Abstract
We conducted a study to evaluate the impact of the exclusive enteral nutrition (EEN) on perioperative outcome in Crohn's disease (CD) patients following immunosuppressive therapy.
Patients with CD followed at a referral center between January 2001 and March 2014 who underwent abdominal surgery were identified. Patients were divided into 4 groups: patients not exposed to immunosuppressive agents in the previous 8 weeks before surgery (group 1); patients received immunosuppressive medications without preoperative drug-free interval (group 2); patients had preoperative immunosuppressants-free interval (group 3); patients treated with adding EEN to preoperative immunosuppressants-free interval regimen (group 4). Urgent operation requirement, stoma creation, postoperative complications, readmission, and reoperation were compared in patients among groups.
Overall, 708 abdominal surgeries performed in 498 CD patients were identified. Three hundred seventy-six (53.11%) surgeries performed in those receiving preoperative immunosuppressive medications. Compared with other groups, group 2 had increased postoperative complications, more frequent urgent operation, and higher rate of stoma creation. Patients in group 4 were found to have better outcome including lower rate of stoma creation (P < 0.05), and decreased incidence of postoperative complications (P < 0.05) compared with group 2 and group 3. Additionally, decreased urgent operation requirement (P < 0.05) and extended preoperative drug-free interval (P < 0.001) were observed in the group 4 than those in the group 3.
Preoperative optimization of CD following immunosuppressive therapy by EEN prolongs the immunosuppressants-free interval, reduces the risk of urgent surgery and reoperation, and most importantly, decreases complications after abdominal surgery.
INTRODUCTION
Crohn's disease (CD) is a relapsing, transmural inflammatory bowel disease (IBD) that can affect the entire gastrointestinal tract from the mouth to the anus.1 Development of complications including strictures, abscesses, or fistulas is one of the typical presentations of CD.2 Surgical resection is an almost inevitable event through the course of CD and the rate of surgical intervention increases with disease duration.3 The rate of surgery is 20%–40% during the first year of the disease, 30%–70% at 10 years after diagnosis, and 70%–90% after 15 years of diagnosis.4–6 Surgery in CD is frequently performed in those with factors associated with the increased risk of postoperative complications such as malnutrition status, presence of abdominal abscess, and preoperative immunomodulators use.7–9
Currently, the medical management for CD is directed suppressing the overactive immune response, and typically relies on combination therapy with immunosuppressive medications which can be generally categorized into 3 classes: steroids, immunomodulators, and biologics.10 Although studies evaluating the impact of preoperative immunosuppressive therapy on postoperative outcome in CD patients undergoing abdominal surgery have revealed conflicting results, immunosuppressive agents are potentially associated with the increased incidence of postoperative complications. Combination immunosuppressive therapy before surgery in patients with CD appears to be associated with an increase in postoperative morbidity.11,12 Some reports also found the increased incidences in both mortality and morbidity after biologic treatment.13–15
To minimize the potential influence of immunosuppressive therapy, withdrawal of immunosuppressive agents before surgical intervention to provide the drug window with the purpose of drug wash out is recommended in actual clinical practice. The importance of a preoperative drug-free interval which may reduce the risk of infections after surgery is suggested.11 However, because of the immunosuppressive agent withdrawal, the disease might exacerbate during the preoperative drug-free interval which may subsequently result in urgent surgical intervention. Urgent surgery is suspected to be associated with increased postoperative complications and the increased incidence of stoma creation.16,17 Therefore, medical alternatives for disease control during the immusupressants-free interval is required. Unfortunately, medications such as 5-ASA is less effective in patients receiving previous chronic immunomodulators although the association between 5-ASA and postoperative adverse outcome was rarely reported.
Exclusive enteral nutrition (EEN), which can provide 100% nutrients needs of a patient and is largely free from significant side effects.18,19 EEN therapy is shown to be beneficial and efficacious in inducing and maintaining remission for CD.20 In our institute, EEN is increasingly used in adult CD patients especially for preoperative optimization. For those with fistulizing disease, preoperative EEN therapy was associated with the reduced risk of intra-abdominal septic complications after operation in CD.21 For those with stricture disease, EEN therapy can effectively relieve inflammatory bowel stricture in CD.22 We recently found EEN could effectively induce the disease remission and improve health-related quality of life in adults with active CD which may be associated with its activity in abdominal fat modification.23–25
Preoperative optimization of CD patients following immunosuppressive treatment may decrease postoperative complications, and may avoid temporary stoma creation.26 However, there are few reports evaluating the effects of preoperative optimization on the perioperative outcomes in patients with CD. Furthermore, how to optimize these patients is an important and difficult issue for both gastroenterologist and colorectal surgeons, and which is still not well answered. In the routine clinical practice at our IBD center, patients are generally recommended to receive adding EEN to preoperative immunosuppressants-free interval regimen although some patients may refuse to use EEN because of personal choice or economic issue. According to our experience, patients receiving preoperative EEN regimen rarely develop postoperative morbidity. However, evidence of the efficacy and safety of the EEN regimen in the preoperative drug-free interval is largely unknown for CD patients undergoing abdominal surgery. The aim of this study was to evaluate whether preoperative optimization by EEN can result in a better outcome in immunosuppressants-treated CD patients.
METHODS
Patients
From January 2001 to March 2014, information of all patients with CD who underwent abdominal surgery in Department of General Surgery of Jinling Hospital were collected from a prospectively maintained IBD database. The Institutional Review Board of Jinling Hospital approved the project. All CD patients have a verified diagnosis according to conventional clinical, radiological, and endoscopic criteria, confirmed by the histological findings.27,28
Preoperative immunosuppressive medication use within 8 weeks before surgery was recorded and categorized into 3 classes: steroids, immunomodulators, and biologics. Patients were divided into 4 groups: patients not exposed to immunosuppressive agents in the previous 8 weeks before surgery (group 1); patients received immunosuppressive medications without preoperative drug-free interval (group 2); patients had preoperative immunosuppressants-free interval (group 3); patients treated with adding EEN to preoperative immunosuppressants-free interval regimen (group 4). Adding EEN to drug-free interval regimen was defined as EEN treatment from the time of immunosuppressive medication withdrawal to 1 day before surgical intervention. Patients were generally recommended to receive EEN treatment for 4 weeks. The EEN therapy was performed as previously described.23,25 During the period of EEN therapy, any other food and drink except water was forbidden. The enteral formula composing of maltodextrin, hydrolyzed whey protein peptide, fat, vitamins and trace elements was a commercially available Peptisorb Liquid (Nutricia, Amsterdam, the Netherlands). The enteral formula was infused continuously through a nasogastric tube. The daily calorie intake was 25–30 kcal/kg body weight and was gradually increased from one-third of amount to the full amount.
Data Collection
Patients character such as age, disease duration, age at diagnose, disease location, disease behavior, current smoking status, preoperative medical therapy, CD-related surgical history, and body mass index (BMI) were collected. Operation-related information including indication for surgery, type of surgery (open or laparoscopic), stoma creation, number of anastomoses, emergent surgery, principle surgical procedure, operative time, and estimated blood loss were also collected. We were particularly interested in the duration of immunosuppressants-free interval, postoperative 30-day complications. Special interesting was also given to the reoperation and readmission.
Outcome Measurement
Thirty-day postoperative complications were collected and divided into infectious complications (wound infection, intaabdominal abscess, anastomotic leak, fistula, urinary tract infection, pulmonary infection) and noninfectious complications (eg, ileus, bleeding, thrombolic events, dehydration, other). The primary outcome assessed was the infectious complication rate up to 30 days after surgery. Secondary outcome was the rate of noninfectious complications within 30 days postoperatively. We were also interested in the rate of stoma creation, urgent operation requirement, reoperation, and readmission.
Statistical Analysis
All data were entered into a standardized database computer program and the statistical analysis was performed using SPSS 16.0 software (SPSS, Inc., Chicago, IL). A χ2 test or Fisher's exact test was used to compare the categorical variables. Continuous variables were expressed as the mean and standard deviation (SD) and were compared by using a Student's t-test test. One-way analysis of variance (ANOVA) was used for multiple group comparisons. Multivariate analysis using was used to identify the independent risk factors for postoperative complications. Independent variables for multivariate analysis included those with a significant influence in univariate analysis (P < 0.05). The odds ratio (OR) and 95% confidence interval were calculated for all the variables. All hypothesis testing was 2-sided with a P value of <0.05 was considered significant in all the analyses.
RESULTS
Patient's Character
There were a total of 708 surgeries performed in 498 patients. Patients in group 1 were operated between 2001 and 2013, patients in group 2 received surgery from 2002 to 2012, patients in group 3 underwent surgical intervention between 2001 and 2004, and patients in group 4 were operated from 2001 to 2014. Three hundred seventy-six (53.11%) of these surgeries were performed among patients who received perioperative immunosuppressive agent. Clinical characteristics of the study population in each group are shown in Table 1. No significant differences were observed among patient groups in gender, age at surgery, disease duration, age at diagnosis, disease location, disease behavior, or previous CD-related surgery. In addition, there were no significant differences among group 2, group 3, and group 4 in terms of preoperative medical therapy or the number of immunosuppressive medications. However, patients in group 4 had increased BMI at the time of surgery when compared with those in group 1 (P = 0.003) and group 3 (P = 0.03). Furthermore, our data indicated patients in group 4 had longer preoperative drug-free interval (Figure 1).
TABLE 1.
Clinical Features by Number of Classes of Immunosuppressive Medications
FIGURE 1.
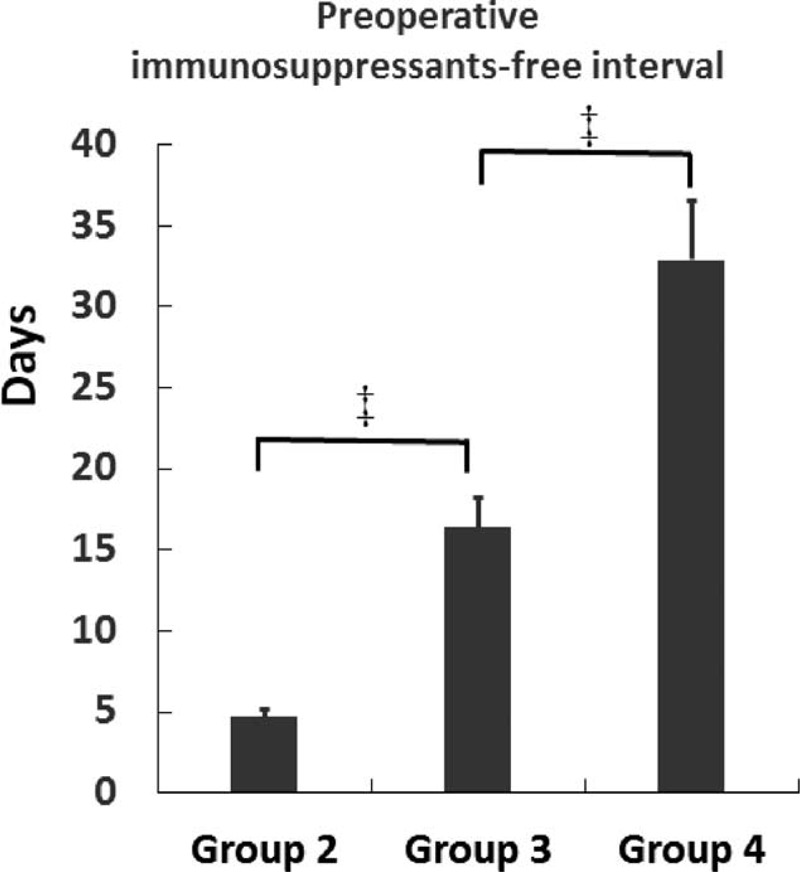
The preoperative immunosuppressants-free interval in group 2, group 3, and group4. ‡P < 0.001.
Operative details are presented in Table 2. There was no difference in surgical indication, type of surgery, number of anastomoses, principle surgical procedure, operative time, or estimated blood loss among groups. However, patients in group 2 were more likely to have fecal diversion, while patients in group 4 had a lower frequency of stoma creation (Figure 2). In addition, patients in group 2 had more requirement of emergent surgical intervention. However, decreased incidence of urgent operation was observed in group 4 (Figure 2).
TABLE 2.
Operative Details of Abdominal Surgeries in Crohn's Disease Patients
FIGURE 2.
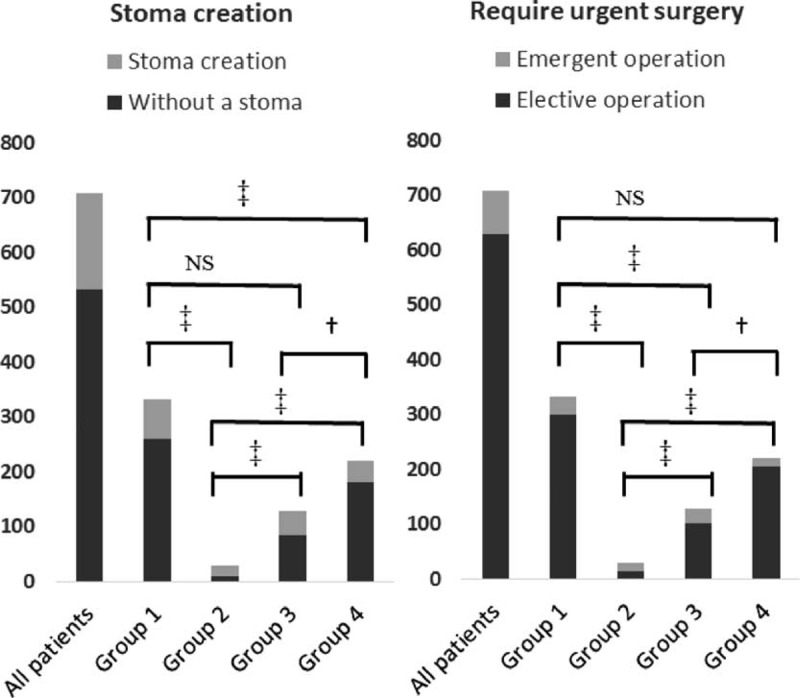
Stoma creation and urgent surgery requirement among groups. †P < 0.05, ‡P < 0.001, and NS means not significant.
Postoperative Infectious Complications
All 30-day postoperative infectious complications are shown in Table 3. There was a significant difference in the number of patients developing postoperative infectious complications among groups (Figure 3). There were significant differences among groups in wound infection, intra-abdominal abscess, and anastomotic leak. Higher frequencies of total infectious complications, wound infection, intra-abdominal abscess, and anastomotic leak were observed in group 2 and group 3. However, lower incidences of total infectious complications or any each specific infectious complication were found in group 4.
TABLE 3.
Postoperative Complications
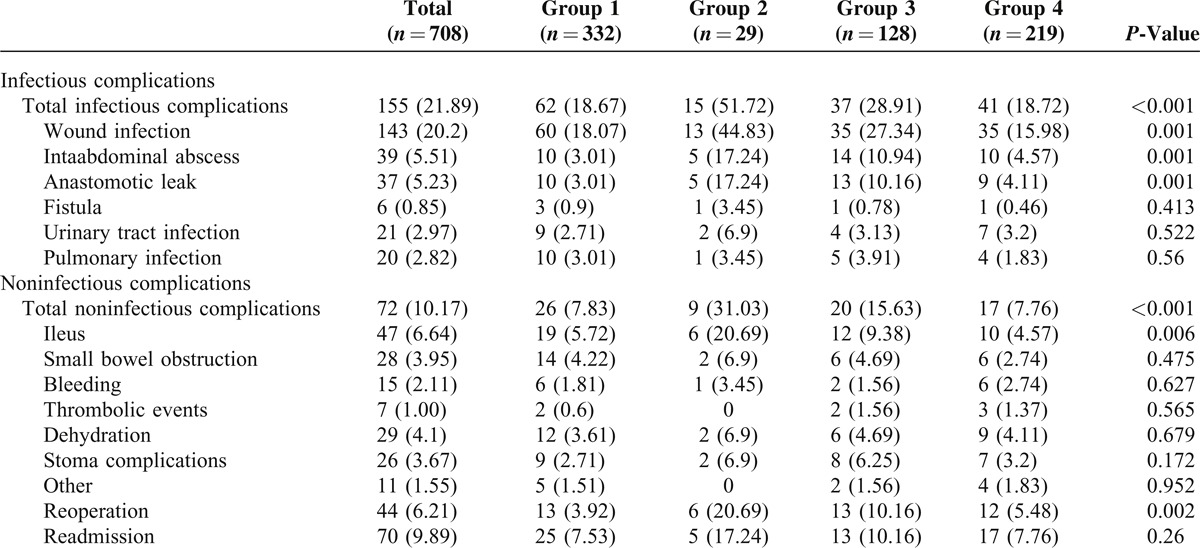
FIGURE 3.
Postoperative infectious complications among groups. †P < 0.05, ‡P < 0.001, and NS means not significant. UTI, urinary tract infection.
Noninfectious Complications
Postoperative noninfectious complications occurring within 30 days are presented in Table 3.
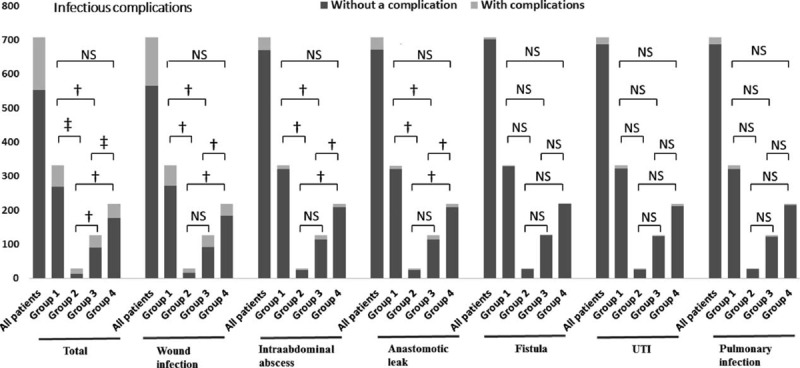
There was a significant difference in respect to total noninfectious complications among groups (Figure 4). The group 2 and group 3 had higher frequencies of total noninfectious complications compared with group 1. However, no significant difference was found between group 1 and group 4 in respect to total noninfectious complications or any each specific infectious complication.
FIGURE 4.
Postoperative noninfectious complications among groups. †P < 0.05, ‡P < 0.001, and NS means not significant. SBO, small bowel obstruction.
Postoperative Reoperation and Readmission
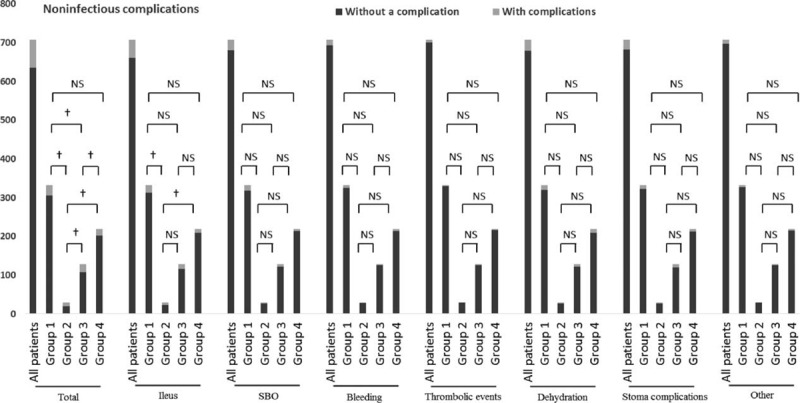
The overall reoperation rate is 6.21% (44/708). The rate of reoperation in group 1, group 2, group 3, and group 4 is 3.92% (13/332), 20.69% (6/29), 10.16% (13/128), 5.48% (12/219), respectively. There was a statistically significant difference among groups for reoperation (Figure 5). In our series, the overall readmission rate is 9.89% (70/708). The rate of readmission in group 1, group 2, group 3, and group 4 is 7.53% (25/332), 17.24% (5/29), 10.16% (13/128), 7.76% (17/219), respectively. There was no statistically significant difference among groups for readmission (Figure 5).
FIGURE 5.
Reoperation and readmission among groups. †P < 0.05, NS means not significant.
Independent Risk Factors of Postoperative Complications
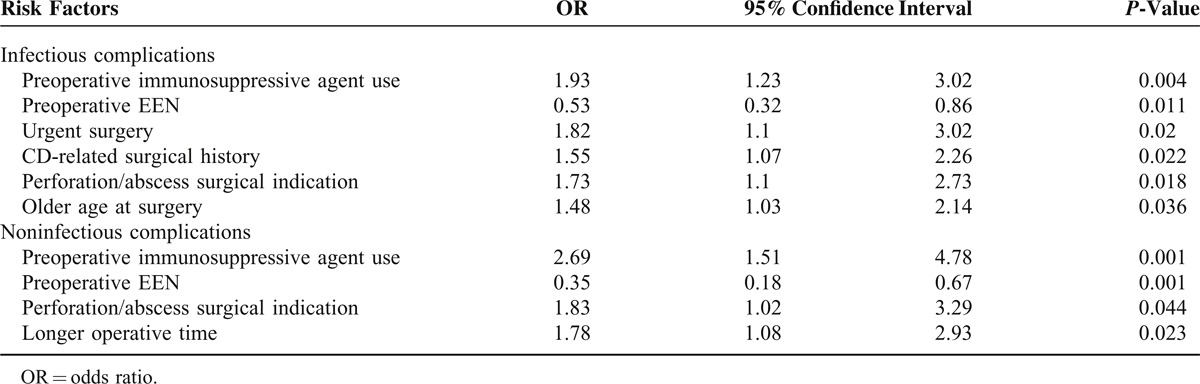
To identify the independent factor for postoperative complications. Multivariate logistic regression analysis model was used, and the results were shown in Table 4. The multivariate logistic regression analysis including risk factors such as preoperative immunosuppressive agent use, preoperative EEN, emergent surgery, CD-related surgical history, perforation/abscess surgical indication, longer operative time, and older age at surgery was performed. Our data indicated preoperative immunosuppressive agent use, emergent surgery, perforation/abscess surgical indication, and older age at surgery were found to be independent predictors for postoperative infectious complications, while preoperative EEN demonstrated to be a risk-reducing factor for infectious complications after surgery. In addition, in multivariate analysis, preoperative immunosuppressive agent use, perforation/abscess surgical indication, and longer operative time was independently associated with postoperative noninfectious complications, while preoperative EEN was found to be a protective factor for noninfectious complications after surgery.
TABLE 4.
Multivariate Analysis of Factors Associated With Postoperative Complications
DISCUSSION
A growing number of reports indicate the relative high postoperative morbidity and frequent infectious complications in patients with CD.29–31 Currently, immunosuppressive medications are often used in combination with one another for the preoperative treatment of CD.32 However, the safety of these immunosuppressive agents in perioperative setting is unclear. Accumulated data indicated that preoperative therapy with immunosuppressive agents increased the incidence of postoperative complications, particularly infectious and anastomotic leak.11,33 Therefore, preoperative drug window is needed for patients exposed to immunosuppressive agents with the purpose of drug wash out.
Our study found that, compared with those in other groups, patients without preoperative drug-free interval had increased incidences of both infectious and noninfectious complications, and higher risk of stoma creation. Preoperative EEN therapy provided longer drug-free interval, reduced the requirement of urgent surgical intervention, and decreased the rate of fecal diversion. In addition, postoperative complications in patients receiving EEN regimen were significantly decreased. We also found a higher rate of reoperation in patients without preoperative immunosuppresants-free interval although no significant difference was found between those with and without EEN regimen during the preoperative drug-free interval.
To date, few studies have evaluated the impact of preoperative drug-free interval on perioperative outcomes in patients with CD. The 1-month wash out period for azathioprine was reported based on the pharmacokinetics for the metabolite of the agent.33–35 For infliximab, 8-week wash out period was recommended according to pharmacokinetics and the treatment profile of the agent.33,36 In a meta-analysis, the authors concluded that patients with CD on preoperative immunosuppressive agents are at higher risk for complications, and a preoperative drug-free interval was suggested to reduce the risk of infections.11 In this study, we found preoperative immunosuppressants-free interval played an important role in decreased complications after surgery, and interestingly, we found the EEN regimen was associated with better outcome comparing with those without EEN during the drug-free interval.
Preoperative optimization of CD, when possible, may decrease postoperative complications, and avoid formation of stomas for fecal diversion.37 It is also reported that enhancing nutritional status and streamlining immunomodulator therapy before surgery may improve postoperative outcomes.26 EEN is the provision of 100% of a person's nutritional requirements which is effective in inducing remission in adults with active CD and proposed as an alternative to steroids therapy.19 Beside induction of remission, CD treatment is associated with remission maintenance as well as health-related quality of life.24,38 However, few studies were performed to evaluate the potential association between EEN and perioperative outcome in patients following immunosuppressive therapy although there are several studies focused on the association between total parenteral nutrition (TPN) and postoperative complications in CD.39–41 we found patients receiving adding EEN to preoperative drug-free interval regimen had a longer drug window. We may thus speculated that the longer preoperative immunosuppressants-free interval may contribute to the decreased incidence of postoperative complications. However, it was recently reported that increasing the washout period for biologicals to 12 weeks did not modify the frequency of postoperative surgical site infection.42 We tentatively put forward that EEN itself may play an important role in the decreased postoperative complications through inducing disease remission, attenuating the inflammatory response, and improving the nutrition status in patients with CD. Indeed, preoperative nutrition treatment was found to be associated with the reduced postoperative intra-abdominal septic complications.21 Based on our results, in clinical practice, for patients receiving immunosuppressive agents, preoperative drug-free interval should be recommended. If possible, patients can be treated with EEN regimen which is associated with longer drug window, decreased incidence of perioperative adverse events such as stoma creation and postoperative complications.
It should be noted that this study has inherent limitations. It is possible that those patients without preoperative EEN regimen are sicker patients who are intolerant to EEN therapy and are more likely to have postoperative complications. However, we think this is less likely considering the cohort study nature, and no difference was observed in respect to disease characters or operative information among groups. Second, <10% of our patients had a laparoscopic procedure, which may result in bias. This is probably a consequence of limited experience of laparoscopic procedure in patients with CD in China. Third, as the retrospective nature of the study, the abstraction of data especially medical records, may be subject to bias. In addition, because of its retrospective nature and no sample size calculation has been done, this long-term operated study may result in a potential selection bias. However, the data were extracted from the prospectively maintained IBD database, in which all variables were prospectively collected. Finally, there is a difference in immunosuppressive agents use for patients between China and other countries. None of our patients has been treated with budesonide or 6-mercaptopurine. Our results should be interpreted with caution when they are extrapolated to other patients.
In conclusion, despite its preliminary character, this study can clearly indicate that preoperative immunnosuppressants-free interval is required for Crohn's patients at increased risk for postoperative complications associated with the use of immunosuppressive agents. We found that preoperative optimization of CD following immunosuppressive therapy by EEN prolongs the immunosuppressants-free interval, reduces the risk of urgent surgery and reoperation, and most importantly, decreases complications after abdominal surgery. Our data offer a strategy to extend the preoperative immunosuppressants-free interval and thereby might be of benefit for a patient population at increased risk for postoperative complications associated with the use of immunosuppressive agents.
Acknowledgments
This work was partly supported by National Natural Science Foundation of China (Grant 81200263 and 81170365) and National Ministry of Health for the Digestive Disease (Grant 201002020).
Footnotes
Abbreviations: BMI = body mass index, CD = Crohn's disease, EEN = exclusive enteral nutrition, IBD = inflammatory bowel disease, OR = odds ratio, SBO = small bowel obstruction, SD = standard deviation, TPN = total parenteral nutrition, UTI = urinary tract infection.
National Natural Science Foundation of China (Grant No. 81200263 and 81170365); National Ministry of Health for the Digestive Disease (Grant No. 201002020).
The authors declare that they have no competing financial interests.
REFERENCES
- 1.Podolsky DK. Inflammatory bowel disease. N Engl J Med 2002; 347:417–429. [DOI] [PubMed] [Google Scholar]
- 2.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet 2007; 369:1641–1657. [DOI] [PubMed] [Google Scholar]
- 3.Aratari A, Papi C, Leandro G, et al. Early versus late surgery for ileo-caecal Crohn's disease. Aliment Pharmacol Ther 2007; 26:1303–1312. [DOI] [PubMed] [Google Scholar]
- 4.Bernell O, Lapidus A, Hellers G. Risk factors for surgery and postoperative recurrence in Crohn's disease. Ann Surg 2000; 231:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao X, Yang RP, Chen MH, et al. Risk factors for surgery and postoperative recurrence: analysis of a south China cohort with Crohn's disease. Scand J Gastroenterol 2012; 47:1181–1191. [DOI] [PubMed] [Google Scholar]
- 6.Shivananda S, Hordijk ML, Pena AS, Mayberry JF. Crohn's disease: risk of recurrence and reoperation in a defined population. Gut 1989; 30:990–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aszodi A, Ponsky JL. Effects of corticosteroid on the healing bowel anastomosis. Am Surg 1984; 50:546–548. [PubMed] [Google Scholar]
- 8.Hesp FL, Hendriks T, Lubbers EJ, de Boer HH. Wound healing in the intestinal wall. Effects of infection on experimental ileal and colonic anastomoses. Dis Colon Rectum 1984; 27:462–467. [DOI] [PubMed] [Google Scholar]
- 9.Tartter PI, Driefuss RM, Malon AM, et al. Relationship of postoperative septic complications and blood transfusions in patients with Crohn's disease. Am J Surg 1988; 155:43–48. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn's disease in adults. Am J Gastroenterol 2009; 104:465–483.quiz 464, 484. [DOI] [PubMed] [Google Scholar]
- 11.Ahmed Ali U, Martin ST, Rao AD, Kiran RP. Impact of preoperative immunosuppressive agents on postoperative outcomes in Crohn's disease. Dis Colon Rectum 2014; 57:663–674. [DOI] [PubMed] [Google Scholar]
- 12.White EC, Melmed GY, Vasiliauskas E, et al. Does preoperative immunosuppression influence unplanned hospital readmission after surgery in patients with Crohn's disease? Dis Colon Rectum 2012; 55:563–568. [DOI] [PubMed] [Google Scholar]
- 13.Appau KA, Fazio VW, Shen B, et al. Use of infliximab within 3 months of ileocolonic resection is associated with adverse postoperative outcomes in Crohn's patients. J Gastrointest Surg 2008; 12:1738–1744. [DOI] [PubMed] [Google Scholar]
- 14.Ljung T, Karlen P, Schmidt D, et al. Infliximab in inflammatory bowel disease: clinical outcome in a population based cohort from Stockholm County. Gut 2004; 53:849–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Syed A, Cross RK, Flasar MH. Anti-tumor necrosis factor therapy is associated with infections after abdominal surgery in Crohn's disease patients. Am J Gastroenterol 2013; 108:583–593. [DOI] [PubMed] [Google Scholar]
- 16.Lombardo F, Maggini M, Gruden G, Bruno G. Temporal trend in hospitalizations for acute diabetic complications: a nationwide study, Italy, 2001-2010. PLoS ONE 2013; 8:e63675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Slater G, Kreel I, Aufses AH., Jr Temporary loop ileostomy in the treatment of Crohn's disease. Ann Surg 1978; 188:706–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupont B, Dupont C, Justum AM, et al. Enteral nutrition in adult Crohn's disease: present status and perspectives. Mol Nutr Food Res 2008; 52:875–884. [DOI] [PubMed] [Google Scholar]
- 19.Wall CL, Day AS, Gearry RB. Use of exclusive enteral nutrition in adults with Crohn's disease: a review. World J Gastroenterol 2013; 19:7652–7660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Day AS, Whitten KE, Lemberg DA, et al. Exclusive enteral feeding as primary therapy for Crohn's disease in Australian children and adolescents: a feasible and effective approach. J Gastroenterol Hepatol 2006; 21:1609–1614. [DOI] [PubMed] [Google Scholar]
- 21.Li G, Ren J, Wang G, et al. Preoperative exclusive enteral nutrition reduces the postoperative septic complications of fistulizing Crohn's disease. Eur J Clin Nutr 2014; 68:441–446. [DOI] [PubMed] [Google Scholar]
- 22.Hu D, Ren J, Wang G, et al. Exclusive enteral nutritional therapy can relieve inflammatory bowel stricture in Crohn's disease. J Clin Gastroenterol 2014; 48:790–795. [DOI] [PubMed] [Google Scholar]
- 23.Feng Y, Li Y, Mei S, et al. Exclusive enteral nutrition ameliorates mesenteric adipose tissue alterations in patients with active Crohn's disease. Clin Nutr 2013; 33:850–858. [DOI] [PubMed] [Google Scholar]
- 24.Guo Z, Wu R, Zhu W, et al. Effect of exclusive enteral nutrition on health-related quality of life for adults with active Crohn's disease. Nutr Clin Pract 2013; 28:499–505. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Zhu W, Gong J, et al. Influence of exclusive enteral nutrition therapy on visceral fat in patients with Crohn's disease. Inflamm Bowel Dis 2014; 20:1568–1574. [DOI] [PubMed] [Google Scholar]
- 26.Efron JE, Young-Fadok TM. Preoperative optimization of Crohn's disease. Clin Colon Rectal Surg 2007; 20:303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn's disease. Gastroenterology 1990; 99:956–963. [DOI] [PubMed] [Google Scholar]
- 28.Rutgeerts P, Geboes K, Vantrappen G, et al. Natural history of recurrent Crohn's disease at the ileocolonic anastomosis after curative surgery. Gut 1984; 25:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heimann TM, Greenstein AJ, Mechanic L, Aufses AH., Jr Early complications following surgical treatment for Crohn's disease. Ann Surg 1985; 201:494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamamoto T, Allan RN, Keighley MR. Risk factors for intra-abdominal sepsis after surgery in Crohn's disease. Dis Colon Rectum 2000; 43:1141–1145. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto T, Keighley MR. Factors affecting the incidence of postoperative septic complications and recurrence after strictureplasty for jejunoileal Crohn's disease. Am J Surg 1999; 178:240–245. [DOI] [PubMed] [Google Scholar]
- 32.Cosnes J, Nion-Larmurier I, Beaugerie L, et al. Impact of the increasing use of immunosuppressants in Crohn's disease on the need for intestinal surgery. Gut 2005; 54:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Colombel JF, Loftus EV, Jr, Tremaine WJ, et al. Early postoperative complications are not increased in patients with Crohn's disease treated perioperatively with infliximab or immunosuppressive therapy. Am J Gastroenterol 2004; 99:878–883. [DOI] [PubMed] [Google Scholar]
- 34.Chan GL, Erdmann GR, Gruber SA, et al. Azathioprine metabolism: pharmacokinetics of 6-mercaptopurine, 6-thiouric acid and 6-thioguanine nucleotides in renal transplant patients. J Clin Pharmacol 1990; 30:358–363. [DOI] [PubMed] [Google Scholar]
- 35.Sandborn WJ, Tremaine WJ, Wolf DC, et al. Lack of effect of intravenous administration on time to respond to azathioprine for steroid-treated Crohn's disease. North American Azathioprine Study Group. Gastroenterology 1999; 117:527–535. [DOI] [PubMed] [Google Scholar]
- 36.Sandborn WJ, Hanauer SB. Antitumor necrosis factor therapy for inflammatory bowel disease: a review of agents, pharmacology, clinical results, and safety. Inflamm Bowel Dis 1999; 5:119–133. [DOI] [PubMed] [Google Scholar]
- 37.Zerbib P, Koriche D, Truant S, et al. Pre-operative management is associated with low rate of post-operative morbidity in penetrating Crohn's disease. Aliment Pharmacol Ther 2010; 32:459–465. [DOI] [PubMed] [Google Scholar]
- 38.Afzal NA, Van Der Zaag-Loonen HJ, Arnaud-Battandier F, et al. Improvement in quality of life of children with acute Crohn's disease does not parallel mucosal healing after treatment with exclusive enteral nutrition. Aliment Pharmacol Ther 2004; 20:167–172. [DOI] [PubMed] [Google Scholar]
- 39.Gouma DJ, von Meyenfeldt MF, Rouflart M, Soeters PB. Preoperative total parenteral nutrition (TPN) in severe Crohn's disease. Surgery 1988; 103:648–652. [PubMed] [Google Scholar]
- 40.Jacobson S. Early postoperative complications in patients with Crohn's disease given and not given preoperative total parenteral nutrition. Scand J Gastroenterol 2012; 47:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steffes C, Fromm D. Is preoperative parenteral nutrition necessary for patients with predominantly ileal Crohn's disease? Arch Surg 1992; 127:1210–1212. [DOI] [PubMed] [Google Scholar]
- 42.Serradori T, Germain A, Scherrer ML, et al. The effect of immune therapy on surgical site infection following Crohn's Disease resection. Br J Surg 2013; 100:1089–1093. [DOI] [PubMed] [Google Scholar]


