Supplemental Digital Content is available in the text
Abstract
As the number of cardiac implantable electronic devices (CIEDs) is increasing annually, CIED-related complications are becoming increasingly important. The aim of the study was to assess the risks associated with CIEDs by a nationwide database.
Patients were selected from the Taiwan National Health Insurance Database. Admissions for CIED implantation, replacement, and revision were evaluated and the evaluation period was 14 years. Endpoints included CIED-related infection, pneumothorax, and heart perforation.
The study included 40,608 patients with a mean age of 71.8 ± 13.3 years. Regarding infection, the incidence rate was 2.45 per 1000 CIED-years. Male gender, younger age, device replacement, and previous infection were risks for infection while old age and high-volume centers (>200 per year) were protectors. The incidence of pneumothorax was 0.6%, with an increased risk in individuals who had chronic obstructive lung disease (COPD) and cardiac resynchronized therapy (CRT). The incidence of heart perforation was 0.09%, with an increased risk in individuals who had pre-operation temporal pacing and steroid use.
High-volume center was found to decrease infection rate while male gender, young people, and individuals who underwent replacements were associated with an increased risk of infection. Additionally, pre-operation temporal pacing and steroid use should be avoided if possible. Furthermore, COPD patients or those who accept CRTs should be monitored closely.
INTRODUCTION
Cardiovascular implantable electronic devices (CIEDs), which include pacemakers (PM), implantable cardioverter-defibrillators (ICD), and cardiac resynchronized therapy (CRT), are standard therapy for bradyarrhythmias, tachyarrhythmias, and systolic heart failure with left bundle branch block.1–3 Though the numbers of these devices have increased annually over the last decade,4,5 as the population with implanted devices continues to grow, CIED-related complications such as infection, pneumothorax and cardiac perforation have increased as well.6,7 Such complications not only result in prolonged hospitalization and increased costs, but also worse outcomes and mortality.8 For this reason, many studies have attempted to investigate the factors causing CIED-related complications by evaluating baseline characteristics, procedure types, and medications.
Among the device-related complications, infection has attracted most of our attention. Some studies have reported some factors contributing to infection, such as diabetes mellitus (DM), end-stage renal disease (ESRD), corticosteroid use, early re-intervention, temporary pacing, as well as physician experience in CIED implantation and revision/replacement procedures.9–15 However, the conclusions have been inconsistent. Baddour et al16 pointed out that these studies had various limitations such as relatively small numbers of CIED infection patients, and individual single-center.
In addition to CIED-related infection, evaluating the risks of other complications such as pneumothorax and heart perforation is also important. Pneumothorax after implantation has supposedly shown a correlation with subclavian vein puncture, old age, female gender, and operator experience,17 causing increased patient morbidity and substantial cost.18 Another associated complication is heart perforation, though relatively rare with an incidence reported from between 0.09%19 to 1.2% in the medical literature.20 Some studies have pointed out certain risk factors for heart perforation such as temporary pacemakers, steroid use within 7 days prior to implantation, and helical screws.20 However, compared to studies of device-related CIED infections, there has been little research assessing the risk of pneumothorax and heart perforation in CIED patients.
In order to fill this lacuna in the literature and evaluate CIED-related complications in Asian populations, a nationwide database was analyzed to identify possible risk factors and incidences of CIED-related complications.
MATERIALS AND METHODS
Data Source and Design of outcomes
This retrospective national population-based cohort study was retrieved from the National Health Insurance Research Database (NHIRD) released by the Taiwan National Health Research Institute. The NHIRD enrols 99.91% of the Taiwanese population (about 23.20 million in 2012) and consists of all enrollees health care data. Previous studies have described it in detail21 and validated the accuracy of the NHIRD diagnostic data.22 The insurance reimburses all the CIED implantation, replacement, revision, and removal expenses with appropriate indication according to the clinical practice guidelines of CIEDs from 1996 to the present. All CIED procedures were enrolled including new implantation, replacement due to any cause, revisions or removal procedures, but procedures that could not be clearly defined were excluded. The CIEDs include pacemakers, ICD, and CRT. The study period was between January 1, 1997 and December 31, 2010. We inspected all admissions after CIED procedures to evaluate any CIED-related complications. The end points included device-related infection, pneumothorax, and heart perforation. The cohort was followed up until either death or December 31, 2010. The ethical approval is not necessary (approved by The Institutional Review Board of Chang Gung Memorial Hospital) because the data from NHIRD was consists of deidentified secondary data and used for research purposes only.
Definitions
The cohort was divided into 3 main groups based on the outcomes: CIED infection, CIED pneumothorax, and CIED heart perforation. The CIED infection was defined as an infection that occurred during admission for CIED-related procedures, which included implantation, replacement, revision, and removal. CIED pneumothorax was defined as a pneumothorax that occurred during admission for any CIED-related procedure. CIED heart perforation was defined as a heart perforation treated with heart repair surgery during admission for any CIED-related procedure.
Risk Factor Assessment
The analyzed risk factor parameters were separated into 4 components, including patient, device, medication, and provider factors. Patient factors included gender, age, and comorbidities, which was identified based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) (as listed in Table 1). Device factors referred to CIED and procedure types. Medication factors referred to the usage of antibiotics, steroid, antiplatelets, and warfarin. Finally, the hospital procedure volume and hospital level were defined as provider factors. The hospital procedure volume was a time-dependent variable and was indicative of the annual hospital CIED procedure numbers.
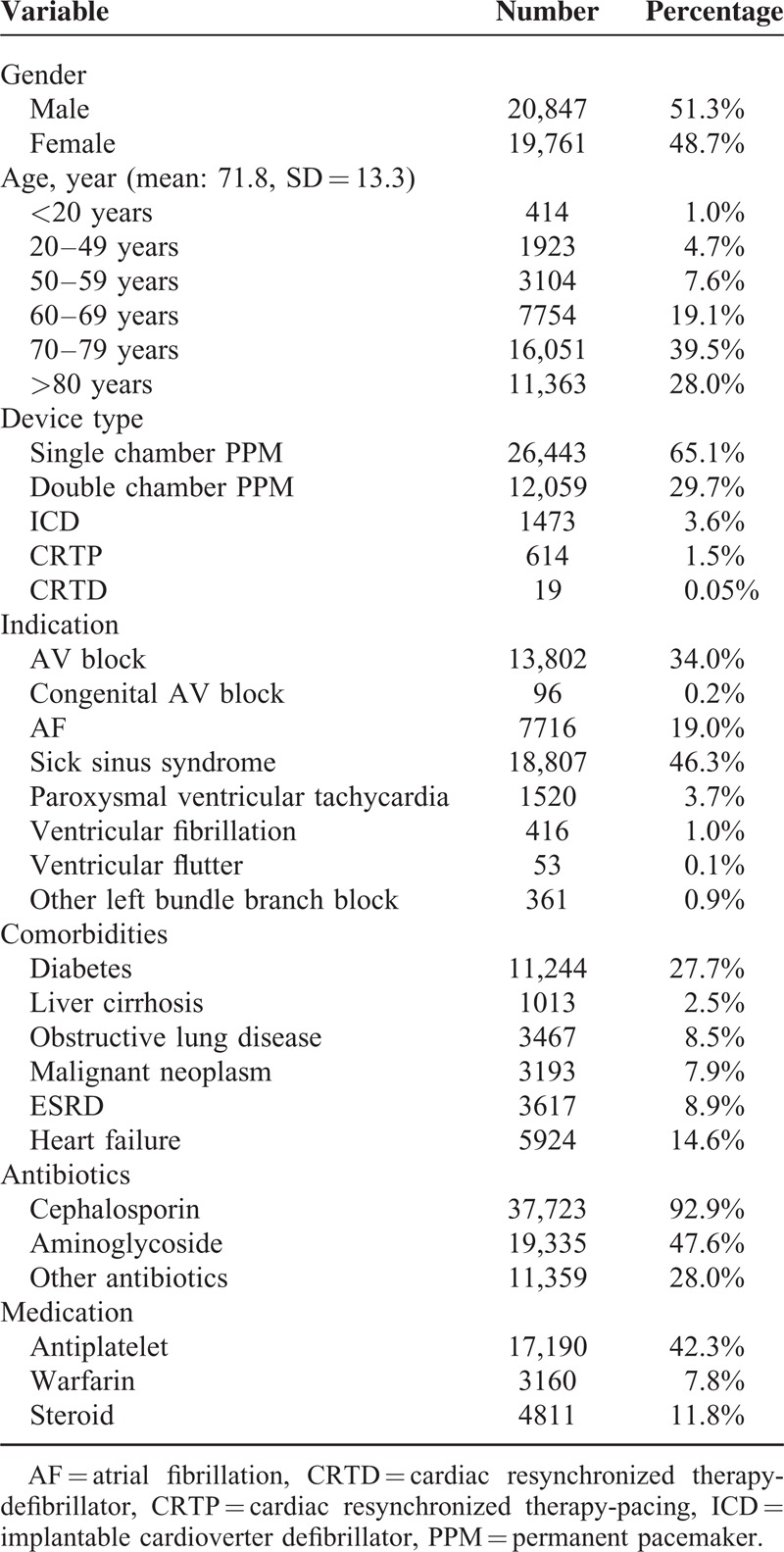
TABLE 1.
Patient Characteristics at First Onset (n = 40,608)
Statistics
Our study noted the possibility that 1 subject might suffer 1 or more infections related to CIED procedures and replacements during the study period. Hence, independent replacement risk factors due to CIED infection were identified using multiple-event per subject Cox proportional hazard analyses. This model allowed for evaluation of time-dependent prognostic factors and multiple events. The associated complications (pneumothorax and heart repair) were identified by multivariate logistic regression analyses. The results were presented as an odds ratio (OR) for logistic regression or hazard ratio (HR) for Cox regression with corresponding 95% confidence intervals (CIs). All data analyses were conducted using SPSS software version 15.0 (SPSS Inc., Chicago, IL).
RESULTS
From January 1, 1997 to December 31, 2010, this study included 46,506 CIED procedures, which comprised of 40,608 patients during the study period (Supplementary, http://links.lww.com/MD/A84). There were 35,308 patients accepted for the first CIED implantation during the study period; 10,731 replacement procedures were carried out in 4840 patients. Reoperations consisting of lead replacements without generator replacement were performed in 467 procedures (out of 461 patients). In those patients with mechanical complications during the same CIED procedure admission, there were 279 episodes of pneumothorax and 43 episodes of heart perforation requiring surgical correction. The total follow-up was 170,299 device-years; mean follow-up for each subject was 4.19 person-years. A total of 417 CIED-related procedures due to infection were found during the study period: 290 after first CIED implantation (incidence 2.19/1000 CIED-years), 120 after replacement (incidence 3.39/1000 CIED-years), and 7 after revision/replacement (incidence 3.08/1000 PPM-years). The infection incidence rate was 2.45/1000 CIED-years and the most events happened in the first 6 months. The time to the CIED infection was illustrated by Kaplan–Meier plots in Figure 1.
FIGURE 1.
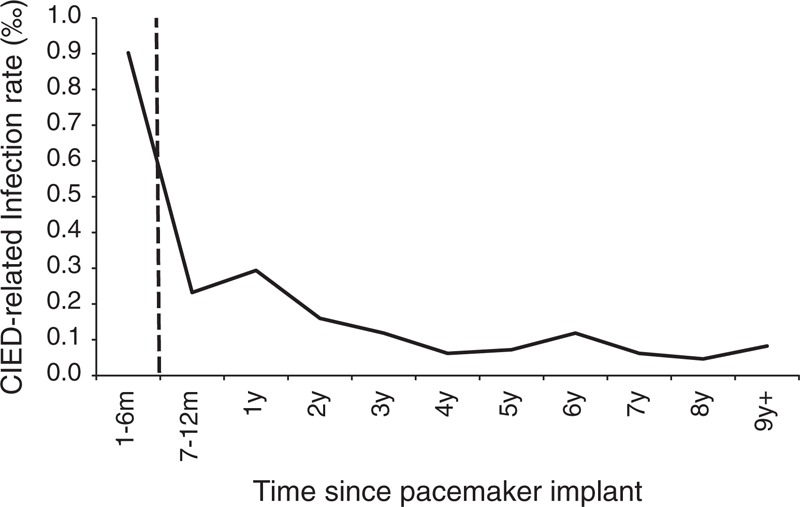
Time to cardiac implantable electronic device (CIED) infection event.
The baseline characteristics of patients are shown in Table 1. The mean age of CIED patients was 71.8 ± 13.3 years old (y/o) and the age distribution skewed toward elderly participants (>70 y/o; 67.5%). Male gender was predominant (51.3%). Patients who underwent pacemaker implantations were in the majority (94.8%), and the major criteria for CIED were sick sinus syndrome (46.3%) and atrioventricular (AV) block (34%). In addition, prophylactic antibiotics were prescribed in nearly all the types of procedures.
Risk Factors Associated With Infection
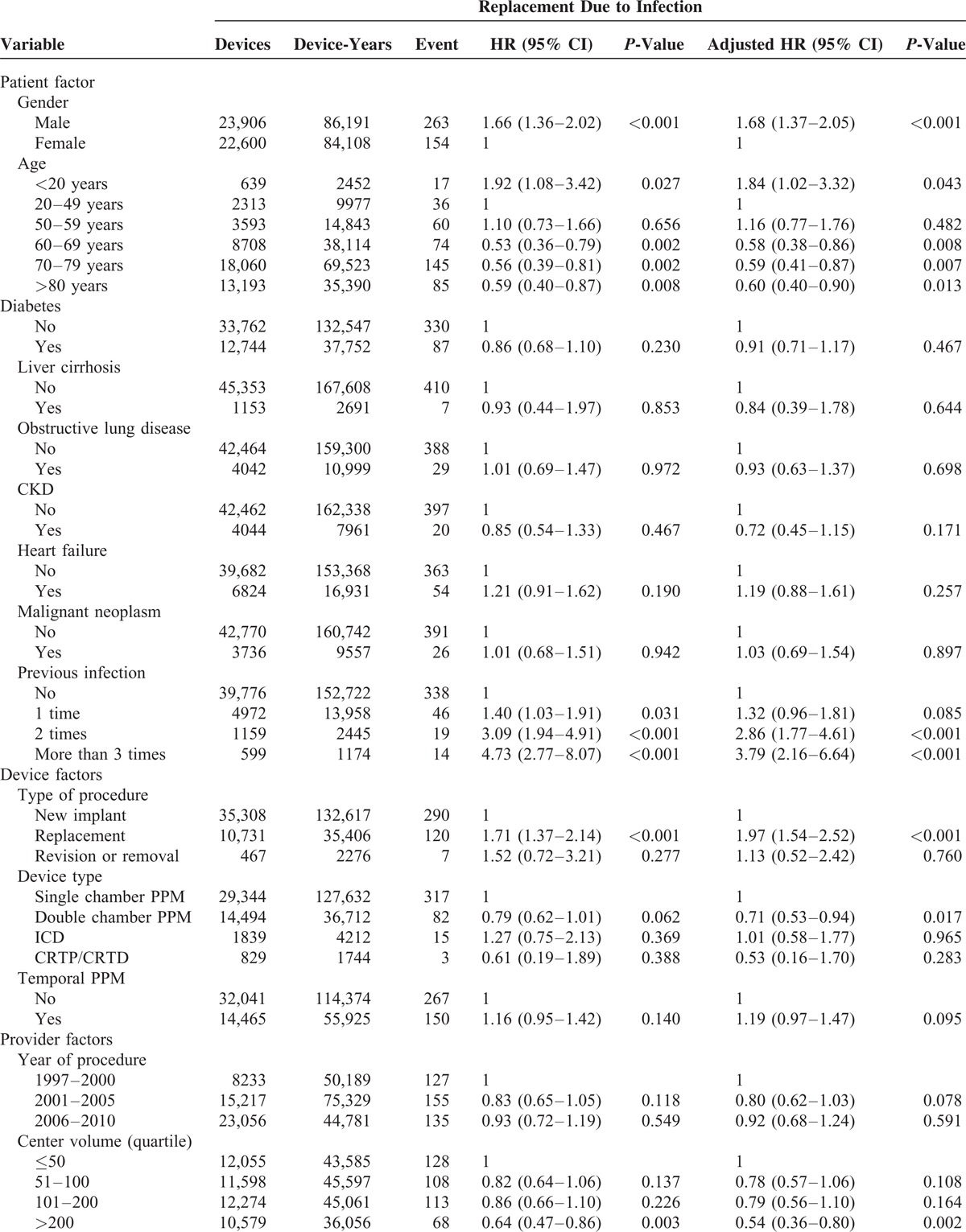
Several CIED infection risks were found (shown in Table 2 ). There were no significant differences in patient factors, except for gender and age: male patients had higher incidence of infection than females (P < 0.001; HR: 1.68, 95% CI: 1.37–2.05); Relative to middle age (20–49 y/o), young age (<20 y/o) (P = 0.027; HR: 1.84, 95% CI: 1.02–3.32) was a risk for infection, while old age (all staged aged period >60 y/o; all P < 0.01; HR: around 0.53–0.59) prevented patients from CIED-related infection. In addition, patients with a greater frequency of previous CIED infections had a higher incidence of CIED infection. Regarding device factors, replacement procedures (P < 0.001; HR: 1.71, 95% CI: 1.37–2.14) were an increased risk for CIED infection when compared to new implantation; dual chamber PM was a protector for CIED infection when compared to single chamber PM (P = 0.021; HR: 0.79, 95% CI: 0.62–1.01). Among provider and medication factors, high center volume (>200 vs ≤50; P = 0.002; HR: 0.54, 95% CI: 0.36–0.80) was a protector from infection; cephalosporin also trended toward preventing devices from infection (P = 0.078; HR: 0.74, 95% CI: 0.54–1.03).
TABLE 2.
Associated Factors Related to CIED Infection
Risk Factors Associated With Complications—Pneumothorax and Heart Perforation
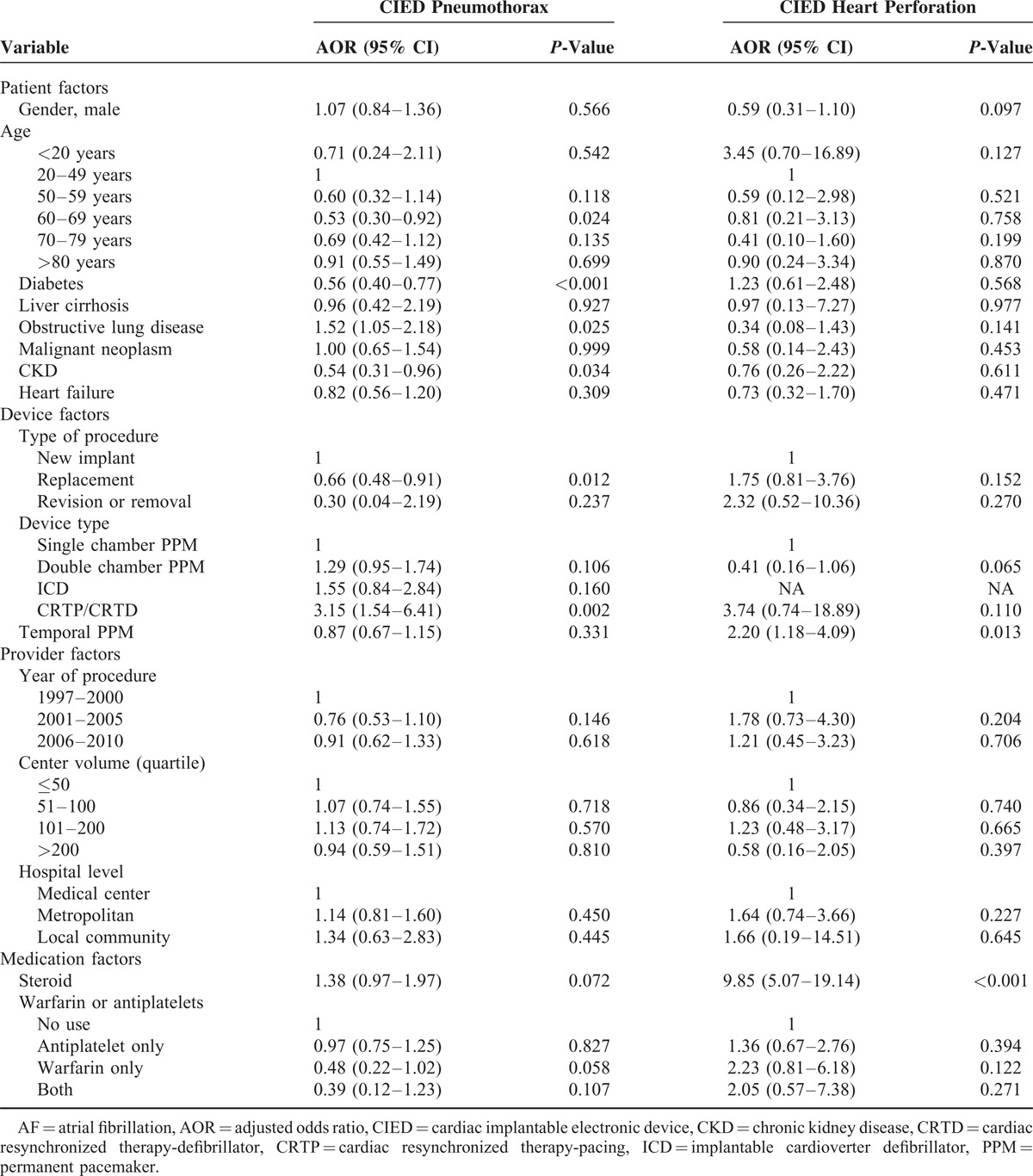
The CIED pneumothorax incidence was 0.60% (279/46,506) and CIED heart perforation incidence was 0.09% (43/46,506) in this study. Regarding CIED pneumothorax, chronic obstructive lung disease (COPD) (P = 0.025; HR: 1.52, 95% CI: 1.05–2.18) was a risk while DM (P < 0.001; HR: 0.56, 95% CI: 0.40–0.77) and chronic kidney disease (CKD) (P = 0.034; HR: 0.54, 95% CI: 0.31–0.96) might be protectors (see the left panel in Table 3). The CIED pneumothorax incidence was lower in patients accepting CIED replacement than those accepting new devices (P = 0.012; OR: 0.66 CI: 0.48–0.91): the incidence was also lower in patients accepting pacemakers than those accepting cardiac resynchronized therapy-pacing/cardiac resynchronized therapy-defibrillator (CRT-P/CRT-D) (P = 0.02; OR: 3.15 CI: 1.54–6.41). Regarding CIED heart perforation, there were only 2 risks identified under logistic regression analyses. One was the pre-operation temporal pacing system (P = 0.013; OR: 2.20 CI: 1.18–4.09) and the other was steroid use (P = < 0.001; OR: 9.85 CI: 5.07–19.14), as seen in the right panel of Table 3.
TABLE 2 (Continued).
Associated Factors Related to CIED Infection
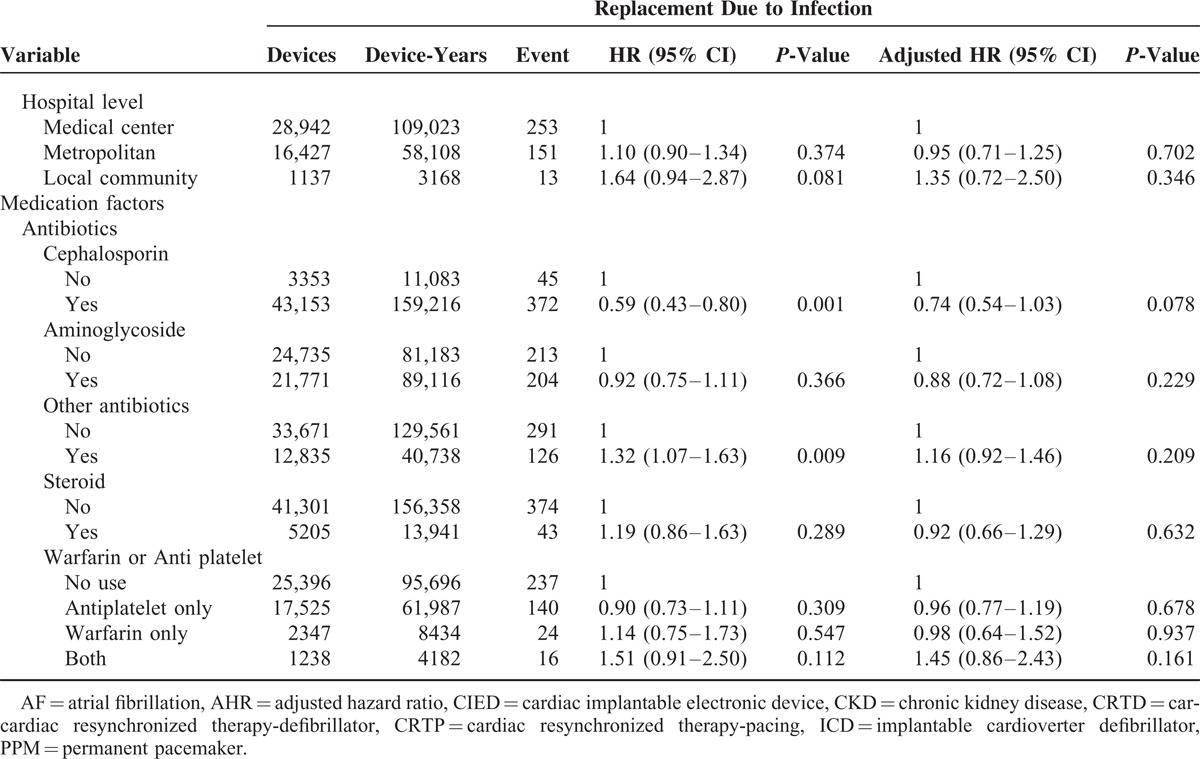
TABLE 3.
Associated CIED-Related Complication Factors: CIED Pneumothorax and CIED Heart Perforation
DISCUSSION
In this nationwide cohort study, some risk factors of the 3 catastrophes (CIED related infection, pneumothorax, and cardiac rupture) were found. Male gender, young age, CIED replacement, and previous CIED-related infection were contributors of CIED infection while old age and high-volume center were protectors. In individuals with pneumothorax, the incidence was higher in COPD patients than those without COPD, as well as the incidence was higher in patients who accepted CRT-P/CRT-D than those who accepted single chamber PM. A pre-operation temporal pacing and steroid use increased the risk of heart perforation.
Factors Contributing to Infection
The incidence of CIED infection has been evaluated since the early 1970s and has varied widely between 0.13% and 19.9%. Gould et al23 reported that a study demonstrated increasing infection rates year by year. Baddour et al16 also observed that the numbers of CIED infection-related hospitalizations increased out of proportion to rates of new device implantation. Although these conditions were not evident in our study, which showed constant infection rates per year (the incidence was around 1%) (Supplementary, http://links.lww.com/MD/A84), our findings corresponded to a large registered study from Danish.11 In terms of risk factors of CIED infection, more comprehensive parameters, such as baseline characteristics, the type of procedures, providers and medications, were analyzed in our study when comparison with another nation cohort study (a registry study in Danish).11
Regarding patient factors, male gender was important and an inverse relationship between age and infection risk also was found, showing that the infection rate was highest in children and lowest in the elderly. Although several hypotheses have been proposed explaining the increased pediatric infection risk, including more abdominal approaches in children and a greater frequency of replacement, no studies have decidedly verified any hypothesis. In our study, we found CIED replacement is an independent risk for infection and more epicardial leads and higher prevalence of replacement were noted in younger-aged participants than older-aged ones (Supplementary, http://links.lww.com/MD/A85, and http://links.lww.com/MD/A86). This study can thus indirectly confirm 1 hypothesis. As for provider factors, high risk of ICD infection associated with lower physician volume was reported by Al-Khatib et al24 but high hospital volume was proven to decrease the risk of ICD implantation complications in a retrospective study.25 Unfortunately, no previous study could establish an effect of hospital volume in other CIEDs.11,14 In this study, we showed that hospital volume had a significant effect on overall CIED-related infection. When combining the Danish registry data11 and ours, both hospital volume and operator experience were important factors in CIED-related infection control.
As for medication factors, a meta-analysis study26 concluded that prophylactic antibiotics could reduce CIED-related infections and guideline for CIED infection16 also recommends pre-procedure antibiotics. But no large study has concluded which antibiotics is the most beneficial in reducing infection rates. Therefore, this is the first large study to mention what antibiotics impact on the CIED-related infection and to find that the cephalosporins might be more efficacious than others, especially in replacement procedures (Supplementary, http://links.lww.com/MD/A87). At last, other potential risks of infection published in the literature, such as DM, heart failure,9,27,28, corticosteroid13 and anticoagulation,29 did not achieve statistical significance but they nonetheless did correspond to another large prospective multicenter study.14
Factors Contributing to Pneumothorax
The incidence of CIED related pneumothorax was reported as 1.7% in a single center 30 and was 0.60% over a 14-year observation period in a national registry.17 In this study, our result (0.60% (279/46,506)) was comparable to presented incidence in the literature. Regarding of risks for pneumothorax, the Danish registry showed that COPD and dual chamber device were important risks for pneumothorax and they also supposed CRT may be a risk. In our study, CRT-P/D was proven as a risk of pneumothorax. Similarly, our study also was able to confirm the hypothesis that replacement is a protector (given the absence of lead insertions). Other protective factors for pneumothorax were in this study, such as diabetes and CKD, but no evidence has emerged to show why the above variables became predictors. A possible explanation is that the previously mentioned comorbidities are notorious risks for cardiovascular disease, making cardiologists pay greater attention to patients undergoing procedures with these comorbidities.
Factors Contributing to Heart Perforation
Heart perforation has been a rare but significant complication after CIED implantation. Khan et al31 summarized event rates ranging from 0.1% to 0.8% for pacemakers and 0.6–5.2% for ICD. In our study, the event rate for heart perforation was lower than rates reported in literature at 0.09%, though the major reason may be our restricted definition focusing on the complications requiring intervention. In addition, some studies have mentioned that heart perforation incidence may be underestimated due to the fact that many perforations lack clinical symptoms.32 Regarding the risk of heart perforation, a cohort study from the Mayo clinic20 noted 2 strong predictors: temporary transvenous pacemaker installation and steroid use. These 2 risks were proven again in our study. In the Mayo clinic study, the researchers proposed that temporary pacing leads were usually placed in emergencies and more leads in the right ventricle20 increased the perforation risk. In our clinical practice, we agree with that explanation. In terms of steroid use, although Mahapatra et al20 proposed that steroid use will make cardiac muscle weaker and atrophy as it does to skeletal muscle,33 there has been no published research to support this hypothesis, and thus requires further study.
Study Limitations
This retrospective analysis bears the inherent limitations of these types of studies. First, the information of the severity of the complications and procedures was not available, since the data were from an insurance system where diseases were classified according to ICD-9 codes and payment was made according to procedure type. Therefore, some complications may have been underestimated. Nonetheless, the complications requiring treatment were likely to be more important clinically than those without therapy. Second, information for some potential risks, including preoperative infections and procedure type (ie, cut-down vs puncture method) were not available in the analysis. However, the data were from the national insurance system that covers nearly every citizen in a country that may reduce systematic bias caused by a single operator or center and uncover factors that may not be readily found in studies of single clinics or with a small sample size. Therefore, such large number of patients and the complete follow-up should counterbalance the inherent study weaknesses.
CONCLUSION
In the CIED related infection, high-volume center can lower it and clinicians should pay more attention to young male patients and those who undergo device replacements. Pre-procedure antibiotics remain important and cephalosporins should be considered a priority, especially in CIED replacement patients. As for heart perforation, temporary pacing should be avoided whenever possible after taking into account patient stability and timing to permanent pacing device. Careful attention should be paid when patients on steroids receive CIED implantations, due to the higher risk of heart perforation and it is recommended that steroids be discontinued if possible. Clinicians also should pay more attention to COPD patients and CRT patients in order to prevent pneumothorax.
Footnotes
Abbreviations: CIED = cardiac implantable electronic device, CRT = cardiac resynchronized therapy, CRT-P = cardiac resynchronized therapy-pacing, CRT-D = cardiac resynchronized therapy-defibrillator, COPD = chronic obstructive lung disease, CKD = chronic kidney disease, DM = diabetes mellitus, ESRD = end-stage renal disease, ICD = implantable cardioverter-defibrillator, PM = Pacemaker.
The authors have no funding or conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Vardas PE, Auricchio A, Blanc JJ, et al. Guidelines for cardiac pacing and cardiac resynchronization therapy: The Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology. Developed in collaboration with the European Heart Rhythm Association. Eur Heart J 2007; 28:2256–2295. [DOI] [PubMed] [Google Scholar]
- 2.Abikhzer G, Turpin S, Bigras JL. Infected pacemaker causing septic lung emboli detected on FDG PET/CT. J Nucl Cardiol 2010; 17:514–515. [DOI] [PubMed] [Google Scholar]
- 3.Dickstein K, Vardas PE, Auricchio A, et al. 2010 Focused Update of ESC Guidelines on device therapy in heart failure: an update of the 2008 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure and the 2007 ESC guidelines for cardiac and resynchronization therapy. Developed with the special contribution of the Heart Failure Association and the European Heart Rhythm Association. Eur Heart J 2010; 31:2677–2687. [DOI] [PubMed] [Google Scholar]
- 4.Mond HG, Irwin M, Ector H, et al. The world survey of cardiac pacing and cardioverter-defribrillators: calendar Year 2005 an International Cardiac Pacing and Electrophysiology Society (ICPES) project. PACE 2008; 31:1202–1212. [DOI] [PubMed] [Google Scholar]
- 5.Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009: a World Society of Arrhythmia's project. PACE 2011; 34:1013–1027. [DOI] [PubMed] [Google Scholar]
- 6.Cabell CH, Heidenreich PA, Chu VH, et al. Increasing rates of cardiac device infections among Medicare beneficiaries: 1990–1999. Am Heart J 2004; 147:582–586. [DOI] [PubMed] [Google Scholar]
- 7.Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. PACE 2010; 33:414–419. [DOI] [PubMed] [Google Scholar]
- 8.Sohail MR, Henrikson CA, Braid-Forbes MJ, et al. Mortality and cost associated with cardiovascular implantable electronic device infections. Arch Intern Med 2011; 171:1821–1828. [DOI] [PubMed] [Google Scholar]
- 9.Tompkins C, McLean R, Cheng A, et al. End-stage renal disease predicts complications in pacemaker and ICD implants. J Cardiovasc Electrophysiol 2011; 22:1099–1104. [DOI] [PubMed] [Google Scholar]
- 10.Nery PB, Fernandes R, Nair GM, et al. Device-related infection among patients with pacemakers and implantable defibrillators: incidence, risk factors, and consequences. J Cardiovasc Electrophysiol 2010; 21:786–790. [DOI] [PubMed] [Google Scholar]
- 11.Johansen JB, Jorgensen OD, Moller M, et al. Infection after pacemaker implantation: infection rates and risk factors associated with infection in a population-based cohort study of 46299 consecutive patients. Eur Heart J 2011; 32:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romeyer-Bouchard C, Da Costa A, Dauphinot V, et al. Prevalence and risk factors related to infections of cardiac resynchronization therapy devices. Eur Heart J 2010; 31:203–210. [DOI] [PubMed] [Google Scholar]
- 13.Sohail MR, Uslan DZ, Khan AH, et al. Risk factor analysis of permanent pacemaker infection. Clin Infect Dis 2007; 45:166–173. [DOI] [PubMed] [Google Scholar]
- 14.Klug D, Balde M, Pavin D, et al. Risk factors related to infections of implanted pacemakers and cardioverter-defibrillators: results of a large prospective study. Circulation 2007; 116:1349–1355. [DOI] [PubMed] [Google Scholar]
- 15.Raad D, Irani J, Akl EG, et al. Implantable electrophysiologic cardiac device infections: a risk factor analysis. Eur J Clin Microbiol Infect Dis 2012; 31:3015–3021. [DOI] [PubMed] [Google Scholar]
- 16.Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation 2010; 121:458–477. [DOI] [PubMed] [Google Scholar]
- 17.Kirkfeldt RE, Johansen JB, Nohr EA, et al. Pneumothorax in cardiac pacing: a population-based cohort study of 28,860 Danish patients. Europace 2012; 14:1132–1138. [DOI] [PubMed] [Google Scholar]
- 18.Tobin K, Stewart J, Westveer D, et al. Acute complications of permanent pacemaker implantation: their financial implication and relation to volume and operator experience. Am J Cardiol 2000; 85:774–776. [DOI] [PubMed] [Google Scholar]
- 19.Piekarz J, Lelakowski J, Rydlewska A, et al. Heart perforation in patients with permanent cardiac pacing—pilot personal observations. Arch Med Sci 2012; 8:70–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mahapatra S, Bybee KA, Bunch TJ, et al. Incidence and predictors of cardiac perforation after permanent pacemaker placement. Heart Rhythm 2005; 2:907–911. [DOI] [PubMed] [Google Scholar]
- 21.Wu C-Y. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection HBV nucleoside analogues, and HCC recurrence. JAMA 2012; 308:1906–1914. [DOI] [PubMed] [Google Scholar]
- 22.Cheng CL, Kao YH, Lin SJ, et al. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Safety 2011; 20:236–242. [DOI] [PubMed] [Google Scholar]
- 23.Gould PA, Gula LJ, Yee R, et al. Cardiovascular implantable electrophysiological device-related infections: a review. Curr Opin Cardiol 2011; 26:6–11. [DOI] [PubMed] [Google Scholar]
- 24.Al-Khatib SM, Lucas FL, Jollis JG, et al. The relation between patients’ outcomes and the volume of cardioverter-defibrillator implantation procedures performed by physicians treating Medicare beneficiaries. J Am Coll Cardiol 2005; 46:1536–1540. [DOI] [PubMed] [Google Scholar]
- 25.Freeman JV, Wang Y, Curtis JP, et al. The relation between hospital procedure volume and complications of cardioverter-defibrillator implantation from the implantable cardioverter-defibrillator registry. J Am Coll Cardiol 2010; 56:1133–1139. [DOI] [PubMed] [Google Scholar]
- 26.Darouiche R, Mosier M, Voigt J. Antibiotics and antiseptics to prevent infection in cardiac rhythm management device implantation surgery. PACE 2012; 35:1348–1360. [DOI] [PubMed] [Google Scholar]
- 27.Bloom H, Heeke B, Leon A, et al. Renal insufficiency and the risk of infection from pacemaker or defibrillator surgery. PACE 2006; 29:142–145. [DOI] [PubMed] [Google Scholar]
- 28.Dasgupta A, Montalvo J, Medendorp S, et al. Increased complication rates of cardiac rhythm management devices in ESRD patients. Am J Kidney Dis 2007; 49 5:656–663. [DOI] [PubMed] [Google Scholar]
- 29.Lekkerkerker JC, van Nieuwkoop C, Trines SA, et al. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart 2009; 95:715–720. [DOI] [PubMed] [Google Scholar]
- 30.Pakarinen S, Oikarinen L, Toivonen L. Short-term implantation-related complications of cardiac rhythm management device therapy: a retrospective single-centre 1-year survey. Europace 2010; 12:103–108. [DOI] [PubMed] [Google Scholar]
- 31.Khan MN, Joseph G, Khaykin Y, et al. Delayed lead perforation: a disturbing trend. PACE 2005; 28:251–253. [DOI] [PubMed] [Google Scholar]
- 32.Hirschl DA, Jain VR, Spindola-Franco H, et al. Prevalence and characterization of asymptomatic pacemaker and ICD lead perforation on CT. PACE 2007; 30:28–32. [DOI] [PubMed] [Google Scholar]
- 33.Twycross R. The risks and benefits of corticosteroids in advanced cancer. Drug Saf 1994; 11:163–178. [DOI] [PubMed] [Google Scholar]


