Abstract
The inferior colliculus is a mesencephalic structure endowed with serotonergic fibers that plays an important role in the processing of acoustic information. The implication of the neuromodulator serotonin also in the aetiology of sudden unexplained fetal and infant death syndromes and the demonstration in these pathologies of developmental alterations of the superior olivary complex (SOC), a group of pontine nuclei likewise involved in hearing, prompted us to investigate whether the inferior colliculus may somehow contribute to the pathogenetic mechanism of unexplained perinatal death. Therefore, we performed in a wide set of fetuses and infants, aged from 33 gestational weeks to 7 postnatal months and died of both known and unknown cause, an in-depth anatomopathological analysis of the brainstem, particularly of the midbrain. Peculiar neuroanatomical and functional abnormalities of the inferior colliculus, such as hypoplasia/structural disarrangement and immunonegativity or poor positivity of serotonin, were exclusively found in sudden death victims, and not in controls. In addition, these alterations were frequently related to dysgenesis of connected structures, precisely the raphé nuclei and the superior olivary complex, and to nicotine absorption in pregnancy. We propose, on the basis of these results, the involvement of the inferior colliculus in more important functions than those related to hearing, as breathing and, more extensively, all the vital activities, and then in pathological conditions underlying a sudden death in vulnerable periods of the autonomic nervous system development, particularly associated to harmful risk factors as cigarette smoking.
INTRODUCTION
The inferior colliculi (ICs), that are the caudal eminences of the corpora quadrigemina in the tectal region of the midbrain, are the principal mesencephalic nuclei of the auditory pathway, and play an important role in a number of pathophysiological conditions that involve the hearing.1–3
The IC has been shown to be an integrating station that filters sounds from vocalization, chewing, and breathing via input from different neuromodulatory systems that originate in specific brainstem nuclei. Among the neuromodulators demonstrated in the IC, indoleamine-5-hydroxytryptamine (5HT or serotonin) has received the closest attention.4–8 Different methodologic approaches, such as histological, immunohistochemical, chromatographic techniques and electrical/chemical stimulation, have confirmed the presence in the IC of many mammalian species of serotonin fibers arising from both the rostral and the caudal raphé nuclei groups in the brainstem, and more frequently from the dorsal raphé nucleus.9,10 The presence of serotonin in the IC is in general considered essential to modulate the brain responses to auditory stimuli.
Moreover, serotonin is implicated in wider functions, including trophic effects during central nervous system (CNS) development, the regulation of cardiovascular and respiratory activities and sleep behavior.11–13
Previously, we have reported its involvement also in sudden intrauterine unexplained death syndrome (SIUDS) and sudden infant death syndrome (SIDS) pathogenesis14. We highlighted that, in addition to causing morphological developmental alterations of the raphé nuclei, serotonin network dysfunctions result in failure of autonomic and respiratory responses to hypoxia and/or hypercapnia, triggering a death mechanism in both intrauterine and postnatal life.
In addition, in victims of SIUDS and SIDS we have demonstrated a high incidence of developmental alterations of the superior olivary complex (SOC), a group of pontine nuclei likewise involved in multiple aspects of hearing, with dense projections terminating in the IC.15
In this study, we proposed to evaluate whether the presence of serotonin in the IC is directed not only to integrating auditory inputs but also to mediating basic vital functions. Therefore we aimed to: (1) clearly delineate the cytoarchitecture of the human IC in a cohort of fetuses and infants who died of both known and unknown causes (2) validate the presence of serotonin fibers in this structure, as in other mammalian species, by applying the specific immunohistochemical methods; (3) highlight possible morphological and functional alterations of the IC in the study subjects, and their relation with developmental defects of the connected brainstem centers, particularly the raphé nuclei and the SOC.
MATERIALS AND METHODS
Study Subjects
The study included 81 fetuses and infants, sent to our Research Center and diagnosed according to the application of the guidelines stipulated by Italian law n.31/2006 “Regulations for Diagnostic Post Mortem Investigation in Victims of Sudden Infant Death Syndrome (SIDS) and Sudden Intrauterine Unexpected Death Syndrome (SIUDS).” This law decrees that all infants with suspected SIDS who died suddenly in Italian regions within the first year of age, as well as all fetuses who died without any apparent cause (SIUDS), must undergo an in-depth anatomo-pathological examination, particularly of the autonomic nervous system. Parents of all subjects provided written informed consent to the examination under protocols approved by the institutional review board of the Milan University L. Rossi Research Center.
Table 1 summarizes the groups of subjects analyzed in this study. In particular, after the routine autopsy and clinical history analysis, the death remained unexplained in 53 cases. A diagnosis of “SIUDS” was made for 24 fetuses (group II), 14 females and 10 males, aged 33–41 gestational weeks (mean age: 37 ± 3 gestational weeks), and a diagnosis of “SIDS” for 29 infants (group IV), 15 females and 14 males, who died within the first 7 months of life (mean age: 3 ± 2 months). In the remaining 28 cases, 13 stillbirths, and 15 infants, a precise cause of death was formulated at autopsy. These cases, age-matched with the SIUDS/SIDS groups, were regarded as “controls.” Specific diagnoses among the fetal controls (group I) were severe chorioamnionitis (n = 7) and congenital heart disease (n = 6). Infant control deaths (group III) were caused by congenital heart disease (n = 7), severe bronchopneumonia (n = 3), myocarditis (n = 1), pulmonary dysplasia (n = 2), pneumonia with acute respiratory distress (n = 1), and mucopolysaccharidosis type I (n = 1).
TABLE 1.
Case profile of the study (N = 81)
Neuropathological Examination
In all cases an in-depth histological examination of the ANS was performed, with the principal aim of detecting even fine developmental alterations of the brainstem structures controlling the vital functions, particularly in victims of sudden unexplained death, for which a clear pathological explanation has not yet been gained. The study methodology is reported in the protocol of the Lino Rossi Research Center of the Milan University available at the website: http://users.unimi.it/centrolinorossi/en/guidelines.html.
Specifically, after fixation in 10% phosphate-buffered formalin, the brainstem was processed and embedded in paraffin. Then 3 specimens were taken. The first specimen included the upper third of the pons and the adjacent caudal portion of midbrain, below the superior colliculus and including the inferior colliculus, the main focus of this study; the second specimen extended from the upper third of the medulla oblongata to the adjacent caudal portion of the pons; the third specimen included the obex. Transverse serial sections from the three samples were made at intervals of 60 μm. For each level, six 4 μm sections were obtained, three of which were routinely stained for histological examination using hematoxylin–eosin, Klüver-Barrera and Bielschowsky's silver impregnation technique. The remaining sections were subjected to immunohistochemical study of the serotonin neurotransmitter.
Histological Examination of the Brainstem
The routine histological evaluation of the brainstem was focused on the locus coeruleus, the parafacial/facial complex, the SOC, the Kölliker-Fuse, median and magnus raphé nuclei in the pons; on the inferior colliculus, the substantia nigra, the dorsal and caudal linear raphé nuclei in the caudal mesencephalon; on the hypoglossus, the dorsal motor vagal, the tractus solitarius, the ambiguus, the inferior olivary, the -Bötzinger, the arcuate and the obscurus and pallidus raphé nuclei in the medulla oblongata. All histological analyses were carried out by 2 independent and blinded observers and comparison of the results was performed to evaluate the inter-observer reproducibility.
Immunohistochemical Study
Serotonergic immunohistochemistry was performed to map the 5-HT-containing neurons and fibers, using a specific 5-HT monoclonal antibody. Selected tissue sections, after deparaffinization and rehydration, were immersed in EDTA 0.05 M pH8, boiled 3 times for 5 minutes in a pressure cooker and washed with TBS buffer. Each section was placed on the Dako cytomation automated immunostainer and incubated with the specific monoclonal antibody at room temperature for 45 minutes, washed with TBS pH 7.6 and incubated in biotinylated goat anti-mouse anti-rabbit immunoglobulins (Dako REAL™, cod.K5005, Dako cytomation, Glostrup, Denmark) at room temperature for 30 minutes. After incubation with the secondary antibody and further washing with TBS pH 7.6, sections were incubated with streptavidin conjugated to alkaline phosphatase at room temperature for 30 minutes. A red chromogen solution was prepared as indicated by the Dako REAL™ datasheet and used as enzyme substrate. Finally, each section was counterstained in Mayer's hematoxylin solution and coverslipped.
A set of sections from each study group was used as negative control. The slides were stained using the same procedure but omitting the primary antibody to check that the immunolabeling was not due to nonspecific labeling by the secondary antibody.
Risk Factors Information
For each case, all available information about pregnancy, fetal development and delivery and, in cases of infant death, about the environmental and familial situation where the death occurred, besides information related to microorganisms and bacterial toxin investigations, genetic analyses and potential both “preventable” and “unpreventable” risk factors,16 were collected and categorized during post-mortem family interviews. Among the preventable risk factors, great attention was paid to cigarette smoke, and especially to a maternal smoking habit already before the beginning of pregnancy. In all, 36 of the 54 mothers of victims belonging to groups II and IV of Table 1 (SIUDS and SIDS) (66%) and 8 (29%) belonging to the control groups (I and III) had been active smokers since they were quite young. All the information sheets were recorded in the registry of a dedicated data bank, as stipulated by law 31.
Statistical Analysis
Results were tabulated and differences assessed by analysis of variance (ANOVA), comparing pairs of groups. Statistical calculations were carried out with SPSS statistical software (version 11.0; SPSS, Inc., Chicago, IL). The threshold level set for statistical significance was P < 0.05.
RESULTS
Morphological Examination of the Inferior Colliculus (IC)
Control Cases (Groups I and III of Table 1)
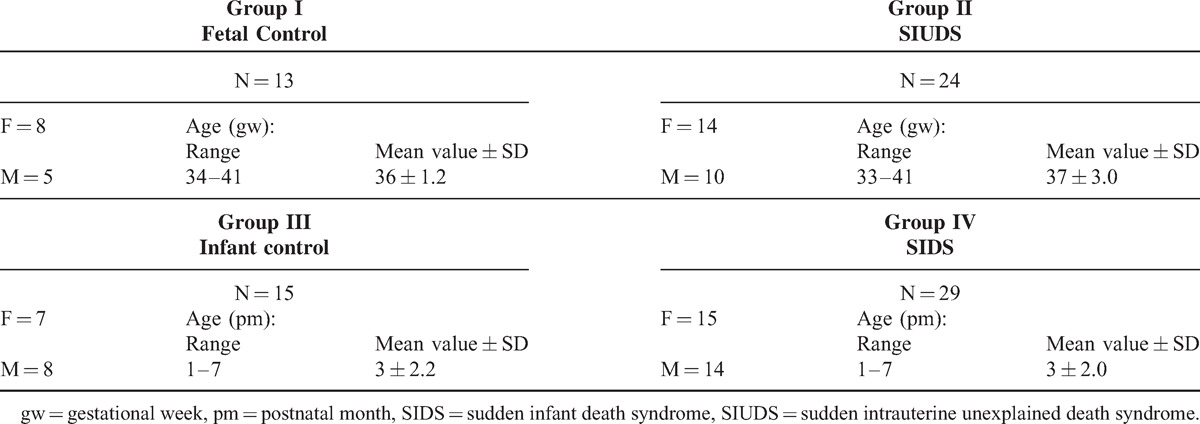
Firstly, we proceeded to define the cytoarchitecture of the IC, the target of this study, in transverse sections of caudal midbrain in control cases. In fetuses and, in the same way in infants, the IC showed a predominant central, large, dark core, oval in shape with its long axis directed dorso-laterally, surrounded by a light peripheral layer. Similarly to the IC definition in animal studies, we recognized 3 histological subdivisions: a central nucleus (CN), a dorsal cortex (DC), and an external cortex (EC) located laterally (Figure 1).
FIGURE 1.
Transverse histological hemisection of caudal midbrain showing the localization of the inferior colliculus (IC) and its subdivisions: the central nucleus (CN); the external cortex (EC) and the dorsal cortex (DC). Control newborn (2 months old). Klüver Barrera staining. Magnification 4×.
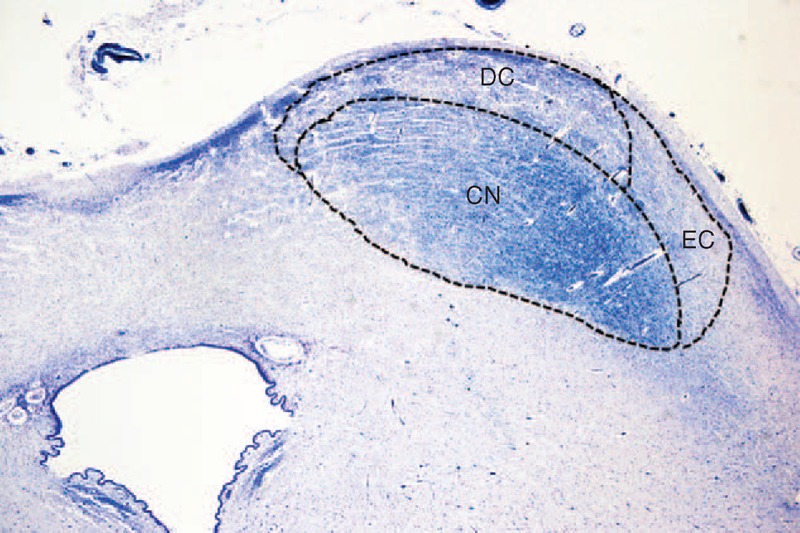
The CN was characterized by a marked cellularity with 2 main cell types randomly arranged in an intense tangle of myelinized fibers. The majority of cells were small, oval, spindle or triangular shaped, without a distinct nucleus. There were also, but less widespread, large/medium-sized round, oval or triangular cells with an evident nucleus and nucleolus, both usually centrally placed. In both the DC and EC there were few small neurons, prevalently fusiform in shape with sketchy processes (Figure 2).
FIGURE 2.
Histological aspects of the IC subnuclei. (A) Central nucleus—marked cellularity with small to large neurons with different morphologies (oval, round, triangular) and presence of myelinized fibers. (B) Dorsal cortex—small cells, prevalently fusiform in shape with a poor neuropil. (C) Microphotograph of the IC, indicating where the images in (A) and (B) came from. Control fetus (34 gestational weeks). Bielschowsky's silver impregnation technique. Magnification (A) and (B) 40× and (C) 2×.
SIUDS and SIDS Cases (Groups II and IV of Table 1)
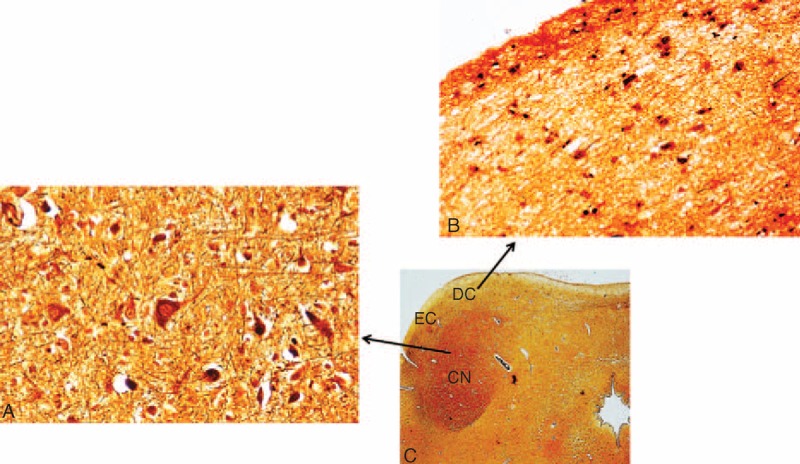
Developmental alterations of the IC were observed in a large number of victims of sudden death, precisely in 62% of SIUDS and 59% of SIDS. In particular, the CN was unidentifiable or scarcely identifiable in all the serial histological sections of caudal midbrain in 11 SIUDS and 13 SIDS cases (IC hypoplasia) (Figure 3A). In these cases, widespread neurons with poor dendritic arborization were homogeneously distributed in the entire area of the colliculus. A marked disarrangement of the CN, frequently subdivided into 2 or 3 portions by the insertion of tongues protruding from the DC, was detected in 4 SIUDS and 4 SIDS cases (Figure 3B and C).
FIGURE 3.
Morphological alterations of the IC. (A) Lack of the central nucleus. (B) and (C) Subdivision of the central nucleus in 2 and 3 portions, respectively, due to the insertion of strips from the dorsal cortex. (A) SIDS case (3 months old); (B) SIUDS case (35 gestational weeks); (C) SIDS case (2 months old). Klüver Barrera staining. Magnification 4×.
Serotonin Immunohistochemical Examination in the Inferior Colliculus (IC)
Control Cases (Groups I and III of Table 1)
Serotonin-immunopositivity was observed in 25 of the 28 control cases. Staining was mainly localized in fiber structures, mostly consisting of dot-like varicosities having a typical beads-in-a-row morphology with densities ranging from low to very high. Serotonergic fibers were most plentiful in the CN, forming a dense network, and less dense in the DC and EC (Figure 4). The positive fibers generally surrounded non-immunoreactive neurons, even if very few immunoreactive perikarya were sometimes observed. In 3 fetal control deaths (group I) 5-HT immunoreactivity was not clearly evident.
FIGURE 4.
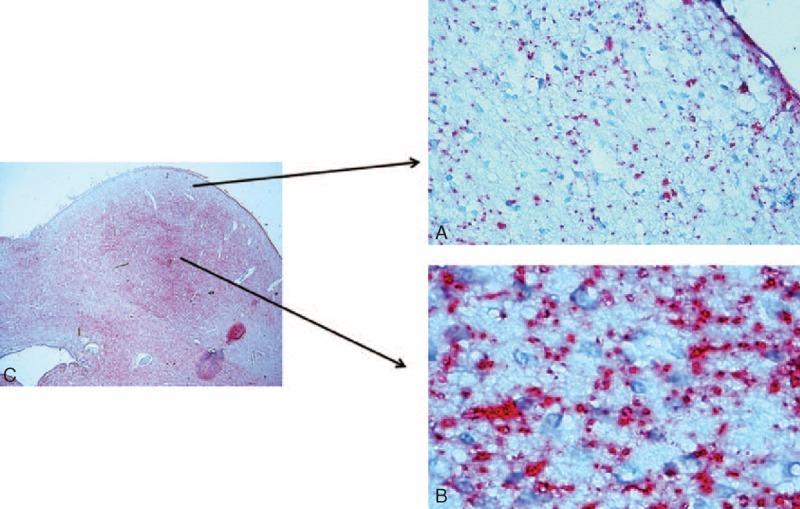
Serotonin-immunopositive fibers in the IC showing the typical dot-like varicosities. They are less diffuse in the cortex (A) than in the central nucleus (B). Note the general immunonegativity of the neuronal bodies. In (C) Microphotograph of the inferior colliculus indicating where the images in (A) and (B) came from. Control infant (6 months old). 5-HT immunohistochemistry. Magnification (A) and (B) 40× and (C) 4×.
SIUDS and SIDS Cases (Groups II and IV of Table 1)
Unlike in the greater part of the control groups cases, immunoreactive serotonergic fibers were absent or very reduced in all the subdivisions of the IC, if present, in 15 SIUDS and 19 SIDS cases (Figure 5).
FIGURE 5.
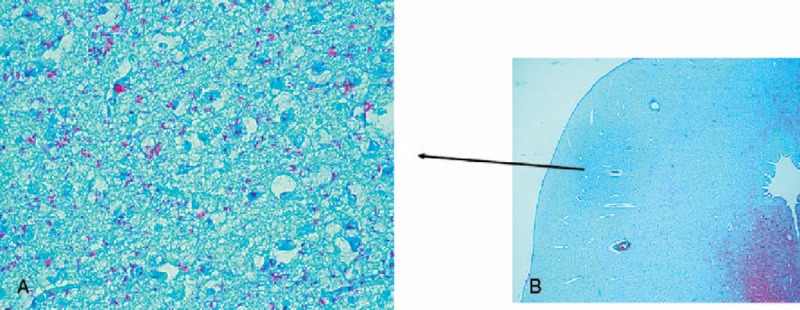
Poor serotonin-immunopositivity in the central nucleus of the IC of a SIDS case (4 months old). Note on the contrary the intense 5-HT immunostaining in the periaqueductal area. 5-HT immunohistochemistry. Magnification (A) 20x; (B) 4x.
Additional Results on Brainstem
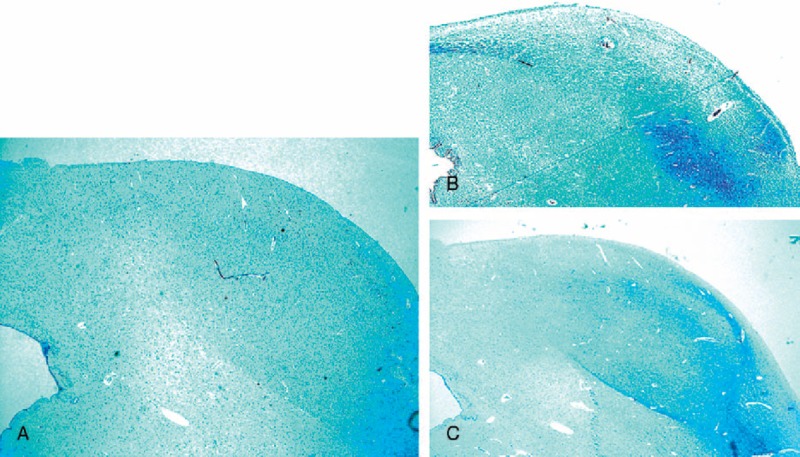
Histological examination of the overall brainstem structures showed frequent hypodevelopment of one or more nuclei of both the serotonergic rostral and caudal raphé nuclei in 28 SIUDS/SIDS cases. The presence of hypoplasia of the mesencephalic dorsal raphé nucleus (DRN) in 11 SIUDS and 10 SIDS with morphofunctional anomalies of the IC was very interesting (Figure 6). The finding in these same SIUDS/SIDS cases of dysgenesis of the medial superior olivary nucleus (MSON), the main component of the SOC, was also noteworthy. This nucleus was largely devoid of fibers, with few scattered immature neurons (Figure 7). In 4 control cases (2 in group I and 2 in group III) there was hypoplasia of the raphé obscurus nucleus. Structural alterations of the MSON were observed only in one control infant (group III).
FIGURE 6.
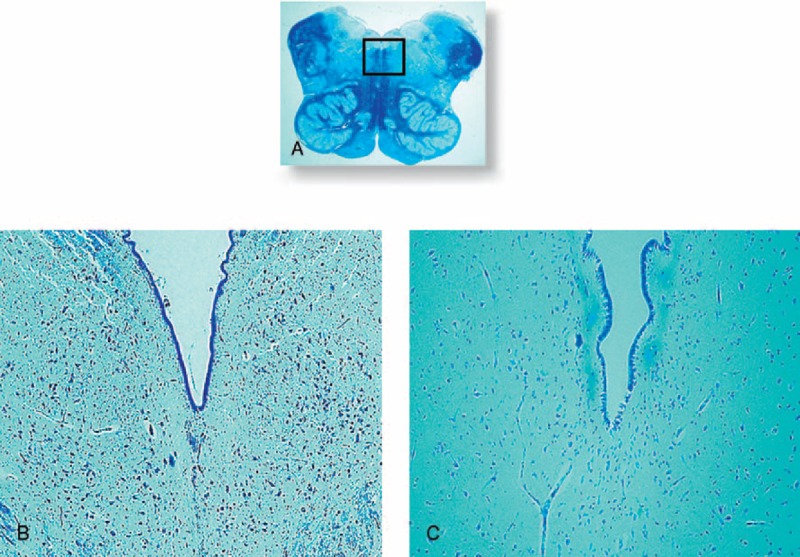
Dorsal raphé nucleus. In (A) histological section of medulla oblongata showing in the framed area the localization of the DRN. In (B) normal structure in a control fetus (39 gestational weeks) with many neurons. In (C) hypoplasia of the DRN with few neurons in an age-matched SIUDS case. Klüver Barrera staining. Magnification (A) 0.5x; (B) and (C) 10x.
FIGURE 7.
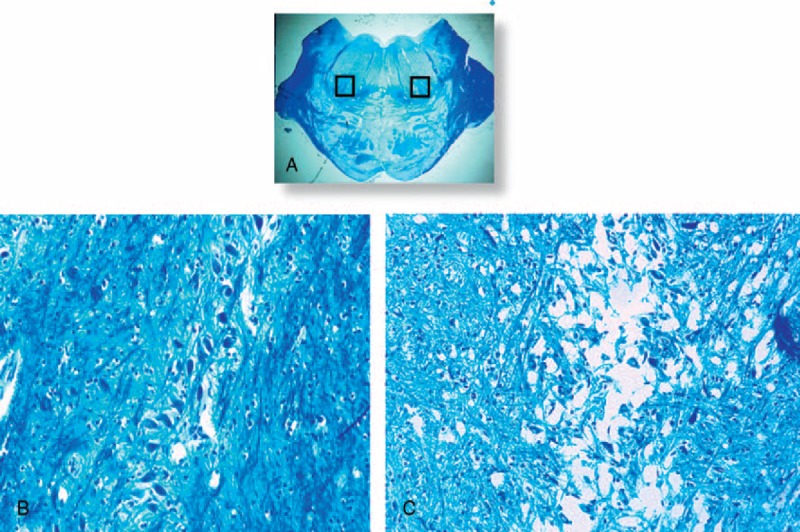
Medial superior olivary nucleus. In (A) histological section of caudal pons showing in the framed area the localization of the MSON. In (B) the normal structure in a control infant (3 months old) showing a compact layer of neurons included in a narrow column. In (C) hypoplasia with few scattered neurons and a lack of fibers in an age-matched SIDS case. Klüver Barrera staining. Magnification (A) 0.5x; (B) and (C) 20x.
Table 2 summarizes all the neuropathological results.
TABLE 2.
Neuropathological Findings in SIUDS, SIDS and Controls§
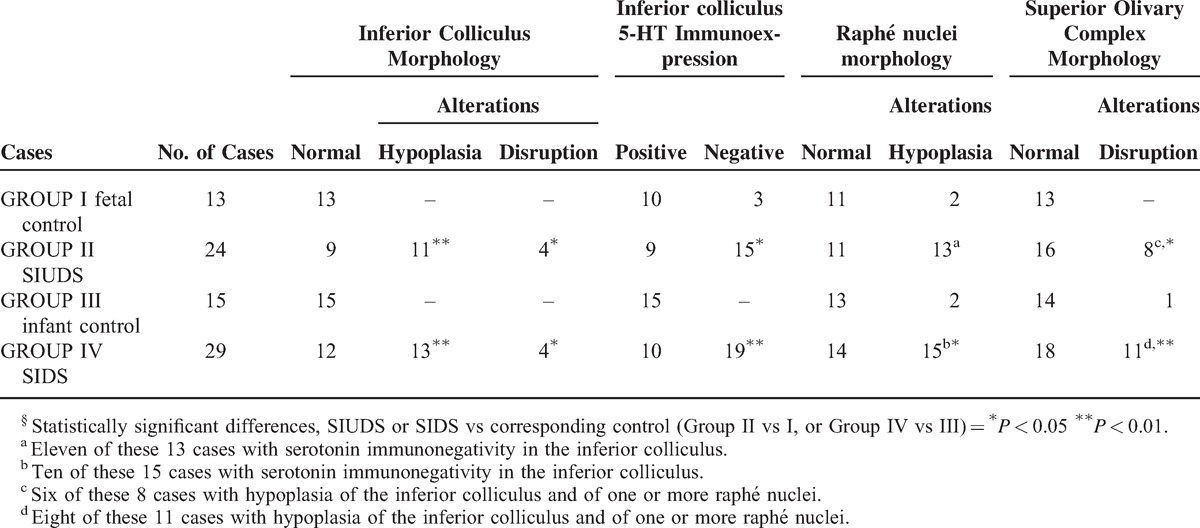
Correlation of Findings With Risk Factors
We observed a statistically significant correlation between maternal smoking, the most important among the “preventable” risk factors, and the presence of developmental alterations of many brainstem nuclei (P < 0.01). In particular, 26 of the 36 victims of sudden death with a smoker mother (72%) showed one or more defects of the IC, frequently associated with hypoplasia of both the DRN and the MSON. Among the controls, 2 of the 3 cases of the Group I (Fetal Control) with a deficient serotonin expression in the IC, as well as the 4 cases of the Group III (Infant Control) with SOC hypoplasia, had a smoker mother, confirming the role of smoke absorption during pregnancy in pathogenic development of the autonomic nervous system, already pointed out in our previous works in this field.17–20
DISCUSSION
The present study is the first to report the morphological and functional characterization of the inferior colliculus (IC), a component of the mesencephalic corpora quadrigemina, in perinatal life, and various pathological aspects observed in SIUDS/SIDS cases.
Because the IC is known to be an important site of convergence of auditory information coming from both ears,1,2 we undertook this research as a sequel to our previous paper on the SOC, a group of pontine nuclei that are likewise important in the processing of acoustic information, and that have dense projections terminating in the IC.14 In that study, we highlighted the involvement of the SOC also in more important survival functions than those related to hearing, such as breathing and, more extensively, all the vital activities, as well as in pathogenic mechanisms underlying sudden death in a vulnerable period of perinatal autonomic nervous system development. Therefore, in the present study we proposed to evaluate whether the IC, morphologically and functionally linked to the SOC, could have similar features and functions.
In addition, the knowledge gained from the Conrad Simon Memorial Research Initiative,21 demonstrating that the IC shows a comparatively higher rate of metabolic activity than several other areas of the brain, supported our hypothesis that neuronal developmental disarrangements of this structure might play a fundamental causal role in SIUDS and SIDS. The Conrad Simon Memorial Research Initiative measured the blood flow of the IC in the cat brain and put the number at 1.80 cc/g/minute. As reference, the runner-up in the included measurements was the somatosensory cortex, running at 1.53. This indicates that the inferior colliculus is metabolically more active than many other parts of the brain.
We recognized, in a wide set of brainstems obtained from fetuses and newborns, 3 compartments in the IC, characterized by a dyshomogeneous distribution of serotonin-immunoreactive fibers, with a beads-in-a-row morphology, similar to those described in findings from experimental studies. However, the fiber concentration is higher in both the dorsal and external cortices of the IC, particularly in rat brain, while it is poorer in the CN.9,22,23 On the contrary, our observations indicated that in humans the serotonergic fibers are more dense in the CN as compared to the external and dorsal IC compartments.
Frequently, we observed serotonin fiber varicosities closely apposed to unstained cells, suggesting that they are associated with the neuronal cell bodies in a synaptic relationship. Accordingly, several authors have proposed that serotonin in the IC neurons could be directly released from these varicosities, unlike the classical neurotransmitter synapses.24,25
These results, besides showing a dissimilar density and distribution of serotonergic fibers in the IC among different species, provide anatomical evidence that some neuron types receive more serotonergic input and therefore may be more subject to serotonergic modulation than others. The differential distribution of serotonin in the IC subnuclei suggests that this transmitter system might influence different functional circuits and differentially interact with vital brainstem centers.
The serotonergic fibers of the IC originate in the chain of raphé nuclei distributed along the midline of the brainstem from the medulla oblongata to the midbrain. In particular, several authors have reported that serotonergic neurons of the mesencephalic dorsal raphé nucleus specifically innervate, across the forebrain, auditory centers including the IC.9,10
We demonstrated that immunoreactive serotonergic fibers, very evident in the IC in controls and particularly in the CN, as reported above, were greatly reduced in SIUDS/SIDS cases. Our data indicate that serotonin expression in the IC may play a role not only in central auditory processing, but also in the control of brainstem centers that are essential for vital functions.
In particular, the relation of sensory systems with the sleep–wake cycle has been frequently proposed.26 Acoustic stimulation has always been used to lull babies, and sleep facilitated by reducing noisy inputs afferent to the brain.27,28 On the contrary, one of the triggering events of SIDS has been identified as a loud noise that causes a sudden waking from sleep.16,29 This event is, in fact, associated with a rapid change in sympathetic activity that predisposes the heart to arrhythmias, leading to a lethal reflexogenic mechanism (the so-called “cardioauditory death”).30 A similar mechanism can also occur in adults.31
Already in 1935, Bremer32 had emphasized the relevance of sensory stimulations in maintaining the “central tonus” supporting wakefulness and the vital activities. In the waking state, in fact, the brainstem controls the main physiological parameters, such as the metabolism, blood flow, oxygen distribution, breathing, temperature, and endocrine system.
Serotonin in the IC is closely dependent on behavioral changes occurring in the dorsal raphé neurons in relation to the sleep–wake cycle, with cells firing at a higher rate during wakefulness and at a lower rate during sleep.33 Therefore, the alterations of the serotonin fibers in the IC of victims of sudden death observed in this study may indicate a disruption of the excitatory/inhibitory inputs reaching this nucleus from the dorsal raphé neurons. This hypothesis is supported by the frequent association observed in the present study between morphofunctional alterations of the IC and hypoplasia of the DRN.
Functional and neuroanatomical abnormalities of the IC have also been reported in patients with schizophrenia, a severe brain disorder that causes a range of different psychological symptoms, including hearing hallucinations.34–36 In particular, both in vivo molecular neuroimaging and post-mortem neurohistological studies in this syndrome have demonstrated a deficient serotonin function in IC, associated to an increased rDNA transcriptional activity in the DRN, as a compensatory mechanism to offset the serotonergic hypofunction.37,38
Thus, the common alterations of the IC we observed in this study, and those reported in schizophrenia, may indicate a similar disruption of the excitatory/inhibitory inputs reaching this nucleus, but with different degrees of severity and pathophysiological consequences. This means, in particular, that the serotonin system may be highly plastic during the IC development, and easily suffer a failure of control of a broad spectrum of functions, ranging from auditory processing to vital potentials. Therefore, the IC may be considered an important center for the convergence of both auditory and non-auditory inputs. This is also supported by our observation of a correlation between alterations of the IC and alterations of both the SOC, an important complex collecting acoustic information, and the DRN, known for its specific involvement in autonomic responses to hypoxia and/or hypercapnia.
In addition to our previous works showing developmental alterations of the classical brainstem structures controlling the main vital functions, such as breathing and cardiovascular activity, in SIUDS/SIDS cases (available at the website http://users.unimi.it/centrolinorossi/en/publications.html), the findings here presented provide new insights into the association of these sudden deaths also with IC alterations. They support our recent emphasis on the importance of all acoustic brainstem centers in triggering sudden lethal reflexes in perinatal life, when the development of the autonomic nervous system is in a particularly sensitive and vulnerable phase.
Acknowledgments
The authors thank Ms. Mary Victoria Candace Pragnell, B.A. for English revision of this manuscript. This study was supported by the Italian Health's Ministry in accordance with the Law 31/2006 “Regulations for Diagnostic Post Mortem Investigation in Victims of Sudden Infant Death Syndrome (SIDS) and Unexpected Fetal Death”, by the project “Investigating the genetic and environmental basis of sudden infant death syndrome (SIDS) and sudden intrauterine unexplained death (SIUD)” founded by the Italian University's Ministry (PRIN 2009, grant no. 2009KE5N9K) and by the Convention with the ASL—Provincia autonoma of Trento.
Authors’ contributions: AML planned the study, analyzed the data and wrote the manuscript with collaborative input and extensive discussion with TP and LM.
Footnotes
Abbreviations: CN = central nucleus, CNS = central nervous system, DC = dorsal cortex, DRN = dorsal raphé nucleus, EC = external cortex, IC = inferior colliculus, MSON = medial superior olivary nucleus, SIDS = sudden infant death syndrome, SIUDS = sudden intrauterine unexplained death syndrome, SOC = superior olivary complex.
All authors declare that they have no conflicts of interest, financial or otherwise to declare.
REFERENCES
- 1.Brugge JF. Popper AN, Fay RR. An overview of central auditory processing. The Mammalian Auditory Pathway: Neurophysiology. New York: Springer-Verlag; 1992. 1–33. [Google Scholar]
- 2.Oliver DL, Huerta MF. Webster DB, Popper AN. Inferior and superior colliculi. The Mammalian Auditory Pathway: Neuroanatomy. New York: Springer-Verlag; 1992. 168–222. [Google Scholar]
- 3.Schaette R, Kempter R. Development of tinnitus-related neuronal hyperactivity through homeostatic plasticity after hearing loss: a computational model. Eur J Neurosci 2006; 23:3124–3138. [DOI] [PubMed] [Google Scholar]
- 4.Hurley LM, Pollak GD. Serotonin differentially modulates responses to tones and frequency-modulated sweeps in the inferior colliculus. J Neurosci 1999; 9:8071–8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hurley LM, Pollak GD. Serotonin effects on frequency tuning of inferior colliculus neurons. J Neurophysiol 2001; 85:828–842. [DOI] [PubMed] [Google Scholar]
- 6.Johnson R, Stevens KE, Rose GM. 5-Hydroxytryptamine2 receptors modulate auditory filtering in the rat. J Pharmacol Exp Ther 1998; 285:643–650. [PubMed] [Google Scholar]
- 7.Obara N, Kamiya H, Fukuda S. Serotonergic modulation of inhibitory synaptic transmission in mouse inferior colliculus. Biomed Res 2014; 35:81–84. [DOI] [PubMed] [Google Scholar]
- 8.Bohorquez A, Hurley LM. Activation of serotonin 3 receptors changes in vivo auditory responses in the mouse inferior colliculus. Hear Res 2009; 251:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klepper A, Herbert H. Distribution and origin of noradrenergic and serotonergic fibers in the cochlear nucleus and inferior colliculus of the rat. Brain Res 1991; 557:190–201. [DOI] [PubMed] [Google Scholar]
- 10.Moore R, Halaris A, Jones B. Serotonin neurons of the midbrain raphe: ascending projections. J Comp Neurol 1978; 180:417–438. [DOI] [PubMed] [Google Scholar]
- 11.Ozawa Y, Okado N. Alteration of serotonergic receptors in the brainstems of human patients with respiratory disorders. Neuropediatrics 2002; 33:142–149. [DOI] [PubMed] [Google Scholar]
- 12.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 2004; 5:449–461. [DOI] [PubMed] [Google Scholar]
- 13.Zhou FC, Sari Y, Zhang JK. Expression of serotonin transporter protein in developing rat brain. Dev Brain Res 2000; 119:33–45. [DOI] [PubMed] [Google Scholar]
- 14.Lavezzi AM, Casale V, Oneda R, Weese-Mayer DW, Matturri L. Sudden infant death syndrome and sudden intrauterine unexplained death: correlation between hypoplasia of raphé nuclei and serotonin transporter gene promoter polymorphism. Ped Res 2009; 66:22–27. [DOI] [PubMed] [Google Scholar]
- 15.Lavezzi AM, Matturri L. Neuroanatomical dysmorphology of the medial superior olivary nucleus in sudden fetal and infant death. Front Hum Neurosci 2012; 6:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guntheroth WG. The Sudden Infant Death Syndrome. 1995; Armonk, NY: Futura Pub Co, 201. [Google Scholar]
- 17.Lavezzi AM, Corna MF, Matturri L. Ependymal alterations in sudden intrauterine unexplained death and sudden infant death syndrome: possible primary consequence of prenatal exposure to cigarette smoking. Neural Dev 2010; 5:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavezzi AM, Matturri L, Del Corno G, et al. Vulnerability of fourth ventricle choroid plexus in sudden unexplained fetal and infant death syndromes related to smoking mothers. Int J Dev Neurosci 2013; 31:319–327. [DOI] [PubMed] [Google Scholar]
- 19.Lavezzi AM, Mecchia D, Matturri L. Neuropathology of the area postrema in sudden intrauterine and infant death syndromes related to tobacco smoke exposure. Auton Neurosci 2012; 166:29–34. [DOI] [PubMed] [Google Scholar]
- 20.Lavezzi AM, Ottaviani G, Mauri M, et al. Biopathology of the dentate-olivary complex in sudden unexplained perinatal and sudden infant death syndrome related to maternal cigarette smoking. Neurol Res 2007; 29:525–532. [DOI] [PubMed] [Google Scholar]
- 21.Conrad Simon Memorial Research Initiative. Homepage. <http://www.conradsimon.org/InferiorColliculus.shtml> [Google Scholar]
- 22.Morest D, Oliver D. The neuronal architecture of the inferior colliculus in the cat: defining the functional anatomy of the auditory midbrain. J Comp Neurol 1984; 222:209–236. [DOI] [PubMed] [Google Scholar]
- 23.Hurley LM, Thompson AM, Pollak GD. Serotonin in the inferior colliculus. Hear Res 2002; 168:1–11. [DOI] [PubMed] [Google Scholar]
- 24.Bunin M, Wightman R. Quantitative evaluation of 5-hydroxytryptamine (serotonin) neuronal release and uptake: an investigation of extrasynaptic transmission. J Neurosci 1998; 18:4854–4860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vergé D, Calas A. Serotonergic neurons and serotonin receptors: gains from cytochemical approaches. J Chem Neuroanat 2000; 18:41–55. [DOI] [PubMed] [Google Scholar]
- 26.Velluti R. Interactions between sleep and sensory physiology. J Sleep Res 1997; 6:61–77. [DOI] [PubMed] [Google Scholar]
- 27.Anderssen SH, Nicolaisen RB, Gabrielsen GW. Autonomic response to auditory stimulation. Acta Paediatr 1993; 82:913–918. [DOI] [PubMed] [Google Scholar]
- 28.Szczepaniak WS, Mǿller AR. Evidence of neuronal plasticity within the inferior colliculus after noise exposure: a study of evoked potentials in the rat. Electroencephalogr Clin Neurophysiol 1996; 100:158–164. [DOI] [PubMed] [Google Scholar]
- 29.Chan RS, McPherson B, Zhang VW. Neonatal otoacoustic emission screening and sudden infant death syndrome. Int J Pediatr Otorhinolaryngol 2012; 76:1485–1489. [DOI] [PubMed] [Google Scholar]
- 30.Rossi L, Matturii L. Rognum TO. Anatomo-histological features of the heart's conduction system and innervation in SIDS. Sudden Infant Death Syndrome: New Trends in the Nineties. Oslo: Scandinavian University Press; 1995. 207–212. [Google Scholar]
- 31.Rossi L. Bulbospinal pathology in neurocardiac sudden death of adults: pragmatic approach to a neglected problem. Int J Legal Med 1999; 112:83–90. [DOI] [PubMed] [Google Scholar]
- 32.Bremer F. Cerveau ‘isole’ et physiologie du sommeil. C R Soc Biol 1935; 118:1235–1241. [Google Scholar]
- 33.Trulson ME, Trulson VM. Differential effects of phasic auditory and visual stimuli on serotonergic neurons in the nucleus raphe dorsalis and nucleus raphe pallidus in freely moving cats. Neurosci Lett 1982; 32:137–142. [DOI] [PubMed] [Google Scholar]
- 34.van Os J, Kapur S. Schizophrenia. Lancet 2009; 374:635–645. [DOI] [PubMed] [Google Scholar]
- 35.Picchioni MM, Murray RM. Schizophrenia. J Br Med 2007; 335:91–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schroder J, Niethammer R, Geider FJ, et al. Neurological soft signs in schizophrenia. Schizophr Res 1991; 6:25–30. [DOI] [PubMed] [Google Scholar]
- 37.Martinez-Granados B, Martinez-Bisbal MC, Sanjuan J, et al. Study of the inferior colliculus in patients with schizophrenia by magnetic resonance spectroscopy. Rev Neurol 2014; 59:1–7. [PubMed] [Google Scholar]
- 38.Krzyżanowska M, Steiner J, Brisch R, et al. Ribosomal DNA transcription in the dorsal raphe nucleus is increased in residual but not in paranoid schizophrenia. Eur Arch Psychiatry Clin Neurosci 2014; DOI 10.1007/s00406-014-0518-4. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


