Abstract
Insufficient data are available regarding the validation of long-term survival in patients with T2 (solitary tumor with microvascular invasion [MVI] or multiple tumors, none >5 cm) hepatocellular carcinoma (HCC) after primary hepatectomy. We aim to evaluate the survival and relevant risk factors for T2 HCC patients. Between 2001 and 2007, 312 T2 HCC patients who underwent primary hepatectomy were included. Survival was estimated using the Kaplan–Meier method and compared using Cox proportional hazard model with adjusted independent prognostic factors. The 1, 3, and 5-year overall survival rates of patients with MVI were 85.7%, 68.7%, and 64.8%, respectively; these were inferior to the rates in patients without MVI, which were 93.0%, 89.3%, and 73.7%, respectively (P = 0.037). Within the with-MVI group, the survival rate of patients with tumor sizes ≥5 cm was inferior to that of patients with tumors <5 cm (overall, P = 0.01; recurrence-free, P < 0.0001). For patients with the largest tumors in the <5-cm group, those without MVI tended to have a higher probability of recurrence for 2 years after resection (P = 0.088) but a similar overall survival rate relative to those with MVI (P = 0.31). The crude metastasis-free survival was higher in the without-MVI group than in the with-MVI group (P = 0.012). The T2 HCC category comprised heterogeneous patients with differences in survival rates. Extrahepatic recurrence occurred more frequently in patients with MVI than in those without MVI. These results provide evidence for an updated definition of T2 HCC.
INTRODUCTION
Hepatocellular carcinoma (HCC) is among the most common malignancies worldwide,1 and the outcomes of HCC patients after curative resection are far from satisfactory because of the high recurrence rate.2–7 The accurate staging of HCC is of paramount importance because patients classified as having a high risk of recurrence warrant a policy of close monitoring and aggressive treatment. In Taiwan, the most commonly accepted pathologic staging system for HCC was developed by the American Joint Committee on Cancer (AJCC), which is the T (tumor), N (node), and M (metastasis) as parameters for HCC staging.8
The category of T2 HCC, which is defined by either a solitary tumor with vascular invasion or multiple tumors, none >5 cm, was adopted from a study cohort of 557 patients (from Houston, Rochester, Paris, and Kyoto) who underwent complete resection of HCC between 1980 and19989; however, the prognostic accuracy of this category has not been widely externally validated.10–12 Potentially, this categorization would introduce a high level of heterogeneity because solitary HCC cases with microvascular invasion (MVI) are triaged into this category, regardless of the tumor size. Additionally, the survival rates of HCC patients have improved in recent decades.12 Herein, we aimed to examine the validity of the prognostic outcomes of T2 HCC in our cohort.
PATIENTS AND METHODS
Subjects
The institutional review board of the National Taiwan University Hospital, Taipei, Taiwan approved this study (NTUH REC: 201104066RC). Patients who underwent primary curative resection for pathologically proven T2 HCC at the National Taiwan University Hospital between January 2001 and December 2007 were included in this study. T2 HCC was defined as a solitary tumor with MVI or multiple tumors, none >5 cm, in accordance with the seventh AJCC TNM staging system.8 MVI was defined as the presence of tumor emboli within the tributaries of the hepatic vein or the branches of the portal vein. HCC patients with major vascular invasion, defined as tumor invasion into the first branch of the portal vein or first tributary of the hepatic vein, were staged as T3b and excluded. The follow-up programs for HCC patients after primary resection has been previously described.3 HCC recurrence was defined as the detection via imaging studies of a new lesion with features typical of HCC that was diagnosed either by pathology/cytology or by the appearance of a typical hypervascular tumor >1 cm with washout in the venous phase in a liver with chronic hepatitis or cirrhosis (intrahepatic) or the detection of extrahepatic metastases using computed tomography (CT), magnetic resonance imaging (MRI), or bone scintigraphy. If necessary, a biopsy was performed for suspicious lesions.
Management of Recurrence
The treatment provided upon the detection of intrahepatic recurrence during follow-up after primary tumor resection was previously described.3 For extrahepatic metastases, surgical resection was first evaluated and if declined, systemic chemotherapy or target therapy was considered. Supportive treatment was provided if no further therapeutic intervention was undertaken.
Study Outcome
The follow-up protocol was the same as that used to evaluate patients with primary HCC after resection; patient death was confirmed using the mortality data bank of the National Death Registry as previously described.3 A patient's death, if it occurred, was identified as the study outcome. Cancer-related death was defined as a patient deceased with severe uncontrolled tumor progression at the time of death, irrespective of the immediate cause of death. The data of patients who survived to the end of the study were censored in December 2009.
Survival differences between the 2 groups (with-MVI and without-MVI) and differences among the without-MVI group and the subgroups of the with-MVI group (primary tumor ≥5 cm, S1; primary tumor <5 cm, S2) were further compared.
Other Prognostic Variables
Data for the other prognostic variables of survival were retrospectively collected by reviewing the medical records at the time of primary HCC diagnosis and the first HCC recurrence. Data regarding the following variables were collected at the time of HCC diagnosis: sex; age; viral hepatitis B surface antigen (HBsAg) status; anti-hepatitis C virus (HCV) antibody status; liver cirrhosis; presence of tumor capsule, central necrosis, satellite nodules (defined by nodule(s) found at <1 cm from the main tumor), infiltrating tumor type, or MVI; primary tumor pathologic grade, size, and number; α-fetoprotein (AFP) serum level; and primary resection type. Cirrhosis was defined as the detection of compatible imaging and pathologic findings. Major resection was defined as resection involving >3 segments. The time to disease recurrence was defined as the period from the date of primary surgical resection to the detection of the first HCC recurrence; the following data were collected accordingly: size and number of recurrent tumors, presence of intra- or extrahepatic recurrence, the date of local treatment for first recurrence, and treatment of recurrence. Resection included liver reresection, liver transplantation, or any surgical resections of tissues other than the liver, such as video-assisted thoracic surgery (VATS) or brain resection. Systemic chemotherapy or target therapy was classified as other.
Statistical Analysis
The Student t or χ2 test was used to compare the variables where appropriate. The cumulative survival rates of the study groups were estimated using the Kaplan–Meier method and compared with the log-rank test. Cox proportional hazard model was used for multivariate analysis to adjust for the potential confounding of the treatment effects by other factors and identify independent prognostic factors. Adjusted hazard ratios (aHRs) and the associated 95% confidence intervals (95% CIs) were based on the results of the Cox model analysis of variables that differed significantly among the study groups. The survival difference between the with-MVI and without-MVI groups was compared. Furthermore, the survival difference among the without-MVI group and the S1 and S2 subgroups was compared. A 2-sided P value <0.05 was considered to indicate statistical significance. Analyses were performed with the Statistical Package for Social Sciences version 21.0 (SPSS Inc, Chicago, IL).
RESULTS
Patient Groups and Clinicopathologic Characteristics
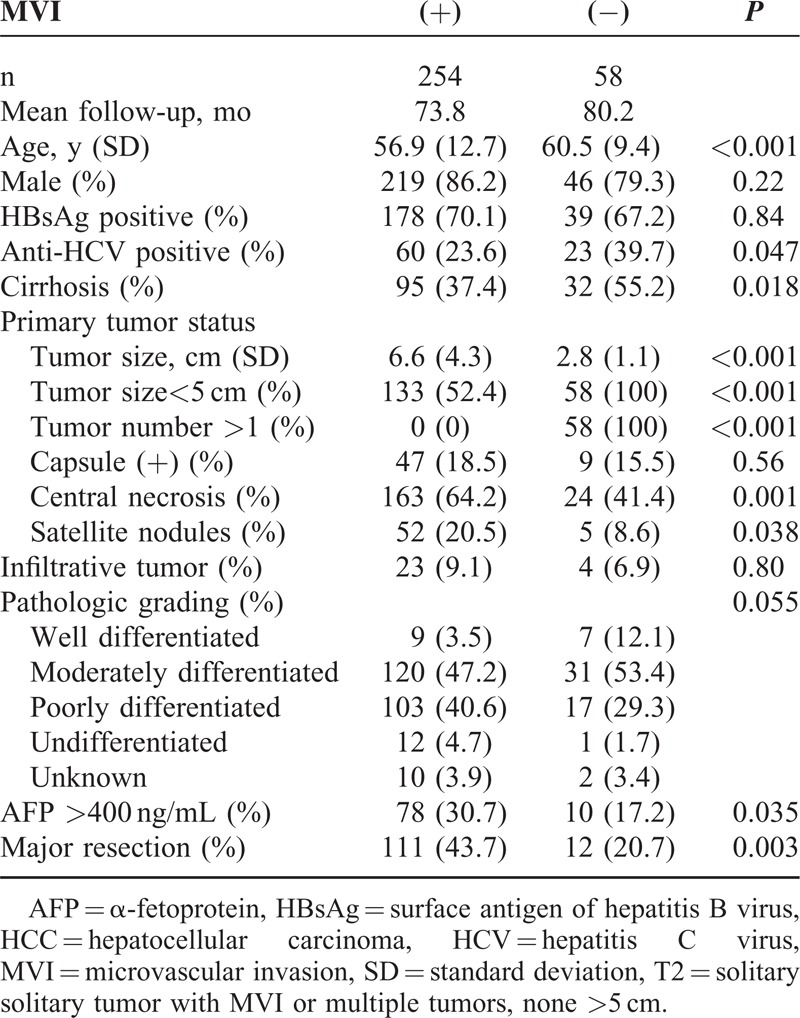
A total of 313 patients with T2 HCC were identified among the 980 patients who underwent primary resection for HCC before the end of the follow-up period in December 2009. One patient was excluded because of missing survival information. Accordingly, 312 patients with T2 HCC were included in this study (Figure 1). MVI was found in 254 patients, 136 of whom experienced recurrence. Fifty-eight patients had T2 HCC without MVI and among them, 36 developed recurrent disease (Figure 1). Table 1 lists the patients’ clinicopathologic characteristics. The 2 study groups (with and without MVI) did not differ in terms of sex, HBsAg status, some characteristics of the primary tumor status (capsule and infiltrative nature), or the pathologic grade of the primary HCC. Compared with the without-MVI group, the with-MVI group included younger patients, fewer carriers of HCV, a lower incidence of cirrhotic changes around the primary HCC tumors, higher number of primary tumors, larger primary tumor size, increased central tumor necrosis, higher frequencies of satellite nodules, elevated serum AFP levels (>400 ng/mL), and a higher incidence of major resections.
FIGURE 1.
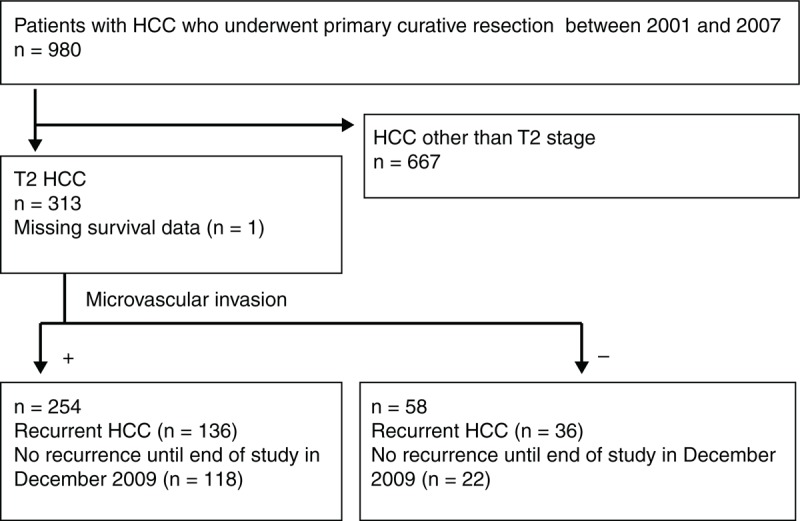
Patient selection process and inclusion/exclusion criteria. HCC = hepatocellular carcinoma, MVI = microvascular invasion, T2 = solitary tumor with MVI or multiple tumors, none >5 cm.
TABLE 1.
Clinicopathologic Features of T2 HCC Patients With or Without MVI Who Underwent Primary Curative Resection
Mortality, Causes of Death, and Patient Survival
Eighty-two patients, including 72 in the with-MVI group and 10 in the without-MVI group, died during the follow-up period. In the with-MVI group, 15 of these deaths (20.8%) were cancer-related and 16 (22.2%) were because of various noncancer related reasons (eg, sepsis, gastrointestinal bleeding, respiratory failure, liver failure, or cerebrovascular accident); the causes of death in the other 41 cases (56.9%) were unknown. In the without-MVI group, 1 death (10%) was cancer-related, 5 (50%) were because of various non-HCC related reasons (eg, heart failure, liver failure, or lung cancer), and 4 (40%) had unknown causes. The 1-year, 3-year, and 5-year survival rates in the with- and without-MVI groups were 85.7%, 68.7%, and 64.8% and 93.0%, 89.3%, and 73.7%, respectively (Figure 2). The crude patient survival rate was higher in the without-MVI group than in the with-MVI group (P = 0.037).
FIGURE 2.
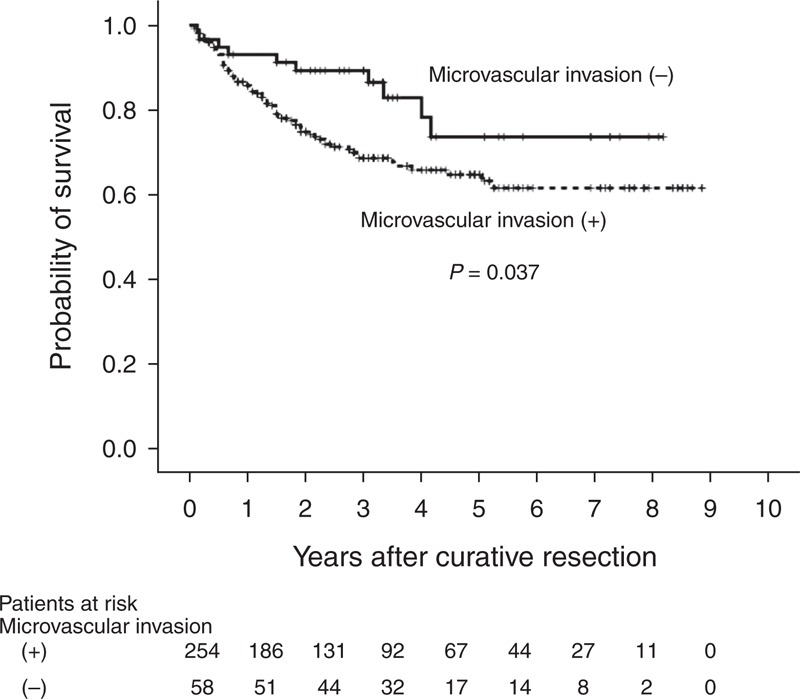
Kaplan–Meier curves for overall survival in patients who underwent primary curative resection for T2 HCC. HCC = hepatocellular carcinoma, MVI = microvascular invasion, T2 = solitary tumor with MVI or multiple tumors, none >5 cm.
Recurrence, Recurrence-Free, and Postrecurrence Survival
During the follow-up period, 172 patients (55.1%) developed recurrences. Most recurrent cases (82.6%, 142/172); occurred <2 years of the primary resection; of these, 118 (out of 136 cases, 86.8%) were in the with-MVI group and 24 (out of 36 cases, 66.7%) were in the without-MVI group. Among the recurrent cases, the with-MVI group presented with a higher number of recurrent tumors than did the without-MVI group (P = 0.016, Table 2). The recurrence-free survival curves are shown in Figure 3A. The median time intervals from primary resection to the first HCC recurrence were 23.0 months (range, 0–66 months) in the with-MVI group and 26.0 months (range, 4–58 months) in the without-MVI group. The 1-year postrecurrence survival rates in the with-MVI and without-MVI groups were 70.2% and 85.1%, respectively (Figure 3B). The crude postrecurrence survival rate was higher in the without-MVI group than in the with-MVI group (P = 0.007). The mean follow-up durations after the detection of recurrence in the with-MVI and without-MVI groups were 47.3 months (range, 1–84 months; median, 53.0 months) and 74.1 months (range, 0–86 months, median survival time was lacking as >80% patients survived), respectively.
TABLE 2.
Recurrence Characteristics and Treatment in Patients With T2 HCC
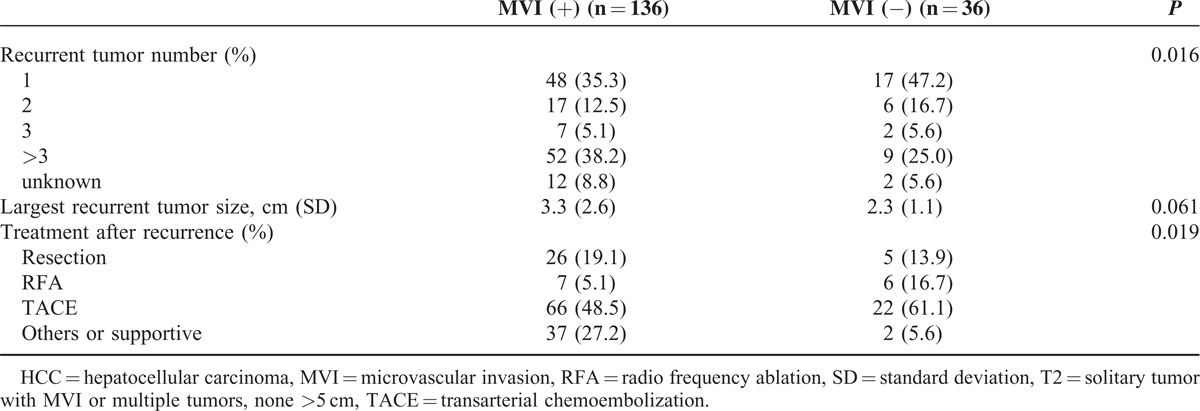
FIGURE 3.
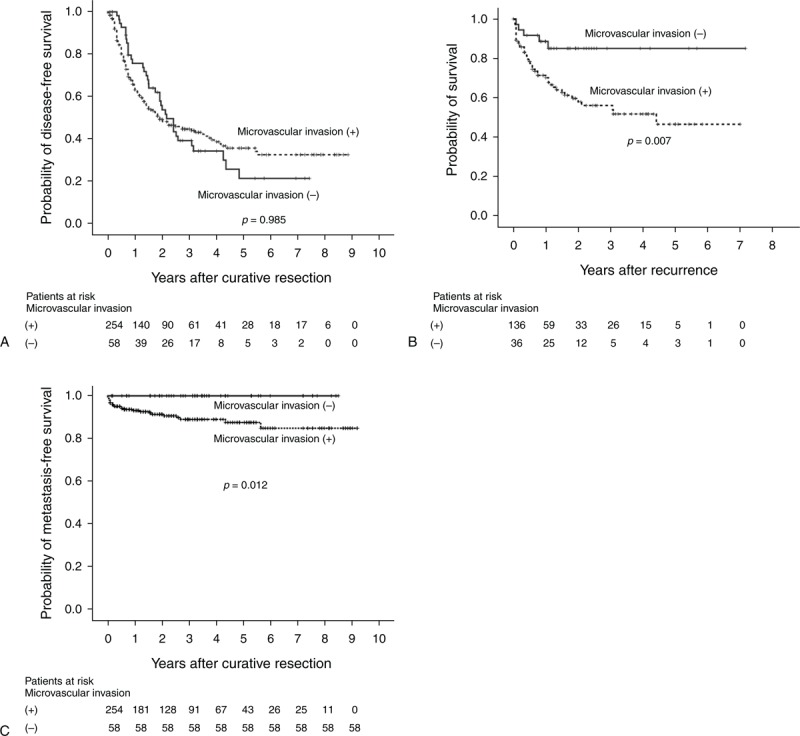
(A) Kaplan–Meier curves for disease-free survival in patients who underwent primary curative resection for T2 HCC. (B) Kaplan–Meier curves for postrecurrence survival in patients who underwent primary curative resection for T2 HCC. (C) Kaplan–Meier curves for metastasis-free survival in patients who underwent primary curative resection for T2 HCC. HCC = hepatocellular carcinoma, MVI = microvascular invasion, T2 = solitary tumor with MVI or multiple tumors, none >5 cm.
There were no extrahepatic recurrences in the without-MVI group, whereas there were 24 event cases (17.6%) in the with-MVI group. The 1-year, 3-year, 5-year, and 7-year metastasis-free survival rates in the with-MVI group were 93.4%, 89.4%, 88.0%, and 85.4%, respectively (Figure 3C). The crude metastasis-free survival rate was higher in the without-MVI group than in the with-MVI group (P = 0.012).
The management of recurrence differed significantly between the 2 groups (P = 0.019, Table 2). In the with-MVI group, 26 patients underwent resection (15 liver reresections, 5 VATSs, 1 brain surgery, 1 liver transplantation, 3 liver reresections and VATSs, and 1 VATS and brain surgery), whereas in the without-MVI group, 5 patients underwent resection (4 liver reresections and 1 liver transplantation). Supportive or other treatments for recurrences were more frequently administered to patients in the with-MVI group (3 chemotherapies, 1 target therapy [sorafenib], 1 radiotherapy, and 32 supportive treatments) than to those in the without-MVI group (1 chemotherapy and 1 supportive treatment).
Multivariate Risk Factor Analysis of Recurrence and Patient Survival
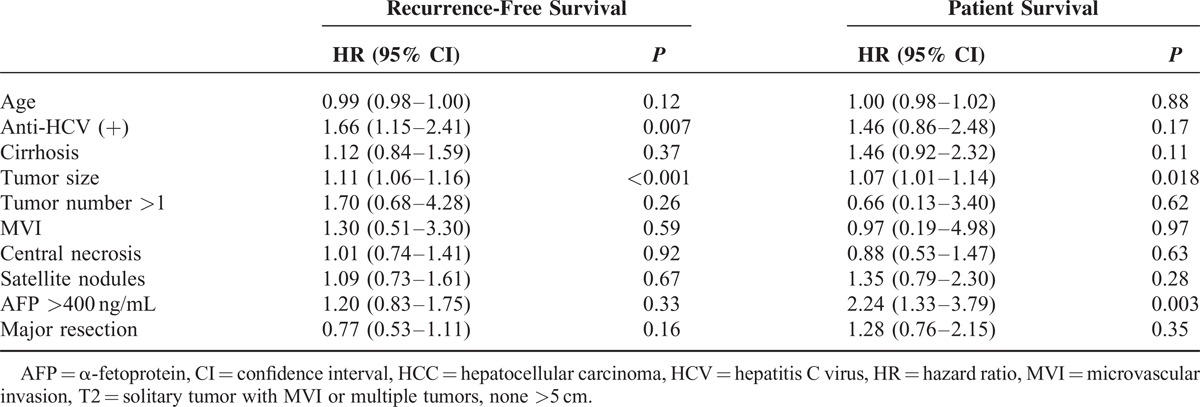
A multivariate analysis (Table 3) was conducted according to the Cox proportional hazard model to identify the potential independent risk factors. In T2 HCC, the tumor size was found to be an independent risk factor for both recurrence and patient survival with aHR values of 1.11 (95% CI, 1.06–1.16) and 1.07 (95% CI, 1.01–1.14), respectively. The presence of anti-HCV antibodies was another risk factor for recurrence, with an aHR of 1.66 (95% CI, 1.15–2.41). An AFP level >400 ng/mL was another risk factor for patient survival, with an aHR of 2.24 (95% CI, 1.33–3.79). The statistical significance of the presence of MVI decreased for both recurrence (P = 0.59) and patient survival (P = 0.97). This finding was validated by evidence that the HR for patient survival decreased from the crude value of 2.06 (95% CI, 1.06–39.8; univariate analysis, P = 0.031) to the adjusted value of 1.59 (95% CI, 0.80–3.15; P = 0.18) when the additional factor of “tumor size” was entered into the Cox proportional model. This adjusted value was further decreased to 0.97 (95% CI, 0.19–4.98; P = 0.97) when multiple factors were considered together.
TABLE 3.
Multivariate Analysis of Recurrence-Free and Patient Survival in Patients With T2 HCC Patients Who Underwent Primary Curative Resection
Survival Among T2 HCC Cases Stratified by Tumor Size
Compared with the without-MVI group (all tumors <5 cm), the subgroups of the with-MVI group (single tumor ≥5 cm, S1; single tumor <5 cm, S2) behaved differently with respect to survival. The difference in patient survival between the with-MVI and without-MVI groups was attributed to the S1 subgroup (without-MVI vs S2, P = 0.31; S1 vs S2, P = 0.01; Figure 4A). S1 and S2 differed significantly in terms of recurrence-free survival (P < 0.0001, Figure 4B). Although the without-MVI group and S2 subgroup did not significantly differ with respect to the recurrence-free survival duration (P = 0.088), the curves of these 2 groups became widely separated for 2 years after curative resection. Interestingly, the without-MVI group had a higher probability of recurrence than did the S2 subgroup for 2 years after resection (Figure 4B). However, the postrecurrence survival duration was longer in the without-MVI group than in the S1 and S2 subgroups (without-MVI vs S2, P = 0.024; S2 vs S1, P = 0.13; Figure 4C).
FIGURE 4.
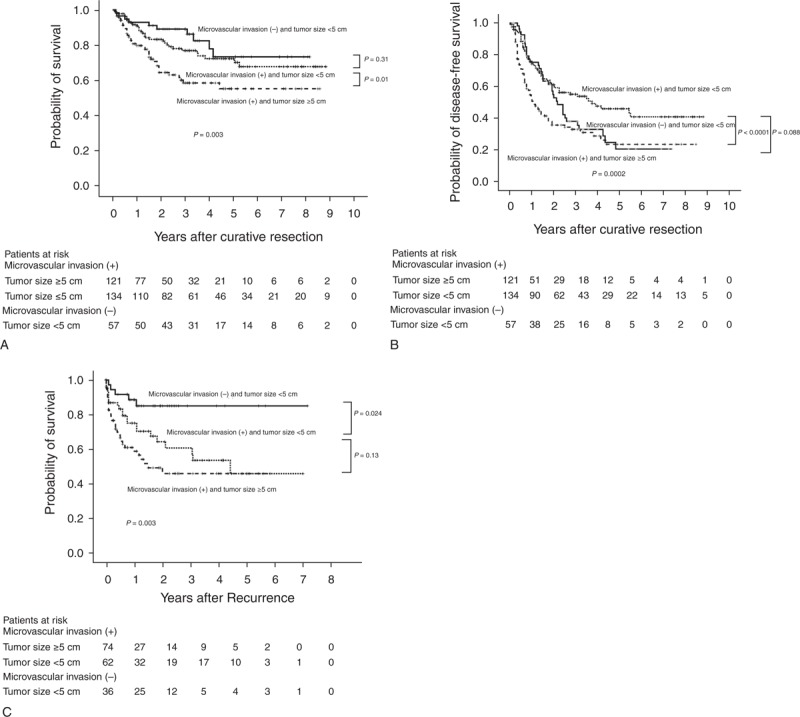
(A) Comparison of the Kaplan–Meier curves for overall survival in patients who underwent primary curative resection for T2 HCC. (B) Comparison of the Kaplan–Meier curves for disease-free survival in patients who underwent primary curative resection for T2 HCC. (C) Comparison of the Kaplan–Meier curves for postrecurrence survival in patients who underwent primary curative resection for T2 HCC. HCC = hepatocellular carcinoma, MVI = microvascular invasion, T2 = solitary tumor with MVI or multiple tumors, none >5 cm.
DISCUSSION
In the present study, the analysis of our T2 HCC hospital-based surgical cohort yielded 4 main findings. First, the overall survival of patients with MVI was inferior to that of patients without MVI, even though the recurrence-free survivals of the 2 groups were similar. Furthermore, within the with-MVI group, the survival rates (overall and recurrence-free) of patients with tumor sizes ≥5 cm were inferior to those of patients with tumor sizes <5 cm. Second, for patients with the largest tumors in the <5-cm group, the survival rates (overall and recurrence-free) of patients in the with-MVI subgroup S2 and without-MVI group were similar; specifically, both groups exhibited similar recurrence-free survival rates <2 years after resection and a trend toward a higher probability of recurrence in the without-MVI group (multiple HCCs initially) after 2 years. Third, the with-MVI group had a higher probability of extrahepatic recurrence during the follow-up period relative to the without-MVI group. Finally, a review of the univariate and multivariate analyses suggested that the significance of MVI had decreased and was influenced greatly by tumor size.
In the without-MVI group and the with-MVI S1 and S2 subgroups, the 1-year, 3-year, and 5-year overall survival rates were 93.0%, 89.3%, and 73.7%; 79.7%, 58.5%, and 55.2%; and 91.4%, 80.7%, and 73.1%, respectively. The 1-year, 3-year, and 5-year recurrence-free survival rates for the same groups were 74.2%, 55.2%, and 46.0%; 50.7%, 33.0%, and 23.6%; and 74.5%, 49.6%, and 37.9%, respectively. In the HCC surgical cohort (1989–2005) of Hong Kong, the 1-year and 5-year overall survival rates in patients with solitary tumors >5 cm and MVI (similar to our S1 subgroup) were 68.2% and 26.5%, respectively, and the 1-year and 5-year recurrence-free survival rates were 29.4% and 11.2%, respectively.7 The previously reported 5-year overall survival rate for patients with single tumors ≤5 cm and MVI (similar to our S2 subgroup) was 30.4%.10 Both previous studies demonstrated survival rate differences within the broad T2 HCC category. The definition of T2 HCC in the seventh AJCC was adopted without modification from the sixth edition.9 The rationale for this definition was based on a study cohort of 557 patients (from Houston, Rochester, Paris, and Kyoto) who underwent complete resection of HCC between 1980 and 1998.9 As advances in the treatment modalities for recurrence (eg., radio frequency ablation3) and adjuvant therapy (eg., antiviral agents13–15) have continued to revolutionize treatments in recent decades, the survival outcomes have improved in the specific population of patients with T2 HCC.12 In our study, the pattern of recurrence (intrahepatic or extrahepatic) likely played a major role in the observed survival differences. Accumulating evidence suggests that adjuvant therapy after resection for hepatitis virus-associated HCC could reduce the recurrence rate.13–15 For example, HCV-related HCCs were characterized by higher incidence of tumor multicentricity16 and associated with active viral replication, hepatic inflammation, and cirrhosis.17 Therefore, it can be expected that the presence of anti-HCV antibodies is another risk factor for recurrence after curative hepatic resection, which was also supported by the study by Ariizumi et al.18 Recent dramatic advances in anti-HCV therapies for chronic HCV infection have led to evolutions in the treatment schema from interferon-based to interferon-free and even ribavirin-free all-oral and pan-genotype treatment combinations.19 It is therefore reasonable to expect that the recurrence rates of HCC patients with HCV would further decrease in the future. The definition of T2 HCC will require updating in order to better predict the true survival differences in the future.
Our study demonstrated that the tumor size played a more important role than did MVI with respect to T2 HCC stratification. Some studies have demonstrated that the presence of MVI associated strongly with an increased tumor size.20–23 This is biologically plausible because MVI indicates an advanced stage of tumor growth and occurs once the HCC cell phenotype has evolved sufficiently (eg, protease production and decreased cell-to-cell adhesion consequent to reduced E-cadherin expression) to invade blood vessels and initiate metastasis.24 The larger of the tumor size, which is associated with higher risk of MVI, would, therefore, result in less favorable patient outcome. This finding is consistent with the identification of a statistically significant association between the preoperative CT identification of a nonsmooth tumor margin, which signifies active tumor growth, and MVI.25 Shirabe et al26 further proposed a scoring system comprising the tumor size, serum des-γ-carboxy prothrombin levels, and the maximum standardized uptake value on 2-[18F]-fluoro-2-deoxy-d-glucose positron emission tomography for the preoperative prediction of MVI and achieved good sensitivity and specificity with this system in their HCC validation cohort. The factors other than tumor size that were used by Shirabe et al, however, are not widely used in routine practice worldwide. Shindoh et al also demonstrated that a small HCC size (≤2 cm) was associated with excellent prognosis independently of the presence of MVI.27 The accumulating evidence therefore suggests that the tumor size is the most important preoperative prognostic factor.
Our study had some limitations. The study population was a retrospective hospital cohort and therefore might have included some biases. Compared with our T2 HCC cohort, the whole population in the study by Vauthey et al9 seemed to be similar in age, sex, and cirrhosis except less in hepatitis B virus (HBV) percentage (36% vs 67%–70%). Some studies suggested that patients with HBV-related HCCs had better long-term disease-free survival than those with HCV-related HCCs.17,28 Given the complex of different populations, population bias, which was adjusted in the analysis, might still exist. However, in the study by Ariizumi et al,18 of which T2 HCC population had high percentage of HCV carriers, the presence of anti-HCV antibody was associated with tumor recurrence, consistent with our study results. Nonetheless, caution needs to be taken when applying our results externally to other populations. Further confirmatory studies from other populations are needed. We focused solely on T2 HCC and did not perform a comparison of all HCC patients, which might limit a more general application of these findings. However, this particular T2 category (an intermediate stage between early and advanced HCC) most likely harbors an inhomogeneous population that requires further triage. In fact, because small multiple HCCs (<5 cm) without MVI are grouped together with solitary large HCC (≥5 cm) with MVI, statistical modeling would place more weight on the tumor size than on MVI if patients with large HCCs exerted a significant impact on the survival rates. The effects of tumor number were not analyzed because the factor was used for grouping in the first place (single tumor with MVI vs multiple tumor without MVI). The cause of mortality was unknown in approximately half of the patient deaths, which rendered determinations of causal contributions difficult because these deaths were confirmed via the mortality data bank of the National Death Registry without the provision of further information. We could only imply that intrahepatic recurrences did not result in patient deaths in the short-term. Further investigation will be needed to validate this assumption.
In conclusion, the T2 HCC category includes heterogeneous patients with differences in survival. Among the T2 HCC cases, extrahepatic recurrence occurred more frequently in patients with MVI than in those without MVI. These results provide evidence to suggest updating the definition of T2 HCC.
Footnotes
Abbreviations: AFP = α-fetoprotein, aHR = adjusted hazard ratio, AJCC = American Joint Committee on Cancer, HBsAg = hepatitis B surface antigen, HBV = hepatitis B virus, HCC = hepatocellular carcinoma, HCV = hepatitis C virus.
This study was supported by the National Taiwan University Hospital under grant MG259. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Available at: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx Accessed June 2014. [Google Scholar]
- 2.Ho CM, Lee PH, Chen CL, et al. Long-term outcomes after resection versus transplantation for hepatocellular carcinoma within UCSF criteria. Ann Surg Oncol 2012; 19:826–833. [DOI] [PubMed] [Google Scholar]
- 3.Ho CM, Lee PH, Shau WY, et al. Survival in patients with recurrent hepatocellular carcinoma after primary hepatectomy: comparative effectiveness of treatment modalities. Surgery 2012; 151:700–709. [DOI] [PubMed] [Google Scholar]
- 4.EI-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011; 365:1118–1127. [DOI] [PubMed] [Google Scholar]
- 5.Shah SA, Cleary SP, Wei AC, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery 2007; 141:330–339. [DOI] [PubMed] [Google Scholar]
- 6.Lang H, Sotiropoulos GC, Brokalaki EI, et al. Survival and recurrence rates after resection for hepatocellular carcinoma in noncirrhotic livers. J Am Coll Surg 2007; 205:27–36. [DOI] [PubMed] [Google Scholar]
- 7.Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg 2014; doi: http://dx.doi.org/10.1097/SLA.0000000000000710. [Epub ahead of print.]. [DOI] [PubMed] [Google Scholar]
- 8.Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC Cancer Staging Manual. 7th ed.New York, NY: Springer; 2010. 237–246. [Google Scholar]
- 9.Vauthey JN, Lauwers GY, Esnaola NF, et al. Simplified staging for hepatocellular carcinoma. J Clin Oncol 2002; 20:1527–1536. [DOI] [PubMed] [Google Scholar]
- 10.Chan AC, Fan ST, Poon RT, et al. Evaluation of the seventh edition of the American Joint Committee on Cancer tumor-node-metastasis (TNM) staging system for patients undergoing curative resection of hepatocellular carcinoma: implications for the development of a refined staging system. HPB (Oxford) 2013; 15:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang J, Zhang Y, Peng Z, et al. A modified TNM-7 staging system to better predict the survival in patients with hepatocellular carcinoma after hepatectomy. J Cancer Res Clin Oncol 2013; 139:1709–1719. [DOI] [PubMed] [Google Scholar]
- 12.Kee KM, Wang JH, Lin CY, et al. Validation of the 7th edition TNM staging system for hepatocellular carcinoma: an analysis of 8,828 patients in a single medical center. Dig Dis Sci 2013; 58:2721–2828. [DOI] [PubMed] [Google Scholar]
- 13.Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver resection. JAMA 2012; 308:1906–1914. [DOI] [PubMed] [Google Scholar]
- 14.Kubo S, Takemura S, Sakata C, et al. Adjuvant therapy after curative resection for hepatocellular carcinoma associated with hepatitis virus. Liver Cancer 2013; 2:40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzaferro V, Romito R, Schiavo M, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology 2006; 44:1543–1554. [DOI] [PubMed] [Google Scholar]
- 16.Miyagawa S, Kawasaki S, Makuuchi M. Comparison of the characteristics of hepatocellular carcinoma between hepatitis B and C viral infection: tumor multicentricity in cirrhotic liver with hepatitis C. Hepatology 1996; 24:307–310. [DOI] [PubMed] [Google Scholar]
- 17.Huang YH, Wu JC, Chen CH, et al. Comparison of recurrence after hepatic resection in patients with hepatitis B vs. hepatitis C-related small hepatocellular carcinoma in hepatitis B virus endemic area. Liver Int 2005; 25:236–241. [DOI] [PubMed] [Google Scholar]
- 18.Ariizumi S, Katagiri S, Katsuragawa H, et al. Sectionectomy is suitable for patients with T2 hepatocellular carcinoma according to the modified international union against cancer TNM Classification. Dig Surg 2007; 24:342–348. [DOI] [PubMed] [Google Scholar]
- 19.Sarkar S, Lim JK. Advances in interferon-free hepatitis C therapy: 2014 and beyond. Hepatology 2014; 59:1641–1644. [DOI] [PubMed] [Google Scholar]
- 20.Jonas S, Bechstein WO, Steinmüller T, et al. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology 2001; 33:1080–1086. [DOI] [PubMed] [Google Scholar]
- 21.Esnaola NF, Lauwers GY, Mirza NQ, et al. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg 2002; 6:224–232. [DOI] [PubMed] [Google Scholar]
- 22.Pawlik TM, Delman KA, Vauthey JN, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl 2005; 11:1086–1092. [DOI] [PubMed] [Google Scholar]
- 23.Du M, Chen L, Zhao J, et al. Microvascular invasion (MVI) is a poorer prognostic predictor for small hepatocellular carcinoma. BMC Cancer 2014; 14:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu ZY, Ding SM, Zhou L, et al. FOXC1 contributes to microvascular invasion in primary hepatocellular carcinoma via regulating epithelial-mesenchymal transition. Int J Biol Sci 2012; 8:1130–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou CT, Chen RC, Lee CW, et al. Prediction of microvascular invasion of hepatocellular carcinoma by pre-operative CT imaging. Br J Radiol 2012; 85:778–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirabe K, Toshima T, Kimura K, et al. New scoring system for prediction of microvascular invasion in patients with hepatocellular carcinoma. Liver Int 2014; 34:937–941. [DOI] [PubMed] [Google Scholar]
- 27.Shindoh J, Andreou A, Aloia TA, et al. Microvascular invasion does not predict long-term survival in hepatocellular carcinoma up to 2 cm: reappraisal of the staging system for solitary tumors. Ann Surg Oncol 2013; 20:1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roayaie S, Haim MB, Emre S, et al. Comparison of surgical outcomes for hepatocellular carcinoma in patients with hepatitis B versus hepatitis C: a western experience. Ann Surg Oncol 2000; 7:764–770. [DOI] [PubMed] [Google Scholar]


