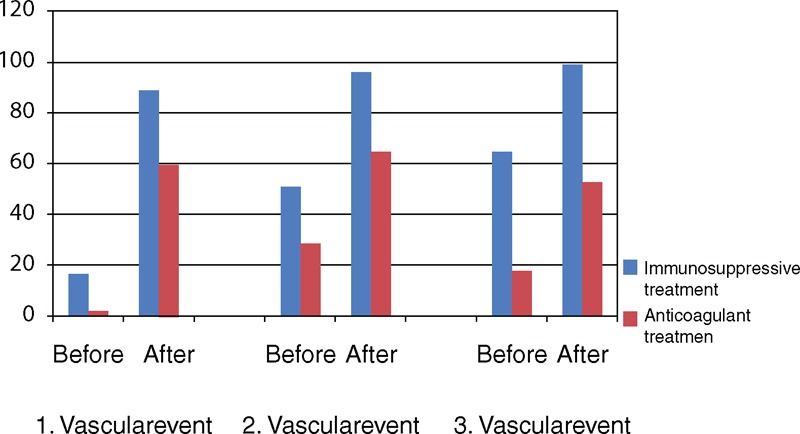
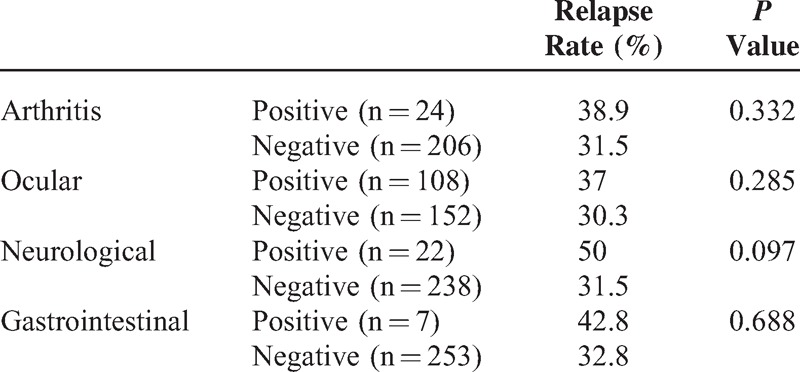
Fatma Alibaz-Oner
Fatma Alibaz-Oner, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Aslı Karadeniz
Aslı Karadeniz, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Sema Yılmaz
Sema Yılmaz, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Ayşe Balkarlı
Ayşe Balkarlı, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Gezmiş Kimyon
Gezmiş Kimyon, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Ayten Yazıcı
Ayten Yazıcı, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Muhammet Çınar
Muhammet Çınar, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Sedat Yılmaz
Sedat Yılmaz, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Fatih Yıldız
Fatih Yıldız, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Şule Yaşar Bilge
Şule Yaşar Bilge, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Emre Bilgin
Emre Bilgin, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Belkis Nihan Coskun
Belkis Nihan Coskun, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Ahmet Omma
Ahmet Omma, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Gözde Yıldırım Çetin
Gözde Yıldırım Çetin, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Yonca Çağatay
Yonca Çağatay, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Yaşar Karaaslan
Yaşar Karaaslan, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Mehmet Sayarlıoğlu
Mehmet Sayarlıoğlu, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Yavuz Pehlivan
Yavuz Pehlivan, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Umut Kalyoncu
Umut Kalyoncu, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Ömer Karadağ
Ömer Karadağ, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Timuçin Kaşifoğlu
Timuçin Kaşifoğlu, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Eren Erken
Eren Erken, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Salih Pay
Salih Pay, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Ayşe Çefle
Ayşe Çefle, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Bünyamin Kısacık
Bünyamin Kısacık, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Ahmet Mesut Onat
Ahmet Mesut Onat, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Veli Çobankara
Veli Çobankara, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1,
Haner Direskeneli
Haner Direskeneli, MD
1From the Marmara University, School of Medicine, Department of Rheumatology Istanbul (FA-O, AK, HD); Selçuk University, School of Medicine, Department of Rheumatology Konya (SY); Pamukkale University, School of Medicine, Department of Rheumatology Denizli (AB, VÇ); Gaziantep University, School of Medicine, Department of Rheumatology Gaziantep (GK, BK, AMO); Kocaeli University, School of Medicine, Department of Rheumatology Kocaeli (AY, AÇ); Gulhane Military School of Medicine, Department of Rheumatology Ankara (MÇ, SY, SP); Çukurova University, School of Medicine, Department of Rheumatology Adana (FY, EE); Osmangazi University, School of Medicine, Department of Rheumatology Eskişehir (ŞYB, TK); Hacettepe University, School of Medicine, Department of Rheumatology Ankara (EB, UK, ÖK); Uludağ University, School of Medicine, Department of Rheumatology Bursa (BNC, YP); Ankara Numune Training and Research Hospital, Department of Rheumatology, Ankara (AO); Sütçü İmam University, School of Medicine, Department of Rheumatology, Kahramanmaraş (GYÇ); Bilim University, School of Medicine, Department of Rheumatology, Istanbul (YÇ); Hitit University Medical Faculty, Department of Rheumatology, Çorum (YK); and Ondokuz Mayıs University, School of Medicine, Department of Rheumatology, Samsun, Turkey (MS).
1