Abstract
In the setting of central precocious puberty (CPP), the motivation for hormonal intervention is to help the child to reach a taller adult stature than she would achieve otherwise. While gonadotropin-releasing hormone analogs (GnRHa) constitute an established treatment for improving adult stature in girls presenting with CPP up to age 6 (true precocious puberty), it is not yet clear whether or not the same is true in the setting of CPP presented in girls beyond age 6 (advance puberty). GnRHa may slow growth velocity, offsetting the anticipated improvement in final height that should have resulted from the increased time before growth plate fusion. Consequently, it's been suggested that growth hormone (GH) should be combined with GnRHa to improve the results.
Few controlled prospective studies have been performed with GnRHa in children and many conclusions rely in part on collective expert opinion. Therefore, the literature was searched and relevant studies were selected using the search terms “gonadotropin releasing hormone agonist,” “precocious puberty/early puberty,” and “GnRH analogue.” After selected articles were screened for relevance, the process yielded 8 studies, the results of which were then pooled in a meta-analysis aimed at evaluating the effects of GnRHa therapy both with and without added GH in the setting of early puberty.
A significant difference was elucidated in final height and predicted adult height comparing GnRHa and combined GnRHa/GH groups. However, no significant difference was elucidated in final height standard deviation scores (SDS) and initial height SDS when comparing GnRHa and control groups. At the same time, the final analysis revealed no significant difference in final height SDS and initial height SDS when GnRHa and combined GnRHa/GH groups were compared.
The results suggest GnRHa therapy may have a positive effect on final adult height in girls with early puberty, while adding GH to the treatment may suggest more advantage. Interpretation of the results requires extreme caution, given the complexity of the outcome analysis. Final height gain may prove to be a more appropriate measure of treatment efficacy in any case.
INTRODUCTION
Occurring in approximately 1 out 5000 to 10,000 children, precocious puberty is 10 times more common in females than males, and the number of diagnosed cases is on the rise.1,2 In a minority of cases, the condition results from a peripheral etiology, such as an ovarian or adrenal tumor or cyst or exposure to estrogen from an external source.3,4 However, most cases occur as central precocious puberty (CPP), resulting from disruption of the hypothalamic-pituitary-gonadal (HPG) axis.5 In rare cases, the HPG axis disruption may present with an identifiable cause, such as a central nervous system (CNS) neoplasm, hemartoma, or hydrocephalus, CNS injury, or hypothyroidism.6 Additionally, both peripheral precocious puberty and CPP may be associated with genetic disorders, such as McCune–Albright syndrome.7 In the majority of cases, however, precocious puberty is idiopathic (with no identifiable cause).8–10
In some cases of idiopathic CPP, the motivation for intervention is to mitigate the continued early development of secondary sexual characteristics that otherwise would lead to psychosocial maladjustment, although the majority of cases present with only 1 premature secondary sex characteristic3 Thus, in most cases, the motivation for treatment is not sexual precocity per se, but rather to help the child to reach a taller adult stature than she would achieve otherwise,10 although final height can influence psychosocial adjustment apart from secondary sex characteristics.11 The target height for such children is based on population, gender-specific growth curves that have been evolving since the 1940s,12 and that have been augmented with various height prediction tools, such as the Bayley–Pinneau method12–14 and the Roche–Wainer–Thissen and Tanner methods.14 Although parental desire for their children to achieve tall stature is influenced strongly by cultural factors, all of the height prediction methods cited above and the hormonal treatment that will be discussed below address final stature in the context of the child's genetic disposition.9–11,13,15 Thus, from the perspective of precocious puberty, a child reaching short final stature is one who ends up significantly shorter than her parents. Untreated, precocious puberty leads to short final stature, because it increases the growth velocity (GV), which in turn causes early fusion of the epiphyseal growth plates of long bones.12,14,16,17 Consequently, the clinical strategy of choice is to administer gonadotropin-releasing hormone (GnRH) analogs (GnRHa), which down-regulate and desensitize of pituitary GnRH receptors, inhibiting the HPG axis, thereby slowing the onset of puberty.18 This, in turn, reduces the GV, giving the long bones more time to lengthen before the grow plates fuse, thus maximizing the adult height that the child obtains.18
When considering intervention for CPP, it is important to distinguish patients according to age. In girls, true precocious puberty is the appearance of secondary sex characteristics before 8 years of age, or the onset of menarche before age 9. However, many patients are referred to pediatric endocrinology clinics for advanced puberty, which refers to the onset of pubertal signs between ages 8 and 10.19 While GnRHa treatment is established for improving adult stature in girls presenting with CPP up to age 6 several controversies remain for older age groups.10 Firstly, not enough data have been published to differentiate between outcomes from different hormonal treatments, or for long-term effects on reproductive health or on offspring, although it now appears that GnRHa does not lead to losses in bone mineral density, nor to polycystic ovarian disease (PCOD).20 Secondly, more study is needed as to the psychosocial effects of treatment,10 and, moreover, a concerted effort is needed to assess the efficacy of GnRHa and variants of the treatment in the setting of advanced puberty.10 In particular, in certain cases, the use of GnRHa slows the GV to such an extent as to offset the anticipated improvement in final height that should have resulted form the increased time before growth plate fusion.18 Consequently, it's been suggested that growth hormone (GH) should be combined with GnRHa to improve the results.18 Few controlled prospective studies have been performed with GnRHa in children and many conclusions rely in part on collective expert opinion.10 Therefore, the aim of the current meta-analysis is to evaluate the effect of GnRHa therapy both with and without added GH.
METHODS
Search Strategy
The Medline, Cochrane, EMBASE, Google Scholar databases were searched for studies published up to March 1, 2014 using the key phrases “gonadotropin releasing hormone agonist,” “precocious puberty/early puberty,” and “GnRH analogue.” The reference lists of relevant studies were also hand searched for relevancy. Reports were included, if they were randomized controlled trials or 2-arms prospective studies, and if the patients had presented with precocious puberty. Additionally, for inclusion in the meta-analysis, patients had to have been grouped into either GnRHa versus combined GH/GnRHs or GnRHa versus control. Papers were excluded, if they were case reports, letters, comments, or editorials, if they were retrospective in design, if patient groups were stratified based on predicted adult height (PAH), if they compared efficacy of varying dosages and/or varying GnRHa protocol, or if no quantitative outcomes were reported.
Study Selection and Data Extraction
Using search and screening approach outlined above, articles were selected by 2 independent reviewers. In cases of uncertainty regarding eligibility of a publication, a third reviewer was consulted. Two independent reviewers also performed the data extraction; as with the article selection process, a third reviewer was consulted in cases of uncertainty. The following data were extracted from studies that met the inclusion criteria: the name of the first author; the year of publication; study design; number of participants in each treatment group; subject demographic data; diagnostic criteria; the agent/s used; dosage and length of treatment for each group; clinical results.
Quality Assessment and Outcome Measures
The studies included at the end of the selection process were assessed for risk of bias using the “Risk of Bias” assessment tool, Review Manager 5.1. Recommendations for judging risk of bias were provided in Chapter 8 of the Cochrane Handbook for Systematic Reviews Interventions [Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org]. Height gain, defined as difference between initial height prediction for PAH and attained final height, was the primary outcome measure of the current study. Secondary outcome was difference between final height standard deviation scores (SDS) and initial height SDS.
Statistical Analysis
For both the primary and secondary outcomes described above, standard difference in means was calculated to compare between control groups, GnRHa only groups, and combined GnRHa/GH groups. The Cochran Q and the I2 statistic, were applied to assess heterogeneity among the studies. For the Q statistic, P < 0.10 was considered statistically significant for heterogeneity. Indicative of the observed variability between studies (due to heterogeneity rather than chance), the I2 statistic was assessed using the following ranges as guidelines: no heterogeneity (I2 = 0–25%); moderate heterogeneity (I2 = 25–50%); large heterogeneity (I2 = 50–75%); and extreme heterogeneity (I2 = 75–100%). When either Q statistics (P < 0.1) or I2 statistic (>50%) indicated heterogeneity between studies, the random-effects model was preferred (DerSimonian–Laird method). Otherwise, the fixed-effect model (Mantel–Haenszel method) was recommended. Pooled standard difference in means of the 2 outcomes was calculated, and a 2-sided P-value <0.05 was considered indicative of statistical significance. Sensitivity analysis was carried out based on the leave-one-out approach of difference between final height and PAH for GnRH analogue group compared to the control group. All statistical analyses were performed using the statistical software Comprehensive Meta-Analysis, version 2.0 (Biostat, Englewood, NJ).
RESULTS
Literature Search
The article screening and selection process is summarized as a flow chart (Figure 1). The initial literature search yielded 174 articles after the removal of duplicates. Of these, 158 were excluded as not meeting inclusion criteria or non-relevant, leaving 16 reports for full-text review to assess eligibility. Two of the full-review studies were then excluded because they did not involve an intervention of interest, while 2 others were excluded because no outcomes of interest were assessed. Two more studies were excluded because they recruited patients with normally timed puberty, despite short stature.21,22 Two other studies were removed because they involved the same patients.23,24 Summarized as a flow chart in Figure 1, the article screening yielded 8 studies for meta-analysis.17,18,25–30 The characteristics of the meta-analyzed studies are summarized in Table 1, while the assessed outcomes of the studies are shown in Figure 2. For each trial, the risk of bias is detailed as a “risk of bias” summary (Figure 2A), and an overall assessment of risk of bias is depicted as a “risk of bias graph” (Figure 2B).
FIGURE 1.
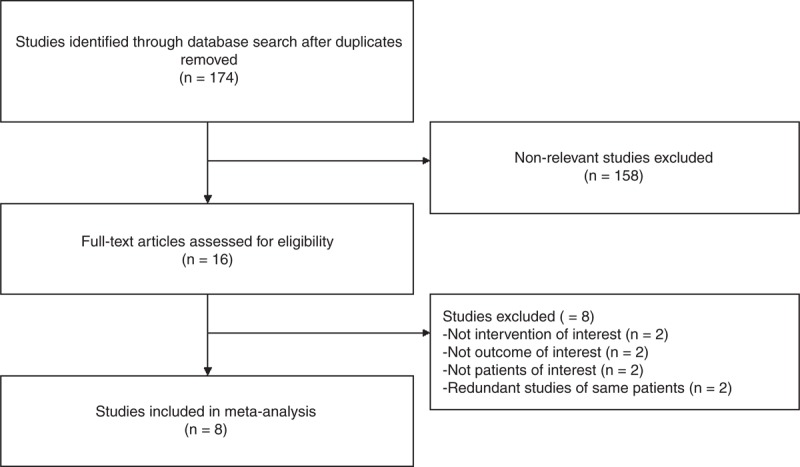
Flow chart summarizing study selection.
TABLE 1.
Summary of Meta-Analyzed Studies on Treatment of Girls With Advanced Puberty
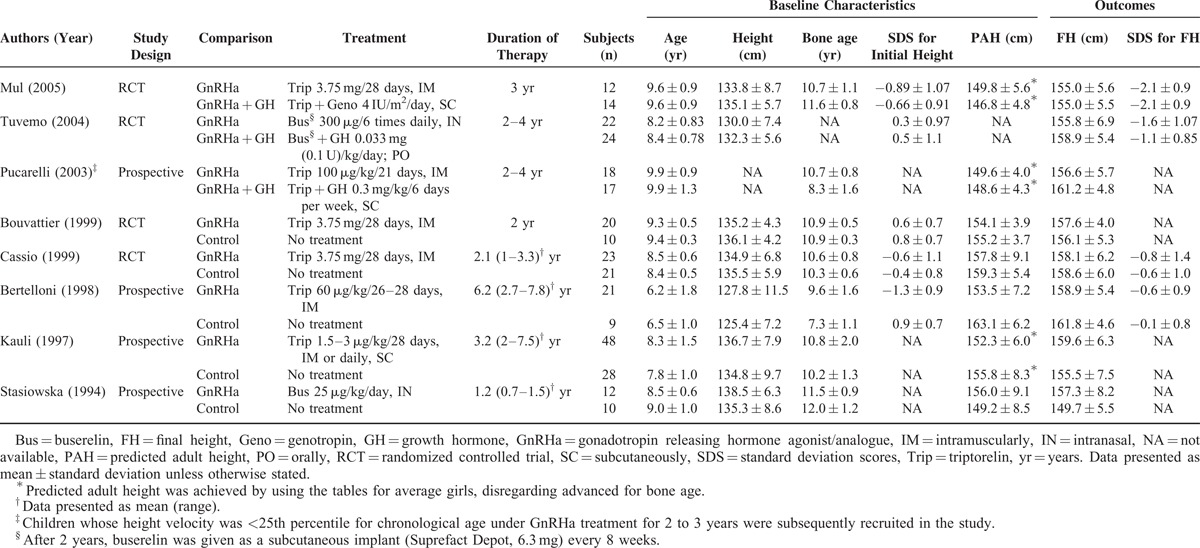
FIGURE 2.
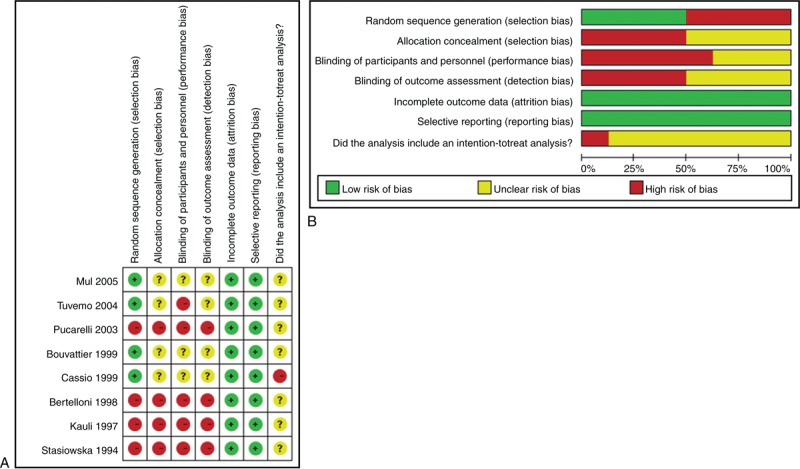
(A) The quality assessment for each included study as “risk of bias summary.” (B) Outcomes presented as percentages across all meta-analyzed studies, each depicted as a “risk of bias graph” (B).
Study Characteristics and Clinical Outcome
Basic Characteristics
The characteristics of the meta-analyzed studies are summarized in Table 1. Five studies compared GnRHa treatment with an untreated control group,8,17,28–30 while 3 studies compared GnRHa alone with combined GnRHa/GH treatment.18,25,26 Total numbers of participants ranged from 12 to 48 in the GnRH analogue group and from 9 to 28 in the comparison group. Mean age ranged from 6.2 to 9.9 years in the GnRH analogue group and from 6.5 to 9.9 years in the comparison group. All participants were female. SDS for initial height ranged from −1.3 to 0.6 in the GnRH analogue group and −0.7 to 0.9 in the comparison group. PAH ranged from 149.6 to 157.8 cm in the GnRH analogue group and from 146.8 to 163.1 cm in the comparison group. Final height ranged from 155.0 to 159.6 cm in the GnRH analogue group and from 149.7 to 161.8 cm in the comparison group. SDS for final height ranged from −2.1 to −0.6 in the GnRH analogue group and from −2.1 to −0.1 in the comparison group.
Outcome Evaluation
Difference Between Final Height and Predicted Adult Height
There was significant heterogeneity when data from the 5 studies were pooled (heterogeneity test: Q = 8.74, df = 4, P = 0.068, I2 = 54.23%); therefore, a random-effects model of analysis was used (Figure 3A). The overall analysis revealed significant difference in final height and PAH comparing the GnRHa and control groups (pooled standard difference in means = 0.63, 95% CI = 0.17–1.08, Z = 2.71, P = 0.007).
FIGURE 3.
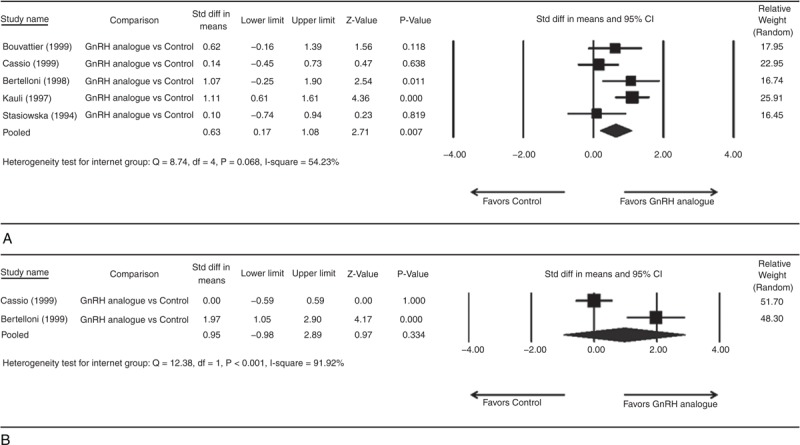
Forest plots showing results for the meta-analysis of (A) difference between final height and predicted adult height; (B) difference between final height SDS and initial height SDS for GnRH analogue group compared to the control group. CI = confidence interval.
One study was not included in analysis because no outcome available for analysis.26 There was no significant heterogeneity when data from the 2 studies were pooled (heterogeneity test: Q = 1.23, df = 1, P = 0.268, I2 = 18.50%); therefore, a fixed-effect model of analysis was used (Figure 4A). The overall analysis revealed significant difference in final height and PAH comparing GnRHa and combined GnRHa/GH groups (pooled standard difference in means = −0.89, 95% CI = −1.42 to −0.36, Z = −3.28, P = 0.001).
FIGURE 4.
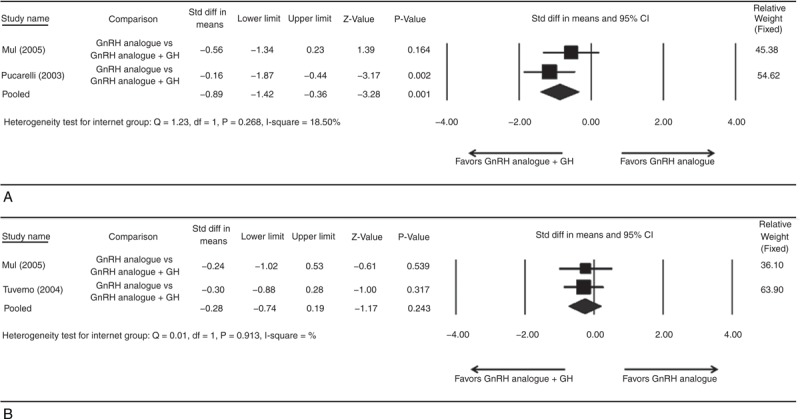
Forest plots showing results for the meta-analysis of (A) difference between final height and predicted adult height; (B) difference between final height SDS and initial height SDS incidence for GnRH analogue group compared with the GnRH analogue plus GH group. CI = confidence interval.
Difference Between Final Height SDS and Initial Height SDS
Three studies were not included in analysis because no outcome available for analysis.17,29,30 There was significant heterogeneity when data from the 2 studies were pooled (heterogeneity test: Q = 12.38, df = 1, P < 0.001, I2 = 91.92%); therefore, a random-effects model of analysis was used (Figure 3B). The overall analysis revealed no significant difference in final height SDS and initial height SDS comparing GnRHa and control groups (pooled standard difference in means = 0.95, 95% CI = −0.98 to 2.89, Z = 0.97, P = 0.334).
One study was not included in analysis because no outcome available for analysis.18 There was no significant heterogeneity when data from the 2 studies were pooled (heterogeneity test: Q = 0.01, df = 1, P = 0.913, I2 = 0%); therefore, a fixed-effect model of analysis was used (Figure 4B). The overall analysis revealed no significant difference in final height SDS and initial height SDS comparing GnRHa and combined GnRHa/GH groups (pooled standard difference in means = −0.28, 95% CI = −0.74 to 0.19, Z = −1.17, P = 0.243).
Sensitivity Analysis
Figure 5 summarizes the results of difference between final height and PAH for GnRHa group compared with the control group using the leave-one-out approach to assess sensitivity for random-effects model. The direction and magnitude of pooled estimates did not vary considerably, indicating that the meta-analysis had good reliability.
FIGURE 5.
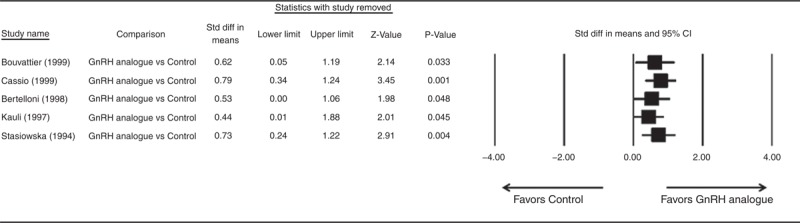
Results of sensitivity analysis to examine the influence of individual studies on pooled estimates as determined use the leave-one-out approach of difference between final height and predicted adult height for GnRH analogue group compared to the control group. CI = confidence interval, OR = odds ratio.
Publication Bias
Publication bias was not assessed for the above outcomes because more than 5 studies are required to detect funnel plot asymmetry.31
DISCUSSION
When untreated, CPP leads to short final stature in comparison with the stature dictated by the child's genetic disposition. GnRHa is the gold standard treatment and is well established in cases of true precocious puberty.10 However, more uncertainty exists regarding girls with advanced puberty, characterized by the appearance of sexual characteristics as early as 8 years of age and/or menarche by age 9. It has been suggested that GH should be combined with GnRHa to improve the results,18 but few controlled prospective studies have been performed with GnRHa in children.10 Therefore, the current meta-analysis was conducted to evaluate the effect of GnRHa therapy both with and without added GH. Evaluation was conducted using final height gain and the difference between the initial and final height SDS as outcome measures. In terms of height gain, pooled results show a significant advantage for girls treated with GnRHa compared with control patients. However, the addition of GH is associated with further increases in height. No significant difference was elucidated in comparing the initial and final height SDS. Height SDS was calculated as (height − mean)/SD where height is the actual height of a child, and mean and SD are the height and standard deviation for girls of age corresponding to that child.16 No significant adverse events were recorded during the treatment period,18,26,27 nor were there any negative effects on bone mineral density.28
Although the current study is the first meta-analysis addressing the efficacy of GnRHa compared with no treatment and with combined GnRHa/GH, a recent review touches on the same topic by examining outcomes of GnRHa treatment in terms of potential long-term adverse effects.20 The aforementioned review assesses outcomes through the post-treatment period and into adulthood. No significant adverse effects on ovarian function or bone density were recorded during the treatment period,20 nor was GnRHa treatment found to affect reproductive function significantly in other reviews.8,15 These importance of the findings of these other reviews notwithstanding, the present study offers a novelty advantage, since it pools studies relevant specifically to the efficacy of GnRHa and GH treatment in terms of final adult stature. As commented in a recent consensus report on hormonal treatment of early puberty, no randomized, controlled trials quantifying the effect of therapy on adult height are available. To our knowledge, this is the first meta-analysis collecting data from RCT and prospective trials have been published summarizing the effect of GnRHa therapy on height gain. Outcome analysis of this treatment is complicated, particularly when given to girls older than 6 years of age, because of a variety of factors, such as psychosocial issues and the fact that many conclusions published thus far have been founded on expert opinion rather than objective data.10 Furthermore, only indirect methods have been employed to assess the long-term effects of GnRHa and other hormonal treatments for precocious puberty.9,10 Additionally, the issue is complicated by differences in methodology of those assessing bone maturity vis-à-vis the PAH; for instance the rate of a child's height change does not always correlate appropriately with observations related to bone age (the predicted age of the child based on epiphyseal plate staging).9 Typically, height is predicted using the Bayley-Pinneau method,12 which is considered to be the most accurate in the setting of precocious puberty.14 In instances, however, this method can lead to overestimation.13,26,29 Interpretation of outcome results must therefore take the methodology of height prediction into account. For consistency in this regard, whenever possible, in the present meta-analysis, data of PAH using the “average tables” of Bayley and Pinneau were selected.29
Notwithstanding the novelty of the present meta-analysis, it was limited by its focus on early puberty in females, as no data were available regarding outcomes in boys treated for precocious puberty. Moreover, none of the meta-analyzed studies reported on the psychosocial impact of GnRHa therapy on young children. The studies also were not stratified to assess associations between patient age and PAH at initiation of therapy and the final height achieved. Examining results of the overall analyses leads to the following conclusions: In the setting of early puberty in girls, for the outcome measure of height gain, this pooled analysis shows that GnRHa treatment is associated with a significant gain compared with no treatment (ie, the control group). Furthermore, addition GH to the GnRHa treatment significantly increases in height gain compared with GnRHa treatment alone. However, in terms of the final vs. initial height SDS outcome measure, the 2 comparison groups did not differ significantly. These results should be taken with extreme caution, given the complexity of the outcome analysis. Although the meta-analysis did not elucidate a significant difference between the initial and final height SDS, this result could merely be a reflection of a lack of sensitivity, given the small number of studies that were meta-analyzed. Moreover, final height gain may prove to be a more appropriate outcome measure when evaluating this type of treatment. Clearly, a definitive evaluation of the efficacy of both GnRHa and combined GnRHa/GH as treatment for precocious puberty in girls will require an expanded and concerted effort.
Footnotes
Abbreviations: CPP = central precocious puberty, CNS = central nervous system, GH = growth hormone, GnRH = gonadotropin-releasing hormone, GnRHa = gonadotropin-releasing hormone analogs, GV = growth velocity, HPG = hypothalamic-pituitary-gonadal, PAH = predicted adult height, PCOD = polycystic ovarian disease, SDS = standard deviation scores.
Shanghai Science and Technology Committee (12411950403, 12411950400, 009411963100). NSFC (Natural Science Foundation of China, State Natutual Sciences Foundation, 81370686)
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Cesario SK, Hughes LA. Precocious puberty: a comprehensive review of literature. J Obstet Gynecol Neonatal Nurs 2007; 36:263–274. [DOI] [PubMed] [Google Scholar]
- 2.Partsch CJ, Sippell WG. Pathogenesis and epidemiology of precocious puberty. Effects of exogenous oestrogens. Hum Reprod Update 2001; 7:292–302. [DOI] [PubMed] [Google Scholar]
- 3.Sultan C, Gaspari L, Kalfa N, Paris F. Clinical expression of precocious puberty in girls. Endocr Dev 2012; 22:84–100. [DOI] [PubMed] [Google Scholar]
- 4.Buck Louis GM, Gray LE, Jr, Marcus M, et al. Environmental factors and puberty timing: expert panel research needs. Pediatrics 2008; 121:S192–S207. [DOI] [PubMed] [Google Scholar]
- 5.Antoniazzi F, Zamboni G. Central precocious puberty: current treatment options. Paediatr Drugs 2004; 6:211–231. [DOI] [PubMed] [Google Scholar]
- 6.Chauhan A, Grissom M. Disorders of childhood growth and development: precocious puberty. FP Essent 2013; 410:25–31. [PubMed] [Google Scholar]
- 7.Brito VN, Latronico AC, Arnhold IJ, Mendonça BB. Update on the etiology, diagnosis and therapeutic management of sexual precocity. Arq Bras Endocrinol Metabol 2008; 52:18–31. [DOI] [PubMed] [Google Scholar]
- 8.Cassio A, Bal MO, Orsini LF, et al. Reproductive outcome in patients treated and not treated for idiopathic early puberty: long-term results of a randomized trial in adults. J Pediatr 2006; 149:532–536. [DOI] [PubMed] [Google Scholar]
- 9.Kelnar CJ, Stanhope R. Height prognosis in girls with central precocious puberty treated with GnRH analogues. Clin Endocrinol 2002; 56:295–296. [DOI] [PubMed] [Google Scholar]
- 10.Carel JC, Eugster EA, Rogol A, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics 2009; 123:e752–e762. [DOI] [PubMed] [Google Scholar]
- 11.Schoevaart CE, Drop SLS, Otten BJ, et al. Growth analysis up to final height and psychosocial adjustment of treated and untreated patients with precocious puberty. Horm Res 1990; 34:197–203. [DOI] [PubMed] [Google Scholar]
- 12.Bayley N, Pinneau SR. Tables for predicting adult height from skeletal age: revised for use with the Greulich and Pyle hand standards. J Pediatr 1952; 40:423–441. [DOI] [PubMed] [Google Scholar]
- 13.Bar A, Linder B, Sobel EH, et al. Bayley-Pinneau method of height prediction in girls with central precocious puberty: correlation with adult height. J Pediatr 1995; 126:955–958. [DOI] [PubMed] [Google Scholar]
- 14.Zachmann M, Sobradillo B, Frank M, et al. Bayley-Pinneau, Roche-Wainer-Thissen, and Tanner height predictions in normal children and in patients with various pathologic conditions. J Pediatr 1978; 93:749–755. [DOI] [PubMed] [Google Scholar]
- 15.Heger S, Partsch C-J, Sippell WG. Long-term outcome after depot gonadotropin-releasing hormone agonist treatment of central precocious puberty: final height, body proportions, body composition, bone mineral density, and reproductive function. J Clin Endocrinol Metab 1999; 84:4583–4590. [DOI] [PubMed] [Google Scholar]
- 16.Karlberg P, Taranger J, Engström I, et al. Physical growth from birth to 16 years and longitudinal outcome of the study during the same age period. Acta Paediatr Scand Suppl 1976; 258:7–76. [DOI] [PubMed] [Google Scholar]
- 17.Bouvattier C, Coste J, Rodrigue D, et al. Lack of effect of GnRH agonists on final height in girls with advanced puberty: a randomized long-term pilot study. J Clin Endocrinol Metab 1999; 84:3575–3578. [DOI] [PubMed] [Google Scholar]
- 18.Pucarelli I, Segni M, Ortore M, et al. Effects of combined gonadotropin-releasing hormone agonist and growth hormone therapy on adult height in precocious puberty: a further contribution. J Pediatr Endocrinol Metab 2003; 16:1005–1010. [DOI] [PubMed] [Google Scholar]
- 19.Chulani VL, Gordon LP. Adolescent growth and development. Prim Care 2014; 41:465–487. [DOI] [PubMed] [Google Scholar]
- 20.Thornton P, Silverman LA, Geffner ME, et al. Review of outcomes after cessation of gonadotropin-releasing hormone agonist treatment of girls with precocious puberty. Pediatr Endocrinol Rev 2014; 11:306–317. [PubMed] [Google Scholar]
- 21.Yanovski JA, Rose SR, Municchi G, et al. Treatment with a luteinizing hormone-releasing hormone agonist in adolescents with short stature. N Engl J Med 2003; 348:908–917. [DOI] [PubMed] [Google Scholar]
- 22.Municchi G, Rose SR, Pescovitz OH, et al. Effect of deslorelin-induced pubertal delay on the growth of adolescents with short stature and normally timed puberty: preliminary results. J Clin Endocrinol Metab 1993; 77:1334–1339. [DOI] [PubMed] [Google Scholar]
- 23.Tuvemo T, Gustafsson J, Proos LA. Growth hormone treatment during suppression of early puberty in adopted girls. Swedish Growth Hormone Advisory Group. Acta Paediatr 1999; 88:928–932. [DOI] [PubMed] [Google Scholar]
- 24.Pasquino AM, Pucarelli I, Segni M, et al. Adult height in girls with central precocious puberty treated with gonadotropin-releasing hormone analogues and growth hormone. J Clin Endocrinol Metab 1999; 84:449–452. [DOI] [PubMed] [Google Scholar]
- 25.Mul D, Oostdijk W, Waelkens JJ, Drop SL. Final height after treatment of early puberty in short adopted girls with gonadotrophin releasing hormone agonist with or without growth hormone. Clin Endocrinol (Oxf) 2005; 63:185–190. [DOI] [PubMed] [Google Scholar]
- 26.Tuvemo T, Jonsson B, Gustafsson J, et al. Final height after combined growth hormone and GnRH analogue treatment in adopted girls with early puberty. Acta Paediatr 2004; 93:1456–1462. [DOI] [PubMed] [Google Scholar]
- 27.Cassio A, Cacciari E, Balsamo A, et al. Randomised trial of LHRH analogue treatment on final height in girls with onset of puberty aged 7.5-8.5 years. Arch Dis Child 1999; 81:329–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertelloni S, Baroncelli GI, Sorrentino MC, et al. Effect of central precocious puberty and gonadotropin-releasing hormone analogue treatment on peak bone mass and final height in females. Eur J Pediatr 1998; 157:363–367. [DOI] [PubMed] [Google Scholar]
- 29.Kauli R, Galatzer A, Kornreich L, et al. Final height of girls with central precocious puberty, untreated versus treated with cyproterone acetate or GnRH analogue. A comparative study with re-evaluation of predictions by the Bayley-Pinneau method. Horm Res 1997; 47:54–61. [DOI] [PubMed] [Google Scholar]
- 30.Stasiowska B, Vannelli S, Benso L. Final height in sexually precocious girls after therapy with an intranasal analogue of gonadotrophin-releasing hormone (buserelin). Horm Res 1994; 42:81–85. [DOI] [PubMed] [Google Scholar]
- 31.Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000; 320:1574–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]


