Supplemental Digital Content is available in the text
Abstract
Our previous study indicated overexpression of metadherin (MTDH) is an adverse prognostic factor in squamous cell carcinoma of the head and neck (SCCHN) and promotes SCCHN cell proliferation and invasion. However, its mechanism remains unclear. Recent studies have indicated that MTDH is a cancer-metastasis-associated molecule that participates in the process of angiogenesis. Therefore, the study is aimed to investigate that whether vascular endothelial growth factor (VEGF), as one of the most potent proangiogenic cytokines, is regulated by MTDH and the role of the phosphatidylinositide 3-kinases/Protein Kinase B (PI3K/Akt) pathway in this process of regulation and the clinical significance of both MTDH and VEGF in SCCHN.
Immunohistochemistry was used to assay the expression of MTDH and VEGF in a cohort of 189 SCCHN patients with intact follow-up information. The expression of MTDH was then upregulated or inhibited by lentivirus-mediated MTDH Complementary deoxyribonucleic acid or MTDH short hairpin ribonucleic acid (shRNA) to observe the resulting alterations in VEGF expression and the PI3K/Akt signaling pathway in SCCHN cell lines. In addition, the PI3K/Akt pathway was modulated to observe the resulting changes in the MTDH-mediated expression of VEGF.
The immunohistochemistry data showed that MTDH expression is positively correlated with VEGF expression in SCCHN tissues. Moreover, the overexpression of MTDH in SCCHN Tu686 and 5-8F cells led to increases in the expression of VEGF, and this effect was accompanied by activation of the PI3K/Akt pathway. Conversely, shRNA-mediated knockdown of MTDH led to decreased VEGF expression. In addition, inhibition of the Akt signaling pathway reversed the upregulation of VEGF resulting from MTDH overexpression. Moreover, the survival analysis revealed that VEGF is an independent prognostic factor, and a combined survival analysis based on both MTDH and VEGF showed synergistic effects in the prognosis evaluation of SCCHN patients.
The findings of the present study demonstrate that MTDH regulates the expression of VEGF via the PI3K/Akt signaling pathway, indicating the potential role of the MTDH-mediated activation of VEGF signaling pathway in SCCHN angiogenesis and metastasis.
INTRODUCTION
Squamous cell carcinoma of the head and neck (SCCHN) is the sixth most frequently occurring malignancy worldwide, and is a serious global health threat.1 Despite the improvements and refinements in surgical, chemotherapeutic, and radiotherapeutic regimens that have occurred in the past few decades, the survival quality and ultimate prognosis remain unsatisfactory. Local or distant metastasis rather than the primary tumor represents the primary cause of poor outcome in patients with SCCHN.2 Metastasis is a complex and highly regulated process that includes local invasion, intravasation, extravasation, and metastatic colonization at distant sites. In metastatic cascades, angiogenesis recruits the newly formed blood vessels to offer nutrition and oxygen as well as an ability to evacuate metabolic wastes and carbon dioxide, to sustain tumor cell survival and provide metastatic advantages.3 Tumor cells start an angiogenic switch by disturbing the local balance of proangiogenic and antiangiogenic factors.4 Abundant evidence has demonstrated that tumor angiogenesis is critical to cancer metastasis, including SCCHN, and that angiogenesis inhibition appears to be a valuable and promising strategy for anticancer therapy.5,6
As a critical proangiogenic mediator, vascular endothelial growth factor (VEGF) functions directly or indirectly through binding to its tyrosine kinase receptors. Numerous studies indicated that VEGF is significantly increased in subsets of human malignancies including breast cancer,7 lung cancer,8 and SCCHN.9–11 Large-scale investigations on multiple types of cancers further confirm the prognostic significance of VEGF. Importantly, VEGF has been reported to promote cancer metastasis via angiogenesis both in vitro and in vivo.12 The inhibition of VEGF can effectively reverse the angiogenic switch and thereby block cancer metastasis.13,14 Taken together, targeting VEGF-mediated cancer angiogenesis using agents such as bevacizumab has been recognized as a promising potential therapeutic strategy in human cancer metastasis.15
Metadherin (MTDH), a recently discovered oncogene that is also known by the names Astrocyte elevated gene-1 and Lyric, has been located in human chromosome 8q22 and cloned as a human immunodeficiency virus-1 and tumor necrosis factor α-inducible gene in primary human fetal astrocytes.16 Except as a the target of miRNA-375 in SCCHN,17 MTDH regulates various signaling networks implicated in tumorigenesis, such as NF-kB,18,19 phosphatidylinositide 3-kinases/Protein Kinase B (PI3K/Akt),20,21 MAPK,22 and Wnt/β-catenin.23 Structural insights into the tumor-promoting function of MTDH–staphylococcal nuclease domain containing 1 complex in breast cancer have been reported recently.24,25 Among the above signaling transduction pathways, PI3K/Akt activation is frequently observed in a variety of tumor types, and is a major pathway activated by MTDH overexpression, which modulates numerous Akt downstream factors that are essential for cancer cell proliferation, apoptosis, and survival.26 These MTDH-regulating Akt downstream factors primarily utilize apoptosis-associated proteins such as Bcl-2, caspase-3,27 p27,28 and Forkhead box protein O1.29 In addition to its ability to boost growth, the PI3K/Akt pathway also participates in cancer angiogenesis by controlling the production of angiogenesis-associated factors including VEGF. Previous studies have revealed that increased MTDH expression is closely associated with poor prognosis in various types of cancers including breast cancer,30 esophageal squamous cell carcinoma,28 hepatocellular carcinoma,23 and nonsmall cell lung cancer.31 The majority of previous studies have focused on the modulation of MTDH in cancer metastasis. Our previous studies in cell and tissue revealed that MTDH regulates the metastasis of SCCHN via epithelial–mesenchymal transition (EMT).32,33 However, the potential influence of MTDH on cancer angiogenesis related protein such as VEGF has not been researched previously in SCCHN. Therefore, the present study aimed to investigate the correlation between MTDH and VEGF in 2 SCCHN cell lines and 189 primary cancerous samples. Furthermore, the potential regulatory role of the Akt pathway in MTDH-mediated VEGF expression in SCCHN also was explored. Finally, the clinical significance of MTDH and VEGF was further analyzed.
MATERIALS AND METHODS
Patient Samples and IHC Staining Evaluation for VEGF
The cohort of paraffin-embedded tissue samples collected from 189 patients used in this study was reported previously.32,33 The clinical information of these patients, including age, clinical stage, T classification, N classification, and distant metastasis status, was reviewed periodically. The study was approved by the Research Ethics Committee of the Central South University.
Immunohistochemical (IHC) staining was performed as previously described.32,33 Briefly, after deparaffinization, retrieval, and blocking, 4-mm-thick sections were incubated with VEGF (1:500) and an appropriate secondary antibody. Phosphate buffer solution was used in lieu of VEGF as a negative control, and the known positive slices in the streptavidin-perosidase Kit (Maxim-Bio, Fuzhou, China) were used as a positive control.
To evaluate the expression of VEGF, a scale criteria corresponding to the sum of the staining intensity8 (0 = negative, 1 = weak, 2 = intermediate, and 3 = strong) and the percentage of positive cells (0 = 0% positive cells, 1 ≤ 25% positive cells, 2 = 26%–50% positive cells, and 3 ≥ 50% positive cells) with a maximum score of 6 was established. A score >2 was considered to define high expression.
Cell Culture and Transfection
Tu686 cells were received from Dr Zhuo Chen of Emory University's Winship Cancer Institute in Atlanta, GA, and maintained as monolayer cultures in Dulbecco's Modified Eagle Media/F12 medium (1:1) supplemented with 10% fetal bovine serum (FBS). 5-8F cells were passed from the Central Experiment Laboratory of Xiangya Medical School (Central South University, Changsha, China) and cultured in Roswell Park Memorial Institute-1640 medium supplemented with 10% FBS. The medium was changed every other day. Exponentially growing cells were used for the following experiments.
To establish stable cell lines, lentiviral particles encoding MTDH Complementary deoxyribonucleic acid (cDNA) or short hairpin ribonucleic acid (shRNA) and the corresponding control plasmids were transfected into Tu686 and 5-8F cell lines according to the manufacturers’ instructions. Tu686 and 5-8F cells stably expressing MTDH were transiently transfected with AKT or control small interfering ribonucleic acid (siRNA) (SC-43609; SC-37007, purchased from Santa Cruz, CA). A western blot analysis was performed to verify the effect of transfection in the cell lines.
Western Blotting Analysis
All of the western blotting analyses were performed as described previously.34 In brief, 30 μg of protein was separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto a Polyvinalidene Fluoride membrane (Millipore, Bedford, MA). The blotted membranes were incubated with primary antibodies against MTDH (1:800; Proteintech Group, Inc, Chicago, IL), VEGF (1:1000; Santa Cruz, CA), pan-Akt (1:1000; Cell Signaling Technology, Inc, Beverly, MA), phospho-Akt (Thr308) (1:800; Cell Signaling Technology, Inc), and then with the corresponding secondary antibody (1:3000; Beyotime, Shanghai, China). A specific anti-β-actin protein (1:1000; Beyotime) was used to detect the quantity of the loading control protein. The Image Lab software (Version 4.1; Bio-Rad Laboratories, Inc, Berkeley, CA) was applied to analyze the intensity of the blotted bands, and each experiment was repeated 3 times.
Enzyme-Linked Immunosorbent Assay
Supernatants from all of the cell cultures were collected and stored at −80°C before analysis by enzyme-linked immunosorbent assay (ELISA). The concentrations of secreted VEGF were quantified using a human VEGF-ELISA kit (R&D, Inc) according to the manufacturer's instructions.
Statistical Analysis
All of the statistical analyses were performed using the SPSS statistical software (version 17.0; SPSS Inc, Chicago, IL). The statistical significance of the difference in the expression of VEGF protein and clinicopathological factors was analyzed by the χ2 test and visualized by R studio, Inc. Boston, MA (version 3.0.2). Survival analyses were performed using the Kaplan–Meier method, and the resulting curves were compared using the log-rank test. The relevant prognostic factors were identified by univariate and multivariate Cox regression analyses. The correlation between VEGF and MTDH proteins in the tissue samples was analyzed by a Spearman analysis, and Student test was applied to compare the data derived from the cell experiments. Differences were considered to be significant if P < 0.05. All of the tests were 2-sided.
RESULTS
Expression of MTDH Is Positively Correlated With VEGF Expression in Archived SCCHN Tissue Specimens
Previous studies from our lab have demonstrated that MTDH is overexpressed and positively correlated with metastasis in SCCHN patients.32,33 In vitro experiments have also revealed that MTDH promotes the metastasis of SCCHN through PI3K/Akt-pathway-mediated-EMT changes.33 To clarify the potential correlation between MTDH and VEGF, the expression of VEGF was assayed in the same 189 SCCHN patient cohort that was used to previously evaluate the expression of MTDH.33 The IHC results showed that VEGF staining was mainly distributed in the cytoplasm of the SCCHN cancer cells (Figure 1C and D). The Spearman rank test further showed that MTDH expression had significantly positively correlated with VEGF in SCCHN tissues (r = 0.319, P < 0.001, Figure 1E), which prompted further investigation of the potential association between MTDH and VEGF.
FIGURE 1.
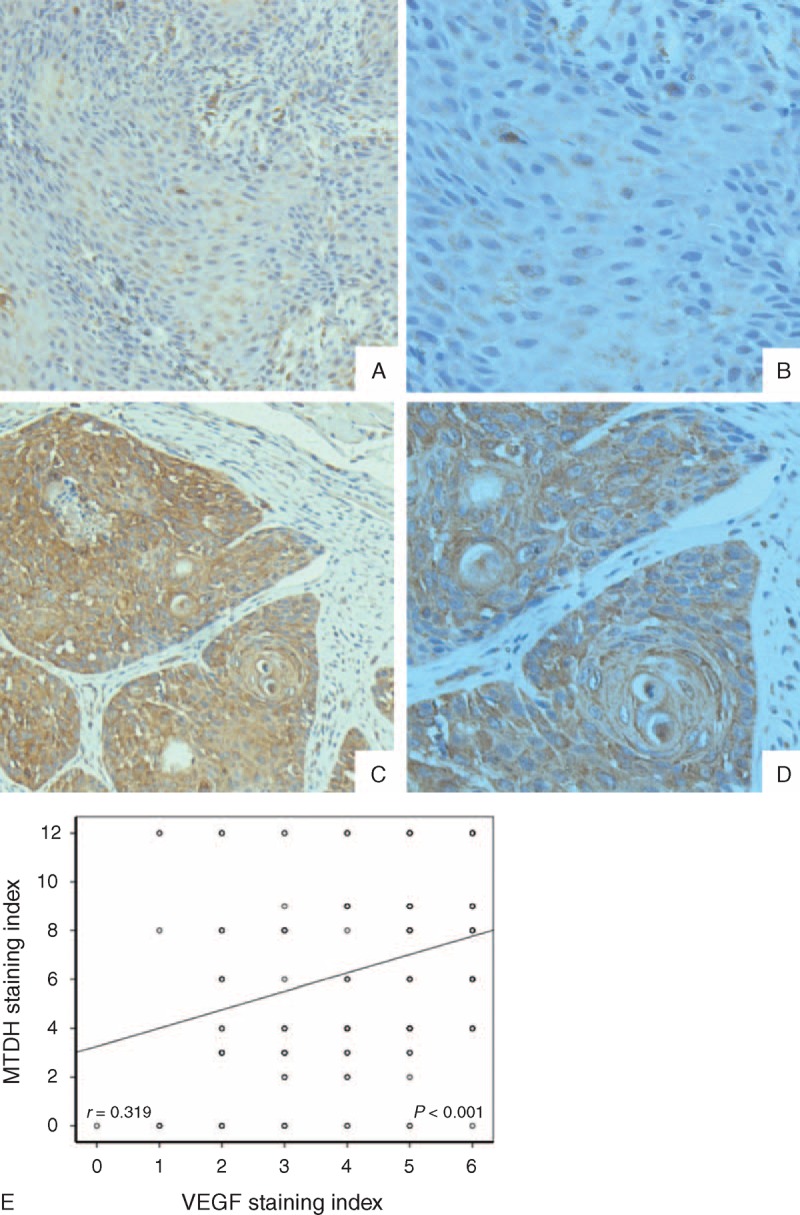
Typical IHC staining showed low (A and B) and high (C and D) expression of VEGF expression in SCCNH patients’ slides (A and C = 200×, B and D = 400×, original resolution), significant correlation between VEGF and MTDH expression in SCCHN patients, and its fitting curve (E). IHC = immunohistochemical, MTDH = metadherin, SCCHN = squamous cell carcinoma of the head and neck, VEGF = vascular endothelial growth factor.
MTDH Regulates the Expression of VEGF Protein in SCCHN Cells
In agreement with our findings in patient samples, the expression of MTDH was changed in 2 SCCHN cell lines (Tu686 and 5-8F cells) allowing observation of VEGF expression alteration in vitro. Both lenti-MTDH-cDNA and lenti-MTDH-shRNA were designed and synthesized to increase and inhibit the expression of MTDH, respectively. The transfection efficiency was finally assayed by western blots. As shown in Figure 2A, the transfection of lenti-MTDH-cDNA successfully upregulated the expression of MTDH by approximately 3-fold in both Tu686 and 5-8F cells compared with the control cells. Conversely, the transfection of lenti-MTDH-shRNA led to >75% inhibition of the expression of MTDH in Tu686 and 5-8F cells (Figure 2B). As predicted from the IHC results, the western blot results revealed that MTDH overexpression caused an increase in the expression of VEGF, whereas MTDH inhibition correspondingly blocked the expression of VEGF in Tu686 and 5-8F cells (Figure 2A and B). Because VEGF is always secreted by tumor cells to the extracellular environment, ELISA findings similarly demonstrated that the overexpression of MTDH resulted in enhanced secretion of VEGF by multiple SCCHN cells in vitro (Figure 2C–F). Taken together, these data indicate that MTDH can modulate the expression of VEGF in SCCHN cells.
FIGURE 2.
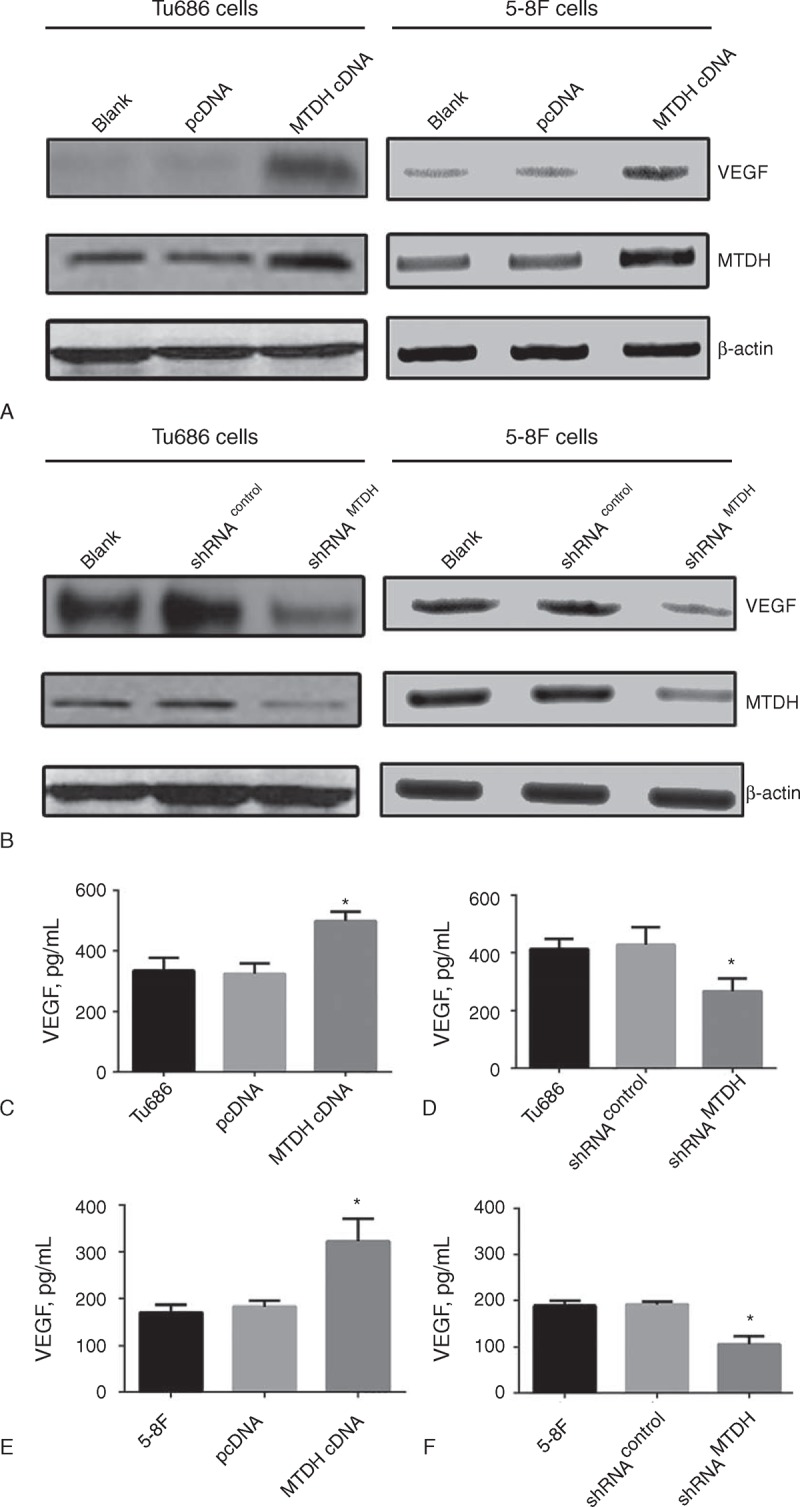
Increasing expression of VEGF in overexpressed MTDH (MTDH cDNA) cells was detected comparing with parental cell (Blank) and vector control (pcDNA) by western blot (A) and ELISA (C and E); reduced VEGF expression was found in MTDH knockdown (shRNAMTDH) cells compared with parental (Blank) and control cells (shRNAcontrol) by western blot (B) and ELISA (D and F). ∗P < 0.05. ELISA = enzyme-linked immunosorbent assay, MTDH = metadherin, VEGF = vascular endothelial growth factor.
PI3K/Akt Signaling Pathway Is Involved in the MTDH-Induced Modulation of VEGF Expression in SCCHN Cells
The above-presented results suggested that MTDH could regulate VEGF expression in SCCHN cells. However, mechanism through which MTDH regulates the expression of VEGF requires more investigation. Previous studies conducted by the authors and other researchers have shown that the PI3K/Akt signaling pathway plays a critical role in MTDH-mediated cancer behaviors.26,33 In the present study, MTDH overexpression was found to successfully activate the PI3K/Akt signaling pathway in SCCHN cells and vice versa (Figure 3A and B). Therefore, to avoid off-target events, 2 methods, one of which involved using Akt plasmid shRNA, and the other of which used a PI3K specific inhibitor (LY294002), were used to block the PI3K/Akt signaling pathway and test its effect on VEGF expression. After PI3K/Akt activity was effectively impeded (Figure 3C and E), the elevated expression of VEGF caused by MTDH overexpression was significantly reversed in SCCHN cells (Figure 3C–F). Considered together, these data indicate that the PI3K/Akt signaling pathway is involved in MTDH-induced modulation of VEGF expression.
FIGURE 3.
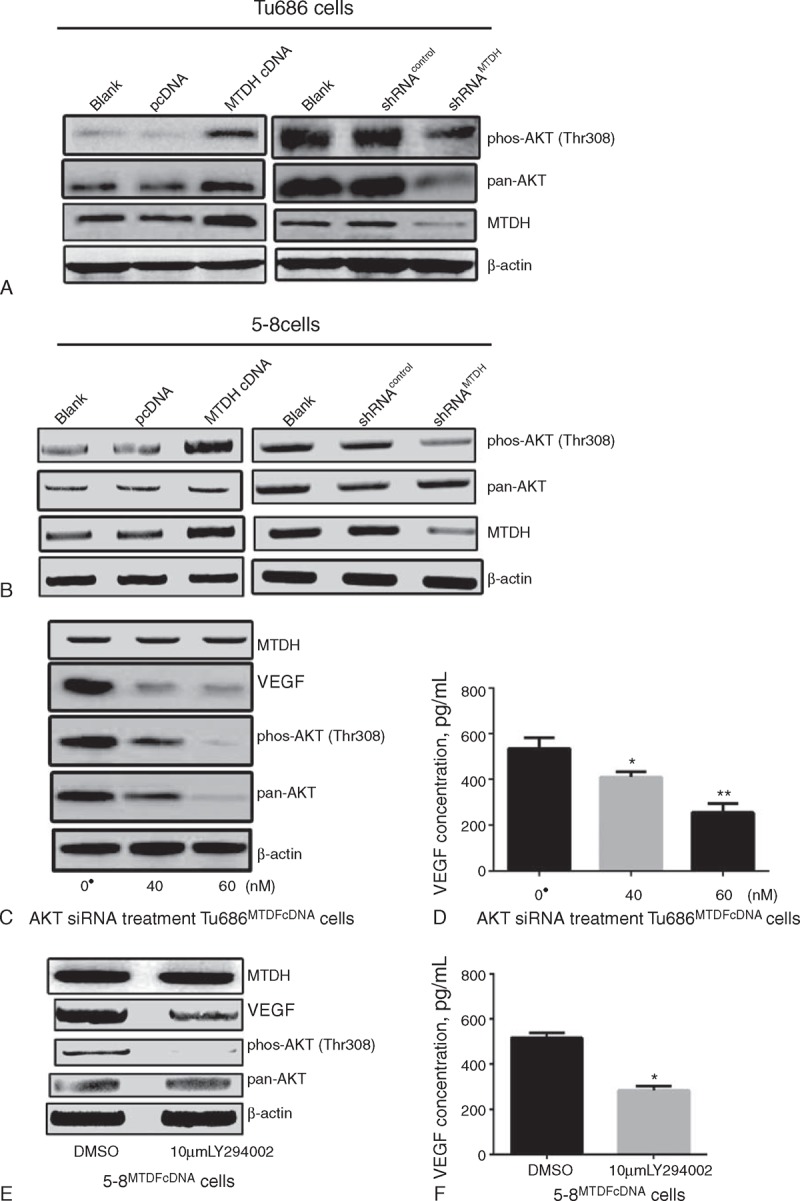
Alteration of phosphorylation on AKT came with MTDH expression (A and B); extra-/intracellular VEGF expression was reduced according to different concentration of AKT siRNA treatment (C and D); VEGF expression was attenuated in extra-/intracellular after PI3K/Akt inhibitor LY294002 stimulation (E and F); ● Control siRNA had added for vector control. ∗P < 0.05, ∗∗P < 0.001. DMSO = dimethyl sulfoxide, MTDH = metadherin, VEGF = vascular endothelial growth factor.
Clinical Significance of VEGF Protein in SCCHN Patients
All of the above-presented results indicated that MTDH could regulate the expression of VEGF via the PI3K/Akt signaling pathway in SCCHN cells. Therefore, χ2 tests were applied to describe the associations between VEGF expression and other clinicopathological parameters in the 189 SCCHN patients. As shown in Figure 4A and B, the VEGF protein was elevated in SCCHN tissues from patients at advanced clinical stages or who presented cervical lymph node metastasis; however, VEGF expression showed no difference between SCCHN tissues with different tumor position and pathological grade.
FIGURE 4.
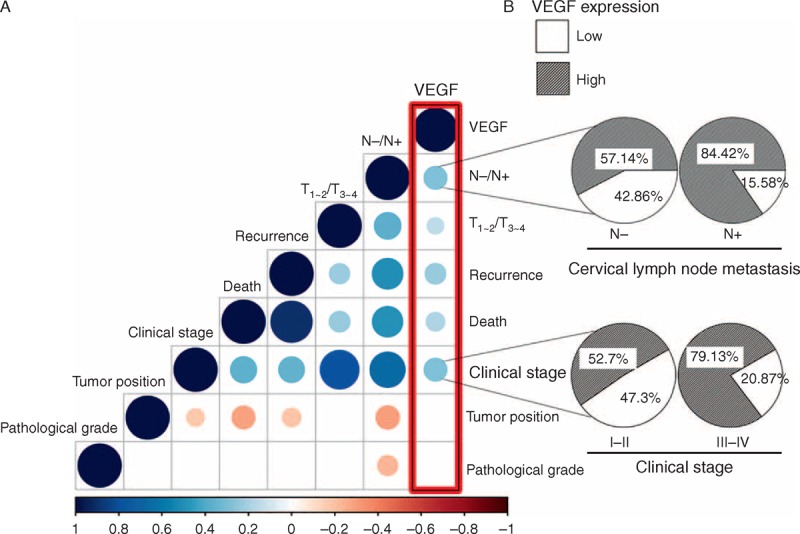
Mutual correlation between several key clinical parameters as shown in matrix (A): blue and red color indicates positive and negative correlation, respectively; the size of the circle and brightness of the color in the intersection of each column and row indicate the extent of correlation between their clinical parameters; the right first panel in (A) represented lymph node metastasis and clinical stage were most significantly associated with VEGF expression. Furthermore, the percentages of high VEGF expression were elevated in the group with cervical lymph node metastasis or late clinical stage (B). VEGF = vascular endothelial growth factor.
Combined Survival Analyses Based on Both MTDH and VEGF Protein Expression
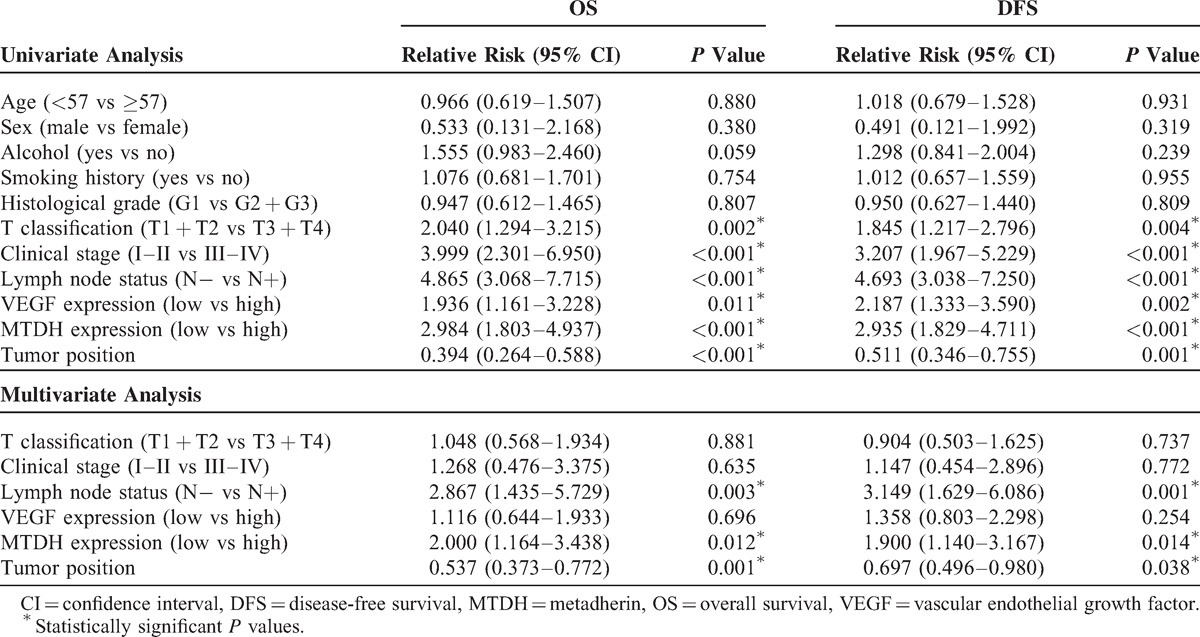
Our previous study revealed that MTDH is negatively associated with the overall survival (OS) and disease-free survival (DFS) times in SCCHN patients.33 Therefore, in this study, the prognostic capacity of VEGF was analyzed in the same 189-patient cohort. Kaplan–Meier survival curves showed that the SCCHN patients who presented high VEGF expression status displayed shorter DFS time and OS time than the SCCHN patients with relatively low VEGF expression (P = 0.006 and P = 0.009, respectively; Figure 5A and B). Univariate Cox regression analyses identified that clinical stage, primary tumor location, tumor classification, lymph node metastasis, MTDH, and VEGF protein expression were prognostic factors influencing both the 5-year OS and DFS in SCCHN patients. Multivariate Cox regression analyses indicated that the tumor location, lymph node metastasis, and MTDH expression were independent prognostic factors (Table 1). However, the present study revealed that VEGF plays a crucial function in determining survival time of SCCHN patients11 and it is found to be regulated by MTDH. Therefore, the expression levels of both MTDH and VEGF were assessed in an effort to enhance their prognostic capability. The results indicated that SCCHN patients who exhibit high expression level of both MTDH and VEGF experienced worse OS and DFS than other patients, including those with a high expression level of either factor alone or low expression of both factors (Figure 5C and D).
FIGURE 5.
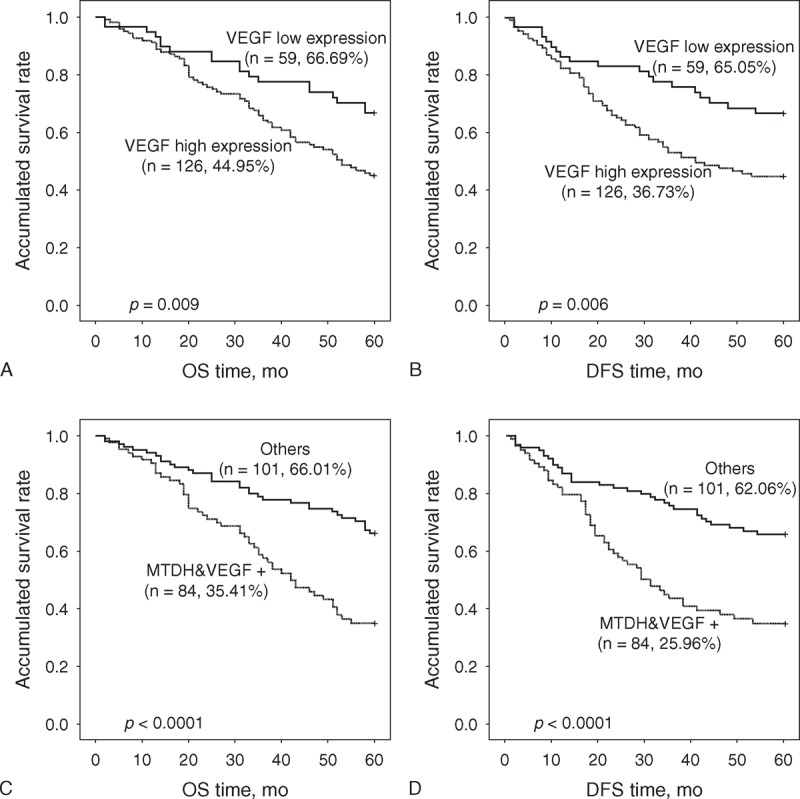
Kaplan–Meier survival curves presented by SCCHN patients with high VEGF expression status displayed shorter OS and DFS time than those with relatively low VEGF expression (A and B). Both high expression of VEGF and MTDH (MTDH&VEGF+) implied worse OS and DFS times than others including high expression of either one and low expression of both (C and D). DFS = disease-free survival, MTDH = metadherin, OS = overall survival, SCCHN = squamous cell carcinoma of the head and neck, VEGF = vascular endothelial growth factor.
TABLE 1.
Cox Model Analysis of DFS and OS
DISCUSSION
In the present study, the underlying correlation between MTDH and VEGF was studied. First, a positive correlation between VEGF and MTDH protein expression levels in archived SCCHN tissues was identified, indicating that MTDH may have a regulatory effect on VEGF. The expression of MTDH in SCCHN Tu686 and 5-8F cells was then induced or reduced to observe any resulting alterations in the VEGF protein level. As expected, changes in MTDH led to corresponding alterations of VEGF, which indicated that MTDH could regulate the expression of VEGF. To further explore the latent mechanism, it was determined that PI3K/Akt is involved in the regulation of VEGF by MTDH. Because MTDH can influence the metastasis of multiple human malignancies containing SCCHN in a previous study,32,33,35 the present research demonstrated that MTDH mediates the expression of VEGF via the PI3K/Akt signaling pathway, which may stimulate SCCHN tumor angiogenesis and thereby facilitate the metastasis process.
MTDH is a versatile molecule, involved in diverse malignant cancer cell behaviors, including uncontrolled proliferation, migration, invasion, metastasis, and drug resistance.26 The authors’ previous study clearly demonstrated that aberrant MTDH expression is tightly associated with aggressive clinical behaviors in human laryngeal carcinoma, a type of SCCHN.32 Further in vitro investigation has revealed that MTDH can promote the EMT, which leads to enhanced invasion and metastasis of SCCHN cells.33 Taken together, the available evidence supports the conclusion that MTDH is a molecule that is tightly associated with cancer metastasis. However, the exact way MTDH promotes cancer metastasis is complicated and remains to be determined. Because angiogenesis is indispensable in cancer metastasis and VEGF is a well-documented pro-angiogensis molecule, the association between MTDH and VEGF was examined in the present study, an association that has been scarcely investigated until now.
The available literature provides direct and indirect evidence of association between MTDH and cancer angiogenesis. Cancer angiogenesis is frequently induced by a hypoxic environment, which also triggers the occurrence of EMT.36,37 EMT-associated transcription factors such Snail and Twist are always overexpressed by endothelial cells in the peritumoral stroma, and have been shown to promote tumor angiogenesis.38 The previous investigation together with studies in breast and hepatocellular carcinoma clearly showed that MTDH stimulates a cancer's EMT process,39,40 which indicates that there may be a connection between MTDH-induction of EMT and angiogenesis. As a well-known potent endothelial-specific mitogen that has a central role in tumor angiogenesis,41 VEGF protein expression is reported to positively correlate with MTDH protein in triple negative breast cancer,42 and cervical carcinoma (vascular formation was also tested),43 which coincides with the results of this study and indicates that MTDH may promote cancer angiogenesis by activating the VEGF signaling pathway.37 The biggest finding of the current study is that MTDH can regulate the expression of the VEGF in vitro, providing mechanistic insight into the potential MTDH-mediating cancer angiogenesis.
Emdad et al44 revealed that PI3K/Akt stimulation is necessary for tube formation in Matrigel and increased invasion of human umbilical vein endothelial cells induced by MTDH upregulation. Therefore, based on the previous finding that MTDH activates PI3K/Akt signaling cascades in SCCHN Tu686 cells and 5-8F cells,33 both Akt siRNA and PI3K/Akt specific inhibitors LY294002 were used to inhibit the PI3K/Akt pathway and successfully ablated the MTDH-mediated upregulation of VEGF. Taken together, these data show that the PI3K/Akt pathway is implicated in MTDH-mediated VEGF expression. However, the mechanism of MTDH activating AKT pathway is scarcely known. Recently, MTDH was reported to interact with Akt2 in cancer, which provides an indicator supporting that MTDH regulates AKT activity by binding to Akt2.45 Long et al46 reported that Aurora Kinase A (AURKA) is a downstream effector of MTDH that regulates of the Akt pathway in Acute myeloid leukemia cell lines. Our supplementary data (Figure S1, http://links.lww.com/MD/A197) presented that alteration of AURKA expression level decreases much more in Tu686 than 5-8F cells after MTDH manipulation. Because AURKA can impact the expression of Akt in cells,46 it may explain the possible reason for the inconsistent tendency of pan-AKT level in 5-8F cells and Tu686 cells in this study. Further work is required to determine the relative roles of AURKA or other MTDH effectors in MTDH-mediated Akt pathway activation in SCCHN.
Clinicopathological variables such as tumor site, histological grade, and clinical stage are beneficial for prognosis evaluation in SCCHN patients. Additionally, the assay of molecular changes in primary tumors has been demonstrated to be able to enhance the clinician's ability to prognosticate. The current results and previous similar reports suggest that both MTDH and VEGF are prognostic factors in SCCHN patients.11,32,33 Finally, combined survival analyses were performed by considering both MTDH and VEGF. The results indicated that patients with the phenotype of both high MTDH and VEGF expression display worst prognoses than any other phenotypes, indicating that combined analyses of MTDH and VEGF provide more accurate and detailed prognosis information in SCCHN patients. With clinical examination, these biomarker examinations may help clinicians to make more reasonable therapeutic plans and periodic reexamination arrangements.
The present study reveals that MTDH can increase the expression of VEGF via activation of the PI3K/Akt signaling pathway, which may facilitate the metastasis of SCCHN. Future studies will use in vitro and in vivo models to study the mechanistic relationship between MTDH-regulated VEGF expression and SCCHN angiogenesis/metastasis. These studies will help to determine whether MTDH may serve as both a prognostic biomarker and valuable therapeutic target in SCCHN.
Acknowledgments
Jonathon Havel, Guo Li et al. (Memorial Sloan-Kettering Cancer Center) is appreciated for his many suggestions.
Footnotes
Abbreviations: DFS = disease-free survival, ELISA = enzyme-linked immunosorbent assay, FBS = fetal bovine serum, IHC = immunohistochemical, MTDH = metadherin, OS = overall survival, SCCHN = squamous cell carcinoma of the head and neck, VEGF = vascular endothelial growth factor.
This study was funded by grants from National High Technology Research and Development Program of China (the Chinese 863 program, No. 2012AA02A201), the National Natural Science Foundation of China (No. 81202128, 81372906, 81372426, 81172558, 81472696, and 81272974), the Research Fund for the Doctoral Program of Higher Education of China (No. 20120162120049), and the Freedom Explore Program of Central South University (No. 2012QNZT099).
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30. [DOI] [PubMed] [Google Scholar]
- 2.Mamelle G, Pampurik J, Luboinski B, et al. Lymph node prognostic factors in head and neck squamous cell carcinomas. Am J Surg 1994; 168:494–498. [DOI] [PubMed] [Google Scholar]
- 3.Welti J, Loges S, Dimmeler S, et al. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J Clin Invest 2013; 123:3190–3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeriswyl V, Christofori G. The angiogenic switch in carcinogenesis. Semin Cancer Biol 2009; 19:329–337. [DOI] [PubMed] [Google Scholar]
- 5.Harlozinska A. Progress in molecular mechanisms of tumor metastasis and angiogenesis. Anticancer Res 2005; 25:3327–3333. [PubMed] [Google Scholar]
- 6.Gallo O, Franchi A, Magnelli L, et al. Cyclooxygenase-2 pathway correlates with VEGF expression in head and neck cancer. Implications for tumor angiogenesis and metastasis. Neoplasia 2001; 3:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown LF, Berse B, Jackman RW, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol 1995; 26:86–91. [DOI] [PubMed] [Google Scholar]
- 8.Mattern J, Koomagi R, Volm M. Association of vascular endothelial growth factor expression with intratumoral microvessel density and tumour cell proliferation in human epidermoid lung carcinoma. Br J Cancer 1996; 73:931–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denhart BC, Guidi AJ, Tognazzi K, et al. Vascular permeability factor/vascular endothelial growth factor and its receptors in oral and laryngeal squamous cell carcinoma and dysplasia. Lab Invest 1997; 77:659–664. [PubMed] [Google Scholar]
- 10.Oc P, Rhys-Evans P, Eccles SA. Expression of vascular endothelial growth factor family members in head and neck squamous cell carcinoma correlates with lymph node metastasis. Cancer 2001; 92:556–568. [DOI] [PubMed] [Google Scholar]
- 11.Zang J, Li C, Zhao LN, et al. Prognostic value of vascular endothelial growth factor in patients with head and neck cancer: a meta-analysis. Head and Neck 2013; 35:1507–1514. [DOI] [PubMed] [Google Scholar]
- 12.Rouhi P, Lee SL, Cao Z, et al. Pathological angiogenesis facilitates tumor cell dissemination and metastasis. Cell Cycle 2010; 9:913–917. [DOI] [PubMed] [Google Scholar]
- 13.Verheul HM, Hammers H, van Erp K, et al. Vascular endothelial growth factor trap blocks tumor growth, metastasis formation, and vascular leakage in an orthotopic murine renal cell cancer model. Clin Cancer Res 2007; 13:4201–4208. [DOI] [PubMed] [Google Scholar]
- 14.Binetruy-Tournaire R, Demangel C, Malavaud B, et al. Identification of a peptide blocking vascular endothelial growth factor (VEGF)-mediated angiogenesis. EMBO J 2000; 19:1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giampieri R, Scartozzi M, Del Prete M, et al. The “angiogenetic ladder”, step-wise angiogenesis inhibition in metastatic colorectal cancer. Cancer Treat Rev 2014; 40:934–941. [DOI] [PubMed] [Google Scholar]
- 16.Kang DC, Su ZZ, Sarkar D, et al. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene 2005; 353:8–15. [DOI] [PubMed] [Google Scholar]
- 17.Hui AB, Bruce JP, Alajez NM, et al. Significance of dysregulated metadherin and microRNA-375 in head and neck cancer. Clin Cancer Res 2011; 17:7539–7550. [DOI] [PubMed] [Google Scholar]
- 18.Emdad L, Sarkar D, Su ZZ, et al. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: implications for tumor progression and metastasis. Cancer Res 2006; 66:1509–1516. [DOI] [PubMed] [Google Scholar]
- 19.Sarkar D, Park ES, Emdad L, et al. Molecular basis of nuclear factor-kappaB activation by astrocyte elevated gene-1. Cancer Res 2008; 68:1478–1484. [DOI] [PubMed] [Google Scholar]
- 20.Lee SG, Su ZZ, Emdad L, et al. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc Natl Acad Sci U S A 2006; 103:17390–17395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SG, Su ZZ, Emdad L, et al. Astrocyte elevated gene-1 activates cell survival pathways through PI3K-Akt signaling. Oncogene 2008; 27:1114–1121. [DOI] [PubMed] [Google Scholar]
- 22.Kikuno N, Shiina H, Urakami S, et al. Knockdown of astrocyte-elevated gene-1 inhibits prostate cancer progression through upregulation of FOXO3a activity. Oncogene 2007; 26:7647–7655. [DOI] [PubMed] [Google Scholar]
- 23.Yoo BK, Emdad L, Su ZZ, et al. Astrocyte elevated gene-1 regulates hepatocellular carcinoma development and progression. J Clin Invest 2009; 119:465–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan L, Lu X, Yuan S, et al. MTDH–SND1 interaction is crucial for expansion and activity of tumor-initiating cells in diverse oncogene- and carcinogen-induced mammary tumors. Cancer cell 2014; 26:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo F, Wan L, Zheng A, et al. Structural insights into the tumor-promoting function of the MTDH–SND1 complex. Cell Rep 2014; 8:1704–1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarkar D, Emdad L, Lee SG, et al. Astrocyte elevated gene-1: far more than just a gene regulated in astrocytes. Cancer Res 2009; 69:8529–8535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ke ZF, Mao X, Zeng C, et al. AEG-1 expression characteristics in human non-small cell lung cancer and its relationship with apoptosis. Med Oncol 2013; 30:383. [DOI] [PubMed] [Google Scholar]
- 28.Yu C, Chen K, Zheng H, et al. Overexpression of astrocyte elevated gene-1 (AEG-1) is associated with esophageal squamous cell carcinoma (ESCC) progression and pathogenesis. Carcinogenesis 2009; 30:894–901. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Yang L, Song L, et al. Astrocyte elevated gene-1 is a proliferation promoter in breast cancer via suppressing transcriptional factor FOXO1. Oncogene 2009; 28:3188–3196. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Zhang N, Song LB, et al. Astrocyte elevated gene-1 is a novel prognostic marker for breast cancer progression and overall patient survival. Clin Cancer Res 2008; 14:3319–3326. [DOI] [PubMed] [Google Scholar]
- 31.Song L, Li W, Zhang H, et al. Over-expression of AEG-1 significantly associates with tumour aggressiveness and poor prognosis in human non-small cell lung cancer. J Pathol 2009; 219:317–326. [DOI] [PubMed] [Google Scholar]
- 32.Zhou YC, Wang XC, Chen Y, et al. [Expression of transcription factor SOX4 and its clinical significance in female lung cancer patients in Xuanwei area, Yunnan Province]. Zhonghua Zhong Liu Za Zhi 2013; 35:202–206. [DOI] [PubMed] [Google Scholar]
- 33.Yu C, Liu Y, Tan H, et al. Metadherin regulates metastasis of squamous cell carcinoma of the head and neck via AKT signalling pathway-mediated epithelial–mesenchymal transition. Cancer Lett 2014; 343:258–267. [DOI] [PubMed] [Google Scholar]
- 34.Zhu G, Cai G, Liu Y, et al. Quantitative iTRAQ LC-MS/MS proteomics reveals transcription factor crosstalk and regulatory networks in hypopharyngeal squamous cell carcinoma. J Cancer 2014; 5:525–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoo BK, Emdad L, Lee SG, et al. Astrocyte elevated gene-1 (AEG-1): a multifunctional regulator of normal and abnormal physiology. Pharmacol Ther 2011; 130:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jiang J, Tang YL, Liang XH. EMT: a new vision of hypoxia promoting cancer progression. Cancer Biol Ther 2011; 11:714–723. [DOI] [PubMed] [Google Scholar]
- 37.Emdad L, Das SK, Dasgupta S, et al. AEG-1/MTDH/LYRIC: signaling pathways, downstream genes, interacting proteins, and regulation of tumor angiogenesis. Adv Cancer Res 2013; 120:75–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez-Tillo E, Liu Y, de Barrios O, et al. EMT-activating transcription factors in cancer: beyond EMT and tumor invasiveness. Cell Mol Life Sci 2012; 69:3429–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li X, Kong X, Huo Q, et al. Metadherin enhances the invasiveness of breast cancer cells by inducing epithelial to mesenchymal transition. Cancer Sci 2011; 102:1151–1157. [DOI] [PubMed] [Google Scholar]
- 40.Zhu K, Dai Z, Pan Q, et al. Metadherin promotes hepatocellular carcinoma metastasis through induction of epithelial–mesenchymal transition. Clin Cancer Res 2011; 17:7294–7302. [DOI] [PubMed] [Google Scholar]
- 41.Ribatti D. The crucial role of vascular permeability factor/vascular endothelial growth factor in angiogenesis: a historical review. Br J Haematol 2005; 128:303–309. [DOI] [PubMed] [Google Scholar]
- 42.Duncan EL, Danoy P, Kemp JP, et al. Genome-wide association study using extreme truncate selection identifies novel genes affecting bone mineral density and fracture risk. PLoS Genet 2011; 7:e1001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long M, Dong K, Gao P, et al. Overexpression of astrocyte-elevated gene-1 is associated with cervical carcinoma progression and angiogenesis. Oncol Rep 2013; 30:1414–1422. [DOI] [PubMed] [Google Scholar]
- 44.Emdad L, Lee SG, Su ZZ, et al. Astrocyte elevated gene-1 (AEG-1) functions as an oncogene and regulates angiogenesis. Proc Natl Acad Sci U S A 2009; 106:21300–21305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu B, Emdad L, Bacolod MD, et al. Astrocyte elevated gene-1 (AEG-1) interacts with Akt isoform 2 to control glioma growth, survival and pathogenesis. Cancer Res 2014; 74:7321–7332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Long M, Hao M, Dong K, et al. AEG-1 overexpression is essential for maintenance of malignant state in human AML cells via up-regulation of Akt1 mediated by AURKA activation. Cell Signal 2013; 25:1438–1446. [DOI] [PubMed] [Google Scholar]


