Abstract
The association of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) has recently been described and suggested to be a new entity within the spectrum of autoinflammatory syndromes, which are characterized by recurrent episodes of sterile inflammation, without circulating autoantibodies and autoreactive T-cells. We conducted an observational study on 5 patients with PASH syndrome, analyzing their clinical features, genetic profile of 10 genes already known to be involved in autoinflammatory diseases (AIDs), and cytokine expression pattern both in lesional skin and serum. In tissue skin samples, the expressions of interleukin (IL)-1β and its receptors I and II were significantly higher in PASH (P = 0.028, 0.047, and 0.050, respectively) than in controls. In PASH patients, chemokines such as IL-8 (P = 0.004), C-X-C motif ligand (CXCL) 1/2/3 (P = 0.028), CXCL 16 (P = 0.008), and regulated on activation, normal T cell expressed and secreted (RANTES) (P = 0.005) were overexpressed. Fas/Fas ligand and cluster of differentiation (CD)40/CD40 ligand systems were also overexpressed (P = 0.016 for Fas, P = 0.006 for Fas ligand, P = 0.005 for CD40, and P = 0.004 for CD40 ligand), contributing to tissue damage and inflammation. In peripheral blood, serum levels of the main proinflammatory cytokines, that is, IL-1β, tumor necrosis factor-α, and IL-17, were within the normal range, suggesting that in PASH syndrome, the inflammatory process is mainly localized into the skin. Four out of our 5 PASH patients presented genetic alterations typical of well-known AIDs, including inflammatory bowel diseases, and the only patient lacking genetic changes had clinically evident Crohn disease. In conclusion, overexpression of cytokines/chemokines and molecules amplifying the inflammatory network, along with the genetic changes, supports the view that PASH syndrome is autoinflammatory in origin.
INTRODUCTION
The clinical triad of pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH) has recently been described as a new entity and proposed to be an autoinflammatory syndrome.1,2 Autoinflammatory diseases (AIDs) represent an emerging group of inflammatory conditions that are distinct from autoimmune, allergic, and infectious disorders.3–5 Pyoderma gangrenosum (PG) and suppurative hidradenitis (hidradenitis suppurativa [HS]) are prototypic neutrophilic dermatoses that are themselves diseases nowadays regarded as autoinflammatory in origin, and whose hallmark is the accumulation of neutrophils in the skin and, rarely, in internal organs.6–8 Acne is a clinically polymorphic disease characterized by a complex pathophysiology in which there is a disorder of keratinization with abnormal sebaceous stem cell differentiation. However, in its pathogenesis, an autoinflammatory component induced by Propionibacterium acnes via inflammasome activation has been recently demonstrated,9–11 linking acne to classic AIDs.
PASH syndrome is similar to the classic autoinflammatory syndrome named pyogenic arthritis, pyoderma gangrenosum, and acne (PAPA), but differs insofar as it lacks in the associated arthritis and a proven genetic basis.1,2 In PAPA syndrome, different mutations involving the proline–serine–threonine phosphatase-interacting protein 1 (PSTPIP1) gene, via an increased binding affinity to pyrin, induce the assembly of inflammasomes. These are molecular platforms involved in the activation of the caspase 1, a protease that cleaves the functionally inactive pro-interleukin (IL)-1β to its active isoform IL-1β.12 IL-1β triggers the release of a number of proinflammatory cytokines and chemokines that are responsible for the recruitment and activation of neutrophils,13 leading to a neutrophil-mediated inflammation. In patient with PASH, no mutations have yet been detected, and the only genetic change was found to be an increased number of CCTG microsatellite repeats in the PSTPIP1 5’UTR region.1 The presence of alleles carrying higher number of the repeats of CCTG motif close to the PSTPIP1 promoter likely deregulates PSTPIP1 expression and may also predispose to forms of neutrophilic inflammation as in aseptic abscesses.14,15
Here, we studied 5 patients with PASH syndrome, analyzing their clinical features, the genetic profile of 10 genes already known to be involved in AIDs, and the cytokine expression pattern both in lesional skin and serum.
PATIENTS AND METHODS
Patients
Five patients, seen in our department from December 2009 to December 2013, were diagnosed as having PASH syndrome on the basis of clinical and histopathological features. All the 5 patients had undergone an incisional biopsy for histological examination and cytokine expression analysis as well as an extensive laboratory work-up, including routine and immunological tests, such as antinuclear antibodies C3 and C4 components of complement, cytoplasmic and perinuclear antineutrophil cytoplasmic antibodies, anticardiolipin antibodies, anti-β2-glycoprotein I antibodies, and lupus anticoagulant.
Cytokine expression analysis was performed in homogenates obtained by skin biopsy specimens taken from lesional skin of the 5 patients with PASH syndrome, which contained both edge and bed of a PG lesion. Normal skin samples taken from 6 subjects who had undergone different interventions of abdominal surgery served as controls.
Peripheral blood samples from the 5 patients with PASH syndrome were collected for genetic studies and serum cytokine measurements.
The protocol was approved by our Institutional Review Board and all of the subjects gave their informed consent before participating in the study.
Methods
Genetic Analysis
In order to exclude mutations in 10 genes most commonly defective in AIDs, the total DNA sequence was tested in genomic DNA samples from the patients under analysis by next-generation sequencing using an Ion AmpliSeq Designer (Life Technologies, Carlsbad, CA) approach followed by Ion personal genome machine (PGM) massive sequencing (Life Technologies). A panel was created to identify possibly disease-causing mutations in 10 genes already known to be involved in AIDs, namely, mediterranean fever (MEFV), mevalonate kinase, tumor necrosis factor (TNF) receptor superfamily member 1A, nucleotide-binding oligomerization domain (NOD)-like receptor family, pyrin domain containing 3 (NLRP3), NLRP12, proline–serine–threonine phosphatase-interacting protein 1 (PSTPIP1), NOD2, proteasome (prosome, macropain) subunit, beta type, 8 (PSMB8), IL-1 receptor antagonist (IL1RN), and lipin 2, for a total of 116 exons corresponding to about 21 kilobases of coding DNA. Ninety-nine percent of the original target is present, and only 1% is missed, in the Ampliseq design of amplicons used for the target DNA capture. Libraries construction and their amplification, as well as emulsion polymerase chain reaction and subsequent sequencing on Ion 314 chips were carried out according to the protocols recommended by Life Technologies, while details about our AID gene panel and validation of the whole procedure, to assess its reliability as a screening tool for AID-associated mutations, are being reported.
The analysis from raw FastQ sequencing data to variant call format tables of annotated variant calls was carried out using 2 different workflows: Ion Torrent Alignment and Ion Reporter 4.0 (Life Technologies, Carlsbad, CA, USA), specific for data generated by PGM; and inhouse pipeline based on free-tools such as Burrows–Wheeler alignment (http://bio-bwa.sourceforge.net/) and genome analysis toolkit (https://www.broadinstitute.org/gatk/).
Variants thus identified and believed to play a causal role were further validated by standard dideoxy sequencing (Sanger seq) using the BigDye Terminator v.3.1 Cycle Sequencing Kit (Life Technologies) and an ABI3730 automatic sequencer (Life Technologies), as already reported.16
Expression Analysis of Cytokines, Chemokines, and Effector Molecules in Skin Specimens
Each tissue sample was weighed and diced into very small pieces using a clean razor blade. Frozen tissue were sliced very thinly and thawed in radioimmunoprecipitation assay (RIPA) buffer (sc-24948) containing protease and phosphatase inhibitors by using 3 mL of ice-cold RIPA buffer per gram of tissue.
Samples were incubated on ice for 30 minutes, transferred to microcentrifuge tubes, and centrifuged at 10,000g for 10 minutes at 4°C. The supernatant was collected and the sample was centrifuged again. The new supernatant fluid was added to the previous one, this mixture representing the total cell lysate. In order to standardize the cell lysate of each tissue sample, we measured the total proteins in each sample by a micro bicinchoninic acid kit (ThermoScientific, Waltham, MA). For each sample, we loaded a volume containing 100 μg of proteins in a glass-slide format of cytokine antibody array (RayBio; RayBiotech, Inc, Norcross, GA). The volume to be loaded was calculated by the following formula: volume (expressed in μL) = 100 μg/protein concentration (expressed in μg/μL). Each glass-slide array contained 14 subarrays and was suitable for 14 samples. Each subarray allowed to evaluate cytokine expression levels in a sample. Normalization of data at the end of the experiment provided semiquantitative results. The subarray was composed by specific antibodies against target molecules coated on the glass slide. After the hybridization of tissue lysate, each antibody bound its target molecule and unbound proteins were washed out. The slide was then incubated with biotin-conjugated antibodies against the same target cytokines, washed, and then incubated with Cy3-conjugated streptavidin, creating a biotin–streptavidin–Cy3 complex detectable using a microarray laser scanner. By using a data-extraction software, we could convert fluorescent signals into numerical data, and after normalization, we obtained an expression value of signal intensity for each molecule in each sample. The tested molecules were the following: IL-1β; IL-1RI; IL-1RII; TNF-α; tumor necrosis factor receptor I (TNFRI); TNFRII; IL-17; IL-17R; leukocyte selectin (L-selectin); endothelial selectin (E-selectin); IL-8; regulated on activation, normal T cell expressed and secreted (RANTES); [C-X-C motif] chemokine ligand 1,2,3 (CXCL 1,2,3); [C = cysteine, X = any amino acid]); CXCL 16; matrix metalloproteinase (MMP)-2; MMP-9; tissue inhibitor of metalloproteinase (TIMP)-1; TIMP-2; sialic acid-binding immunoglobulin-type lectin (Siglec) 5, Siglec 9; Fas (Fas protein also known as cluster of differentiation [CD]95); Fas ligand also known as CD178; CD40; and CD40 ligand.
Expression Analysis of Cytokines in Serum Specimens
Peripheral blood was collected by an antecubital vein of the patients. Blood samples were allowed to clot at room temperature and centrifuged at 2000 g for 10 minutes within 1 hour after blood collection. The serum samples were snap frozen in liquid nitrogen, aliquoted, stored at −80°C, and thawed just for the test. Multiplex bead-based Luminex technology was used to detect IL-1β, TNF-α, and IL-17 in the serum samples of the patients and controls (R&D Systems, Minneapolis, MN), according to the manufacturer's protocol.
Statistics
The results are reported as means with standard deviation. Student t test for unpaired values was used to assess statistical significance of differences between patients and normal controls. The significance level was set at P < 0.05.
RESULTS
Clinical Features
The clinical features of the 5 patients with PASH syndrome are summarized in Table 1. The patients (3 men and 2 women) had a mean age at the time of diagnosis of 32.2 years (range 16–45 years). The duration of the disease ranged from 3 to 7 years (mean, 5 years); the mean follow-up was 3.2 years (range, 2–5 years). All 5 patients exhibited 3 patterns of skin lesions: ulcers and ulcerated nodules (Figure 1, panel B), sometimes with vegetating aspects (Figure 1, panel D and E), typical of PG; papulopustular lesions, abscesses, and fistulas evolving in draining sinuses and scars (Figure 1, panel A), consistent with HS; and acne on the face (Figure 1, panel C). The PG lesions extensively involved the trunk and lower extremities in 4 patients (1, 2, 3, and 5), while were limited to the upper portion of the back in patient 4, in whom they had vegetating features healing with keloidal scars. Hidradenits suppurativa was in Hurley stage III in all 5 patients and the lesions affected the axillary and inguinal folds as well as the anogenital area; in the 2 female patients (patients 2 and 4), the lesions involved also the inframammary folds. Acne was mild in 2 patients (cases 1 and 4), in whom it presented with numerous open comedones and only few erythematosus papules and pustules, whereas it was severe in 2 other cases (cases 2 and 3), who showed a predominance of inflammatory lesions on retentional elements; in patient 5, there were aspects of acne conglobata (Figure 1, panel C). Patient 1 developed his overall clinical picture after bowel bypass surgery for morbid obesity. Two patients complained of rheumatological symptoms: patient 5 had spondyloarthritis and osteitis, while patient 4 suffered from polyarthritis particularly affecting nonaxial joints such as the knee, elbow, shoulder, and hands. In patient 4, intense neutrophilic inflammation of the synovial was documented by means of synovial fluid microscopic examination, and joint radiography revealed asymmetrical destructive polyarthritis. Patient 3 had an underlying Crohn disease, diagnosed 2 years before the onset of the dermatological condition.
TABLE 1.
Summary of Clinical and Laboratory Findings in 5 Patients With PASH Syndrome
FIGURE 1.
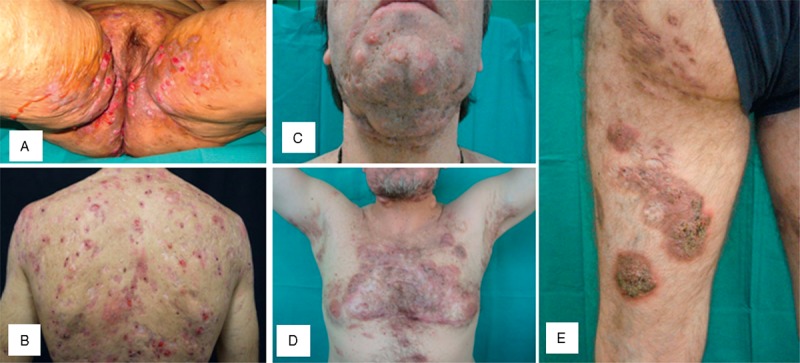
Clinical features of patients with the association of pyoderma gangrenosum, acne and suppurative hidradenitis. Papulopustular lesions, abscesses, and fistulas involving the anogenital area (panel A); multiple ulcerative lesions of the back (panel B), which were histologically diagnosed as pyoderma gangrenosum; acne conglobata on the face (panel C); and ulcerated plaques with vegetating aspects (panel D and E), which were histologically diagnosed as pyoderma gangrenosum.
The family history of PG, HS, Crohn disease, and ulcerative colitis was not relevant in the first-degree relatives of all the 5 patients.
Treatment and Course
In all the 5 patients, different treatments, given in our hospital or in other institutions, had failed to obtain a satisfactory control of the disease; they included topical and systemic antibiotics, topical and systemic corticosteroids, dapsone, and isotretinoin. Given the poor response to previous aforesaid therapies, we decided to give the patients 1, 2, and 5 the TNF-α blocker infliximab intravenously at the dose of 5 mg/kg of body weight at times 0, 2 weeks, and 6 weeks. The 3 patients had a remarkable clinical improvement from the attack cycle of infliximab, with almost complete healing of both PG and acne lesions; in contrast, disease activity persisted in the sites of HS, particularly the perianal area. In patient 5, spondyloarthritis responded well to this treatment. At the time of writing, the patients were in partial remission under a maintenance schedule consisting in 1 infliximab infusion at the same dosage every 8–12 weeks. Patient 3 was treated by gastroenterologists for the underlying Crohn disease with the TNF-α antagonist adalimumab (80 mg subcutaneous at time 0 and then 40 mg every 2 weeks), obtaining also a good control of his cutaneous picture within 4 weeks. Because of the presence of severe arthritis affecting multiple sites, we treated patient 4 with the first choice agent of PAPA syndrome, namely, the IL-1 receptor antagonist, anakinra (100 mg/day subcutaneous), achieving good control of her overall clinical condition.
Laboratory Findings
In all the 5 patients, routine and immunological investigations mentioned above were normal or negative, except for an increased C-reactive protein and anemia (Table 1) at the time of hospitalization; in case 2, anemia required repeated packed red blood cell transfusions. In patients 4 and 5 who suffered from arthritis, rheumatoid factor and anticitrullinated peptide antibodies were within normal limits. Patient 5 was HLA (human leukocyte antigen) B27 positive. In all patients, cultures from recent onset PG ulcers failed to disclose any microorganisms, while those from the skin folds grew a number of agents, particularly Staphylococcus aureus and Gram-negative bacteria such as Proteus mirabilis and Klebsiella pneumoniae. During the course of the disease in patients 1, 2, and 5, there was a temporary mild increase in white blood cells with neutrophilia (11,000–13,000/mm3 and 80%–86%, respectively) due to superinfection of long-lasting lesions.
Genetic Findings
The mean coverage relative to the sequencing run of the 5 libraries, corresponding to the target DNA from the 5 patients, has turned out to be 302X, with 93.8% and 88.6% of the target regions covering >20X and >35X on average, respectively. Given such a read depth, we expect a quite low false-negative rate in the variants call, although undetected variants located in either the low coverage amplicons or the very few missed regions cannot be excluded.
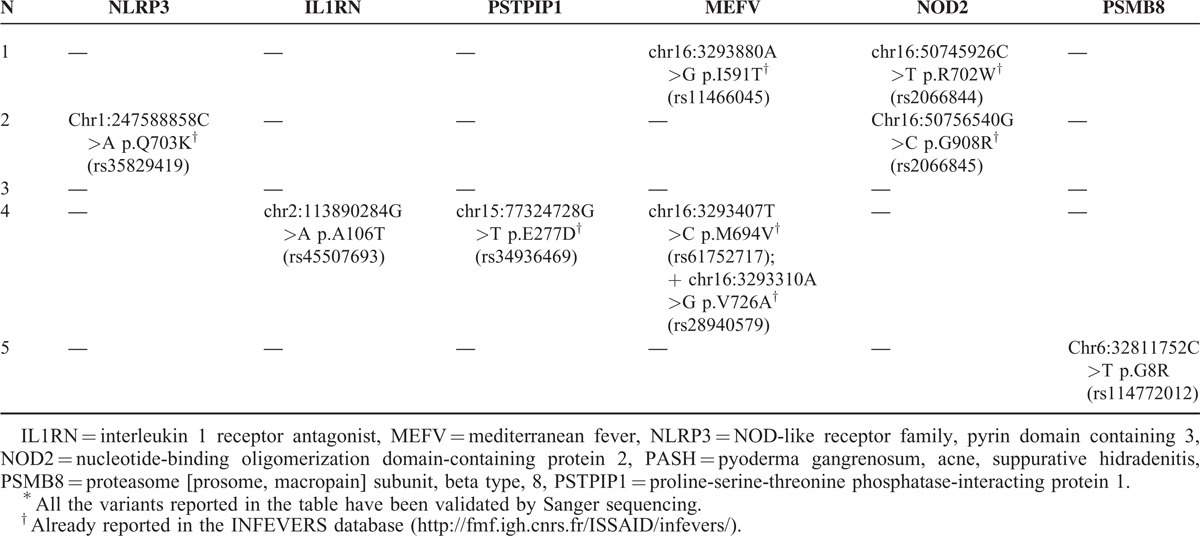
Among the variants called at the 10 genes and confirmed by Sanger sequencing validation, mostly including synonymous and noncoding variants, we took into consideration 9 nonsynonymous changes, representing those still unreported and those already reported either with a minor allele frequency <0.01 or suspected to play a role in AID pathogenesis. These putative causal mutations are reported in Table 2, for each of the 5 patients, in terms of genomic description (chromosome, position in the chromosome, and nucleotide change), codon affected and aminoacid substitution, and the “rs” ID by which the variant is registered in the single-nucleotide polymorphism (dbSNP) database; http://www.ncbi.nlm.nih.gov/snp/). Seven out of our 9 mutations are also present in the registry of hereditary autoinflammatory disorders mutations (Infevers; http://fmf.igh.cnrs.fr/ISSAID/infevers/).
TABLE 2.
Summary of the Variants Found in 10 Genes of Autoinflammatory Diseases Tested in 5 Patients With PASH Syndrome∗
Skin Studies
Cytokine Expression
IL-1β and its receptors (IL-1RI and II) were significantly more expressed in PG lesional skin of the 5 patients with PASH (50.20 ± 44.38, 4.00 ± 2.35, and 15.20 ± 11.81, respectively) than in normal skin (3.33 ± 1.75, 1.66 ± 0.81, and 4.33 ± 1.86; P = 0.028, 0.047, and 0.050, respectively) (Figure 2). The proinflammatory cytokine TNF-α was also overexpressed (3.40 ± 0.54 vs 2.00 ± 0.10; P = 0.005) as well as its receptors TNFRI (18.601 ± 5.68 vs 6.00 ± 3.46; P = 0.001) and TNFRII (17.60 ± 6.06 vs 6.66 ± 3.44; P = 0.004) (Figure 3). Finally, we observed an overproduction of IL-17 (3.20 ± 1.30 vs 1.83 ± 0.41; P = 0.037) and its receptor IL-17R (5.60 ± 2.07 vs 2.83 ± 1.17; P = 0.021) (Figure 4).
FIGURE 2.
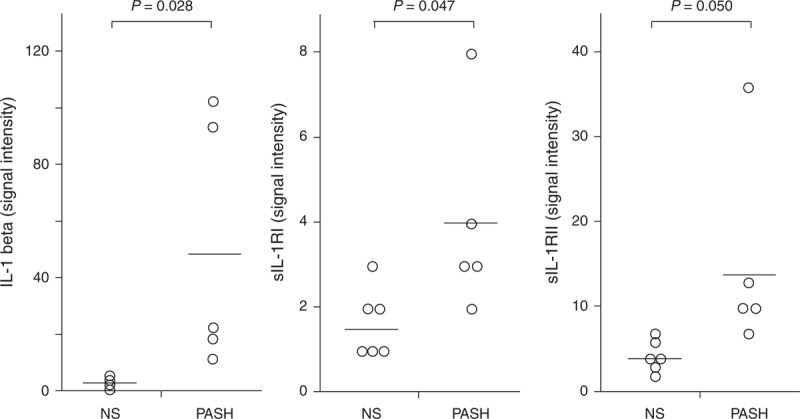
Expression of IL-1-β and its soluble receptors I and II (sIL-1RI and sIL-1RII) in homogenate samples of pyoderma gangrenosum lesional skin from 5 patients with PASH syndrome. Six NS served as controls. Numerical values represent signal intensity in a cytokine array assay. IL-1 = interleukin-1, NS = normal subjects, PASH = pyoderma gangrenosum, acne, and hidradenitis suppurativa.
FIGURE 3.
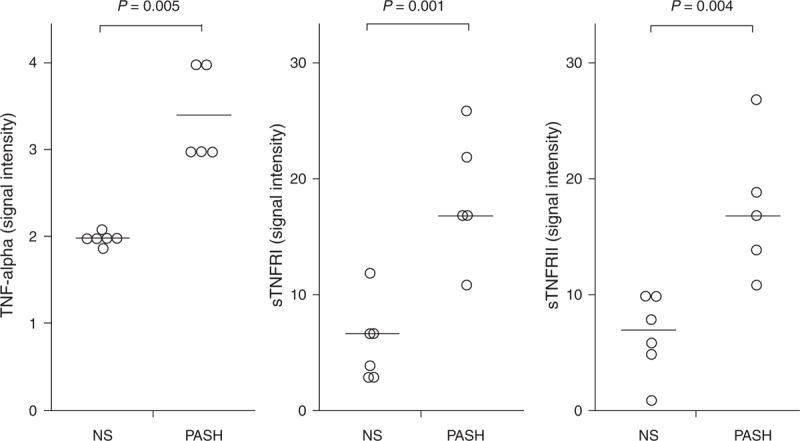
Expression of TNF-α and its soluble receptors I and II (sTNFRI and sTNFRII) in homogenate samples of pyoderma gangrenosum lesional skin from 5 patients with PASH syndrome. Six NS served as controls. Numerical values represent signal intensity in a cytokine array assay. NS = normal subjects, PASH = pyoderma gangrenosum, acne, and hidradenitis suppurativa, TNF = tumor necrosis factor.
FIGURE 4.
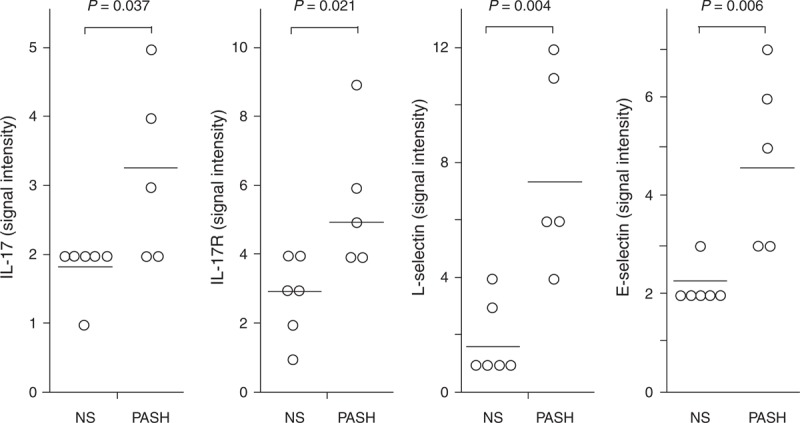
Expression of IL-17, its soluble receptor (sIL17R), L-selectin, and E-selectin in homogenate samples of pyoderma gangrenosum lesional skin from 5 patients with PASH syndrome. Six NS served as controls. Numerical values represent signal intensity in a cytokine array assay. E-selectin = endothelial selectin, IL = interleukin, L-selectin = leukocyte selectin, NS = normal subjects, PASH = pyoderma gangrenosum, acne, and hidradenitis suppurativa.
L-Selectin and E-Selectin Expression
The expression of both E-selectin and L-selectin was significantly higher in PG lesional skin of the 5 patients with PASH syndrome (4.80 ± 1.79 and 7.80 ± 3.49) than in normal skin (2.16 ± 0.40 and 1.83 ± 1.32; P = 0.006 and 0.004) (Figure 4).
Chemokine Expression
As compared to controls, PG lesional skin of PASH patients showed overexpression of chemokines promoting neutrophil transendothelial migration into inflamed tissues, such as IL-8 (21.60 ± 12.28 vs 2.33 ± 1.03; P = 0.004), CXCL 1/2/3 (106.60 ± 91.52 vs 9.17 ± 7.81; P = 0.028), CXCL 16 (5.40 ± 2.41 vs 2.00 ± 0.63; P = 0.008), and RANTES (15.00 ± 7.68 vs 3.33 ± 1.50; P = 0.005) (Figure 5).
FIGURE 5.
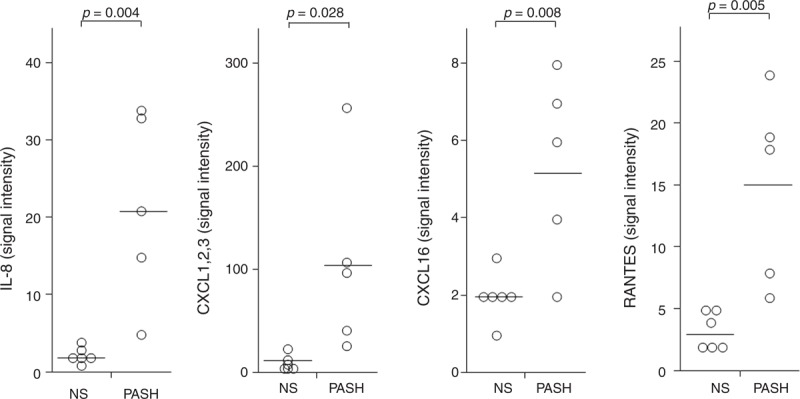
Expression of IL-8, RANTES, CXCL 1,2,3 (C = cysteine, X = any amino acid) and CXCL 16 in homogenate samples of pyoderma gangrenosum lesional skin from 5 patients with PASH syndrome. Six NS served as controls. Numerical values represent signal intensity in a cytokine array assay. CXCL = chemokine [C-X-C motif] ligand, IL = interleukin, NS = normal subjects, PASH = pyoderma gangrenosum, acne, and hidradenitis suppurativa, RANTES = regulated on activation, normal T cell expressed and secreted.
MMP and TIMP Expression
In PG lesional skin of PASH patients, we observed a significant overexpression of molecules involved in tissue damage such as MMP-2 (5.80 ± 2.17 vs 2.00 ± 0.89; P = 0.003) and MMP-9 (341.60 ± 78.27 vs 45.17 ± 68.45; P = 0.0001) (Figure 6). Overproduction of molecules responsible for inhibitory signals aimed at attenuating MMP-mediated inflammation was also demonstrated: TIMP-1 (156.40 ± 129.56 vs 4.17 ± 1.60; P = 0.017) and TIMP-2 (237.00 ± 119.84 vs 53.83 ± 24.28; P = 0.005) (Figure 6).
FIGURE 6.
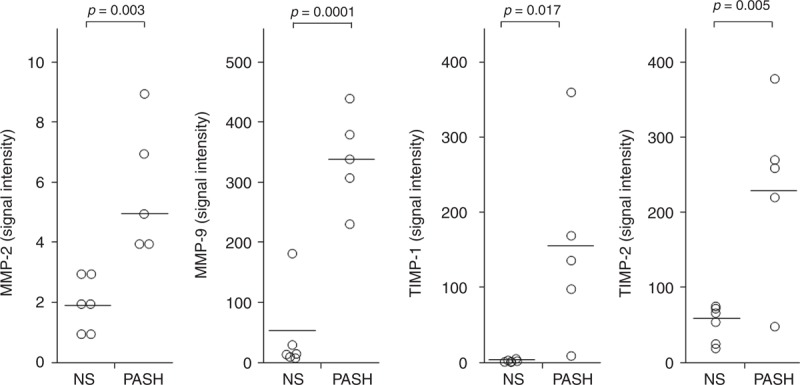
Expression of MMP-2, MMP-9, TIMP-1, and TIMP-2 in homogenate samples of pyoderma gangrenosum lesional skin from 5 patients with PASH syndrome. Six NS served as controls. Numerical values represent signal intensity in a cytokine array assay. MMP = matrix metalloproteinase, NS = normal subjects, PASH = pyoderma gangrenosum, acne, and hidradenitis suppurativa, TIMP = tissue inhibitor of metalloproteinase.
Siglec, Fas/Fas L, and CD40/CD40L Expression
Siglec 5 (126.00 ± 60.07; P = 0.001) and Siglec 9 (59.00 ± 26.71; P = 0.001), which carry inhibitory signals dampening inflammation, were more expressed in PG lesional skin of PASH patients than in normal skin (5.00 ± 2.97 and 7.83 ± 3.60, respectively) (Figure 7). Fas and its ligand were also overexpressed (17.60 ± 7.30 vs 8.50 ± 2.07 with P = 0.016 for Fas; 7.60 ± 2.30 vs 3.50 ± 1.52 with P = 0.006 for Fas ligand) (Figure 7). CD40 and its ligand were significantly more expressed in PG lesional skin of PASH patients than in normal skin (5.20 ± 1.30 vs 3.00 ± 0.63 with P = 0.005 for CD40 and 6.40 ± 2.88 vs 1.83 ± 0.75 with P = 0.004 for CD40 ligand) (Figure 7).
FIGURE 7.
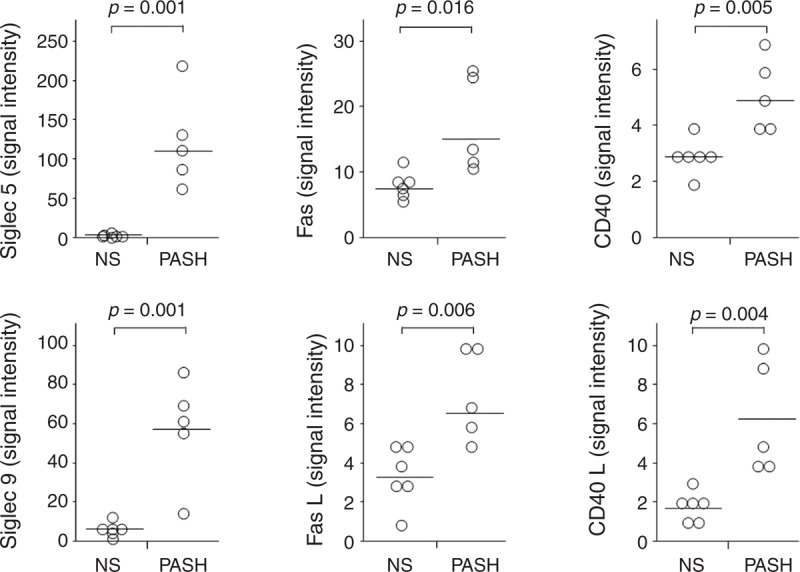
Expression of Siglec 5, Siglec 9, Fas (also known as CD95), FasL (also known as CD178), CD40, and CD40 L (CD40 ligand) in homogenate samples of pyoderma gangrenosum lesional skin from 5 patients with PASH syndrome. Six NS served as controls. Numerical values represent signal intensity in a cytokine array assay. CD40 L = CD40 ligand, Fas = Fas protein, FasL = Fas ligand, NS = normal subjects, PASH = pyoderma gangrenosum, acne, and hidradenitis suppurativa, Siglec = sialic acid-binding immunoglobulin-type lectin.
Serum Studies
No statistically significant differences in serum levels of IL-1β, TNF-α, and IL-17 were found between the 5 patients with PASH syndrome and normal controls (3.47 ± 0.61 vs 3.08 ± 1.10 for IL-1β; 0.19 ± 0.12 vs 0.17 ± 0.05 for TNF-α, and 1.62 ± 2.04 vs 1.24 ± 0.80 for IL-17) (Figure 8).
FIGURE 8.
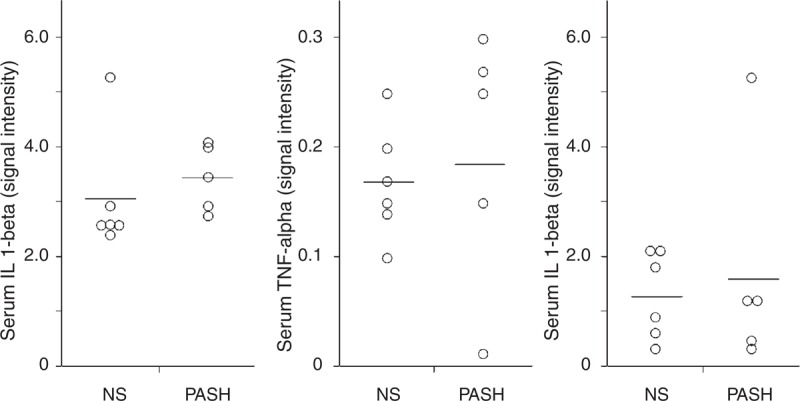
Serum levels of IL-1β, TNF-α, and IL-17 from 5 patients with PASH syndrome. Six NS served as controls. Numerical values represent signal intensity in a cytokine array assay. IL = interleukin, NS = normal subjects, PASH = pyoderma gangrenosum, acne, and hidradenitis suppurativa, TNF = tumor necrosis factor.
DISCUSSION
Herein, we presents 5 unrelated patients with a symptom complex fitting in well with the entity recently described by Braun-Falco et al1 and named PASH syndrome by these authors. Three out of our 5 patients completely share the clinical features of the Braun-Falco's cases; however, compared to the 2 PASH cases of the first description,1 in which there were neither triggering events nor associated conditions, 2 of our patients, albeit typical in their presentation, developed PASH syndrome as complication of bariatric surgery for morbid obesity (patient 1) and in association with Crohn disease (patient 3). The first condition represents the so-called bowel bypass syndrome, also known as bowel-associated dermatitis arthritis syndrome (BADAS), which is a well-recognized complication of jejunoileal bypass and biliopancreatic diversion.2 This disorder is characterized by recurrent fever, polyarthralgia/polyarthritis, myalgia, and tenosynovitis, all lacking in our patient, as well as protean cutaneous manifestations2; the latter usually mimic PG or other neutrophilic dermatoses including HS. Thus, a pathogenesis related to neutrophil-mediated inflammation could be suggested for BADAS and, based on our case, PASH syndrome may be included in the list of complications seen after bowel bypass surgery. In our patient 3, PASH syndrome developed during a remission phase of Crohn disease, and the cutaneous manifestations disappeared when adalimumab was given for an intestinal flare. This observation further supports the close links between PG and inflammatory bowel diseases.17,18 The other 2 cases (patients 4 and 5) are unique in that they show overlapping features with PAPA syndrome and synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome, respectively. For the first, who has PASH syndrome with coexisting arthritis, we have previously coined the name pyogenic arthritis, PG, acne, and HS syndrome.16 In the latter, the overlap with SAPHO syndrome is supported by having spondiloarthrtis and osteitis. SAPHO syndrome is a rare AID characterized by osteoarthropathy associated with various dermatological manifestations in different degrees, such as acne and palmoplantar pustulosis, and more rarely PG, HS, and Sweet syndrome.19,20 Thus, the existence of clinical overlap within the spectrum of neutrophilic dermatoses, already suggested by some authors21 and supported by case series, focused specifically on the combination of PG and HS.22,23 The frequent occurrence of acne in the general population may raise some doubts on the existence of PASH syndrome as an autonomous entity. However, an autoinflammatory component has been demonstrated in the pathogenesis of acne,9–11 which links this condition to other well-recognized AIDs. In any case, in a significant number of patients with the association of PG and HS, acne was also described. In fact, Hsiao et al,22 evaluating 11 cases of concomitant PG and HS, and performing a literature review on this association, found acne in 13 out of 26 patients. Moreover, there are additional case reports describing acne associated with PG and HS.1,2,16,24–26
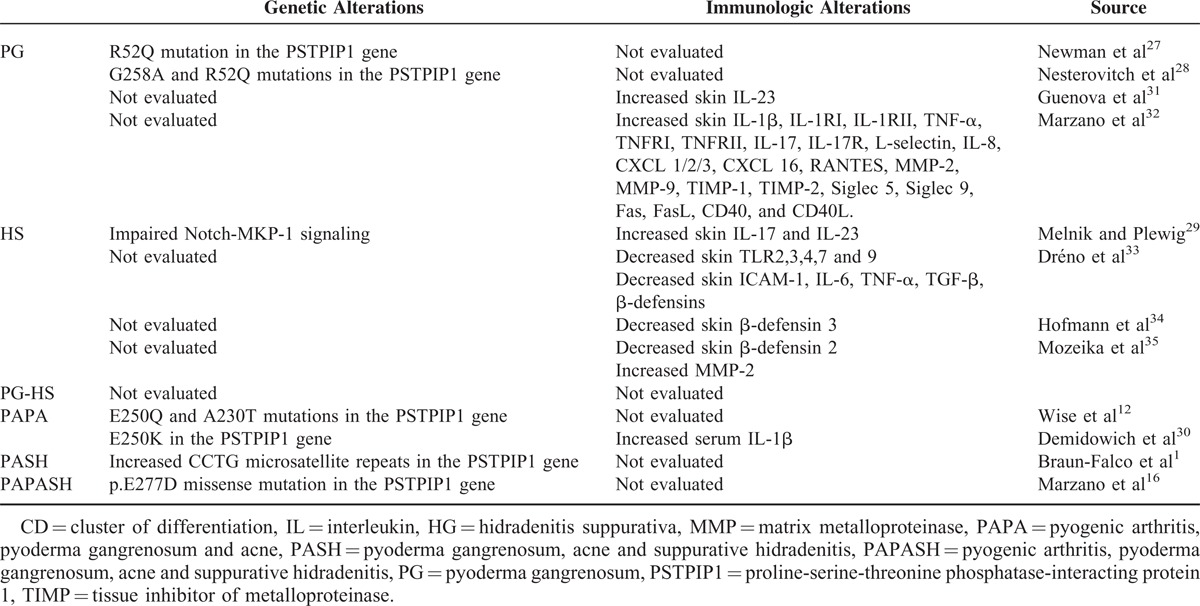
Regarding these entities, an important point is the investigation for presence of mutations and/or other genetic abnormalities involving genes regulating the innate immune responses. A number of genetic alterations involving the innate immunity have been found in PG, HS, and their syndromic forms (Table 3), in particular mutations of the PSTPIP1 gene in PG and impaired Notch–MKP-1 (mitogen-activated protein kinase phosphatase-1) signaling in HS.1,12,16,27–30 Involvement of innate immunity dysfunction in both PG and HS is also supported by immunological studies29–35 (Table 3). All the 9 mutations found in our patients are already reported in the dbSNP, and 7 of them are additionally present in the Infevers registry, meaning that they have already been described in association with an autoinflammatory phenotype. Only variants found in the IL1RN and the PSMB8 genes, p.A106T and p.G8R, respectively, are novel. Of the 7 remaining mutations, p.I591T, p.M694V, and p.V726A of the MEFV gene have already been associated with typical symptoms of recessive familial mediterranean fever (FMF) in the Online Mendelian Inheritance in Man (OMIM) database (OMIM#249100). According to this, patient 4, who is compound heterozygote for 2 of these mutations, is expected to show a typical FMF phenotype, whereas our patient did not show any clinical manifestation of FMF. Of further note, mutations p.R702W and p.G908R of the NOD2 gene were found to be intermittently associated with susceptibility to Crohn disease, representing 32% and 18%, respectively, of the total Crohn disease mutations.36,37 In addition, association studies have proposed these alleles as susceptibility variants for ulcerative colitis, psoriatic arthritis, and sarcoidosis, as reported in the OMIM database (OMIM# 605956). This suggests they may contribute to the disease phenotype in patients 1 and 2, respectively, along with 2 other mutations of known genes of AIDs, the p.I591T of the MEFV gene in the former case and the p.Q703K of the NLRP3 gene in the latter case. The Q703K variant, also known as Q705K, is a single-nucleotide polymorphisms recently associated to a gain-of-function alteration leading to overactive inflammation.38 However, this variant is still considered of uncertain clinical significance.39 Overall, the most intriguing finding of our genetic study was that 4 out of 5 PASH patients presented genetic alterations typical of well-known AIDs, including inflammatory bowel diseases, and the only patient lacking genetic changes had a clinically evident Crohn disease.
TABLE 3.
Genetic and Immunologic Alterations in PG, HS, Their Association (PG–HS), and Their Syndromic Forms (PAPA, PASH, and PAPASH)
With the aim of better understanding the neutrophilic inflammation pathways, we have conducted a study analyzing the expression profile of cytokines, chemokines, and other effector molecules in the skin lesions of our PASH cases by means of a protein array method. Moreover, we have evaluated the main cytokines such as IL-1β, TNF-α, and IL-17 in peripheral blood of the same patients. For the tissue study, we have chosen the ulcerative lesions of PG, believing that they represent a model of neutrophil-mediated inflammation, and so preferring them to the abscesses and scarring lesions of HS as well as to the polymorphic lesions of acne. A cardinal finding in our studies was the overexpressions of IL-1β and its receptors, theoretically due to dysregulation of inflammasome function. As previously emphasized, this highly active pleiotropic cytokine is a leading actor in the AIDs4,13 and plays a pivotal role in triggering the neutrophilic inflammation of the skin. IL-1 promotes the production and release of both classic proinflammatory cytokines, such as TNF-α and IFN-γ, and a number of chemokines, notably IL-8 and RANTES. In fact, TNF-α, which also acts as a key regulator of other proinflammatory cytokines and chemokines, was overexpressed in the present study. Several chemokines such as IL-8, CXCL 1/2/3, CXCL16, and RANTES were overexpressed in our study, promoting neutrophil transendothelial migration into the site of inflammatory process, which was also favored by the upregulation of selectins. This is in line with our previous studies32,40 that highlighted the involvement of IL-8 in the pathogenesis of idiopathic PG.
In the present study, we found an overexpression of IL-17 and its receptor confirming the previously hypothesized role for this T-helper 17-related cytokine in the pathophysiology of the whole spectrum of neutrophilic dermatoses, as well recognized in psoriasis.40,41 IL-17 amplifies the recruitment of neutrophils and induces the production of MMPs,42 a family of endopeptidases to which belong the so-called gelatinases MMP-2 and MMP-9. We found an overexpression of both MMP-2 and MMP-9, suggesting that these preoteinases contribute to induce tissue damage. Interestingly, we also detected an overproduction of TIMP-1 and TIMP-2, which may represent an inhibitory pathway aimed at attenuating the MMP-mediated inflammation. In our cases, we also found an overexpression of Siglec 5 and Siglec 9, which carry inhibitory signals attenuating immune responses and dampening inflammation in PASH syndrome as in autoinflammation in general.43 Our study suggests that 2 other important systems, the Fas/Fas L system and the CD40/CD40 ligand system, may contribute to tissue damage and inflammation in PASH. The Fas/Fas L system belongs to the TNF/TNF receptor superfamily and to date is the best known pathway mediating apoptosis.44 The CD40/CD40L system also belongs to the TNF/TNF receptor superfamily. It represents a costimulatory system that amplifies the immune response and can promote inflammation via upregulation of adhesion molecules and induce production of various cytokines and chemokines.45 The same profile of cytokines and other effector molecules, demonstrated here in PASH, was also previously found in PG and Sweet syndrome occurring outside syndromic forms.32 Although our present data do not support the autonomy of PASH, they support the autoinflammatory nature of this entity.
In peripheral blood, the serum levels of the main proinflammatory cytokines, that is, IL-1β, TNF-α, and IL-17, were within the normal range, suggesting that in PASH syndrome, the inflammatory process is mainly localized into the skin.
The main limitation of our study is the small number of patients, due to the rarity of PASH syndrome, which is, however, counterbalanced by the wide panel of genes and molecules investigated.
CONCLUSIONS
Our data show that patients with PASH have clearly high values of proinflammatory cytokines, chemokines, and tissue damage effector molecules in lesional skin with normal levels of the major proinflammatory cytokines in peripheral blood. These findings support the view that in PASH syndrome, autoinflammation mainly involves the skin with no evidence of proinflammatory signals in the bloodstream. From a genetic point of view, PASH patients are characterized by a polymorphic profile with genetic alterations previously described in other well-known AIDs.
Footnotes
Abbreviations: BADAS = bowel-associated dermatitis arthritis syndrome, BMI = body mass index, CD40 = cluster of differentiation 40, CRP = C-reactive protein, CXCL = C-X-C motif ligand, dbSNP = single nucleotide polymorphism database, E-selectin = endothelial selectin, Hb = hemoglobin, HLA = human leukocyte antigen, IL = interleukin, IL1RN = interleukin 1 receptor antagonist, L-selectin = leukocyte selectin, MEFV = mediterranean fever, MKP-1 = mitogen-activated protein kinase phosphatase-1, MMP = matrix metalloproteinase, NLRP12 = NOD-like receptor family, pyrin domain containing 12, NOD2 = nucleotide-binding oligomerization domain-containing protein 2, PAPA = pyogenic arthritis, pyoderma gangrenosum and acne, PAPASH = pyogenic arthritis, pyoderma gangrenosum, acne and suppurative hidradenitis, PASH = pyoderma gangrenosum, acne and suppurative hidradenitis, PGM = personal genome machine, PSMB8 = proteasome (prosome, macropain) subunit, beta type 8, PSTPIP1 = proline-serine-threonine phosphatase-interacting protein 1, R = receptor, RANTES = regulated on activation, normal T cell expressed and secreted, RIPA = radioimmunoprecipitation assay, SAPHO = synovitis, acne, pustulosis, hyperostosis, osteitis, Siglec = sialic acid-binding immunoglobulin-type lectin, TIMP = tissue inhibitor of metalloproteinase, TNF = tumor necrosis factor.
This work was supported in part by a research grant from “Ricerca corrente,” Fondazione IRCCS Ca’ Granda, Ospedale Maggiore Policlinico, Milano, Italy.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Braun-Falco M, Kovnerystyy O, Lohse P, et al. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)-a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol 2012; 66:409–415. [DOI] [PubMed] [Google Scholar]
- 2.Marzano AV, Ishak RS, Colombo A, et al. Pyoderma gangrenosum, acne and suppurative hidradenitis syndrome following bowel bypass surgery. Dermatology 2012; 225:215–219. [DOI] [PubMed] [Google Scholar]
- 3.Aksentijevich I, Kastner DL. Genetics of monogenic autoinflammatory diseases: past successes, future challenges. Nat Rev Rheumatol 2011; 7:469–478. [DOI] [PubMed] [Google Scholar]
- 4.Kastner DL, Aksentijevich I, Goldbach-Mansky R. Autoinflammatory disease reloaded: a clinical perspective. Cell 2010; 140:784–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doria A, Zen M, Bettio S, et al. Autoinflammation and autoimmunity: bridging the divide. Autoimmun Rev 2012; 12:22–30. [DOI] [PubMed] [Google Scholar]
- 6.Almeida de Jesus A, Goldbach-Mansky R. Monogenic autoinflammatory diseases: concept and clinical manifestations. Clin Immunol 2013; 147:155–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marzano AV, Ishak RS, Saibeni S, et al. Autoinflammatory skin disorders in inflammatory bowel diseases, pyoderma gangrenosum and Sweet's syndrome: a comprehensive review and disease classification criteria. Clin Rev Allergy Immunol 2013; 45:202–210. [DOI] [PubMed] [Google Scholar]
- 8.Wollina U, Haroske G. Pyoderma gangraenosum. Curr Opin Rheumatol 2011; 23:50–56. [DOI] [PubMed] [Google Scholar]
- 9.Kistowska M, Gehrke S, Jankovic D, et al. IL-1β drives inflammatory responses to Propionibacterium acnes in vitro and in vivo. J Invest Dermatol 2014; 134:677–685. [DOI] [PubMed] [Google Scholar]
- 10.Qin M, Pirouz A, Kim MH, et al. Propionibacterium acnes induces IL-1β secretion via the NLRP3 inflammasome in human monocytes. J Invest Dermatol 2014; 134:381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li ZJ, Choi DK, Sohn KC, et al. Propionibacterium acnes activates the NLRP3 inflammasome in human sebocytes. J Invest Dermatol 2014; 134:2747–2756. [DOI] [PubMed] [Google Scholar]
- 12.Wise CA, Gillum JD, Seidman CE, et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum Mol Genet 2002; 11:961–969. [DOI] [PubMed] [Google Scholar]
- 13.Dinarello CA. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur J Immunol 2011; 41:1203–1217. [DOI] [PubMed] [Google Scholar]
- 14.André MF, Aumaître O, Grateau G, et al. Longest form of CCTG microsatellite repeat in the promoter of the CD2BP1/PSTPIP1 gene is associated with aseptic abscesses and with Crohn disease in French patients. Dig Dis Sci 2010; 55:1681–1688. [DOI] [PubMed] [Google Scholar]
- 15.André MF, Piette JC, Kémény JL, et al. Aseptic abscesses: a study of 30 patients with or without inflammatory bowel disease and review of the literature. Medicine (Baltimore) 2007; 86:145–161. [DOI] [PubMed] [Google Scholar]
- 16.Marzano AV, Trevisan V, Gattorno M, et al. Pyogenic arthritis, pyoderma gangrenosum, acne, and hidradenitis suppurativa (PAPASH): a new autoinflammatory syndrome associated with a novel mutation of the PSTPIP1 gene. JAMA Dermatol 2013; 149:762–764. [DOI] [PubMed] [Google Scholar]
- 17.Marzano AV, Trevisan V, Lazzari R, et al. Pyoderma gangrenosum: study of 21 patients and proposal of a “clinicotherapeutic” classification. J Dermatolog Treat 2011; 22:254–260. [DOI] [PubMed] [Google Scholar]
- 18.Langan SM, Groves RW, Card TR, et al. Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: a retrospective cohort study. J Invest Dermatol 2012; 132:2166–2170. [DOI] [PubMed] [Google Scholar]
- 19.Chamot AM, Benhamou CL, Kahn MF, et al. Acne-pustulosis-hyperostosis-osteitis syndrome. Results of a national survey. 85 cases. Rev Rhum Mal Osteoartic 1987; 54:187–196. [PubMed] [Google Scholar]
- 20.Chen W, Obermayer-Pietsch B, Hong JB, et al. Acne-associated syndromes: models for better understanding of acne pathogenesis. J Eur Acad Dermatol Venereol 2011; 25:637–646. [DOI] [PubMed] [Google Scholar]
- 21.Wallach D, Vignon-Pennamen MD. From acute febrile neutrophilic dermatosis to neutrophilic disease: forty years of clinical research. J Am Acad Dermatol 2006; 55:1066–1071. [DOI] [PubMed] [Google Scholar]
- 22.Hsiao JL, Antaya RJ, Berger T, et al. Hidradenitis suppurativa and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol 2010; 146:1265–1270. [DOI] [PubMed] [Google Scholar]
- 23.Ah-Weng A, Langtry JA, Velangi S, et al. Pyoderma gangrenosum associated with hidradenitis suppurativa. Clin Exp Dermatol 2005; 30:669–671. [DOI] [PubMed] [Google Scholar]
- 24.Rosner IA, Richter DE, Huettner TL, et al. Spondyloarthropathy associated with hidradenitis suppurative and acne conglobata. Ann Intern Med 1982; 97:520–525. [DOI] [PubMed] [Google Scholar]
- 25.Shenefelt PD. Pyoderma gangrenosum associated with cystic acne and hidradenitis suppurativa controlled by adding minocycline and sulfasalazine to the treatment regimen. Cutis 1996; 57:315–319. [PubMed] [Google Scholar]
- 26.Koshelev MV, Garrison PA, Wright TS. Concurrent hidradenitis suppurativa, inflammatory acne, dissecting cellulitis of the scalp, and pyoderma gangrenosum in a 16-year-old boy. Pediatr Dermatol 2014; 31:e20–e21. [DOI] [PubMed] [Google Scholar]
- 27.Newman B, Cescon D, Domenchini A, et al. CD2BP1 and CARD15 mutations are not associated with pyoderma gangrenosum in patients with inflammatory bowel disease. J Invest Dermatol 2004; 122:1054–1056. [DOI] [PubMed] [Google Scholar]
- 28.Nesterovitch AB, Hoffman MD, Simon M, et al. Mutations in the PSTPIP1 gene and aberrant splicing variants in patients with pyoderma gangrenosum. Clin Exp Dermatol 2011; 36:889–895. [DOI] [PubMed] [Google Scholar]
- 29.Melnik BC, Plewig G. Impaired Notch-MKP-1 signalling in hidradenitis suppurativa: an approach to pathogenesis by evidence from translational biology. Exp Dermatol 2013; 22:172–177. [DOI] [PubMed] [Google Scholar]
- 30.Demidowich AP, Freeman AF, Kuhns DB, et al. Brief report: genotype, phenotype, and clinical course in five patients with PAPA syndrome (pyogenic sterile arthritis, pyoderma gangrenosum, and acne). Arthritis Rheum 2012; 64:2022–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guenova E, Teske A, Fehrenbacher B, et al. Interleukin 23 expression in pyoderma gangrenosum and targeted therapy with ustekinumab. Arch Dermatol 2011; 147:1203–1205. [DOI] [PubMed] [Google Scholar]
- 32.Marzano AV, Fanoni D, Antiga E, et al. Expression of cytokines, chemokines and other effector molecules in two prototypic autoinflammatory skin diseases, pyoderma gangrenosum and Sweet's syndrome. Clin Exp Immunol 2014; 178:48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dréno B, Khammari A, Brocard A, et al. Hidradenitis suppurativa: the role of deficient cutaneous innate immunity. Arch Dermatol 2012; 148:182–186. [DOI] [PubMed] [Google Scholar]
- 34.Hofmann SC, Saborowski V, Lange S, et al. Expression of innate defense antimicrobial peptides in hidradenitis suppurativa. J Am Acad Dermatol 2012; 66:966–974. [DOI] [PubMed] [Google Scholar]
- 35.Mozeika E, Pilmane M, Nürnberg BM, et al. Tumour necrosis factor-alpha and matrix metalloproteinase-2 are expressed strongly in hidradenitis suppurativa. Acta Derm Venereol 2013; 93:301–304. [DOI] [PubMed] [Google Scholar]
- 36.Wellcome Trust Case Control Consortium. Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 2007; 447:661–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hampe J, Grebe J, Nikolaus S, et al. Association of NOD2 (CARD 15) genotype with clinical course of Crohn's disease: a cohort study. Lancet 2002; 359:1661–1665. [DOI] [PubMed] [Google Scholar]
- 38.Verma D, Särndahl E, Andersson H, et al. The Q705K polymorphism in NLRP3 is a gain-of-function alteration leading to excessive interleukin-1β and IL-18 production. PLoS One 2012; 7:e34977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shinar Y, Obici L, Aksentijevich I, et al. Guidelines for the genetic diagnosis of hereditary recurrent fevers. Ann Rheum Dis 2012; 71:1599–1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marzano AV, Cugno M, Trevisan V, et al. Role of inflammatory cells, cytokines and matrix metalloproteinases in neutrophil-mediated skin diseases. Clin Exp Immunol 2010; 162:100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pène J, Chevalier S, Preisser L, et al. Chronically inflamed human tissues are infiltrated by highly differentiated Th17 lymphocytes. J Immunol 2008; 180:7423–7430. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal S, Misra R, Aggarwal A. Interleukin 17 levels are increased in juvenile idiopathic arthritis synovial fluid and induce synovial fibroblasts to produce proinflammatory cytokines and matrix metalloproteinases. J Rheumatol 2008; 35:515–519. [PubMed] [Google Scholar]
- 43.Pillai S, Netravali IA, Cariappa A, et al. Siglecs and immune regulation. Annu Rev Immunol 2012; 30:357–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wehrli P, Viard I, Bullani R, et al. Death receptors in cutaneous biology and disease. J Invest Dermatol 2000; 115:141–148. [DOI] [PubMed] [Google Scholar]
- 45.Brugnolo F, Annunziato F, Sampognaro S, et al. Highly Th2-skewed cytokine profile of beta-lactam-specific T cells from nonatopic subjects with adverse drug reactions. J Immunol 1999; 163:1053–1059. [PubMed] [Google Scholar]


