Supplemental Digital Content is available in the text
Abstract
The expression of programmed cell death 1 (PD-1) and its ligand (PD-L1) has been observed in various epithelial-originated malignancies. However, whether the expression of PD-L1 on tumor cells or the expression of PD-1 on tumor-infiltrating lymphocytes (TILs) is associated with patients’ survival remains controversial.
Electronic databases were searched for eligible literatures. Data of hazard ratio (HR) for overall survival (OS) with 95% confidence interval (CI) according to the expression status of PD-L1 or PD-1 evaluated by immunohistochemistry were extracted. The outcomes were synthesized based on random-effects model. Subgroup analyses were proposed.
Twenty-nine studies covering 12 types of epithelial-originated malignancies involving 7319 patients (2030/3641 cases for PD-L1 positive/negative, 505/1143 cases for PD-1 positive/negative) with available data of the outcome stratified by PD-L1/PD-1 status were enrolled. Epithelial-originated cancer patients with positive expression of PD-L1 on tumor tissues were associated with significantly poorer OS when compared to those with negative expression of PD-L1 (HR 1.81, 95% CI 1.33–2.46, P < 0.001). Similarly, patients with PD-1 positive expression on TILs had significantly shorter OS than the PD-1 negative group (HR 2.53, 95% CI 1.22–5.21, P = 0.012). In analyses of PD-L1, all subgroups showed consistent trends toward unfavorable prognoses of patients with positive PD-L1 expression, regardless of antibodies and evaluation cutoffs. Subgroup analyses on PD-1 were not available due to limited data.
PD-L1 or PD-1 expression status is a significant prognostic factor in epithelial-originated malignancies.
INTRODUCTION
Improved understanding of the molecular mechanisms that govern the host response to tumors has led to the identification of checkpoint signaling pathways that limit the anticancer immune response.1 Currently, blockade of the programmed cell death 1 (PD-1)/PD-1 ligand 1 (PD-L1) signaling pathway has been proved one of the most promising immunotherapeutic strategies in boosting the immune system to fight against cancer.2,3 Blocking PD-1 on tumor-infiltrating lymphocytes (TILs) or blocking PD-L1 on tumor cells results in the restoration of the functions of tumor-specific T cells. The reactivated T cells can initiate direct killing of tumor cells and secretion of immunostimulatory cytokines such as interferon gamma (IFN-γ), interleukin-2 (IL-2), and tumor necrosis factor alpha (TNF-α).4
PD-L1 expression has been observed in various epithelial-originated malignancies, including carcinomas of the esophagus, gastrointestinal tract, pancreas, breast, lung, and kidney.5,6 Several studies have found PD-L1 expression on tumor cells correlated with poor prognosis7,8; however, not all reports agree with this phenomenon.9,10 In addition, the association between PD-1 expression on TILs and the survival of patients in several tumor types was also controversial.11,12
Therefore, whether the expression of PD-L1 on tumor cells or the expression of PD-1 on TILs is associated with the prognosis of epithelial-originated cancer remains unclear. A comprehensive analysis of the various outcomes is warranted. Since PD-L1/PD-1 is a common pathway that functions in a wide spectrum of cancers, we sought to perform a meta-analysis by incorporating all available evidences to evaluate the overall survival (OS) according to PD-L1/PD-1 status in patients with epithelial-originated cancer.
MATERIAL AND METHODS
Literature Search
All relevant articles were retrieved by searching PubMed, Embase, and Cochrane databases using different combinations of the terms “PD-L1,” “B7-H1,” “CD274,” “PD-1,” “CD279,” “PD-1,” “cancer,” “tumor,”, “survival,” and “prognosis.” An additional search through Google Scholar and a manual search through reference lists of relevant reviews were additionally performed. Three authors (Z.Y., K.S., and S.J.) carried out the search independently. As Chinese investigators, we restricted our searches to studies published in either English or Chinese.
Inclusion and Exclusion Criteria
Eligible studies met the following criteria: investigate the prognosis according to PD-L1/PD-1 status in patients with epithelial-originated cancer; the expression level of PD-L1 or PD-1 was tested by immunohistochemistry (IHC) staining, respectively, on tumor cells or on TILs of the tissue specimens; the primary outcome was available. Studies that failed to meet the inclusion criteria were excluded.
Outcomes Measures, Data Extraction, and Quality Assessment
The primary outcome for this meta-analysis was OS. Data of OS were extracted in the manner of hazard ratios (HRs) with the corresponding 95% confidence interval (CI). If the HR was not displayed directly, it was estimated according to the methods described in the previously published article.13 The data collection and assessment of methodological quality followed the QUORUM and the Cochrane Collaboration guidelines (http://www.cochrane.de). The data on lead author, tumor type, IHC evaluation method, cutoff value for PD-L1/PD-1 positive, primary antibody, PD-L1/PD-1 status, and OS were extracted by 3 investigators (H.J., J.L., and W.W.) independently. Three reviewers (G.Z., P.G., and C.G.) used the Newcastle–Ottawa scale specific to cohort study to assess all included studies.14 Discrepancies were discussed by all investigators to reach consensus. All eligible studies were of high quality (more details in Table S1, http://links.lww.com/MD/A205). All analyses were based on previous published studies, thus no ethical approval and patient consent are required.
Statistical Analysis
HRs for OS with 95% CIs according to the expression status of PD-L1 or PD-1 were pooled. Heterogeneity across the incorporated studies was assessed with a forest plot and the inconsistency statistic (I2). Random-effects model was employed in case of potential heterogeneity and to avoid underestimation of standard errors of pooled estimates in this meta-analysis. All calculations were performed using STATA 11.0 (StataA Corp, College Station, TX). Subgroup analysis was conducted according to IHC evaluation method (even in different cutoff values for PD-L1 positive), tumor type, and primary antibody (subdivided into source, type, and catalog), respectively. An HR that was >1 reflected longer OS for PD-L1 (PD-1) negative patients. All CIs had 2-sided probability coverage of 95%. A statistical test with P value less than 0.05 was considered significant.
Publication Bias
An extensive search strategy was made to minimize the potential publication bias. Graphical funnel plots were generated to visually assess publication bias. The statistical method to detect funnel plot asymmetry was the Begg test.15
RESULTS
Eligible Studies
A total of 1127 records were identified after our initial search. After implementing exclusion criteria, 29 studies were included7–12,16–38 for a total of 7319 epithelial-originated cancer patients (2030/3641 cases for PD-L1 positive/negative, 505/1143 cases for PD-1 positive/negative) with available OS data stratified by PD-L1/PD-1 status. Figure 1 summarized the flow chart. Our study covered 12 types of epithelial-originated malignancies, including breast cancer (BC), cervical carcinoma (CC), clear cell renal cell carcinoma (CRCC), nonclear cell renal cell carcinoma (NCRCC), colorectal cancer (CRC), esophageal cancer (EC), gastric carcinoma (GC), hepatocellular carcinoma (HCC), small cell lung cancer (SCLC), nonsmall cell lung cancer (NSCLC), pancreatic cancer (PC), and urothelial carcinoma (UCC). The percentage of stained cells was one of the most common ways to evaluate the expression of PD-L1/PD-1 among included articles, as well as the H-score method which combines percentage with staining intensity. Mouse-originated monoclonal antibody accounted for the vast majority in terms of primary anti-PD-L1/PD-1 antibody. Table 1 summarized the characteristics of involved studies for meta-analysis.
FIGURE 1.
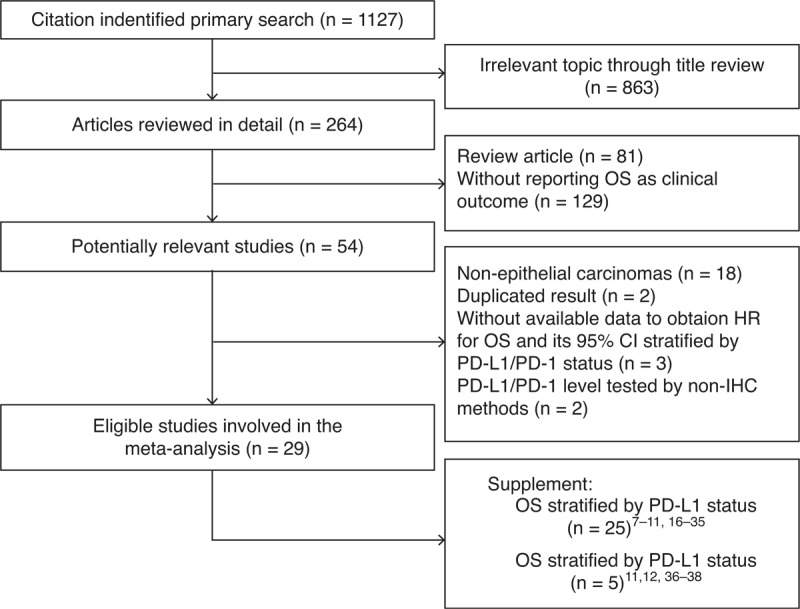
Flow chart of study selection. CI = confidence interval, HR = hazard ratio, IHC = immunohistochemistry, OS = overall survival, PD-1 = programmed cell death 1, PD-L1 = PD-1 ligand 1.
TABLE 1.
Characteristics of Included Studies for Meta-Analyses
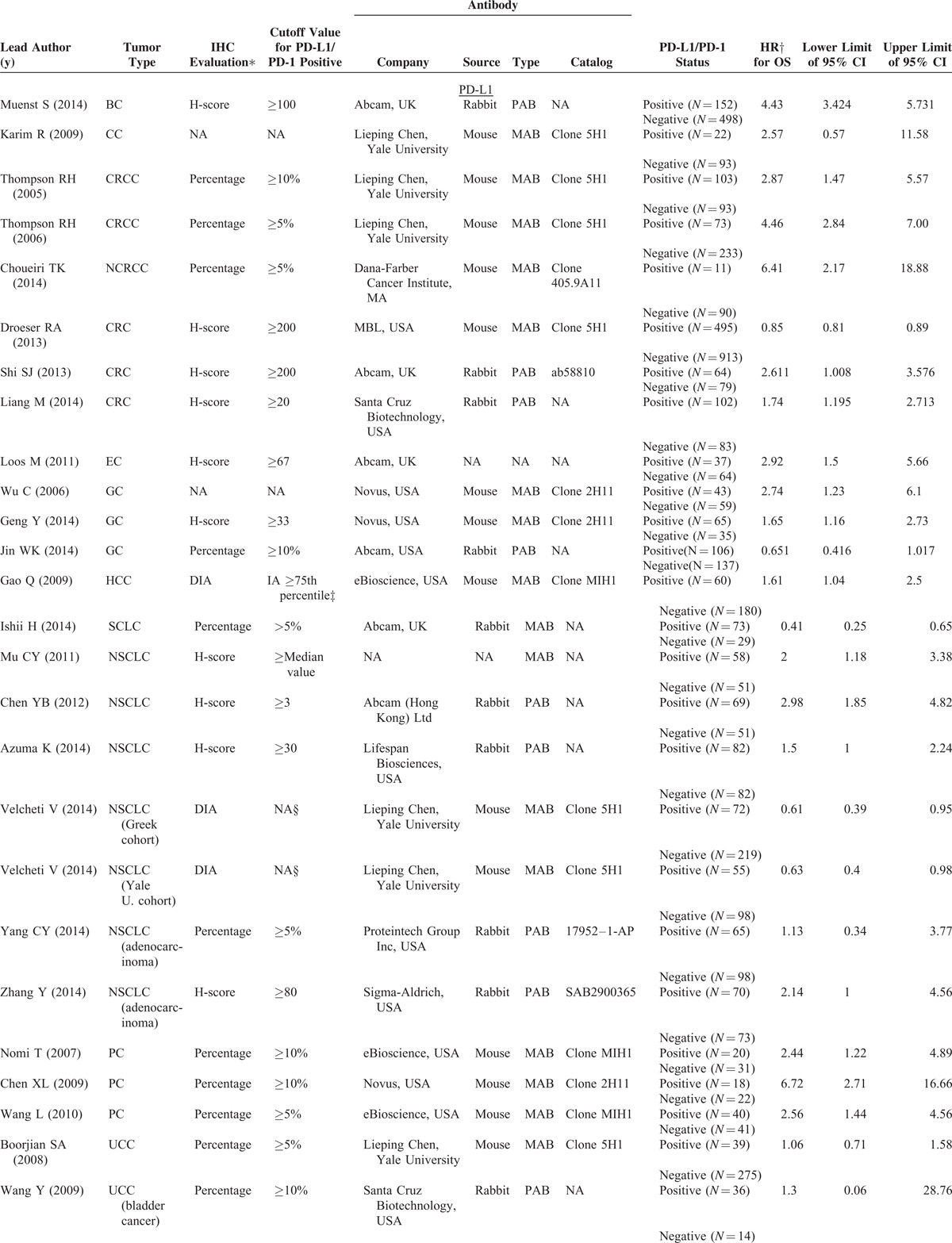
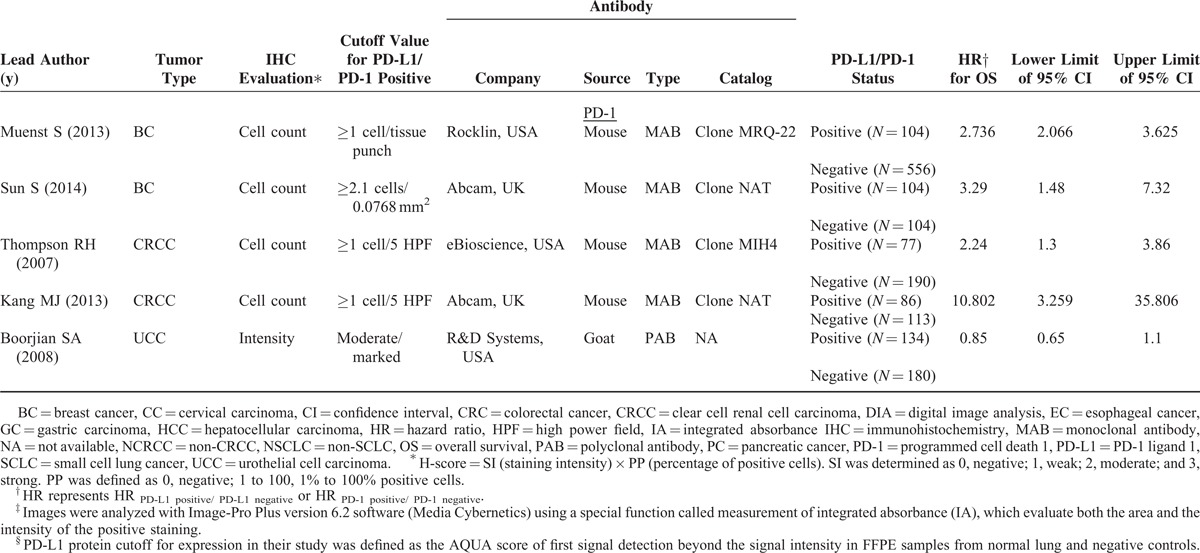
TABLE 1 (Continued).
Characteristics of Included Studies for Meta-Analyses
Meta-Analyses of PD-L1 (PD-1) Positive Versus PD-L1 (PD-1) Negative in Terms of OS
Positive expression of PD-L1 on tumor tissues was associated with significantly poorer OS when compared to those with negative expression of PD-L1 (HR 1.81, 95% CI 1.33–2.46, P < 0.001; Figure 2A) in epithelial-originated cancer patients with an 81% increase in risk for all time mortality. Similarly, patients with PD-1 positive expression on TILs had significantly shorter survival than the PD-1 negative group (HR 2.53, 95% CI 1.22–5.21, P = 0.012; Figure 2B).
FIGURE 2.
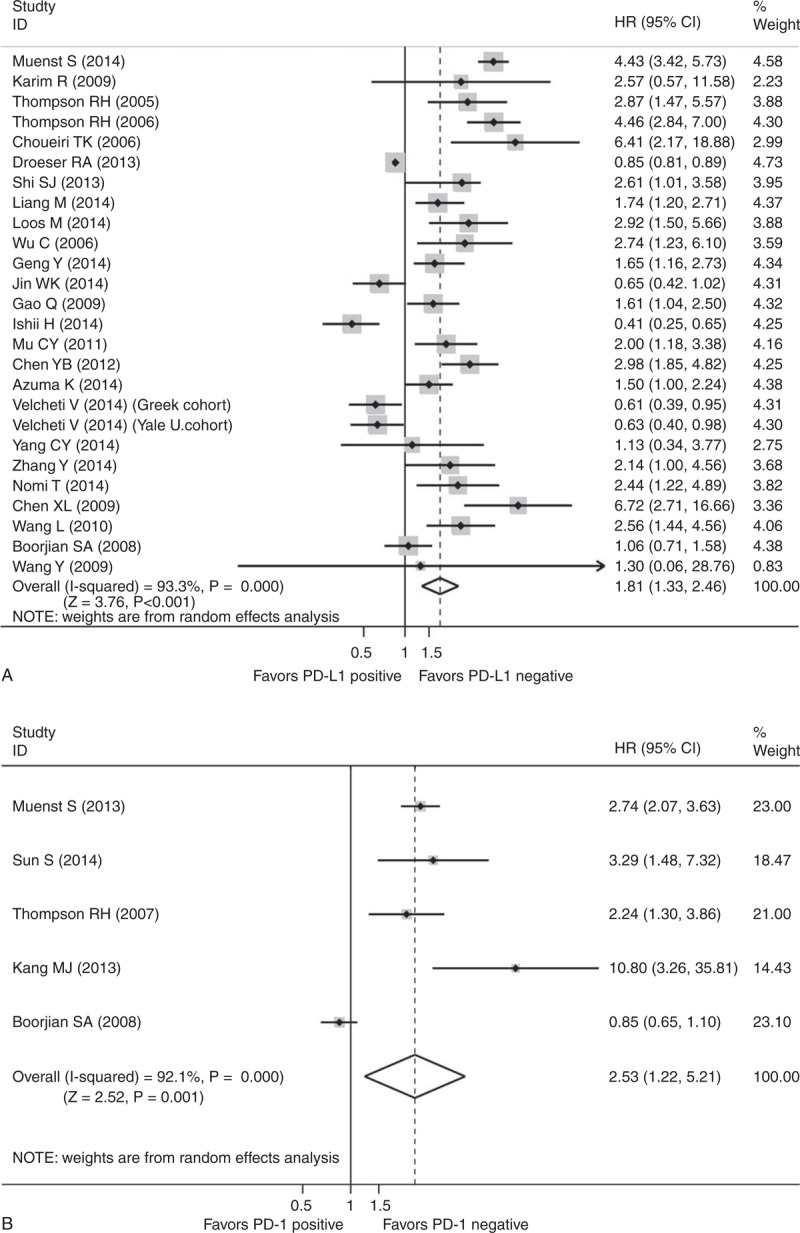
Meta-analysis of PD-L1 positive versus PD-L1 negative on tumor tissues (A) PD-1 positive versus PD-1 negative on tumor infiltrating lymphocytes (B) of epithelial-originated cancer patients in terms of overall survival. CI = confidence interval, HR = hazard ratio, PD-1 = programmed cell death 1, PD-L1 = PD-1 ligand 1.
Subgroup Analyses, Sensitivity Analyses, and Publication Bias
When using percentage evaluation method, it showed numerically inferior survival in PD-L1 positive group if we took 10% (HR 2.16, 95% CI 0.83–5.65, P = 0.115) as the cutoff value, as well as 5% (HR 1.77, 95% CI 0.76–4.15, P = 0.188). The results were similar when using H-score system; both cutoff values presented the adverse prognostic effect of PD-L1 expression (H-score ≤50: HR 1.86, 95% CI 1.40–2.46, P < 0.001; H-score >50: HR 2.26, 95% CI 0.87–5.85, P = 0.093) (Figure 3; Table S2, http://links.lww.com/MD/A205).
FIGURE 3.
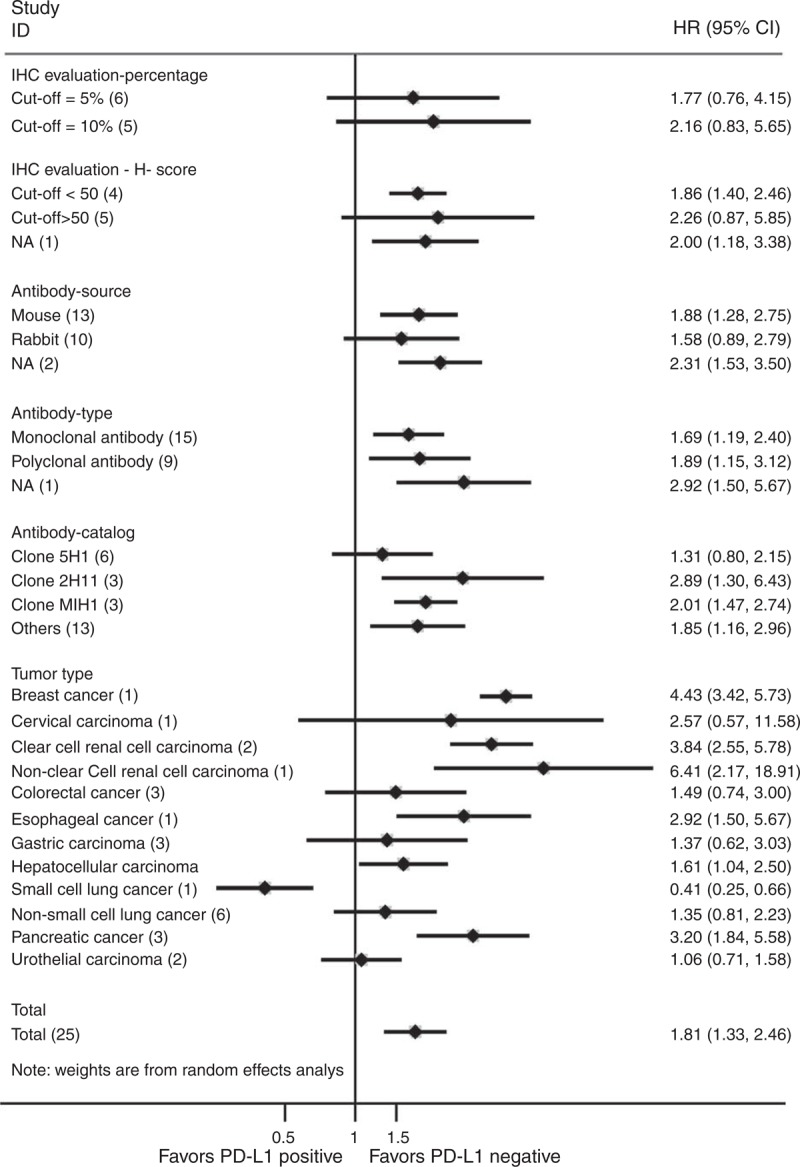
Subgroup analyses of PD-L1 positive versus PD-L1 negative on tumor tissues of epithelial-originated cancer patients in terms of overall survival (number of studies). CI = confidence interval, HR = hazard ratio, IHC = immunohistochemistry, PD-L1 = programmed cell death 1 ligand 1.
Additionally, significantly superior survival was shown in the PD-L1 negative group when murine antibodies were used as primary anti-PD-L1 antibodies (HR 1.88, 95% CI 1.28–2.75, P = 0.001). With respect to the rabbit antibodies, the difference in survival between groups was not significant (HR 1.58, 95% CI 0.89–2.79, P = 0.117). Besides, both monoclonal (HR 1.69, 95% CI 1.19–2.40, P = 0.003) and polyclonal antibodies (HR 1.89, 95% CI 1.15–3.12, P = 0.013) showed statistically different survival between PD-L1 negative and positive groups. As for catalogs, PD-L1 negative patients all presented significantly longer OS when using Clone 2H11 (HR 2.89, 95% CI 1.30–6.43, P = 0.009), Clone MIH1 (HR 2.01, 95% CI 1.47–2.74, P < 0.001), and other antibodies without descriptive details, except for Clone 5H1 (HR 1.31, 95% CI 0.80–2.15, P = 0.291) (more details are in Table S2, http://links.lww.com/MD/A205) (Figure 3).
As an exploratory subgroup analysis, we tried to stratify studies according to tumor type. We observed significant better OS in the PD-L1 negative group for patients with CRCC (HR 3.84, 95% CI 2.55–5.78, P < 0.001) or PC (HR 3.20, 95% CI 1.84–5.58, P < 0.001), while the superiority was not significant in patients with CRC (HR 1.49, 95% CI 0.74–3.00, P = 0.260), GC (HR 1.37, 95% CI 0.62–3.03, P = 0.438), NSCLC (HR 1.35, 95% CI 0.81–2.23, P = 0.252), or UCC (HR 1.06, 95% CI 0.71–1.58, P = 0.761) (Figure 3; Table S2, http://links.lww.com/MD/A205) (The analyses were not available in patients with BC, CC, NCRCC, EC, HCC, or SCLC with only 1 study, each reported PD-L1 status-specific OS).
Only a few studies reported OS stratified by PD-1 status; because of this, subgroup analysis was not suitable or available for this cohort. Funnel plots with the Begg tests are shown in Figure S1, http://links.lww.com/MD/A205.
DISCUSSION
For patients with epithelial-originated malignancies, the association of the expression of PD-L1 or PD-1 and their prognosis remains unclear. A meta-analysis incorporating all available data from correlative studies is a reasonable method to address this question. We conducted this study and found that epithelial-originated cancer patients with positive expression of PD-L1 had significantly poorer survival than with those with negative expression. Additionally, a similar result indicated that PD-1 overexpressed patients had more adverse prognosis compared with the PD-1 negative group. All these results confirmed that activation of PD-L1/PD-1 pathway has a profoundly adverse prognostic impact on cancer patients.
The basis for the above association derived the following interpretations. Firstly, T-cell receptors of TILs recognize tumor-specific antigens when the antigens are presented by the major histocompatibility complex (MHC) on cancer cells. PD-1 is induced to be expressed on T-cells in response to the inflammatory stimuli. Similarly, in response to a normal immune attack, cancer cells can express PD-L1 to inhibit T-cell–mediated antitumor immunity since PD-L1 can recognize and bind the PD-1 on TILs.3 Secondly, PD-L1 expression on tumor cells could lead to tumor cell immune evasion by inducing apoptosis of specific CD8+ cytolytic T cells. In vivo, some tests have proved that the expression of PD-L1 on mouse P815 tumors increased the apoptosis of activated tumor-reactive T cells and promoted the growth of tumors.39 When the pathways of PD-L1/PD-1 activated, cancer cells could evade the immune response and continue to proliferate, which explained poorer survival in PD-L1/PD-1 positive patients.
Subgroup analysis showed that both IHC evaluation methods (the percentage of stained cells and the H-score which combines percentage with staining intensity) displayed consistent prognostic correlation with the overall results. It is notable that the prognostic impact of different PD-L1 status was delineated more greatly when stricter criteria for positive PD-L1 expression was applied. We could see that studies using 10% as cutoff value showed greater difference in OS between PD-L1 positive and negative groups than those using 5%. Since this is a literature-based analysis, we were unable to uniform all the cutoff values across studies. In consideration of big range from >3 to >200 when using H-score method to define the cutoff value for PD-L1 positivity, we conducted a meta-regression analysis to explore the relationship between the cutoff value of PD-L1 positivity and HRPD-L1 positive/PD-L1negative for OS, finding no linear correlation. To manage the diverse H-score values of PD-L1 positivity, we employed a subgroup analysis referring to the cutoff value. Both subgroups showed consistent trends as the general one. Similarly to percentage evaluation, the higher cutoff value (>50) yielded larger HRPD-L1 positive/PD-L1negative. Moreover, the unfavorable prognostic value of PD-L1 was significantly seen in patients with CRCC or PC. Despite some trends observed, we currently cannot draw valid conclusion that PD-L1 status is a predictor of prognosis for patients with CRC, GC, NSCLC, or UCC. In addition, the questions whether PD-L1 status is associated with patient survival in BC, CC, NCRCC, EC, HCC, or SCLC require more clinical evidence. Considering that we do not yet have perfect standardized antibodies for assessment of PD-L1, we conducted relative subgroup analyses according to resources, types, and catalogs of antibodies. We found that 2 kinds of murine monoclonal anti-PD-L1 antibodies, Clone 2H11 and Clone MIH1, were strongly correlated with the prognostic value of PD-L1. The pooled subgroup result also showed unfavorable prognosis in PD-L1 positive patients when using Clone 5H1 as primary anti-PD-L1 antibody. But the statistic was not significant, as a result of controversial results reported by relevant studies.8,11,16–18,29 In future studies, some key issues are the rigorous antibody validation and exclusion of antibodies that cross react with other proteins, as shown by either western blot or IHC. Considering the consistent trends in all subgroups regarding evaluation methods and antibodies, lack of uniform methods and criteria should not be a barrier to a pooled analysis, to illustrate the prognostic significance of PD-L1 in epithelial-originated cancer.
To the best of our knowledge, this is the first study to comprehensively answer the impact of PD-L1 or PD-1 status on patient prognosis in epithelial-originated malignancies. However, several limitations existed: This meta-analysis was mostly based on the extracted data from the survival curves by the indirect method,13 which somehow compromised the precision of data. Cutoff values distinguishing high or low level of PD-L1 expression determined by IHC evaluation and the primary antibodies varied in different types of tumors, which might cause the heterogeneity of the overall results. The subgroup results should have addressed some concerns. We were not able to evaluate the prognostic value of PD-L1 in several tumor types in subgroup analysis due to a lack of data. Few studies mentioned cancer patients’ survival stratified by PD-1 status so that subgroup analyses have not been performed. Most of the eligible studies failed to provide data regarding progression-free survival or recurrence-free survival so we only extracted OS data in our meta-analysis. Researchers might prefer to only report the positive results of the prognostic biomarker, which led to the existence of potential publication bias. In addition, few studies evaluated PD-L1 and PD-1 simultaneously, which prevented us from insightful explanation of mechanism. Further studies are warranted to complete the above information.
Regardless of above limitations, this comprehensive analysis statistically confirmed that epithelial-originated cancer patients with positive expression of PD-L1 or PD-1 were associated with significant shorter OS, especially in PD-L1 positive patients with CRCC and PC. The result gave an important hint that, in clinical trials using anti-PD-L1 or anti-PD-1 antibodies as cancer immunotherapy, enrollment might be preferentially carried out on patients with the tumor types mentioned above. Furthermore, more efforts should be made to investigate the reason why the prognostic value of PD-L1 or PD-1 was in different levels in various epithelial-originated malignancies.
In conclusion, we confirmed that PD-L1 or PD-1 status evaluated by IHC staining is a predictor of patient prognosis in epithelial-originated malignancies.
Footnotes
Abbreviations: BC = breast cancer, CC = cervical carcinoma, CI = confidence interval, CRCC = clear cell renal cell carcinoma, CRC = colorectal cancer, EC = esophageal cancer, GC = gastric carcinoma, HCC = hepatocellular carcinoma, HR = hazard ratio, IFN-γ = interferon gamma, IL-2 = interleukin-2, MHC = major histocompatibility complex, NCRCC = nonclear cell renal cell carcinoma, NSCLC = nonsmall cell lung cancer, OS = overall survival, PC = pancreatic cancer, PD-1 = programmed cell death 1, PD-L1 = PD-1 ligand 1, SCLC = small cell lung cancer, TILs = tumor-infiltrating lymphocytes, TNF-α = tumor necrosis factor alpha, UCC = urothelial carcinoma.
The first 3 authors contributed equally to this article, and all should be considered first author.
YZ, JH, and WL conceived and designed the experiments. YZ, SK, and JS carried out the search. JH, LJ, and WW extracted the data. ZG, GP, and GC assessed the quality of included studies. YZ and SK conducted the statistical analyses. YZ, JH, and WL wrote the manuscript. All of the authors conducted a primary revision.
This study is supported by initiating funding for PhD graduates, Guangzhou Medical University (No. 2014C27).
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Korman AJ1, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol 2006; 90:297–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 2012; 24:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu J, Lee-Gabel L, Nadeau MC, et al. Clinical evaluation of compounds targeting PD-1/PD-L1 pathway for cancer immunotherapy. J Oncol Pharm Pract 2014; pii:1078155214538087. [DOI] [PubMed] [Google Scholar]
- 4.Hamid O, Carvajal RD. Anti-programmed death-1 and anti-programmed death-ligand 1 antibodies in cancer therapy. Expert Opin Biol Ther 2013; 13:847–861. [DOI] [PubMed] [Google Scholar]
- 5.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol 2008; 8:467–477. [DOI] [PubMed] [Google Scholar]
- 6.Flies DB, Chen L. The new B7 s: Playing a pivotal role in tumor immunity. J Immunother 2007; 30:251–260. [DOI] [PubMed] [Google Scholar]
- 7.Muenst S, Schaerli AR, Gao F, et al. Expression of programmed death ligand 1 (PD-L1) is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2014; 146:15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thompson RH, Kuntz SM, Leibovich BC, et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res 2006; 66:3381–3385. [DOI] [PubMed] [Google Scholar]
- 9.Kim JW, Nam KH, Ahn SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer 2014; doi: 10.1007/s10120-014-0440-5. [DOI] [PubMed] [Google Scholar]
- 10.Ishii H, Azuma K, Kawahara A, et al. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol 2014; doi: 10.1097/JTO.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 11.Boorjian SA, Sheinin Y, Crispen PL, et al. T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res 2008; 14:4800–4808. [DOI] [PubMed] [Google Scholar]
- 12.Muenst S, Soysal SD, Gao F, et al. The presence of programmed death 1 (PD-1)-positive tumor-infiltrating lymphocytes is associated with poor prognosis in human breast cancer. Breast Cancer Res Treat 2013; 139:667–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [Google Scholar]
- 15.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 16.Karim R, Jordanova ES, Piersma SJ, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res 2009; 15:6341–6347. [DOI] [PubMed] [Google Scholar]
- 17.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory molecule B7-H1 in primary and metastatic clear cell renal cell carcinoma. Cancer 2005; 104:2084–2091. [DOI] [PubMed] [Google Scholar]
- 18.Droeser RA, Hirt C, Viehl CT, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer 2013; 49:2233–2242. [DOI] [PubMed] [Google Scholar]
- 19.Shi S, Wang L, Wang G, et al. B7-H1 expression is associated with poor prognosis in colorectal carcinoma and regulates the proliferation and invasion of HCT116 colorectal cancer cells. PLoS ONE 2013; 8:e76012.doi: 10.1371/journal.pone.0076012.eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang M, Li J, Wang D, et al. T-cell infiltration and expressions of T lymphocyte co-inhibitory B7-H1 and B7-H4 molecules among colorectal cancer patients in northeast China's Heilongjiang province. Tumour Biol 2014; 35:55–60. [DOI] [PubMed] [Google Scholar]
- 21.Loos M, Langer R, Schuster T, et al. Clinical significance of the costimulatory molecule B7-H1 in Barrett carcinoma. Ann Thorac Surg 2011; 91:1025–1031. [DOI] [PubMed] [Google Scholar]
- 22.Wu C, Zhu Y, Jiang J, et al. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 2006; 108:19–24. [DOI] [PubMed] [Google Scholar]
- 23.Geng Y, Wang H, Lu C, et al. Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3+ Tregs in gastric cancer and its clinical significance. Int J Clin Oncol 2014; doi: 10.1007/s10147-014-0701-7. [DOI] [PubMed] [Google Scholar]
- 24.Gao Q, Wang XY, Qiu SJ, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res 2009; 15:971–979. [DOI] [PubMed] [Google Scholar]
- 25.Choueiri TK, Fay AP, Gray KP, et al. PD-L1 expression in nonclear-cell renal cell carcinoma. Ann Oncol 2014; 25:2178–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mu C, Huang J, Chen Y, et al. High expression of PD-L1 in lung cancer may contribute to poor prognosis and tumor cells immune escape through suppressing tumor infiltrating dendritic cells maturation. Med Oncol 2011; 28:682–688. [DOI] [PubMed] [Google Scholar]
- 27.Chen YB, Mu CY, Huang JA. Clinical significance of programmed death-1 ligand-1 expression in patients with non-small cell lung cancer: a 5-year-follow-up study. Tumori 2012; 98:751–755. [DOI] [PubMed] [Google Scholar]
- 28.Azuma K, Ota K, Kawahara A, et al. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected non-small cell lung cancer. Ann Oncol 2014; 25:1935–1940. [DOI] [PubMed] [Google Scholar]
- 29.Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014; 94:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang C, Lin M, Chang Y, et al. Programmed cell death-ligand 1 expression in surgically resected stage I pulmonary adenocarcinoma and its correlation with driver mutations and clinical outcomes. Eur J Cancer 2014; 50:1361–1369. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Wang L, Li Y, et al. Protein expression of programmed death 1 ligand 1 and ligand 2 independently predict poor prognosis in surgically resected lung adenocarcinoma. Onco Targets Ther 2014; 7:567–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nomi T, Sho M, Akahori T, et al. Clin Cancer Res 2007; 13:2151–2157. [DOI] [PubMed] [Google Scholar]
- 33.Chen XL, Yuan SX, Chen C, et al. Expression of B7-H1 protein in human pancreatic carcinoma tissues and its clinical significance. Ai Zheng 2009; 28:1328–1332. [DOI] [PubMed] [Google Scholar]
- 34.Wang L, Ma Q, Chen X, et al. Clinical significance of B7-H1 and B7-1 expressions in pancreatic carcinoma. World J Surg 2010; 34:1059–1065. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Zhuang Q, Zhou S, et al. Costimulatory molecule B7-H1 on the immune escape of bladder cancer and its clinical significance. J Huazhong Uni Sci Technolog Med Sci 2009; 29:77–79. [DOI] [PubMed] [Google Scholar]
- 36.Sun S, Fei X, Mao Y, et al. PD-1 (+) immune cell infiltration inversely correlates with survival of operable breast cancer patients. Cancer Immunol Immunother 2014; 63:395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson RH, Dong H, Lohse CM, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res 2007; 13:1757–1761. [DOI] [PubMed] [Google Scholar]
- 38.Kang MJ, Kim KM, Bae JS, et al. Tumor-infiltrating PD1-positive lymphocytes and FoxP3-positive regulatory T cells predict distant metastatic relapse and survival of clear cell renal cell carcinoma. Transl Oncol 2013; 6:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dong H, Strome SE, Salomao DR, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med 2002; 8:793–800. [DOI] [PubMed] [Google Scholar]


