Supplemental Digital Content is available in the text
Abstract
Delayed diagnosis of hematological malignancies in immunocompetent patients with fever of unknown origin (FUO) remains an exhausting challenge for non-hematologist physicians. This retrospective cohort study aimed to establish a scoring system, “bone marrow (BM) score”, to identify FUO patients who require early bone marrow biopsy (BMB) to diagnose hematological disease.
Two cohorts, comprising 85 (training) and 20 (validation) eligible immunocompetent patients, with FUOs diagnosed between January 1, 2006 and July 31, 2013, underwent BMBs and were enrolled in the study. Demographic, laboratory, imaging, diagnostic, and outcome data were collected and retrospectively analyzed. Factors associated with hematological etiologies diagnosed using BMBs in the training cohort were identified and scored according to the relative hazards. These were further validated using the validation cohort.
For the training cohort, 29 of 85 (34.1%) patients had hematological etiologies diagnosed using BMB. Seven factors significantly predicted the diagnostic yield of hematological diseases in the BM and were scored, with the 6 points for leucoerythroblastic changes in peripheral blood smears, 5.5 for elevated ferritin level (>1000 ng/mL), 4 for splenomegaly, 2 for thrombocytopenia, 1.5 for each of elevated lactate dehydrogenase levels and anemia, and 1 for neutropenia. When the cut-off value of the scoring system was set to 6, its sensitivity and specificity to diagnose hematological diseases in the BM of immunocompetent FUO patients were 93% and 58%, respectively. For the validation cohort, 7 of 20 (35%) patients had hematological disease, and all had BM scores higher than the cut-off, with the sensitivity and specificity at 100% and 77%, respectively.
As immunocompetent FUO patients with hematological disease have poor prognoses, the “BM score” is valuable for non-hematologist physicians to identify immunocompetent FUO patients requiring early BMB.
INTRODUCTION
The management of fevers of unknown origin (FUOs) remains an exhausting challenge for physicians because of the wide spectrum of potential disease etiologies.1–3 Hematological malignancies make up approximately 11.5% of FUOs, but are responsible for more than half of the associated fatalities, and commonly take longer to establish a definite diagnosis.4 Although bone marrow (BM) biopsies (BMB) are important tools for diagnosing hematological malignancies, the role of BMBs in the diagnostic work-up of FUOs in immunocompetent patients remains controversial because of the paucity of validation studies confirming the utility of BMBs in this patient population.5
Recently, 2 studies analyzed immunocompetent, FUO patients undergoing BMBs as part of their diagnostic work-up. In both studies, the BMB diagnostic yields were up to 25%, with more than 85% of these patients finally being diagnosed with hematological diseases, most of which were malignant with poor prognoses and requiring early diagnosis for timely treatment.6,7 Therefore, we were interested in determining the predictors of hematological disease in immunocompetent patients with FUO, requiring BMB, and in establishing a scoring system that could predict the probability of BM hematological diseases before performing BMB.
We analyzed the final diagnoses of 105 immunocompetent patients with FUOs undergoing BMB patients in a training and validation cohort. Through this analysis, we developed a scoring system (the “BM score”) that helps determine the necessity and timing of BMB in immunocompetent, FUO patients.
MATERIALS AND METHODS
The Taipei Veterans General Hospital (TPE VGH) is a 2941-bed tertiary medical center in Taiwan that treats both veterans and non-veterans, and receives referrals from across the country. At TPE VGH, the Department of Medicine has 11 divisions, including infectious diseases (ID) and general medicine (GM). The majority of FUO patients are initially admitted to the Division of ID, with a minority being admitted to the Division of GM. To develop a predictive model that allows the identification of BM-associated hematological disease in FUO patients, immunocompetent FUO patients admitted to the divisions of ID and GM were categorized as the training and validation cohorts, respectively. The study was approved by the TPE VGH Institutional Review Board.
Enrolment
We retrospectively reviewed the consecutive hematological consultations performed in the divisions between January 2006 and June 2013. Patients undergoing hematological consultation were included if they met the 2 study criteria. First, the primary purpose of each patient's hematological consultation was to evaluate a FUO, which met the 2 primary criteria of the FUO definition8: (1) an illness lasting >3 weeks before diagnosis, and (2) repeated, documented body temperature >38.3°C. Second, a BMB must have been performed as part of the FUO evaluation.
Patients meeting the above criteria were further excluded if they (1) were <18-years-old, (2) had a known human immunodeficiency virus (HIV) infection, (3) were recipients of a solid organ transplant, (4) had received an active immunosuppressive therapy, (5) had a history of malignant hematological disease, (6) were neutropenic (white blood cell [WBC] count <1000/μL, or an absolute neutrophil count [ANC] <500/μL), or (7) were hypogammaglobulinemic (immunoglobulin (Ig) G <50%).
Diagnostic Workup
The minimal work-up included history reviews, physical examinations, routine blood and urine tests, chest radiographs, abdominal ultrasonography, and chest and/or abdominal computed tomography (CT). The routine laboratory tests included complete blood cell counts, with differential leukocyte counts; electrolyte levels; kidney function tests; total/direct bilirubin levels; liver enzyme level determinations; concentrations of alkaline phosphatase, r-glutamyl transpeptidase, C-reactive protein, lactate dehydrogenase (LDH), and ferritin; erythrocyte sedimentation rates; antinuclear antibody levels; rheumatoid factor concentrations; levels of complement components 3 and 4; ≥2 blood cultures while not receiving antibiotics; and serologic tests for cytomegalovirus and HIV. The diagnostic contrast-enhanced chest and/or abdominal CT was done to exclude obvious etiologies (like intra-abdominal abscesses or tumor masses) and to confirm clinical findings of lymphadenopathy, hepatomegaly, or splenomegaly.
Consultations with hematologists occurred at the discretion of the physicians in charge. Testing included peripheral blood (PB) smears and BM studies. The PB smears were used to detect any leucoerythroblastic changes, which refer to the presence of nucleated erythrocytes and immature myeloid cells in the PB.9–11 BM studies included aspiration cytology, biopsy, bacterial culture, acid-fast staining, and mycobacterial culture. BM aspirates were used for cytology, acid-fast staining, and inoculation of bacterial (BacT/ALERT® SA and SN, BioMerieux, Craponne, France) and mycobacterial culture medium (MGIT™, Becton Dickinson, Franklin Lakes, NJ). BMBs were performed by puncture of the posterior iliac crest using a T-lock BMB needle (Angiotech, Vancouver, BC, Canada). Sections of the core biopsy were immersed in formalin and processed in the pathology department; all slides were routinely stained with hematoxylin and eosin, with special immunoperoxidase stains being performed on appropriate specimens.
Data Collection
The clinical and laboratory data for the enrolled patients, including the parameters on (or closest to) the day of admission, were collected. Patients having more than 1 hematological consultation or BMB were analyzed only once, based on the first one (if all of the studies were non-diagnostic) or for the earliest one with diagnostic findings. Overall survival (OS) duration was from the time of the BMB to the date of death from any cause or to the last follow-up in January 2014.
For patients enrolled from the division of ID (training cohort), the BMB was initially categorized as “diagnostic” if the findings provided clues for diagnosing the FUO. Diagnostic BMB was further characterized as being “hematological” or “non-hematological,” based on the deduced disease etiology (Supplemental Figure 1, http://links.lww.com/MD/A112). The “non-hematological etiologies in the diagnostic BMB” and the “non-diagnostic BMBs” were grouped together as “without hematological etiologies.” By comparing the patients with and without hematological etiology, several significant parameters were identified and used to establish the scoring system (BM score) for predicting the probability of hematological disease etiologies in the BM of immunocompetent FUO patients. Patients enrolled from the division of GM acted as a validation group to provide an independent assessment of the BM score.
Statistical Analysis
To compare the results of the patients in the with and without hematological etiology subgroups, we used (1) the t-test for quantitative data, (2) the X2 and Fisher's exact tests for categorical data, and (3) the Kaplan–Meier estimate and log-rank test for OS. In addition, to estimate the odds ratio (OR) for each parameter and develop a scoring system, some quantitative data were converted into categorical data according to defined cut-off levels. Statistically significant predictors in the univariate analysis were further analyzed using a multivariate logistic regression model (the backward-stepwise method) to estimate the OR for each parameter. We utilized the relationship between each predictor's OR to establish a scoring system, with a discriminatory power that was evaluated using the area under the receiver operating characteristics (ROC) curve (AUC). A cut-off value was selected to possess acceptable diagnostic sensitivity and specificity.
All statistical testing was performed using 2-tailed tests; P <0.05 was considered statistically significant. The ORs of each predictor were reported with P-values and 95% confidence intervals (CIs). All analyses were performed using SPSS statistical software, version 17.0 (SPSS, Chicago, IL).
RESULTS
Clinical and Laboratory Features of FUO Patients
Between January 2006 and June 2013, there were 574 and 229 consecutive hematological consultations in the divisions of ID and GM, respectively. Of these consultations, 85 (ID) and 20 (GM) patients met the enrolment criteria. The selection algorithms are shown in Figure 1 (training cohort) and Supplemental Figure 2, http://links.lww.com/MD/A112 (validation cohort). There were no statistically significant differences between the clinical and laboratory features of the training and validation cohorts (Supplemental Table 1, http://links.lww.com/MD/A113).
FIGURE 1.
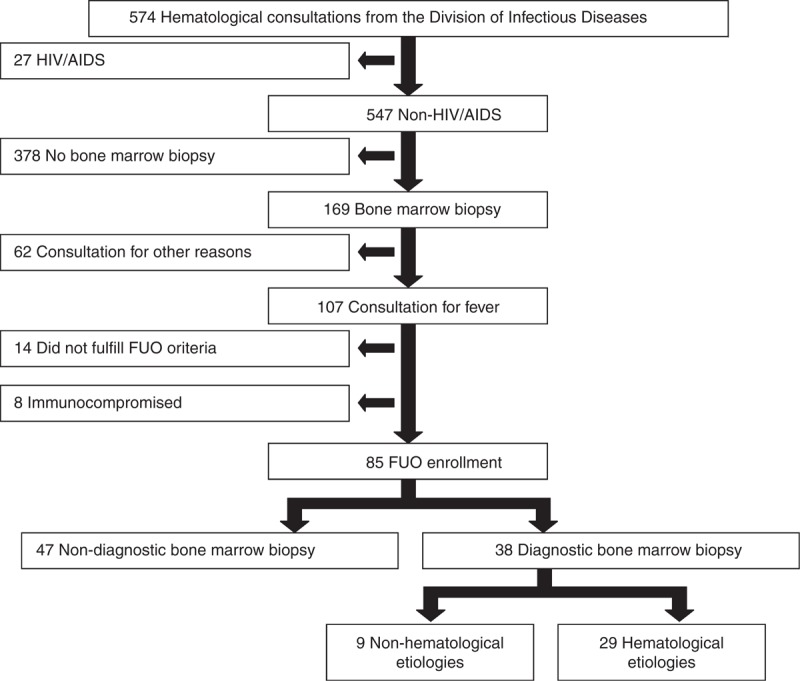
Algorithm of patients with hematological consultations in the Division of Infection Disease (Training cohort). AIDS = acquired immunodeficiency syndrome, FUO = fever of unknown origin, HIV = human immunodeficiency virus.
Final Diagnoses of FUO Patients in the Training Cohort
For the 85 FUO patients in the training cohort, 69 (81.2%) ultimately had their underlying disease diagnosed; these included hematological diseases (36.5%) and infectious diseases (25.9%) in 31 and 22 patients, respectively (Table 1). A total of 38 patients (44.7%) were deemed to have had a diagnostic BMB as the BMB findings provided clues for the final diagnosis. Importantly, patients undergoing diagnostic BMBs had a much higher proportion of hematological diseases than those with non-diagnostic BMBs (76.3% vs 4.3%, P < 0.001).
TABLE 1.
Classification of the Final Diagnoses in 85 Patients With Fever of Unknown Origin in the Training Cohort Based on the Diagnostic Yield of the Bone Marrow Biopsy
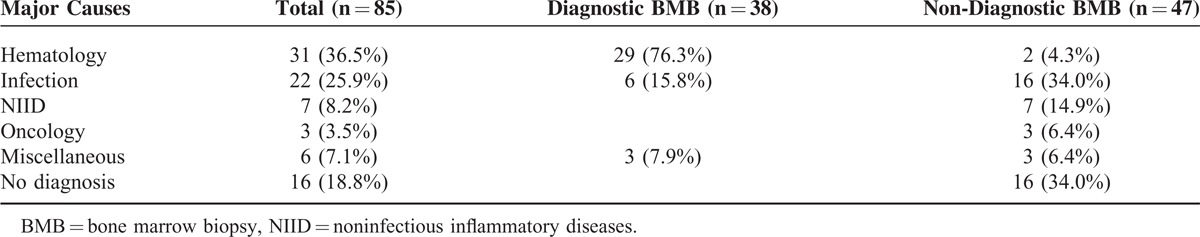
For the 38 patients deemed to have undergone diagnostic BMB (Supplemental Table 2, http://links.lww.com/MD/A113), hematological and non-hematological etiologies accounted for 29 (76.3%) and 9 (23.7%) cases, respectively. Of the hematological etiologies, non-Hodgkin lymphoma was the most prevalent (n = 15, 39.5%), including B-cell (n = 10) and T-cell (n = 5) immunophenotypes. The 9 patients with non-hematological etiologies had granulomas.
For the 47 patients deemed to have undergone non-diagnostic BMB (Supplemental Table 3, http://links.lww.com/MD/A113), infection (n = 16, 34%) was the most commonly described etiology, with 16 (34%) patients not achieving a conclusive diagnosis after their comprehensive work-up. Notably, 2 cases were diagnosed with hematological disease, but without evidence of BM involvement; 1 was diagnosed with Hodgkin disease and the other with splenic lymphoma.
Factors Predicting Hematological Disease in the BMB (Training Cohort)
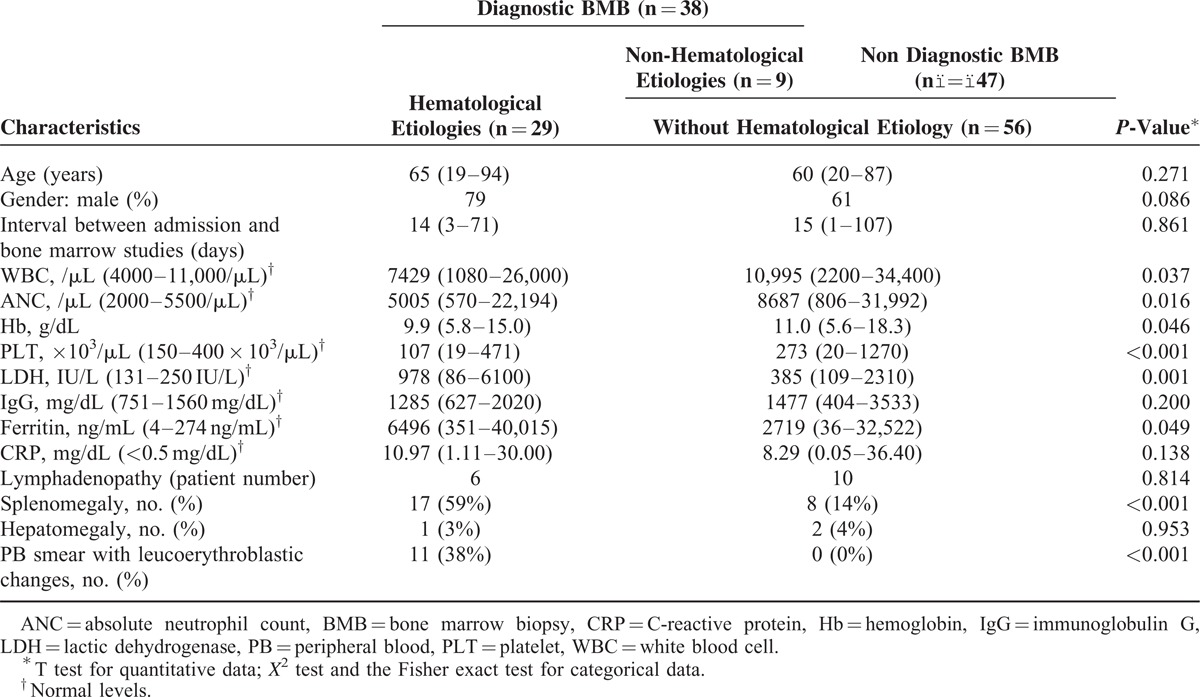
At the last follow-up, the median OS of patients with hematological etiologies was much lower than of those without hematological etiologies (46 days vs 2569 days, P < 0.001; Supplemental Figure 3, http://links.lww.com/MD/A112). Compared to those without hematological etiologies (Table 2), patients with hematological etiologies were found to have significantly lower WBC counts, ANCs, hemoglobin (Hb) levels, and platelet (PLT) counts, whereas they had higher LDH levels, ferritin levels, and higher proportions of leucoerythroblastic changes in the PB smears. Moreover, the proportion of patients with splenomegaly was also higher in those with hematological disease.
TABLE 2.
Baseline Characteristics of Patients From the Training Cohort With and Without Hematological etiologies in the Bone Marrow Biopsies
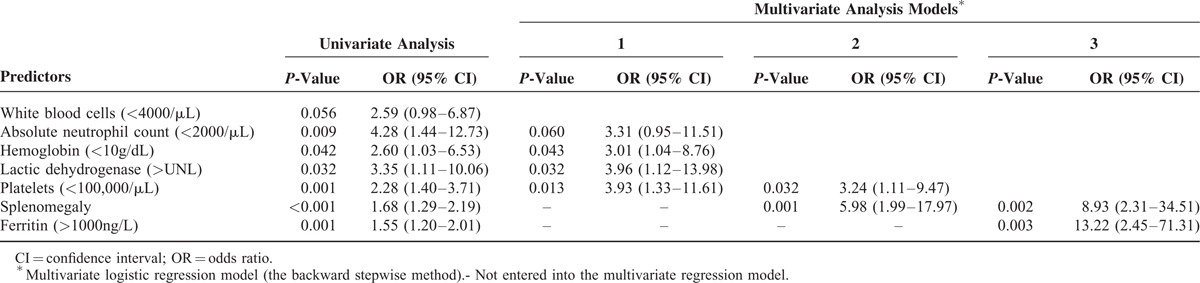
To avoid the loss of potentially useful parameters, the hazards of the above 8 parameters were successively analyzed using different multivariate regression models. As shown in Table 3, 5 factors, WBCs (<4000/μL), ANCs (<2000/μL), Hb (<10 g/dL), LDH (>UNL), and PLTs (<100,000/μL), were initially entered into model 1. The P-values for Hb (<10 g/dL), LDH (>UNL), and PLTs (<100,000/μL) were <0.05, with ANC (<2000/μL) showing a marginally statistical difference (P = 0.06); the ORs were 3.01, 3.96, 3.93, and 3.31, respectively. In model 2, which added the splenomegaly factor, only 2 factors, PLT (<100,000/μL) and splenomegaly, were statistically significant; the ORs were 3.24 and 5.98, respectively. Finally, in model 3, which further added the ferritin (>1000 ng/mL) factor, splenomegaly, and ferritin (>1000 ng/mL) were the only 2 significant predictors, with ORs of 8.93 and 13.22, respectively.
TABLE 3.
Univariate and Multivariate Analyses of Factors Predicting Hematological Disease in Bone Marrow Biopsies of 85 Fever of Unknown Origin Patients From the Training Cohort
BM Score Predicting Hematological Etiologies in BMBs
To develop the scoring system, 6 factors (Supplemental Table 4, http://links.lww.com/MD/A113) were chosen and weighted according to their relative ORs in the models analyzed (Table 3); the AUC was 0.82 (0.71–0.92), with a sensitivity and specificity of 0.83 and 0.58, respectively, when setting the cut-off value to 6 (Supplemental Figure 4, http://links.lww.com/MD/A112). Because the patients with leucoerythroblastic changes in their PB smears were shown to have hematological etiologies, based on their BMBs, “leucoerythroblastic change in PB smears” was directly added and artificially scored as having 6 points. In the final scoring system (BM score, Table 4), the AUC increased to 0.89 (0.81–0.97), and its sensitivity was elevated to 0.93, with the same specificity (0.58) (Figure 2; Supplemental Table 5, http://links.lww.com/MD/A113.) if the cut-off value remained at 6 points.
TABLE 4.
Parameters of Bone marrow score
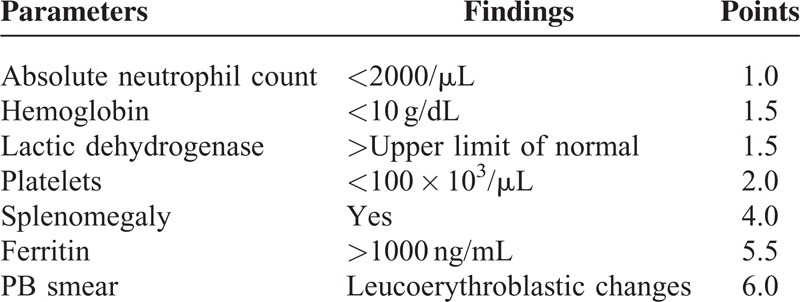
FIGURE 2.
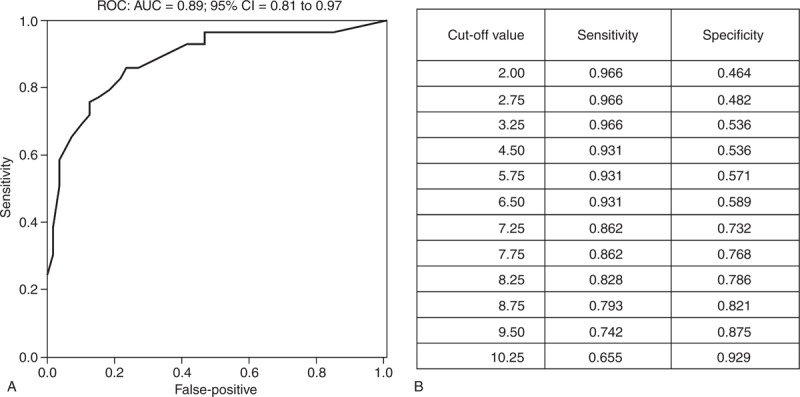
(A) Receiver operating characteristics (ROC) curve and area under the ROC curve for assessing the discriminatory power of the bone marrow score. (B) Cut-off values for the ROC curve. AUC = area under the ROC curve, CI = confidence interval.
Predictive Values of BM Scores in the Validation Cohort
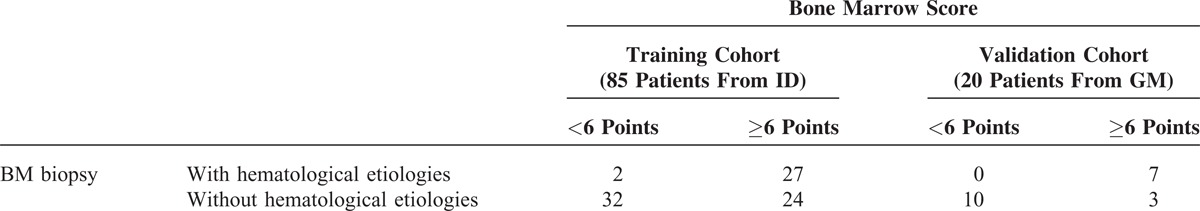
To validate the usefulness of the BM score, the 20 FUO patients in the validation cohort were scored (Supplemental Table 6, http://links.lww.com/MD/A113). Seven (35%) of the 20 patients had hematological diseases, based on their BMB findings, and these 7 patients had BM scores ≥6. None of patients with a BM score <6 were diagnosed with hematological etiologies, based on their BMBs. As shown in Table 5, the sensitivity and specificity were 100% and 77%, respectively. Notably, the negative and positive predictive rates were 100% and 70%, respectively.
TABLE 5.
The Application of Bone marrow Score to Predict “Hematological Disease of Bone Marrow” in FUO Patients From Training and Validation Cohort
DISCUSSION AND CONCLUSIONS
To our knowledge, scoring systems have not been previously suggested to assist in the clinical determination of whether to perform a BMB in immunocompetent FUO patients, although several predictors have been described.6,7 The importance of our proposed system was highlighted by several aspects. First, immunocompetent FUO patients who were finally diagnosed with hematological disease, via BMBs, had very poor OS4; therefore, we recommend early BMBs for immunocompetent FUO patients with BM scores ≥6. Second, the usefulness of the BM score was further validated using an independent cohort. Third, we identified elevated ferritin levels (>1000 ng/mL) as a surrogate predictor of hematological disease in FUO patients. In fact, serum ferritin levels are well known to be nonspecifically elevated in many inflammatory conditions, including infections and malignancies.12–14 In our study, most of the patients had ferritin levels above the upper limit of normal, so we suspect that FUO patients with hematological diseases, diagnosed via BMB, have ferritin levels that are higher than those of patients without hematological etiologies. Fourth, although observed in only 10% of the training cohort patients, a tight association was observed between leucoerythroblastic changes in PB smears and the diagnosis of hematological disease via BMB; however, the value of this association might be limited in hospitals lacking experienced laboratory staff or hematologists.
The proposed scoring system stresses the importance of BMB, instead of mycobacterial and other bacterial isolation methods from BM samples, for evaluating immunocompetent FUO patients. First, lymphoma accounted for approximately half of the patients having a final diagnosis of hematological disease, similar to previous studies,6,7 and its involvement in the BM is well-known to be better evaluated using BM biopsies.15,16 Second, the bacterial yield of the BM cultures has been proven to be as low as 0% to 2%17,18; none of our patients’ BM cultures yielded bacteria (data not shown). Third, isolation of Mycobacterium tuberculosis from the BM denotes a diagnosis of miliary tuberculosis (TB), or the intense systemic dissemination of M tuberculosis into a vascular channel.19 For diagnosing miliary TB, invasive procedures are required to obtain specimens from the lung, peripheral lymph nodes, liver, and BM when noninvasive techniques do not provide a diagnosis.19–21 Currently, prospective study evidence is not available to prove which invasive procedure is better, but liver biopsies are generally associated with higher diagnostic yields than BMB.21–24 Furthermore, blood cultures appear to be as sensitive as BM cultures for diagnosing miliary tuberculosis (sensitivity, 58% vs 54%, respectively).25 According to the findings of our study, the BM score may facilitate choosing between performing a BMB or a liver biopsy when miliary TB is suspected in immunocompetent FUO patients; a BMB may be suggested if the BM score is ≥6, and a liver biopsy if the BM score is <6.
Compared with previous reports of diagnostic yields (24–27%),6,7 those of the BMBs in our patients were relatively high (45% in the training cohort). Several factors might have contributed to this relatively high diagnostic yield. First, some bias would have been derived from patient selection involving inter-hospital referrals and delayed intra-hospital consultations. As described, TPE VGH is a tertiary medical center that receives patient referrals from throughout Taiwan, but not every hospital in the country is able to diagnose hematological disease. In addition, BM studies were usually not done in our hospital until after the completion of other FUO work-ups and hematological consultations. Second, the incidence of BM granulomas in our training cohort (10.5%) was higher than described in previous reports (2.3%6 and 1.3%7), possibly reflecting the high prevalence of TB in Taiwan.26 Excluding the 9 BM granulomas in this study, the diagnostic yield of the BMBs in our patients would be lowered to 34%. In fact, our study focused on “hematological etiologies in BM” and was not affected by the 9 patients with BM granuloma.
Our study has several inherent limitations. First, the enrolment of patients in the training cohort from the ID division might have increased the frequency of infectious diseases in the final diagnoses. Second, although 11 patients with “leucoerythroblastic changes in PB smears” were subgrouped as having hematological etiologies, more patients are required in the future to prove the statistical significance of the multivariate analysis. Finally, a prospective trial will be needed to directly examine the usefulness of the BM score in immunocompetent FUO patients.
In conclusion, immunocompetent FUO patients with hematological diseases have poor prognoses and the “BM score” will be valuable for assisting non-hematologist physicians to identify those requiring early BMBs.
Acknowledgments
We thank Kuo-I Hsiao for preparing the procedure of bone marrow biopsy.
Footnotes
Abbreviations: ANC = absolute neutrophil count, BMB = bone marrow biopsy, BM = bone marrow, FUO = fever of unknown origin, GM = general medicine, ID = infectious diseases, LDH = lactic dehydrogenase, OR = odds ratio, OS = overall survival, PB = peripheral blood, PLT = platelet, TPE VGH = Taipei Veterans General Hospital, ULN = upper limit of normal.
Taiwan Clinical Oncology Research Foundation, Taipei Veterans General Hospital (V103E2–002 to L.-T.), National Science Council (NSC101–2325-B-075–008 and NSC102–2325-B-075–005 to L.-T.), and Ministry of Science and Technology (MOST103–2325-B-075–004 to L.-T.).
The authors declare that they have no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Arnow PM, Flaherty JP. Fever of unknown origin. Lancet 1997; 350:575–580. [DOI] [PubMed] [Google Scholar]
- 2.Hirschmann JV. Fever of unknown origin in adults. Clin Infect Dis 1997; 24:291–300. [DOI] [PubMed] [Google Scholar]
- 3.Knockaert DC, Vanderschueren S, Blockmans D. Fever of unknown origin in adults: 40 years on. J Intern Med 2003; 253:263–275. [DOI] [PubMed] [Google Scholar]
- 4.Vanderschueren S, Knockaert D, Adriaenssens T, et al. From prolonged febrile illness to fever of unknown origin: the challenge continues. Arch Intern Med 2003; 163:1033–1041. [DOI] [PubMed] [Google Scholar]
- 5.Mourad O, Palda V, Detsky AS. A comprehensive evidence-based approach to fever of unknown origin. Arch Intern Med 2003; 163:545–551. [DOI] [PubMed] [Google Scholar]
- 6.Hot A, Jaisson I, Girard C, et al. Yield of bone marrow examination in diagnosing the source of fever of unknown origin. Arch Intern Med 2009; 169:2018–2023. [DOI] [PubMed] [Google Scholar]
- 7.Ben-Baruch S, Canaani J, Braunstein R, et al. Predictive parameters for a diagnostic bone marrow biopsy specimen in the work-up of fever of unknown origin. Mayo Clin Proc 2012; 87:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine 1961; 40:1–30. [DOI] [PubMed] [Google Scholar]
- 9.Clifford G. The clinical significance of leukoerythroblastic anemia. Med Clin N Am 1966; 50:779–790. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz SO, Stansbury F. Significance of nucleated red blood cells in peripheral blood; analysis of 1,496 cases. J Am Med Assoc 1954; 154:1339–1340. [DOI] [PubMed] [Google Scholar]
- 11.Retief FP. Leuco-erythroblastosis in the adult. Lancet 1964; 1:639–642. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Knovich MA, Coffman LG, et al. Serum ferritin: past, present and future. Biochim Biophys Acta 2010; 1800:760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alkhateeb AA, Connor JR. The significance of ferritin in cancer: anti-oxidation, inflammation and tumorigenesis. Biochim Biophys Acta 2013; 1836:245–254. [DOI] [PubMed] [Google Scholar]
- 14.Lee MH, Means RT., Jr Extremely elevated serum ferritin levels in a university hospital: associated diseases and clinical significance. Am J Med 1995; 98:566–571. [DOI] [PubMed] [Google Scholar]
- 15.Stein RS, Ultmann JE, Byrne GE, et al. Bone marrow involvement in non-Hodgkin's lymphoma: implications for staging and therapy. Cancer 1976; 37:629–636. [DOI] [PubMed] [Google Scholar]
- 16.Lai H, Tien H, Hsieh H, et al. Bone marrow involvement in non-Hodgkin's lymphoma. J Formosan Med Assoc 1989; 88:114–121. [PubMed] [Google Scholar]
- 17.Riley UB, Crawford S, Barrett SP, et al. Detection of mycobacteria in bone marrow biopsy specimens taken to investigate pyrexia of unknown origin. J Clin Pathol 1995; 48:706–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volk EE, Miller ML, Kirkley BA, et al. The diagnostic usefulness of bone marrow cultures in patients with fever of unknown origin. Am J Clin Pathol 1998; 110:150–153. [DOI] [PubMed] [Google Scholar]
- 19.Sharma SK, Mohan A, Sharma A, et al. Miliary tuberculosis: new insights into an old disease. Lancet Infect Dis 2005; 5:415–430. [DOI] [PubMed] [Google Scholar]
- 20.Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am J Respir Crit Care Med 2000;161:1376–1395. [DOI] [PubMed] [Google Scholar]
- 21.Sharma SK, Mohan A, Sharma A. Challenges in the diagnosis & treatment of miliary tuberculosis. Ind J Med Res 2012; 135:703–730. [PMC free article] [PubMed] [Google Scholar]
- 22.Kim JH, Langston AA, Gallis HA. Miliary tuberculosis: epidemiology, clinical manifestations, diagnosis, and outcome. Rev Infect Dis 1990; 12:583–590. [DOI] [PubMed] [Google Scholar]
- 23.Maartens G, Willcox PA, Benatar SR. Miliary tuberculosis: rapid diagnosis, hematologic abnormalities, and outcome in 109 treated adults. Am J Med 1990; 89:291–296. [DOI] [PubMed] [Google Scholar]
- 24.Mert A, Bilir M, Tabak F, et al. Miliary tuberculosis: clinical manifestations, diagnosis and outcome in 38 adults. Respirology 2001; 6:217–224. [DOI] [PubMed] [Google Scholar]
- 25.Crump JA, Reller LB. Two decades of disseminated tuberculosis at a university medical center: the expanding role of mycobacterial blood culture. Clin Infect Dis 2003; 37:1037–1043. [DOI] [PubMed] [Google Scholar]
- 26.Chin C, Chen YS, Lee SS, et al. Fever of unknown origin in Taiwan. Infection 2006; 34:75–80. [DOI] [PubMed] [Google Scholar]


