Abstract
Lymphopenia is a useful predictive factor in several cancers. The aim of this study was to determine the prognostic value of lymphopenia in patients with esophageal squamous cell carcinoma (ESCC).
A retrospective analysis of 307 consecutive patients who had undergone esophagectomy for ESCC was conducted. In our study, a lymphocyte count (LC) of fewer than 1.0 Giga/L was defined as lymphopenia. Kaplan–Meier method was used to calculate the cancer-specific survival (CSS). Cox regression analyses were performed to evaluate the prognostic factors. Receiver operating characteristic (ROC) curve was also plotted to verify the accuracy of LC for CSS prediction.
The mean LC was 1.55 ± 0.64 Giga/L (range 0.4–3.7 Giga/L). The incidence of lymphopenia (LC < 1.0 Giga/L) was 16.6% (51/307). Patients with lymphopenia (LC < 1.0 Giga/L) had a significantly shorter 5-year CSS (21.6% vs 43.8%, P = 0.004). On multivariate analysis, lymphopenia (LC < 1.0 Giga/L) was an independent prognostic factor in patients with ESCC (P = 0.013). Lymphopenia had a hazard ratio (HR) of 1.579 [95% confidence interval (CI): 1.100–2.265] for CSS. ROC curve demonstrated that lymphopenia (LC < 1.0 Giga/L) predicts survival with a sensitivity of 86.2% and a specificity of 27.2%.
Lymphopenia (LC < 1.0 Giga/L) is still an independent predictive factor for long-term survival in patients with ESCC.
INTRODUCTION
Esophageal cancer (EC) is one of the most common cancers, with both high incidence and mortality.1 The most common histological types are esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC).2 In China, ESCC is the predominant histologic type (90–95%), in contrast to the predominance of EAC in the West.3,4 There are important biological differences between ESCC and EAC, therefore, a prognostic study that takes into account the predominance of ESCC in China is important.
Inflammation plays an important role in cancer progression.5,6 The inflammatory marker, such as C-reactive protein, neutrophil or platelet count, has been shown to be a prognostic factor in several cancers, including ECs.7–10 Recent studies demonstrated that lymphopenia is associated with prognosis in several cancers, such as hematological malignancy, breast cancer, and renal cell cancer.11–13 However, to the best of our knowledge, no studies regarding the predictive value of lymphopenia in patients with EC are available. Therefore, the aim of this study was to investigate the prognostic value of lymphopenia in patients with ESCC.
PATIENTS AND METHODS
From January 2006 to December 2008, a retrospective analysis was conducted of 307 consecutive patients with ESCC who underwent esophagectomy at Zhejiang Cancer Hospital (Hangzhou, China). All of the patients included in the analysis fit the criteria: (1) ESCC was confirmed by histopathology; (2) patients with curative esophagectomy; (3) patients without preoperative neoadjuvant chemotherapy and/or radiotherapy; and (4) preoperative blood cell counts were obtained before esophagectomy within 1 week. The exclusion criteria were as follows: (1) non-ESCC or gastroesophageal junction carcinoma; (2) patients with previous or concomitant other cancers; (3) patients with incomplete resection with microscopic or macroscopic residual tumors; (4) patients with previous neoadjuvant chemotherapy and/or radiotherapy; or (5) patients with previous anti-inflammatory medicines within 1 week. Ethical approval was obtained from the Ethical Committees of Zhejiang Cancer Hospital.
All patients underwent curative esophagectomy. The standard surgical approach included the Ivor Lewis and the McKeown procedure.14,15 The lymphadenectomy included two-field and three-field lymphadenectomy.14,16 In the current study, most of patients underwent two-field lymphadenectomy. Patients who had received neoadjuvant therapy were excluded in the current study. As the role of postoperative adjuvant chemotherapy and/or radiotherapy was controversial during that period, adjuvant therapy was not mandatory. The most frequent adjuvant chemotherapy included 5-fluorouracil (5-FU) and cisplatin in our institute. Usually, two to four courses of chemotherapy were used, separated by a 3-week interval. The median postoperative radiation dose was 50 Gy. Adjuvant radiation was initially performed through the anteroposterior field to 36 Gy, then through the parallel opposing oblique fields to 14 Gy.
The diagnosis of ESCC was confirmed by histopathology. The fresh specimens were sent for histopathologic examination by routine HE staining. All patients were staged according to the 7th edition of American Joint Committee on Cancer (AJCC) Cancer Staging (TNM stage).17 Then the following data, such as tumor length, vessel invasion, differentiation, T stage, and N stage were recorded according to the results of pathologic reports. Tumor length was measured with a handheld ruler and was recorded in the pathologic reports. Vessel invasion, with HE staining, was considered only if the tumor cells were within an endothelium-lined, vessel-like structure.18 According to the 7th edition staging system, tumors can be divided into well-differentiated tumors, moderately differentiated tumors, and poorly differentiated tumors. The 7th edition defines the T stage into four subclasses: T1, tumors invade lamina propria or submucosa; T2, tumors invade muscularis propria; T3, tumors invade adventitia; and T4, tumors invade adjacent structures (T4a, resectable tumors invade adjacent structures such as pleura, pericardium, diaphragm; T4b, unresectable tumors invade adjacent structures such as aorta, vertebral body, and trachea). The 7th edition defines the N stage according to the number of positive lymph nodes: N0, no positive lymph nodes; N1, one or two positive lymph nodes; N2, three to six positive lymph nodes; and N3, seven or more positive lymph nodes.
Data on preoperative blood cell counts were extracted in our medical records. In our study, a lymphocyte count (LC) of fewer than 1.0 Giga/L was defined as lymphopenia.11,12 Then, it was categorized into two groups: LLC (LC < 1.0 Giga/L) and HLC (LC ≥ 1.0 Giga/L). Based on the medical records, the following data were also collected for each patient: age (≤60 and >60 years), gender (male and female), tumor length (≤3.0 and >3.0 cm), tumor location (upper, middle, and lower), differentiation (well, moderate, and poor), vessel invasion (negative and positive), T stage (T1, T2, T3, and T4a), N stage (N0, N1, N2, and N3), and adjuvant therapy.
Patients were followed up at our outpatient department every 3 to 6 months for the first 2 years, then annually. No patients were missing in our study. The last follow-up was November 30, 2011. As this series described the prognosis of patients with ESCC, therefore, a cancer-specific survival (CSS) was ascertained in the current study.
Statistical Analysis
Independent t tests were used to compare LC of continuous variable. Chi-squared tests were used to determine the significance of differences for patients grouped by LC as a dichotomous variable. The CSS was calculated by the Kaplan–Meier method. A univariate analysis was used to examine the association between prognostic predictors and CSS. Possible prognostic factors associated with CSS were considered in a multivariable Cox regression analysis. Receiver operating characteristic (ROC) curve was also plotted to verify the accuracy of LC for CSS prediction (survival vs death). The area under curve (AUC) was used as an estimation of diagnostic accuracy. A P-value less than 0.05 was considered to be statistically significant. Statistical analysis was conducted with SPSS 17.0 (SPSS, Inc., Chicago, IL).
RESULTS
The baseline characteristics are shown in Table 1. The mean LC was 1.55 ± 0.64 Giga/L (range 0.4–3.7 Giga/L). The histogram of LC is shown in Figure 1. The incidence of lymphopenia (LC < 1.0 Giga/L) was (51/307) 16.6% in our study. As a continuous variable, lower LC correlated with N stage (P = 0.001). As a dichotomous variable, lymphopenia (LC < 1.0 Giga/L) was also associated with N stage (P = 0.038) (Table 1).
TABLE 1.
Comparison of Baseline Clinical Characteristics Based on LC
FIGURE 1.
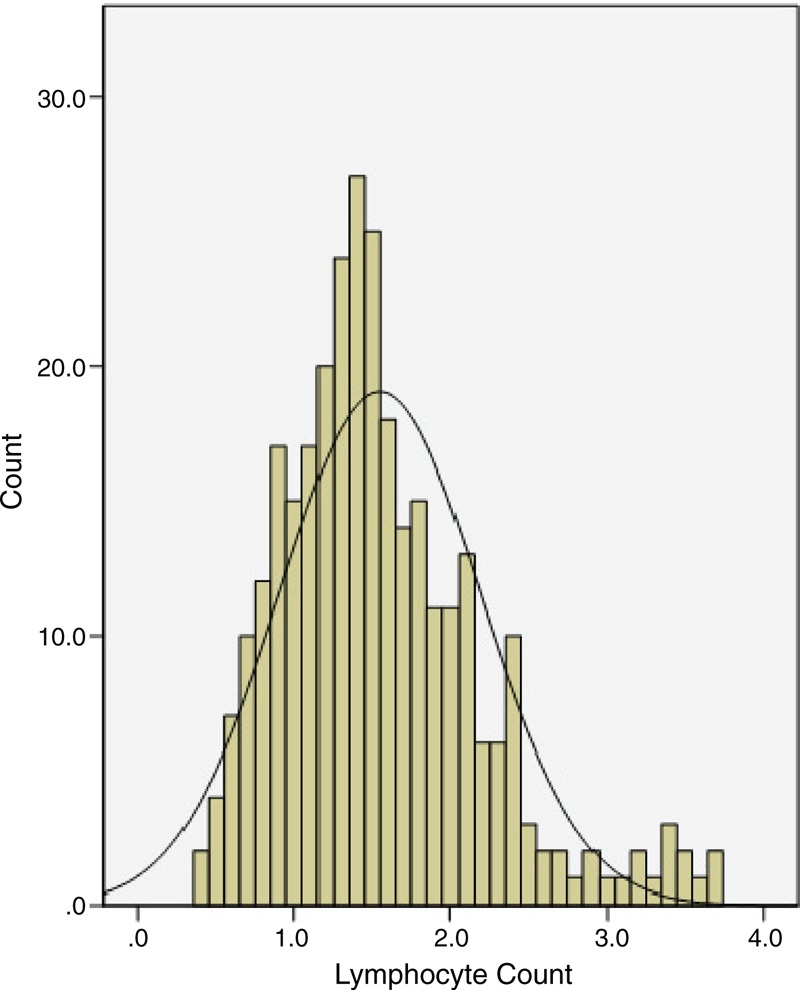
The histogram of the LC (range 0.4–3.7 Giga/L).
The 5-year CSS was 40.1% in our study. Patients with lymphopenia (LC < 1.0 Giga/L) had a significantly shorter 5-year CSS (21.6% vs 43.8%, P = 0.004) (Figure 2). In addition, in our study, there were also significant differences regarding 5-year CSS in tumor length (56.1% vs 34.2%, P < 0.001), vessel invasion (44.2% vs 18.4%, P < 0.001), T stage (57.7% vs 31.0%, P < 0.001), and N stage (59.0% vs 19.2%, P < 0.001). However, no significant difference was found regarding 5-year CSS in adjuvant therapy (40.8% vs 38.5%, P = 0.458) (Table 2).
FIGURE 2.
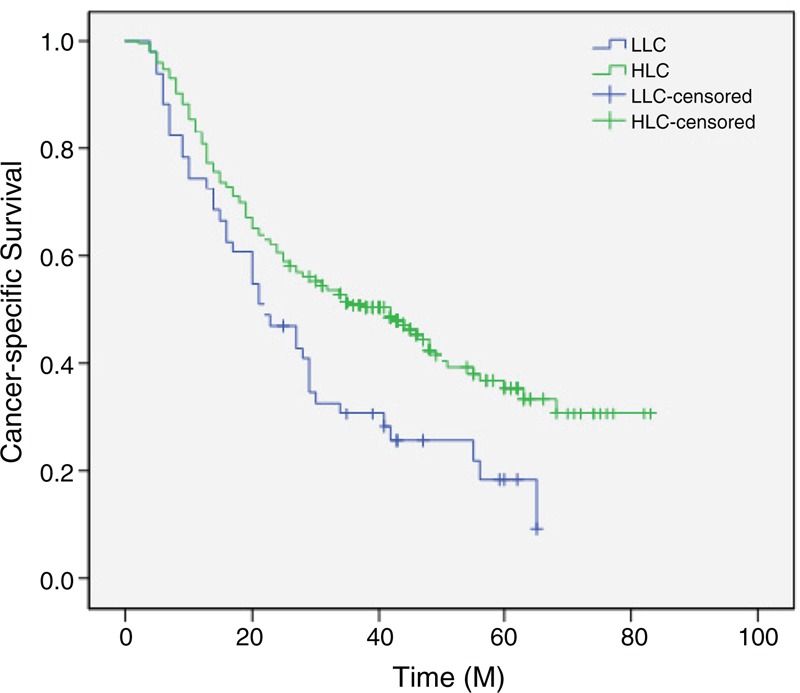
Kaplan–Meier CSS curves stratified by LC. Patients with lymphopenia had a significantly shorter 5-year CSS (21.6% vs 43.8%, P = 0.004). HLC = high lymphocyte count, LLC = low lymphocyte count, M = month.
TABLE 2.
Univariate and Multivariate Analyses of CSS in ESCC Patients
For assessing the confounding effect of LC on T stage (T1–2 vs T3–4a) and N stage (N0 vs N1–3), we further stratified patients into different groups regarding T stage and N stage. The 5-year CSS of patients with LC < 1.0 Giga/L was shorter than that of patients with LC ≥ 1.0 Giga/L in T1–2 group (26.1% vs 66.7%, P = 0.001) and T3–4a group (17.9% vs 33.1%, P = 0.043), respectively (Figure 3A and B). However, as shown in Figure 3C and D, no significant differences were found in N0 (45.0% vs 61.0%, P = 0.208) and N1–3 (6.5% vs 22.6%, P = 0.094) between patients with and without lymphopenia.
FIGURE 3.
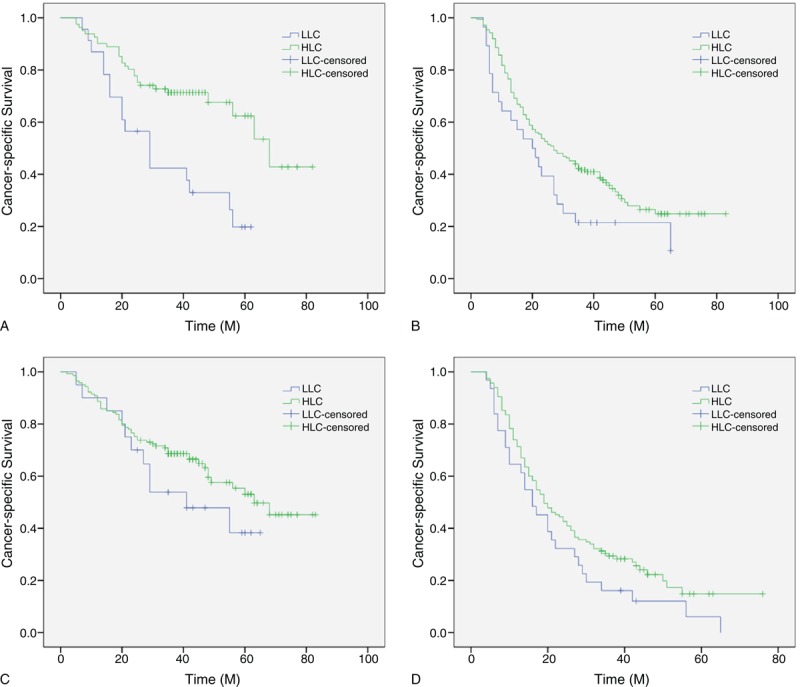
Kaplan–Meier CSS curves stratified by LC in patients with T stage and N stage. The 5-year CSS of patients with LC < 1.0 Giga/L was shorter than that of patients with LC ≥ 1.0 Giga/L in T1–2 group (26.1% vs 66.7%, P = 0.001, [A]) and T3–4a group (17.9% vs 33.1%, P = 0.043, [B]), respectively. However, no significant differences were found in N0 (45.0% vs 61.0%, P = 0.208, [C]) and N1–3 (6.5% vs 22.6%, P = 0.094, [D]) between patients with and without lymphopenia. HLC = high lymphocyte count, LLC = low lymphocyte count, M = month.
By univariate analysis, we found that tumor length, vessel invasion, T stage, N stage, and LC had significant associations with CSS (Table 2). Then all of the 5 variables above were included in a multivariate Cox proportional hazards model to adjust the effects of covariates. In multivariable analysis, we demonstrated that T stage (hazard ratio [HR] = 1.499, 95% confidence interval [CI] 1.015–2.214, P = 0.042), N stage (HR = 2.534, 95% CI 1.814–3.540, P < 0.001) and lymphopenia (HR = 1.579, 95% CI 1.100–2.265, P = 0.013) were independent prognostic factors in patients with ESCC (Table 2). Lymphopenia (LC < 1.0 Giga/L) had an HR of 1.579 (95% CI: 1.100–2.265) for CSS. However, the magnitudes of effect for tumor length (P = 0.284) and vessel invasion (P = 0.340) were reduced between the univariate and multivariate analysis.
ROC curve was also plotted to verify the accuracy of LC for survival prediction. The AUC was 0.699 (95% CI: 0.639–0.759, P < 0.001). It demonstrated that lymphopenia (LC < 1.0 Giga/L) predicts survival with a sensitivity of 86.2% and a specificity of 27.2% (Figure 4).
FIGURE 4.
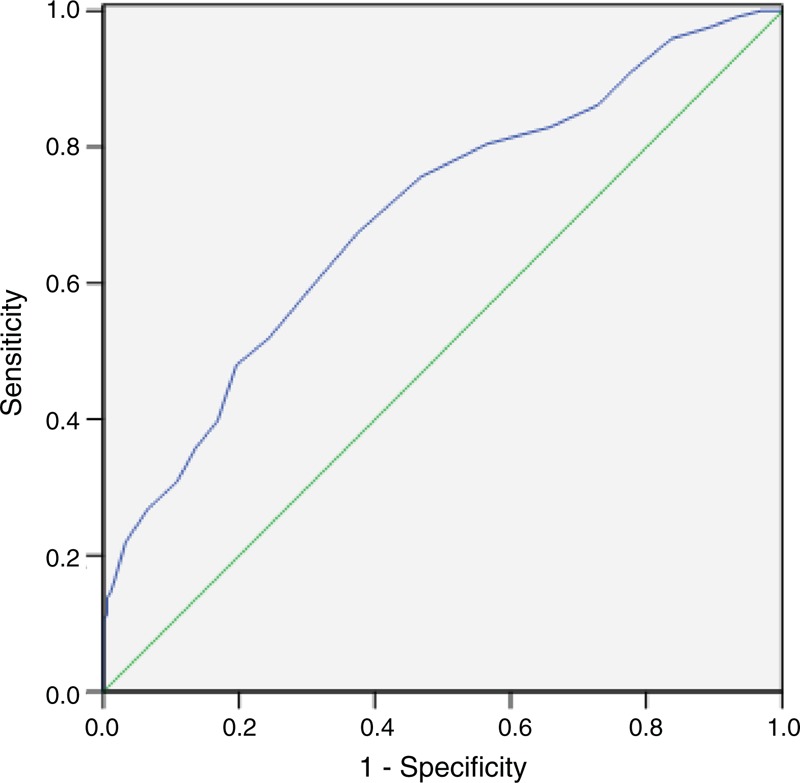
ROC curve for CSS prediction. A ROC curve plots the sensitivity on the y-axis against one minus the specificity on the x-axis. A diagonal line at 45°, known as the line of chance, would result from a test, which allocated subjects randomly. Each point on the ROC curve corresponds to a value of LC. The area under curve (AUC) was used as an estimation of diagnostic accuracy. Lymphopenia (LC < 1.0 Giga/L) predicts survival with a sensitivity of 86.2% and a specificity of 27.2%.
DISCUSSION
To the best of our knowledge, this is the first study to determine the prognostic value of lymphopenia (LC < 1.0 Giga/L) in predicting postoperative survival in patients with ESCC. Our study showed that lymphopenia is associated with prognosis. Patients with lymphopenia had a significantly shorter 5-year CSS (21.6% vs 43.8%, P = 0.004). Multivariate analysis demonstrated that lymphopenia is a significant predictor of CSS. Patients with LC < 1.0 Giga/L had an HR of 1.579 (95% CI: 1.100–2.265, P = 0.013) for CSS.
There is strong linkage between inflammation and cancer.5,6 In our study, we analyzed the prognostic role of lymphopenia in ESCC patients without neoadjuvant treatment mainly because neoadjuvant therapy (chemotherapy and/or radiation) will have an important impact on the inflammation. Therefore, we initially evaluated the usefulness of lymphopenia for predicting postoperative survival in patients with ESCC. Our study showed that lymphopenia was associated with N stage. This observation is in line with data from Saroha et al,13 but is contrary to the result of Mehrazin et al,19 who suggested that lymphopenia is not correlation with the N stage (P = 0.120).
Furthermore, our study showed that patients with lymphopenia had a significantly shorter 5-year CSS (21.6% vs 43.8%, P = 0.004). On multivariate analysis, lymphopenia was a significant predictive factor of CSS (P = 0.013). It is widely agreed that T stage and N stage are strong, independent prognostic factors in EC.20,21 In our study, we also demonstrated that T stage (P = 0.042) and N stage (P < 0.001) were independent prognostic factors. For assessing the confounding effect of LC on T stage (T1–2 vs T3–4a) and N stage (N0 vs N1–3), we further stratified patients into different groups regarding T stage and N stage. In our study, the predictive value of lymphopenia was significant in patients with T stages, but not significant in patients with N stages. Our results clearly demonstrated that lymphopenia can serve as an independent predictor of long-term survival for ESCC patients, especially in T stages.
Tumor length is still a controversial prognostic factor in patients with EC. Several studies have demonstrated that tumor length was related to prognosis but was not an independent prognostic factor in patients with EC.22,23 Eloubeidi et al,24 Yendamuri et al,25 and Feng et al,26 however, showed that tumor length was a prognostic indictor of EC. The presence of vessel invasion has not been found to be a consistent finding. Zafirellis et al27 showed that vessel invasion was an independent prognostic indicator in patients with EC. However, Waraich et al18 analyzed the outcomes of 244 EC patients. Their results demonstrated that vessel invasion was not an independent prognostic factor in patients with EC. In our study, tumor length and vessel invasion related to prognosis but were not independent prognostic factor in patients with ESCC. Since the magnitudes of effect for tumor length and vessel invasion are reduced between the univariate and multivariate analysis and they loss statistical significance, we can suggest that the impact of tumor length and vessel invasion on CSS are confounded by the other variables in the model.
There is no consensus for the standard treatment for EC. However, esophagectomy remains the standard treatment for patients with early stage.28,29 In addition, there is strong evidence to recommend multimodal treatments with adjuvant chemoradiotherapy followed by surgery for patients with resectable locally advanced cancers.2 Resectable locally advanced EC refers to T3–T4a or N1–3 and early stage EC refers to T1–2 or N0 according to the 7th edition of the AJCC.17 Recent studies have demonstrated that postoperative adjuvant chemoradiotherapy significantly improves the long-term survival of patients with EC compared with surgery alone.30,31 However, no significant difference was found regarding 5-year CSS in adjuvant therapy in our study (40.8% vs 38.5%, P = 0.458). Two possible reasons were as follows: Firstly, the postoperative adjuvant chemoradiotherapy was not mandatory in our study. Secondly, although adjuvant therapy was followed by surgery, the survival of locally advanced EC was poor.
The potential limitations of the present study include the use of a retrospective analysis and the short duration of the mean follow-up. Furthermore, in our study, we excluded patients who had received neoadjuvant treatment (chemotherapy and/or radiotherapy), which may have influenced the result. On the one hand, chemotherapy and/or radiation will have a side effect on blood cells, including LC. On the other hand, recent studies revealed that chemotherapy and/or radiotherapy could improve survival before surgery for locally advanced EC, but not for early stage EC.32 In addition, our study revealed that lymphopenia is an independent predictive factor in patients with ESCC, however, it should be kept in mind that lymphopenia itself alone without other variables may not associate with postoperative survival in patients with ESCC. Although we adopted rigorous inclusion and exclusion criteria, it was shown that diabetes mellitus, renal and/or hepatic failure, and many inflammatory diseases may potentially affect the lymphocytes.33,34 In addition, we excluded patients with previous anti-inflammatory, however, anti-diabetic, anti-hypertensive drug, and/or other medications may potentially affect the LC. Therefore, larger prospective studies will need to be performed to confirm these preliminary results.
In conclusion, our study showed that lymphopenia (LC < 1.0 Giga/L) is associated with prognosis and can be considered as an independent marker of prognosis in patients with ESCC. However, larger prospective studies will need to be performed to confirm these preliminary results.
Footnotes
Abbreviations: AUC = areas under the curve, CI = confidence interval, CSS = cancer-specific survival, EAC = esophageal adenocarcinoma, EC = esophageal cancer, ESCC = esophageal squamous cell carcinoma, HR = hazard ratio, LC = lymphocyte count, ROC = receiver operating characteristic.
The authors have no funding information to disclose.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127:2893–2917. [DOI] [PubMed] [Google Scholar]
- 2.Napier KJ, Scheerer M, Misra S. Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014; 6:112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin Y, Totsuka Y, He Y, et al. Epidemiology of esophageal cancer in Japan and China. J Epidemiol 2013; 23:233–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keditsu KK, Jiwnani S, Karimundackal G, et al. Multimodality management of esophageal cancer. Indian J Surg Oncol 2013; 4:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008; 454:436–444. [DOI] [PubMed] [Google Scholar]
- 6.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357:539–545. [DOI] [PubMed] [Google Scholar]
- 7.Karakiewicz PI, Hutterer GC, Trinh QD, et al. C-reactive protein is an informative predictor of renal cell carcinoma-specific mortality: a European study of 313 patients. Cancer 2007; 110:1241–1247. [DOI] [PubMed] [Google Scholar]
- 8.Shimada H, Nabeya Y, Okazumi S, et al. Elevation of pre-operative serum C-reactive protein level is related to poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol 2003; 83:248–252. [DOI] [PubMed] [Google Scholar]
- 9.Donskov F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin Cancer Biol 2013; 23:200–207. [DOI] [PubMed] [Google Scholar]
- 10.Feng JF, Huang Y, Lu WS, et al. Preoperative platelet count in esophageal squamous cell carcinoma: is it a prognostic factor? Langenbecks Arch Surg 2013; 398:1115–1122. [DOI] [PubMed] [Google Scholar]
- 11.Castillo JJ, Morales D, Quinones P, et al. Lymphopenia as a prognostic factor in patients with peripheral T-cell lymphoma, unspecified. Leuk Lymphoma 2010; 51:1822–1828. [DOI] [PubMed] [Google Scholar]
- 12.Manuel M, Tredan O, Bachelot T, et al. Lymphopenia combined with low TCR diversity (divpenia) predicts poor overall survival in metastatic breast cancer patients. Oncoimmunology 2012; 1:432–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saroha S, Uzzo RG, Plimack ER, et al. Lymphopenia is an independent predictor of inferior outcome in clear cell renal carcinoma. J Urol 2013; 189:454–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low DE, Bodnar A. Update on clinical impact, documentation, and management of complications associated with esophagectomy. Thorac Surg Clin 2013; 23:535–550. [DOI] [PubMed] [Google Scholar]
- 15.Levy RM, Wizorek J, Shende M, et al. Laparoscopic and thoracoscopic esophagectomy. Adv Surg 2010; 44:101–116. [DOI] [PubMed] [Google Scholar]
- 16.Ye T, Sun Y, Zhang Y, et al. Three-field or two-field resection for thoracic esophageal cancer: a meta-analysis. Ann Thorac Surg 2013; 96:1933–1941. [DOI] [PubMed] [Google Scholar]
- 17.Rice TW, Rusch VW, Ishwaran H, et al. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Staging Manuals. Cancer 2010; 116:3763–3773. [DOI] [PubMed] [Google Scholar]
- 18.Waraich N, Rashid F, Jan A, et al. Vascular invasion is not a risk factor in oesophageal cancer recurrence. Int J Surg 2011; 9:237–240. [DOI] [PubMed] [Google Scholar]
- 19.Mehrazin R, Uzzo RG, Kutikov A, et al. Lymphopenia is an independent predictor of inferior outcome in papillary renal cell carcinoma. Urol Oncol 2014; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson SK, Ruszkiewicz AR, Jamieson GG, et al. Improving the accuracy of TNM staging in esophageal cancer: a pathological review of resected specimens. Ann Surg Oncol 2008; 15:3447–3458. [DOI] [PubMed] [Google Scholar]
- 21.Wijnhoven BP, Tran KT, Esterman A, et al. An evaluation of prognostic factors and tumor staging of resected carcinoma of the esophagus. Ann Surg 2007; 245:717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Igaki H, Kato H, Tachimori Y, et al. Prognostic evaluation for squamous cell carcinomas of the lower thoracic esophagus treated with three-field lymph node dissection. Eur J Cardiothorac Surg 2001; 19:887–893. [DOI] [PubMed] [Google Scholar]
- 23.Bollschweiler E, Baldus SE, Schröder W, et al. Staging of esophageal carcinoma: length of tumor and number of involved regional lymph nodes. Are these independent prognostic factors? J Surg Oncol 2006; 94:355–363. [DOI] [PubMed] [Google Scholar]
- 24.Eloubeidi MA, Desmond R, Arguedas MR, et al. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer 2002; 95:1434–1443. [DOI] [PubMed] [Google Scholar]
- 25.Yendamuri S, Swisher SG, Correa AM, et al. Esophageal tumor length is independently associated with long-term survival. Cancer 2009; 115:508–516. [DOI] [PubMed] [Google Scholar]
- 26.Feng JF, Huang Y, Zhao Q. Tumor length in elderly patients with esophageal squamous cell carcinoma: is it a prognostic factor? Ups J Med Sci 2013; 118:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zafirellis K, Dolan K, Fountoulakis A, et al. Multivariate analysis of clinical, operative and pathologic features of esophageal cancer: who needs adjuvant therapy? Dis Esophagus 2002; 15:155–159. [DOI] [PubMed] [Google Scholar]
- 28.Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002; 347:1662–1669. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz RE, Smith DD. Clinical impact of lymphadenectomy extent in resectable esophageal cancer. J Gastrointest Surg 2007; 11:1384–1393. [DOI] [PubMed] [Google Scholar]
- 30.Bedenne L, Michel P, Bouché O, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J Clin Oncol 2007; 25:1160–1168. [DOI] [PubMed] [Google Scholar]
- 31.Rawat S, Kumar G, Kakria A, et al. Chemoradiotherapy in the management of locally advanced squamous cell carcinama esophagus: is surgical resection required? J Gastrointest Cancer 2013; 44:277–284. [DOI] [PubMed] [Google Scholar]
- 32.Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol 2014; 32:2416–2422. [DOI] [PubMed] [Google Scholar]
- 33.Alkhouri N, Morris-Stiff G, Campbell C, et al. Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver Int 2012; 32:297–302. [DOI] [PubMed] [Google Scholar]
- 34.Sefil F, Ulutas KT, Dokuyucu R, et al. Investigation of neutrophil lymphocyte ratio and blood glucose regulation in patients with type 2 diabetes mellitus. J Int Med Res 2014; 42:581–588. [DOI] [PubMed] [Google Scholar]


