novel technique combining PCR and flow cytometry. Transplantation 1996; 62:1149–1160. [DOI] [PubMed] [Google Scholar]
- 19.Benner KG, Lee RG, Keeffe EB, et al. Fibrosing cytolytic liver failure secondary to recurrent hepatitis B after liver transplantation. Gastroenterology 1992; 103:1307–1312. [DOI] [PubMed] [Google Scholar]
- 20.Araya V, Rakela J, Wright T. Hepatitis C after orthotopic liver transplantation. Gastroenterology 1997; 112:575–582. [DOI] [PubMed] [Google Scholar]
- 21.Collier J, Heathcote J. Hepatitis C viral infection in the immunosuppressed patient. Hepatology 1998; 27:2–6. [DOI] [PubMed] [Google Scholar]
- 22.Gozzo JJ, Wood ML, Monaco AP. Use of allogenic, homozygous bone marrow cells for the induction of specific immunologic tolerance in mice treated with antilymphocyte serum. Surg Forum 1970; 21:281–284. [PubMed] [Google Scholar]
- 23.Kawai T, Cosimi AB, Spitzer TR, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2008; 358:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scandling JD, Busque S, Dejbakhsh-Jones S, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med 2008; 358:362–368. [DOI] [PubMed] [Google Scholar]
- 25.Kamar N, Ribes D, Izopet J, Rostaing L. Treatment of hepatitis C virus infection (HCV) after renal transplantation: implications for HCV-positive dialysis patients awaiting a kidney transplant. Transplantation 2006; 82:853–856. [DOI] [PubMed] [Google Scholar]


