Abstract
To investigate intraocular pressure (IOP) related patterns before and after selective laser trabeculoplasty (SLT) for normal tension glaucoma (NTG).
In this prospective cohort study, 18 NTG patients underwent SLT. Success was defined as IOP reduction ≥20% by Goldmann applanation tonometry. 24-hour IOP-related pattern recording with a contact lens sensor (CLS) (SENSIMED Triggerfish®, Sensimed, Switzerland) was done before (baseline) and 1 month after SLT. A cosine function was fitted to the mean CLS patterns for each individual in the SLT success and non-success groups and the amplitude before and after SLT was calculated. Diurnal, nocturnal, and 24-hour CLS pattern local variability was determined for pre- and post-SLT sessions. Cosine amplitude and variability were compared before and after SLT by group using paired t-tests, with α = 0.05.
Patients (11 women, 7 men) had a mean age of 65.1 ± 13.7 years. Mean IOP was 15.3 ± 2.2 mm Hg at baseline and was reduced by 17.0% to 12.7 ± 1.8 mm Hg 1 month after SLT (P = 0.001). SLT was successful in 8 patients (44%). The amplitude of the fitted cosine was reduced by 24.6% in the success group, but displayed an amplitude increase of 19.2% post-SLT in the non-success group. Higher diurnal local variability of the CLS pattern was observed after SLT in non-success subjects (P = 0.002), while nocturnal variability showed no significant change. The increase in diurnal variability in the non-success group led to an increase in 24-hour variability in this group (P = 0.001). No change in local variability (diurnal, nocturnal, and 24-hour) was seen in the success group.
The IOP-related pattern cosinor amplitude was reduced in NTG patients with a successful SLT treatment whereas the non-success group exhibited an increase of cosine amplitude. Higher diurnal and 24-hour CLS pattern variability was observed in non-success patients 1 month post-SLT.
INTRODUCTION
Intraocular pressure (IOP) is still one of the most important modifiable risk factors for glaucoma progression,1–3 even in glaucomas where the pressure is normal or low. A single IOP measurement in the clinic is not an accurate depiction of the pressure profile of glaucoma patients, as it does not account for the influence of circadian rhythm and nocturnal posturing on IOP.4,5 Various studies have documented IOP fluctuations throughout the day and night using intermittent IOP measurements taken a few hours apart6–10 but it is still uncertain whether it is the frequency of IOP peaks or the range of IOP fluctuation that leads to glaucoma progression.
Selective laser trabeculoplasty (SLT) is a non-invasive, repeatable, and effective modality of IOP reduction for open angle glaucoma.11–14 It has a similar efficacy to anti-glaucoma eye drops and the former argon laser trabeculoplasty15 and a recent study has affirmed SLT's efficacy in normal tension glaucoma (NTG), reducing the IOP by an additional 20% while using 27% less medication at 6 months compared with pre-treatment levels.16
In terms of IOP fluctuation, Kothy et al17 reported reduced IOP fluctuation over a 24-hour period after SLT and Nagar et al18 found that SLT's ability to reduced diurnal IOP variation was inferior to latanoprost. Prasad et al19 showed that there was less inter-visit IOP fluctuation after 360° SLT treatment compared to 180° SLT treatment. The majority of studies investigating the effects of IOP reduction after SLT only measured IOP over a few sampling periods or during clinical visits.
The SENSIMED Triggerfish® (Sensimed AG, Lausanne, Switzerland) is based on a wireless silicon contact lens sensor (CLS).20 The device allows for the recording of the IOP related pattern over a 24-hour period with minimal disturbance to one's daily routines and sleep cycle. The CLS records IOP-related changes through the detection of biodimensional changes in the corneoscleral area, for 30 seconds every 5 minutes over 24 hours. Each recording “burst” represents 300 data points, of which the median is plotted as a single graph of the 24-hour IOP-related profile. The CLS output is in units of milli-volts equivalent (mVeq). The device was CE-marked and approved for clinical use in 2009. It has been shown to be safe and well tolerated during 24-hour recording of IOP-related patterns in healthy subjects, glaucoma suspects, and glaucoma patients.21,22
The purpose of the study was to analyze the IOP-related fluctuations using the CLS before and after adjuvant SLT in subjects with NTG who were treated with topical anti-glaucoma medications.
PATIENTS AND METHODS
This study adhered to the tenets of the Declaration of Helsinki. Informed patient consent and approval by the Institutional Review Board were obtained prior to study commencement. This study was supported by the provision of SENSIMED Triggerfish® CLS and other device supporting items by Sensimed. There was no financial funding and the authors have no proprietary interests.
This was a prospective cohort study from July 2012 to June 2013, conducted at a Queen Mary Hospital, a university hospital in Hong Kong Special Administrative Region, China. The study recruited consenting adults (age > 18 years old) with unilateral or bilateral NTG who were currently on topical anti-glaucoma medications. NTG was defined by open angle on gonioscopy, progressive thinning of the retinal nerve fiber layer (RFNL) on the Spectralis® (Heidelberg Engineering GmbH, Heidelberg, Germany) Optical Coherence Tomography, and an IOP < 21 mm Hg during office hours. Subjects were excluded for those with previous glaucoma surgery or laser, active or previous corneal diseases, as well as subjects with only one functional eye.
One week prior to SLT, subjects wore the CLS for 24 hours. The CLS was placed on the subject's eye by a single ophthalmologist in the clinic after a slit lamp examination and keratometry by the IOL Master 500 (Carl Zeiss, Oberkochen, Germany) to determine the corneal curvature for selection of the appropriate CLS base curve. For those with unilateral disease, the CLS was placed on the eye with NTG. For those with bilateral disease, a random eye assignment from card shuffling was used to determine the eye for CLS placement while SLT was scheduled for both eyes in subjects with bilateral NTG. The subject then returned home with lubricating eye drops and carried on their routine activities (both indoor and outdoor), apart from showering or swimming (as the device cannot be in contact with water). Subjects continued the same regimen of anti-glaucoma eye drops and slept in their habitual position at night. Each subject carried a logbook to record sleeping and medication instillation times during the 24-hour period. After 24 hours, the subject returned to the clinic to have the contact lens removed followed by a slit lamp examination. The data recorded by the CLS was uploaded into a computer database. Goldmann applanation tonometry (GAT) was performed before and after each CLS wear by a single observer.
All patients then received a single SLT treatment by a single surgeon (JWYL) in the same eye that wore the CLS. A Q-switched Nd:YAG laser (Ellex Solo™, Ellex Medical Pty. Ltd., Adelaide, SA, Australia) was used, with an initial energy of 0.8 mJ and titrated until bubble formation was just invisible. Treatment was delivered in a single burst mode until 360° of the trabecular meshwork was treated.
At 1-month post-SLT, patients wore the CLS in the same eye that received the first CLS recording. As before, the CLS was removed 24 hours later.
All topical anti-glaucoma medications remained unchanged until after the second (post-SLT) CLS wearing. Anti-glaucoma medications were then titrated (decreased or increased) after 1 month of SLT based on an individual target IOP, calculated as a 30% reduction from their documented presenting IOP (prior to starting anti-glaucoma medication) as per the Collaborative Normal Tension Glaucoma Study.23 Patients were followed-up every 3 months thereafter. The order of resuming anti-glaucoma medication included first the use of alpha adrenergic agonists or prostaglandin analogs followed by topical carbonic anhydrase inhibitors or β-blockers. When multiple medications were required, fixed combination medications were given to simplify the drug regime.
The Primary Outcomes Included
-
Local variability: CLS IOP-related variability was measured over 24 hours, diurnally, and nocturnally. This was a measure of local variability of the raw CLS data from the smoothed function obtained using a locally weighted polynomial regression method (shown below).24 This parameter reflects the error of the smoothed function, or the amount of information that is “missed” by the smoothed values.
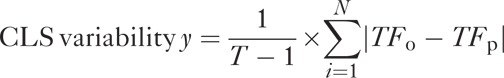
where T is the number of CLS measurements over the recording period, TFO is the observed CLS signal, and TFP is the predicted CLS signal based on the smoothing function selected.
-
Global variability: Cosinor modeling of 24-hour CLS patterns was performed using the below formula. The cosinor model represents the actual amplitude of IOP-related fluctuation over a 24-hour period and is most representative of IOP-related changes
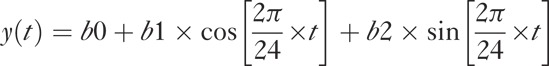
where y is the observed signal in mVeq, t is the time, and b0, b1, and b2 are the regression coefficients, estimated from the data.
SLT success rates: GAT IOP reductions ≥20% from the pre-SLT levels while on the same anti-glaucoma regimen at 1-month post-SLT.
The Secondary Outcomes Included
CLS increase and decrease rates (change in CLS units/change in time): maximum, minimum, median, and mean.
Number of peaks: over 24 hours, <30 minutes, >90 mVeq.
Sleep-to-wake and wake-to-sleep slopes. The calculation of sleep-to-wake and wake-to-sleep slopes has been described previously by Mansouri et al22
The reduction in IOP after SLT was calculated based on the GAT IOP measured at baseline, 1 month, and 3 months after SLT. These GAT IOP readings were taken at approximately 3 pm each visit and taken before the CLS wear at the baseline and 1-month post-SLT visits.
Statistics
All statistical calculations were done using SPSS version 18.0 (SPSS, Inc., Chicago, IL). The differences between the measured parameters detailed above, were compared before and after SLT using the paired t-test or Wilcoxon matched pairs test depending on the normality of the variable distribution. Statistical significance was taken as P ≤ 0.05 and all means were expressed as mean ± standard deviation.
RESULTS
In 18 subjects that were enrolled in the study, there were 7 males and 11 females. The mean age was 65.1 ± 13.7 years. All subjects were ethnic Chinese with pigmented trabecular meshwork and NTG. The mean of the average RNFL thickness was 72.9 ± 9.5 micrometers (μm). One subject did not accurately record his sleeping and waking times, thus, to ensure the accuracy of the data, this subject was excluded from analyses that required the input of sleeping and waking times.
The baseline (pre-SLT) GAT IOP was 15.3 ± 2.2 mm Hg while on 1.7 ± 0.7 types of anti-glaucoma eye drops. All subjects received a single session of SLT with a mean of 198.2 ± 22.9 shots with a mean energy of 0.9 ± 0.09 mJ. There were no complications from SLT.
The distributions of anti-glaucoma eye drops were: β-blockers (32.0%), prostaglandins (20.0%), fixed combination prostaglandin-β-blocker (20.0%), brimonidine (12.0%), and topical carbonic anhydrase inhibitors (8.0%).
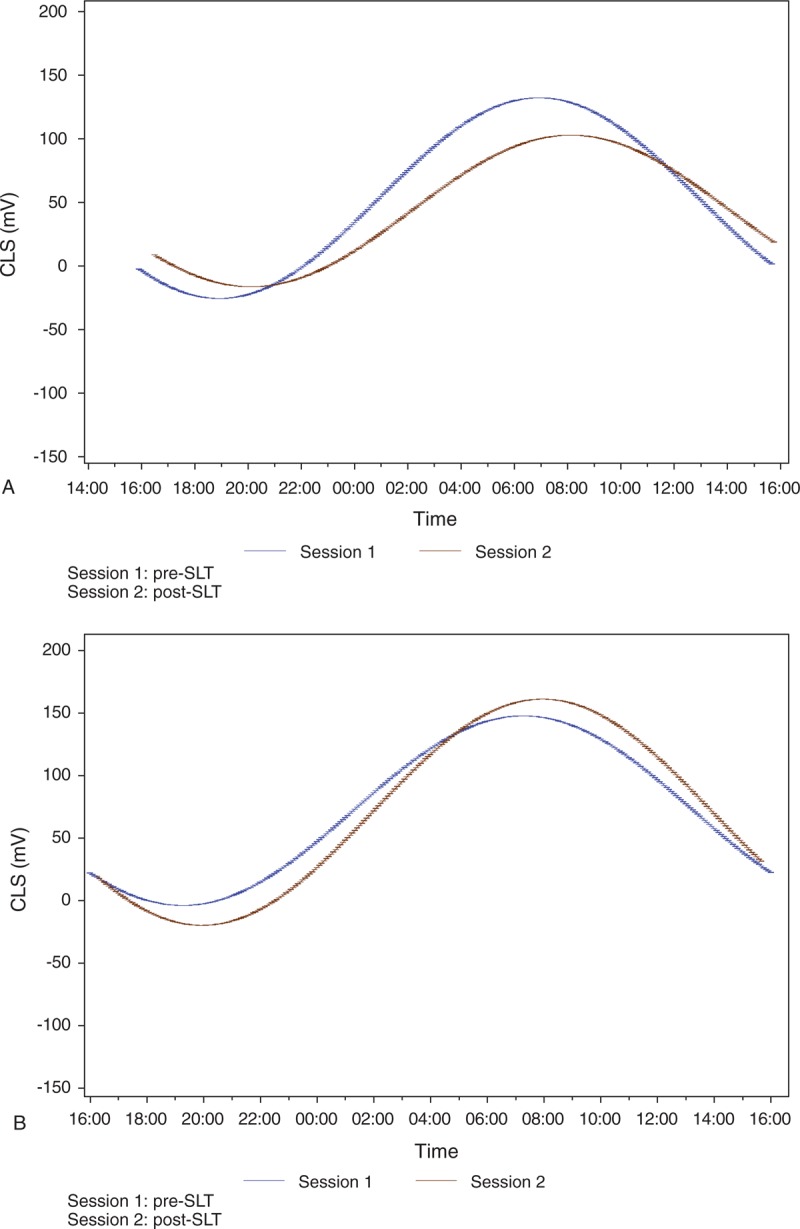
The IOP-related pattern of the 18 subjects was unique in terms of their peaks and slopes. This personal IOP-related pattern remains similar in shape before and after SLT and it is only the CLS pattern amplitude and steepness of slopes that change. Figure 1 shows the 24-hour IOP-related pattern before and after SLT.
FIGURE 1.
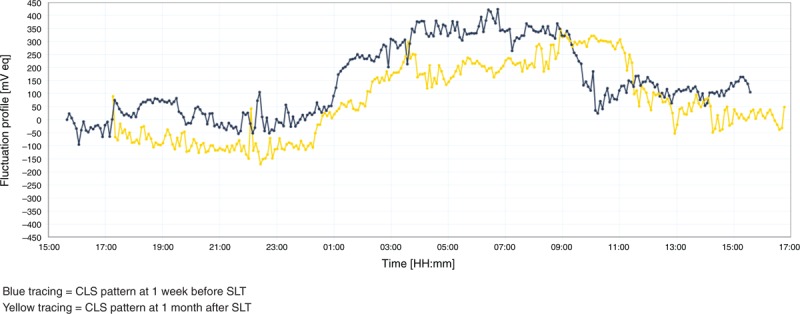
Twenty-four-hour IOP-related pattern before and after SLT.
At 1 month after SLT, the mean GAT IOP measured before the CLS wear was 12.7 ± 1.8 mm Hg while on the same anti-glaucoma medication regimen as before laser, representing a 17.0% reduction in IOP after SLT (P = 0.001). At 3 months post-SLT, the mean GAT IOP was 11.4 ± 1.7 mm Hg while on 1.4 ± 1.2 types of anti-glaucoma eye drops, representing a 25.5% (P = 0.0007) IOP reduction in addition to a 17.6% medication reduction compared to pre-SLT levels. Eight out of 18 (44.4%) subjects fulfilled the criteria of a successful SLT outcome.
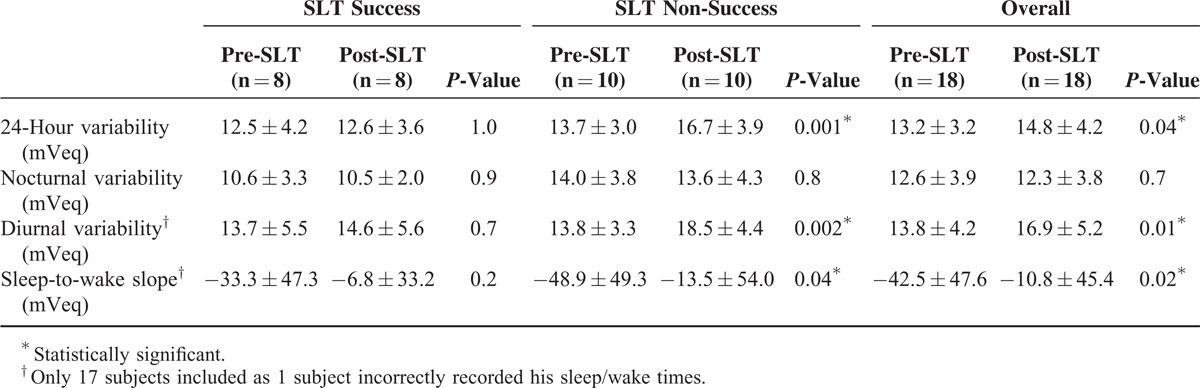
The measured pre- and post-SLT parameters are summarized in Table 1 to 4. From initially similar levels, the mean acrophase amplitude of the fitted cosinor function (global variability) was reduced after SLT by 24.6% in the success subjects (Table 1). In subjects for whom the SLT was unsuccessful, the global variability increased by 19.2% post-SLT, indicating greater 24-hour IOP-related fluctuation after SLT (Figure 2A and B).
TABLE 1.
Mean Global Variability Using the Cosinor Model Before and After SLT
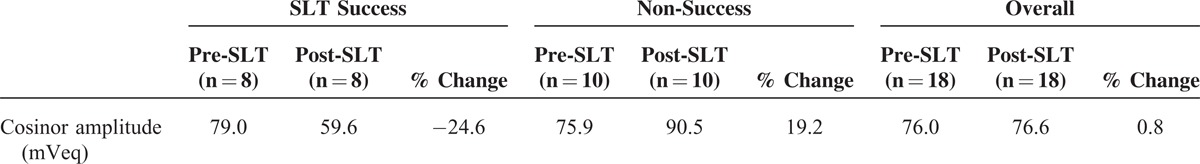
FIGURE 2.
(A) Twenty-four-hour IOP-related pattern (global) variability in success group. (B) Twenty-four-hour IOP-related pattern (global) variability in non-success group.
For the local variability in the non-success group, the 24-hour variability increased by 21.9% (P = 0.001), driven by the magnified 34.1% increase in diurnal variability (P = 0.002), while the nocturnal variability remained unchanged (P = 0.8). For the success group, there was no significant difference in local variability over 24-hours, nocturnal, or diurnal (P > 0.7). For the mean sleep-to-wake slope, the slope was negative in both groups signifying a decrease in CLS output with waking. In the non-success group, the sleep-to-wake slope was flatter after SLT (P = 0.04). No significant changes in variability or sleep-to-wake slope were observed in the SLT success group (P = 0.2) (Table 2).
TABLE 2.
Mean Local Variability and Sleep-to-Wake Slope Before and After SLT
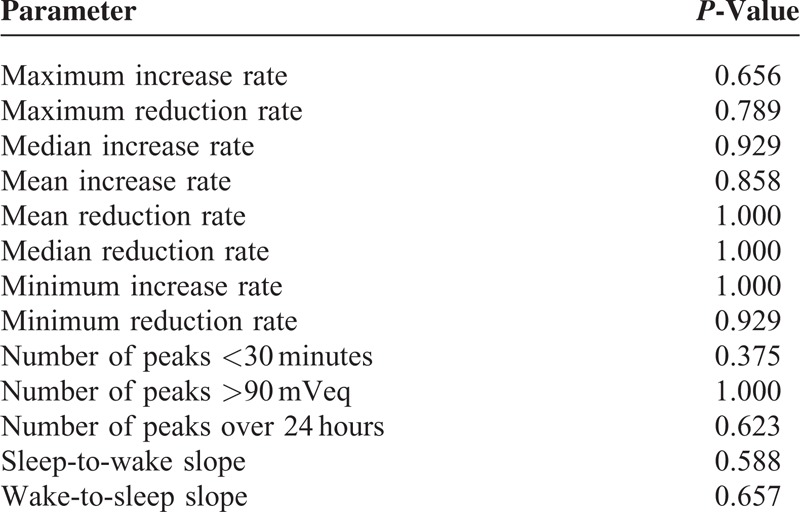
For the overall study population, local diurnal (22.5%, P = 0.01) and 24-hour (12.1%, P = 0.04) variability was higher post-SLT, mainly attributed by the increases in the non-success group detailed above (Table 2). The number of peaks >90 mVeq increased (P = 0.04) and the number of diurnal troughs decreased (P = 0.01) after SLT, independent of SLT success (Table 3). The mean number of peaks over the 24-hour period as well as the number of peaks <30 minutes were not affected by SLT (Table 3). There was no significant difference in the rates of CLS increase or decrease before or after SLT (Table 4). Area under the nocturnal part of the CLS curve was also not significantly different after SLT as compared to before (not shown). Table 5 compared the differences in pre- and post-SLT parameters between the success versus non-success group.
TABLE 3.
Number of Peaks and Troughs Before and After SLT

TABLE 4.
CLS Pattern Increase and Decrease Rates Before and After SLT
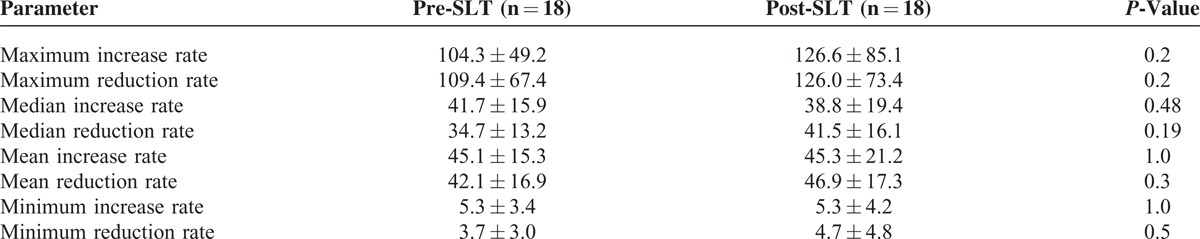
TABLE 5.
Comparison of Differences in Pre- and Post-SLT Parameters Between the Success Versus Non-Success Group
DISCUSSION
Previous studies have reported the significance of IOP fluctuation on glaucoma progression.25,26 While there are studies that have reported otherwise,27,28 much of the existing literature only documented inter-visit IOP variability or daytime IOP variability over a limited number of IOP measurements. To the best of our knowledge, this is one of the first studies investigating the IOP-related fluctuation after SLT for medically treated NTG subjects by the use of a 24-hour continuous IOP related pattern recording device.
SLT was effective in lowering the mean GAT IOP by 17.0% at 1 month and 25.5% at 3 months, in addition to a 17.6% reduction in anti-glaucoma medication compared to pre-SLT baseline levels. Using the CLS for 24-hour continuous IOP-related pattern recording before and 1 month after SLT, we observed a reduction of mean global variability using the cosinor model in the SLT success group, which was anticipated based on previous reports in the literature.17–19,29 The CLS has previously been reported to be an accurate and reproducible method to model IOP rhythm.30 For local variability, we noted a significant 34.1% increase in diurnal variability and a 21.9% increase in the 24-hour variability after non-successful SLT. This increase in diurnal variability caused significant increase in the diurnal and 24-hour variability in the overall study population (22.5% and 12.1%, respectively) while in the SLT success group, there was no significant increase in diurnal, nocturnal, or 24-hour local variability. It should be noted that local variability in this study was defined as the variation of raw CLS data around the smoothed curve, hence it was a measure of the local variability of the CLS as opposed to the global variability that implemented a cosinor model where the amplitude was representative of IOP-related fluctuation over 24 hours. There was also evidence of an overall greater number of peaks >90 mVeq after SLT. Previous studies in rats have demonstrated that rapid IOP spikes can result in greater damage to retinal ganglion cells than persistently elevated IOP levels although the effect on humans is yet to be determined.31
Nagar et al18 previously reported a 41% reduction in IOP fluctuation in 50% of subjects treated with SLT. However, their study subjects consisted of subjects with primary open angle glaucoma (POAG) or ocular hypertension with a pre-SLT IOP of 26.0 mm Hg and IOP was only measured during 4 time intervals at 08:00, 11:00, 14:00, and 18:00 hours.18 In contrast, our population consisted of only NTG subjects with a pre-SLT IOP of 15.3 ± 2.2 mm Hg while on 1.7 ± 0.7 types of anti-glaucoma eye drops and our IOP-related pattern recording was on a continuous basis for 24 hours. Thus, the timing and frequency of IOP measurement is important in accurately assessing the effects of SLT on IOP control. Kothy et al17 reported in their POAG series that after SLT, 5 eyes had IOP reduction >20% during office hour (08:00–12:00 hours) but none of the 26 eyes had a mean IOP reduction of ≥20% when the assessment period was extended from 08:00 to 00:00 hours. The influence of SLT on diurnal and nocturnal IOP fluctuation is also diversified in the literature and probably related to whether or not anti-glaucoma medications were used. Kothy et al17 reported a significant reduction in diurnal IOP fluctuation of 4.3 ± 1.7 and 5.0 ± 1.7 mm Hg at 3 and 6 months after SLT, respectively, in their POAG population after a 4-week washout period prior to SLT. In contrast, Lee et al32 reported in their medically treated POAG patients that after SLT, IOP range was significantly reduced nocturnally but not diurnally. Both of these studies however, only measured IOP at intervals rather than continuously over 24 hours. Therefore, the influence of SLT on IOP fluctuation cannot be fully evaluated using interval or daytime IOP measurements alone.
In this study, SLT was offered as an adjuvant therapy for NTG with the aim of further lowering IOP or to reduce the anti-glaucoma medication requirement. The authors decided not to washout anti-glaucoma medication prior to SLT in order to simulate the realistic clinical scenario where adjuvant SLT is offered to medically controlled NTG patients to reduce medication load or for those with poor adherence or intolerant to topical anti-glaucoma medications. In addition, there is no definite consensus from the literature on the negative influence of topical prostaglandin analogs on SLT outcome. In a retrospective study by Scherer,33 a greater mean IOP reduction was demonstrated following SLT in POAG subjects being treated with topical prostaglandin analogs. Alvarado et al34 on the other hand, showed that prostaglandin analogs might dampen the IOP-lowering effects of SLT while Singh et al35 reported no significant difference in SLT outcome with prostaglandin analog use.
The findings of our study suggest that adjuvant and successful SLT may offer an additional benefit reducing 24-hour IOP-related fluctuation for NTG patients who are already on anti-glaucoma medication. The majority of our patients (40%) was on a once nightly topical prostaglandin analog or fixed combination prostaglandin-β-blocker and 32% were on a topical β-blocker. SLT and prostaglandin analogs have been postulated to share a common IOP-lowering pathway by opening up the intercellular junction and increasing conductivity at level of the Schlemm canal endothelial cells.34 We postulate that after the prostaglandin analogs reach their maximal IOP-lowering effect at 8 to 12 hours after instillation,36 this leads to the rebound in diurnal IOP fluctuation. For those subjects who were responsive to SLT (the success group), their diurnal variability was kept low by the continued effects of the SLT, accounting for the significantly lower diurnal variability as compared to the non-success group.
In all 18 subjects, both before and after SLT, the wake-to-sleep slope was positive and the sleep-to-wake slope was negative, signifying that the supine-posture induced IOP increases are still preserved after adjuvant SLT. There was no significant difference in the wake-to-sleep slope before and after SLT (P = 0.9). However, the sleep-to-wake slope was dampened by 3.9 times, following SLT (P = 0.02) signifying a more gradual drop in IOP upon waking after SLT. It has been demonstrated in animal models that SLT may increase the expression of aqueous endothelin-1 (ET-1) in the early post-laser periods, which can reduce aqueous outflow, explaining the slower drop in IOP upon waking.37 The magnitude and duration of ET-1 influence following SLT has not been well established in human subjects. However, we do not have an account for why only the sleep-to-wake slope was affected as the daytime IOP was reduced after SLT signifying an increase in aqueous outflow overall.
Our study had its limitations. Firstly, the IOP related fluctuation was monitored at 1-month post-SLT. Although it has been established that the post-SLT IOP values as early as 2 weeks were predictive of future IOP control,38 future studies monitoring 24-hour continuous IOP changes at a longer time frame after SLT would provide more long-term results.39 Secondly, at present, the CLS can only measure IOP-related fluctuations in mVeq. The GAT before CLS wear was used as a reference baseline. There are no effective formulae to convert these readings into the gold standard unit of IOP measurement (mm Hg). Further developments in this area would popularize the use of this device in clinical practice. Thirdly, due to the high cost and single use nature of the CLS, the sample size was relatively small due to the constraints of resources. Further studies involving larger samples should be carried out to further strength the preliminary conclusions drawn from this study. Fourthly, the observations from our study are only applicable to medically treated NTG patients and may not be generalizeable to other glaucoma patients or to those without baseline anti-glaucoma medication prior to SLT.
Nevertheless, this study has served to provide objective evidence for the controversies around IOP fluctuations after SLT by evaluating IOP related changes in a 24-hour, continuous manner. It seems that SLT was able to produce a significant IOP reduction measured at a single time point during the day, accompanied by dampened amplitude of the nycthemeral rhythm in patients for whom SLT was successful but an increased in those where SLT was not successful. Our findings confirm the relevance of continuous 24-hour IOP-related pattern monitoring in the accurate evaluation of glaucoma treatments. Further trials involving larger samples, different glaucoma subtypes, and groups with and without baseline anti-glaucoma medications would provide us with a clearer understanding of the influence of SLT on IOP fluctuation.
Acknowledgments
The authors would especially like to thank Ms. Man Yee Lee and her team of devoted nurses, optometrists, and clerical staff at Queen Mary Hospital for dedicating their time and effort in support of clinical research for the betterment of patient care. This study was supported by the provision of SENSIMED Triggerfish® CLS and other device supporting items by Sensimed. The article processing charge was funded by Sensimed. The authors had sole autonomy over all aspects of the clinical research and write-up.
Footnotes
Abbreviations: CLS = contact lens sensor, ET-1 = endothelin-1, GAT = Goldmann applanation tonometry, IOP = intraocular pressure, NTG = normal tension glaucoma, SLT = laser trabeculoplasty.
There was no financial funding and the authors have no proprietary interests.
REFERENCES
- 1.Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol 2002; 120:701–713. [DOI] [PubMed] [Google Scholar]
- 2.Collaborative Normal-Tension Glaucoma Study Group. Effectiveness of intraocular pressure reduction in the treatment of normal-tension glaucoma. C Am J Ophthal 1998; 126:498–505. [DOI] [PubMed] [Google Scholar]
- 3.Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002; 120:1268–1279. [DOI] [PubMed] [Google Scholar]
- 4.Hughes E, Spry P, Diamond J. 24-Hour monitoring of intraocular pressure in glaucoma management: a retrospective review. J Glaucoma 2003; 12:232–236. [DOI] [PubMed] [Google Scholar]
- 5.Barkana Y, Anis S, Liebmann J, et al. Clinical utility of intraocular pressure monitoring outside of normal office hours in patients with glaucoma. Arch Ophthalmol 2006; 124:793–797. [DOI] [PubMed] [Google Scholar]
- 6.Friberg TR, Sanborn G, Weinreb RN. Intraocular and episcleral venous pressure increase during inverted posture. Am J Ophthalmol 1987; 103:523–526. [DOI] [PubMed] [Google Scholar]
- 7.Buguet A, Py P, Romanet JP. 24-Hour (nyctohemeral) and sleep-related variations of intraocular pressure in healthy white individuals. Am J Ophthalmol 1994; 117:342–347. [DOI] [PubMed] [Google Scholar]
- 8.De Vivero C, O’Brien C, Lanigan L, Hitchings R. Diurnal intraocular pressure variation in low-tension glaucoma. Eye (Lond) 1994; 8:521–523. [DOI] [PubMed] [Google Scholar]
- 9.Rota-Bartelink AM, Pitt A, Story I. Influence of diurnal variation on the intraocular pressure measurement of treated primary open-angle glaucoma during office hours. J Glaucoma 1996; 5:410–415. [PubMed] [Google Scholar]
- 10.Tajunisah I, Reddy SC, Fathilah J. Diurnal variation of intraocular pressure in suspected glaucoma patients and their outcome. Graefes Arch Clin Exp Ophthalmol 2007; 245:1851–1857. [DOI] [PubMed] [Google Scholar]
- 11.Lee JW, Chan CW, Wong MO, et al. A randomized control trial to evaluate the effect of adjuvant selective laser trabeculoplasty versus medication alone in primary open-angle glaucoma: preliminary results. Clin Ophthalmol 2014; 8:1987–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JW, Chan JC, Chang RT, et al. Corneal changes after a single session of selective laser trabeculoplasty for open-angle glaucoma. Eye (Lond) 2014; 28:47–52.doi: 10.1038/eye.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JW, Liu CC, Chan J, et al. Predictors of success in selective laser trabeculoplasty for primary open angle glaucoma in Chinese. Clin Ophthalmol 2014; 8:1787–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee JW, Liu CC, Chan JC, Lai JS. Predictors of success in selective laser trabeculoplasty for chinese open-angle glaucoma. J Glaucoma 2014; 23:321–325. [DOI] [PubMed] [Google Scholar]
- 15.Wong MO, Lee JW, Choy BN, et al. Systematic review and meta-analysis on the efficacy of selective laser trabeculoplasty in open-angle glaucoma. Surv Ophthalmol 2014; July 2. pii: S0039-6257(14)00131-3. DOI: 10.1016/j.survophthal.2014.06.006. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 16.Lee JW, Gangwani RA, Chan JC, Lai JS. Prospective study on the efficacy of treating normal tension glaucoma with a single session of selective laser trabeculoplasty. J Glaucoma 2014; July 25. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17.Kothy P, Toth M, Hollo G. Influence of selective laser trabeculoplasty on 24-hour diurnal intraocular pressure fluctuation in primary open-angle glaucoma: a pilot study. Ophthalmic Surg Lasers Imaging 2010; 41:342–347. [DOI] [PubMed] [Google Scholar]
- 18.Nagar M, Luhishi E, Shah N. Intraocular pressure control and fluctuation: the effect of treatment with selective laser trabeculoplasty. Br J Ophthalmol 2009; 93:497–501. [DOI] [PubMed] [Google Scholar]
- 19.Prasad N, Murthy S, Dagianis JJ, Latina MA. A comparison of the intervisit intraocular pressure fluctuation after 180 and 360 degrees of selective laser trabeculoplasty (SLT) as a primary therapy in primary open angle glaucoma and ocular hypertension. J Glaucoma 2009; 18:157–160. [DOI] [PubMed] [Google Scholar]
- 20.Leonardi M, Pitchon EM, Bertsch A, et al. Wireless contact lens sensor for intraocular pressure monitoring: assessment on enucleated pig eyes. Acta Ophthalmol 2009; 87:433–437. [DOI] [PubMed] [Google Scholar]
- 21.Lorenz K, Korb C, Herzog N, et al. Tolerability of 24-hour intraocular pressure monitoring of a pressure-sensitive contact lens. J Glaucoma 2013; 22:311–316. [DOI] [PubMed] [Google Scholar]
- 22.Mansouri K, Medeiros FA, Tafreshi A, Weinreb RN. Continuous 24-hour monitoring of intraocular pressure patterns with a contact lens sensor: safety, tolerability, and reproducibility in patients with glaucoma. Arch Ophthalmol 2012; 130:1534–1539. [DOI] [PubMed] [Google Scholar]
- 23.Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures. Am J Ophthalmol 1998; 126:487–497. [DOI] [PubMed] [Google Scholar]
- 24.Hurvich CM, Simonoff JS, Tsai CL. Smoothing parameter selection in nonparametric regression using an improved Akaike information criterion. J R Stat Soc B 1998; 60:271–293. [Google Scholar]
- 25.Asrani S, Zeimer R, Wilensky J, et al. Large diurnal fluctuations in intraocular pressure are an independent risk factor in patients with glaucoma. J Glaucoma 2000; 9:134–142. [DOI] [PubMed] [Google Scholar]
- 26.Collaer N, Zeyen T, Caprioli J. Sequential office pressure measurements in the management of glaucoma. J Glaucoma 2005; 14:196–200. [DOI] [PubMed] [Google Scholar]
- 27.Bengtsson B, Heijl A. Diurnal IOP fluctuation: not an independent risk factor for glaucomatous visual field loss in high-risk ocular hypertension. Graefes Arch Clin Exp Ophthalmol 2005; 243:513–518. [DOI] [PubMed] [Google Scholar]
- 28.Medeiros FA, Weinreb RN, Zangwill LM, et al. Long-term intraocular pressure fluctuations and risk of conversion from ocular hypertension to glaucoma. Ophthalmology 2008; 115:934–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tojo N, Oka M, Miyakoshi A, et al. Comparison of fluctuations of intraocular pressure before and after selective laser trabeculoplasty in normal-tension glaucoma patients. J Glaucoma 2014; 23:e138–e143.doi: 10.1097/IJG.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 30.Mottet B, Aptel F, Romanet JP, et al. 24-Hour intraocular pressure rhythm in young healthy subjects evaluated with continuous monitoring using a contact lens sensor. JAMA Ophthalmol 2013; 131:1507–1516. [DOI] [PubMed] [Google Scholar]
- 31.Resta V, Novelli E, Vozzi G, et al. Acute retinal ganglion cell injury caused by intraocular pressure spikes is mediated by endogenous extracellular ATP. Eur J Neurosci 2007; 25:2741–2754. [DOI] [PubMed] [Google Scholar]
- 32.Lee AC, Mosaed S, Weinreb RN, et al. Effect of laser trabeculoplasty on nocturnal intraocular pressure in medically treated glaucoma patients. Ophthalmology 2007; 114:666–670. [DOI] [PubMed] [Google Scholar]
- 33.Scherer WJ. Effect of topical prostaglandin analog use on outcome following selective laser trabeculoplasty. J Ocul Pharmacol Ther 2007; 23:503–512. [DOI] [PubMed] [Google Scholar]
- 34.Alvarado JA, Iguchi R, Juster R, et al. From the bedside to the bench and back again: predicting and improving the outcomes of SLT glaucoma therapy. Trans Am Ophthalmol Soc 2009; 107:167–181. [PMC free article] [PubMed] [Google Scholar]
- 35.Singh D, Coote MA, O’Hare F, et al. Topical prostaglandin analogues do not affect selective laser trabeculoplasty outcomes. Eye (Lond) 2009; 23:2194–2199. [DOI] [PubMed] [Google Scholar]
- 36.Russo A, Riva I, Pizzolante T, et al. Latanoprost ophthalmic solution in the treatment of open angle glaucoma or raised intraocular pressure: a review. Clin Ophthalmol 2008; 2:897–905. [PMC free article] [PubMed] [Google Scholar]
- 37.Guzey M, Vural H, Satici A. Endothelin-1 increase in aqueous humour caused by frequency-doubled Nd:YAG laser trabeculoplasty in rabbits. Eye (Lond) 2001; 15:781–785. [DOI] [PubMed] [Google Scholar]
- 38.Johnson PB, Katz LJ, Rhee DJ. Selective laser trabeculoplasty: predictive value of early intraocular pressure measurements for success at 3 months. Br J Ophthalmol 2006; 90:741–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ayala M, Chen E. The influence of topical prostaglandin analogues in inflammation after selective laser trabeculoplasty treatment. J Ocul Pharmacol Ther 2012; 28:118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]


