Abstract
Organophosphate (OP) poisoning is a critical cause of morbidity and mortality worldwide. We conducted a nationwide longitudinal cohort study to investigate the development of deep vein thrombosis (DVT) and pulmonary thromboembolism (PTE) among patients admitted with OP intoxication.
We identified patients with OP intoxication by using the Taiwan National Health Insurance Research Database and enrolled 9223 patients who were hospitalized for OP intoxication between 2000 and 2011. OP intoxication was diagnosed based on a clinical assessment and serum acetylcholinesterase levels at the time of hospital admission. Each patient in the OP intoxication cohort was randomly frequency matched with 4 patients without OP intoxication based on their age, sex, and index year (36,892 patients as control cohort), and all patients were observed from the index date until the appearance of a DVT or a PTE event, or until December 31, 2011. We analyzed the risks of DVT and PTE by using Cox proportional hazards regression models that included the demographic variables of sex, age, and comorbidities (eg, hypertension, diabetes, cerebral vascular disease, heart failure, all cancer types, and lower leg fracture or surgery).
The results revealed a significantly increased risk of developing DVT among patients with OP poisoning (adjusted hazard ratio [HR] = 1.55; 95% confidence interval [CI] = 1.03–2.34) but not PTE (adjusted HR = 1.44; 95% CI = 0.83–2.52). Among the patients without comorbidities, the OP poisoning patients compared with controls had a higher adjusted HR of 2.12 (95% CI = 1.21–3.71) for DVT.
The results of this nationwide cohort study indicate that the risk of developing DVT is markedly higher in patients with OP intoxication compared with that of the general population.
INTRODUCTION
Organophosphates (OPs) are a group of chemicals that are widely used as insecticides in agriculture and household gardens worldwide. Because of the availability of such compounds, the incidence of accidental, occupational, bystander exposure, and suicidal poisoning has gradually increased. OP toxicity primarily targets the nervous system.1 OPs bind covalently and irreversibly at the active site of acetylcholinesterase (AChE).1 Therefore, patients with OP intoxication are typically assessed based on decreased AChE activity. The accumulation of acetylcholine at the peripheral nerve postsynapse cleft may induce sympathetic and parasympathetic reactions through the activation of muscarine and nicotine receptors.2 In addition, high-dose OP exposure may result in severe morbidity and even mortality.3 Previous studies on the toxic effects of OP in nontarget tissues have been limited. However, some studies have indicated that OP poisoning may increase lipid peroxidation, induce oxidative stress, and reduce glutathione levels.4 Under high oxidative stress, tissues may sustain damage and persistently exhibit an inflammatory response, leading to cellular necrosis.5
Deep vein thrombosis (DVT) is the formation of blood clots predominantly in the deep veins of the legs. Severe DVT causes permanent damage to the leg. Pulmonary thromboembolism (PTE) is a potentially life-threatening disease involving pulmonary artery thrombosis or occlusion of the thrombotic occlusion system, and it can lead to the collapse of the respiratory or circulatory systems, resulting in death. Both DVT and PTE constitute venous thromboembolism which, when severe, carries a fatality rate of 11% to 30% within 30 days.6 The triad of Virchow describes the 3 major risk factors that contribute to thrombosis, which are hypercoagulabililty, hemodynamic changes (stasis or turbulence), and endothelial injury or dysfunction.7 Although OP intoxication is not a general risk factor for DVT or PTE, previous studies have shown that chronic inflammatory status is associated with coagulation abnormalities and increased risk of DVT and PTE.8 No study has investigated the association between OP intoxication and DVT or PTE. Therefore, we conducted a nationwide prospective cohort study that involved controlling for potential comorbidities to determine whether OP intoxication increases the risks of DVT and PTE.
METHODS
Data Source
Instituted in 1995, the National Health Insurance (NHI) program is a mandatory health insurance program that offers comprehensive medical care coverage, including outpatient, inpatient, emergency, and traditional Chinese medicine services, to all residents of Taiwan. The NHI program covers >99% of the population of Taiwan (23.74 million people). The NHI Research Database (NHIRD) contains comprehensive information regarding clinical visits, including prescription details and diagnostic codes based on the International Classification of Diseases, Ninth revision, Clinical Modification (ICD-9-CM). The NHIRD is managed by the National Health Research Institutes (NHRI) and the confidentiality is maintained according to the directives of the Bureau of the NHI. This database is used for administrative and research purposes. To protect the privacy of all persons registered in the program, the NHRI encrypts and converts the identification numbers of all NHIRD records before releasing them for researchers. Thus, our study was exempted from full review by our institutional research ethics committee (CMU-REC-101–012).
Sample Participants
We identified patients from the NHIRD who were hospitalized with OP poisoning (ICD-9 Code 989.3) for the first time between 2000 and 2011. The date of first hospitalization was used as the index date. For the OP poisoning cohort, we extracted 9223 patients with OP poisoning, including information on their age and sex, who had no history of DVT (ICD-9-CM 453.8) or PTE (ICD-9-CM 415.1, excluding ICD-9-CM 415.11) before the index date. Records indicating a history of DVT or PTE before the index date or those with incomplete age or sex information and missing variables were excluded from the control group. For each OP poisoning patient in the OP poisoning cohort, 4 controls without OP poisoning were randomly selected, frequency matched by sex, age (every 5-year span), and the year of the index date. A total of 9223 patients with OP poisoning and 36,892 people without OP poisoning were followed up until diagnosis of DVT or PTE, loss to follow-up, death, withdrawal from the insurance program, or the end of 2011, whichever occurred first.
Comorbidities
The baseline comorbidity history of each participant was determined based on the inpatient claims data. We considered several well-known risk factors for DVT and PTE as comorbidities: atrial fibrillation (ICD-9 Code 427.31), hypertension (ICD-9 Codes 401–405), diabetes (ICD-9 Code 250), cerebral vascular disease (CVA) (ICD-9 Codes 430–438), heart failure (ICD-9 Code 428), all cancer types (ICD-9 Codes 140–208), and lower leg fracture or surgery (ICD-9 Codes 820–823 and procedure codes 81.51–81.54). These comorbidities were selected to clarify the independent influence of OP poisoning on the risk of DVT and PTE.
Statistical Analysis
We described and compared the distributions of age, sex, and comorbidities (%) between the 2 cohorts according to the results of χ2 tests. A Student t test was used to examine the mean ages of the 2 cohorts. The cumulative incidence curves of DVT and PTE and the Kaplan–Meier curves of the 2 cohorts were compared using a log-rank test. Incidence-density rates (per 10,000 person-years) were estimated for both of the cohorts based on associated demographic variables and comorbidities. Univariable and multivariable Cox proportional hazards regression models were used to examine the effect of OP poisoning on the risk of DVT and PTE based on hazard ratios (HRs) with 95% confidence intervals (CIs). Only confounding variables found significantly in the univariable model were further included in the multivariable model. All data processing and statistical analyses were performed using SAS Version 9.3 (SAS Institute, Inc, Cary, NC). A 2-tailed P value of <0.05 was considered statistically significant.
RESULTS
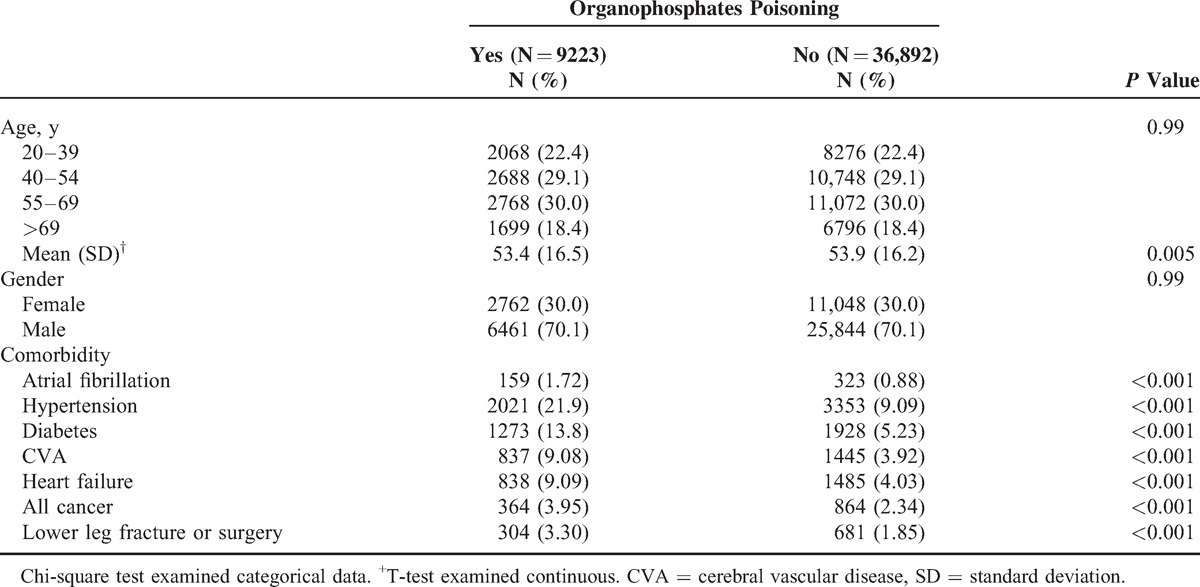
Table 1 shows the distributions of the demographic variables and comorbidities for the OP poisoning and control cohorts. The mean (± standard deviation) age of the OP poisoning cohort was 53.4 (±16.5) years and that of the control cohort was 53.9 (±16.2) years; 51.5% of them were aged 20 to 54 years and women were outnumbered by men (30.0% vs 70.1%). Comorbidities were more prevalent in the OP poisoning cohort than in the control cohort (all P < 0.001).
TABLE 1.
Comparison of Demographics and Comorbidity Between Organophosphates Poisoning Patients and Controls
A Kaplan–Meier analysis was used to reveal the risk of DVT during follow-up in both the cohorts. The cumulative incidence of DVT was significantly higher in the OP poisoning cohort than in the control cohort (log-rank P = 0.006) (Figure 1). For the sake of comparison, Figure 2 shows the cumulative incidence of PTE of the OP poisoning and control cohorts. The difference in cumulative incidence of PTE between the OP poisoning and control cohorts was not significant (log-rank P = 0.13).
FIGURE 1.
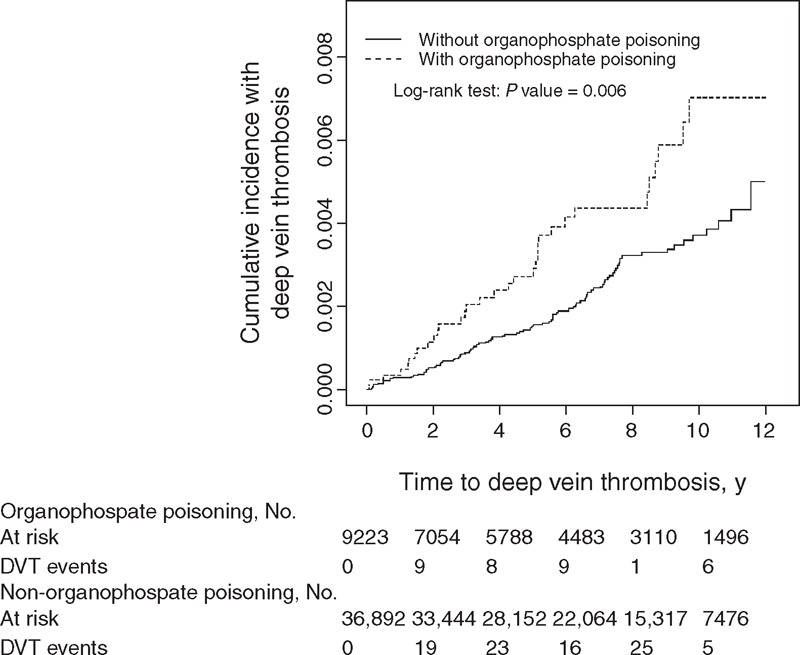
Cummulative incidence of deep vein thrombosis in patients with organophosphate poisoning and comparison patients.
FIGURE 2.
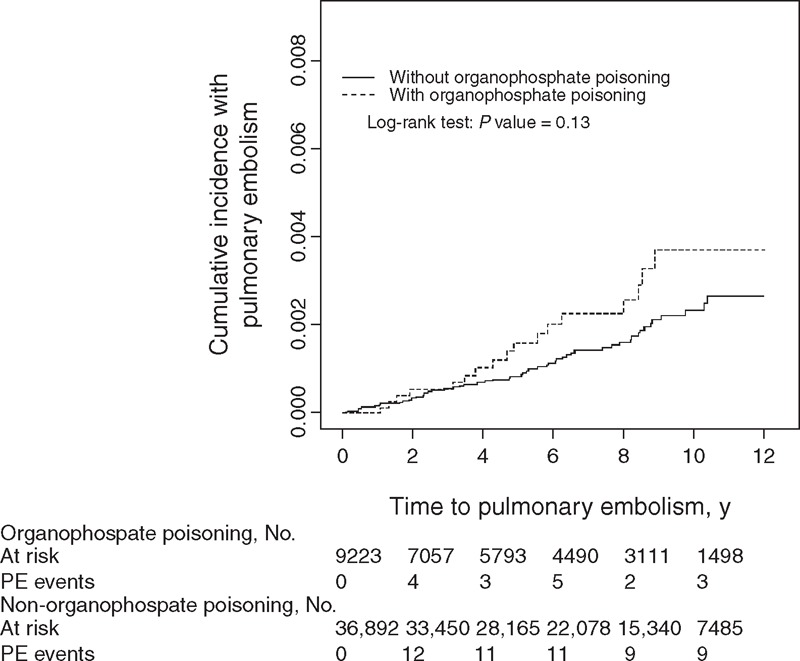
Cummulative incidence of pulmonary thromboembolismin in patients with organophosphate poisoning and comparison patients.
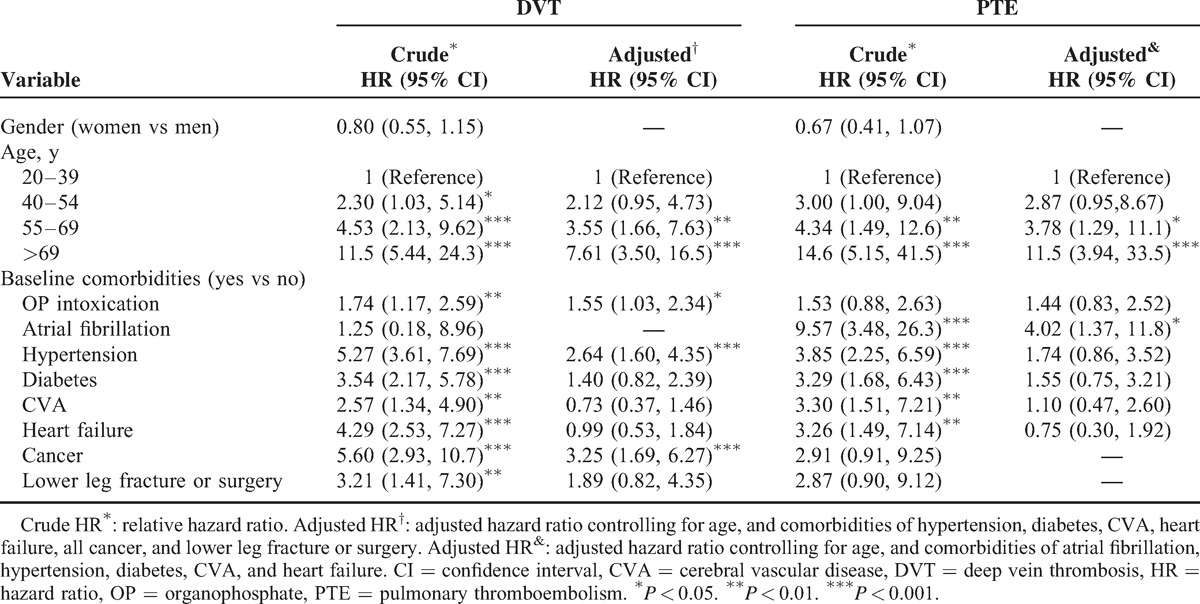
Table 2 shows the results of the univariable and multivariable Cox proportion hazards regression models for the risk of related variables contributing to DVT and PTE. After adjustment for age and comorbidities of hypertension, diabetes, CVA, heart failure, all cancer types, and lower leg fracture or surgery, the risk of developing DVT was significantly higher for patients with OP poisoning (adjusted HR = 1.55; 95% CI = 1.03–2.34) compared with the control cohort. The risk factors contributing to DVT included older age (adjusted HR = 3.55, 95% CI = 1.66–7.63 for 55–69 years of age; adjusted HR = 7.61, 95% CI = 3.50–16.5 for >69 years of age), hypertension (adjusted HR = 2.64, 95% CI = 1.60–4.35), and cancer (adjusted HR = 3.25, 95% CI = 1.69–6.27). After adjustment for age and the comorbidities of atrial fibrillation, hypertension, diabetes, CVA, and heart failure, the risk of developing PTE was not significantly higher for patients with OP poisoning (adjusted HR = 1.44; 95% CI = 0.83–2.52). The risk factors contributing to PTE included older age (adjusted HR = 3.78, 95% CI = 1.29–11.1 for 55–69 years of age; adjusted HR = 11.5, 95% CI = 3.94–33.5 for >69 years of age) and atrial fibrillation (adjusted HR = 4.02, 95% CI = 1.37–11.8).
TABLE 2.
Cox Proportional Hazard Regression Analysis for the Hazard Ratio of OP Intoxication-Associated DVT and PTE Stratified by Age and the Interaction With Comorbidity
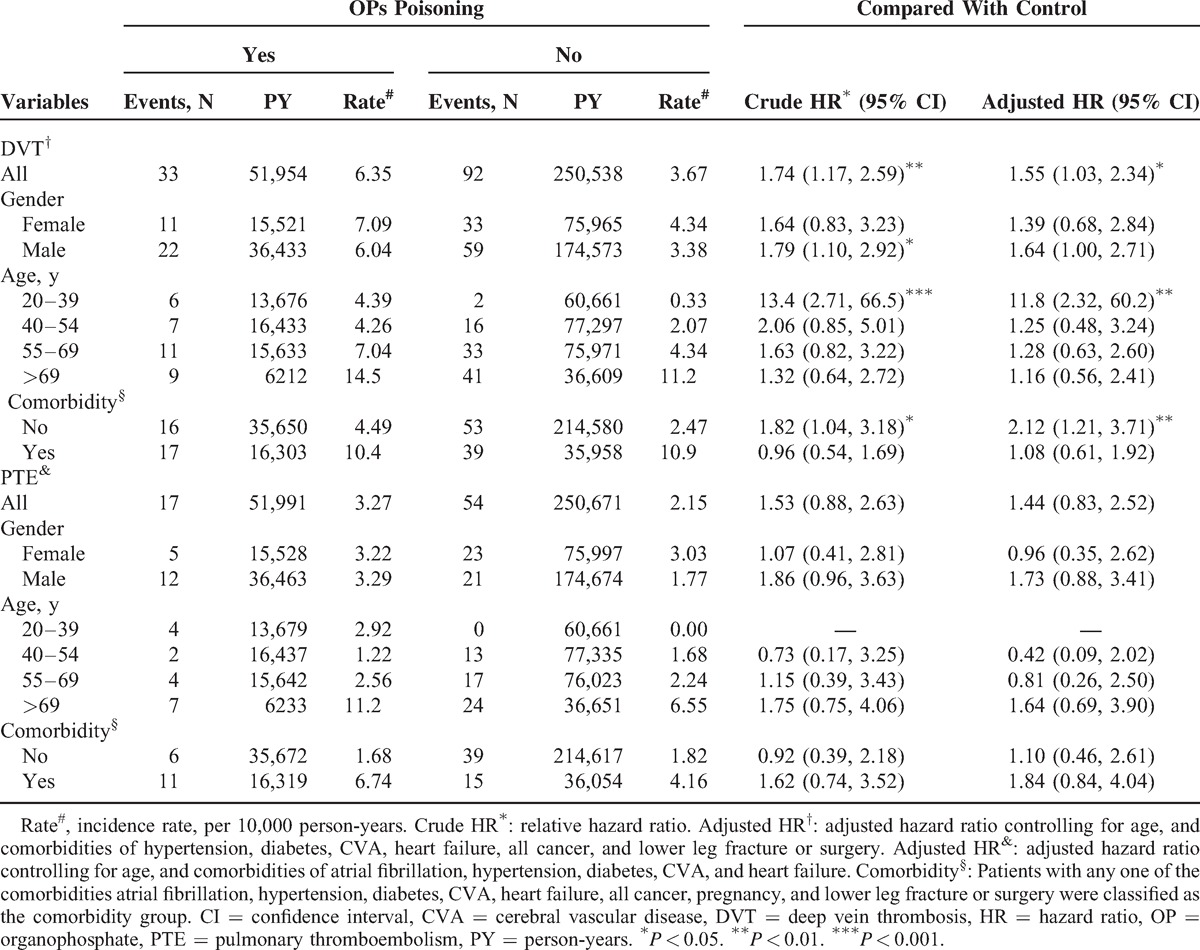
For the subgroup analysis, during 51,954 and 250,538 person-years of follow-up, the overall incidence density of DVT was 1.74-fold higher in the OP poisoning patients than in the control cohort (6.35 vs 3.67 per 10,000 person-years, crude HR = 1.74, 95% CI = 1.17–2.59) (Table 3). The incidence of DVT was greater in women than in men in both cohorts, and DVT incidence increased with age in both cohorts. However, the results of the age-specific analysis of the OP poisoning and control cohorts indicated that the relative risk was highest among those aged 20 to 39 years (adjusted HR = 11.8; 95% CI = 2.32–60.2). Among the patients without comorbidities, the OP poisoning patients compared to controls had a higher adjusted HR of 2.12 (95% CI = 1.21–3.71) for DVT. The overall incidence density of PTE was not significantly higher in the OP poisoning patients than in the control cohort (3.27 vs 2.15 per 10,000 person-years), with an HR of 1.44 (95% CI = 0.83–2.52).
TABLE 3.
Comparison of Incidence and Hazard Ratio of DVT and PTE Stratified by Sex, Age, and Comorbidity Between OP Poisoning Patients and Controls
DISCUSSION
This is the first nationwide longitudinal population-based cohort study to investigate whether a population with OP intoxication exhibited an increased risk of DVT and PTE. Clinical data on AChE levels were used as indicators for the intoxication diagnoses. To avoid selection bias, we estimated the risk of DVT and PTE only for those patients who received a first diagnosis of OP intoxication and had no previous history of DVT or PTE. All of the patients were assigned unique identification numbers in the NHI beneficiary system, and every patient could be traced through the NHIRD in the study period. Compared to the control cohort, the risk of developing DVT was significantly higher among patients with OP poisoning (adjusted HR = 1.55; 95% CI = 1.03–2.34). Moreover, we demonstrated that the incidence of DVT was 6.35 per 10,000 person-years among patients with OP intoxication in Taiwan. Additionally, we found that there was an increased risk of DVT in OP intoxication patients without comorbidity (adjusted HR = 2.12, 95% CI = 1.21–3.71). Therefore, providing adequate care for patients with OP intoxication and comorbidities is essential to prevent further development of DVT; assessment, treatment, and additional care must be provided to patients with OP intoxication.
OPs are lipophilic and may accumulate in various parts of tissues and organs after intoxication. OPs are subsequently released from these tissues into the bloodstream, and intoxication relapse may be prolonged, leading to various clinical manifestations.9 A recent report showed that OP intoxication may be associated with increased reactive oxygen species (ROS) production and lipid peroxidation.10 ROS may act as an initiator of cell apoptosis and organ lesions through various pathways. Under high oxidative stress, cells typically undergo necrosis as a result of tissue damage. Such tissue damage can include subchronic and chronic toxicity and biochemical and histopathologic changes in various tissues, such as those in the liver, kidneys, pancreas, vascular walls, and endometrium,11 as well as an intense inflammatory response.12 Inflammation may cause thrombotic tendencies and microvascular thrombosis by increasing procoagulant factors and inhibiting natural anticoagulant pathways.13 In addition, with chronic inflammatory response, the properties of the endothelium may become dysfunctional with multiple outcomes, including the loss of anticoagulant, antiaggregant, and vasodilatory properties.13 Another acute phase reactant, C-reactive protein (CRP), is substantially altered during infection, trauma, surgery, and tissue necrosis, as well as in patients with burns, inflammatory disease, or advanced cancer.14 Lee et al15 determined that serum CRP levels are highly correlated with the severity of acute OP intoxication but not with serum AChE levels. Therefore, CRP may be an effective alternative index for assessing the intoxication status of patients. These mechanisms may explain why the patients with OP intoxication in this study exhibited significantly higher DVT and PTE rates, particularly in the presence of any comorbidity, compared with the control cohort.
The strength of this study is that it is the first nationwide population-based cohort longitudinal study on the risk of DVT and PTE development to be conducted on patients with OP intoxication in Taiwan. However, several limitations must be considered when interpreting these findings. First, the NHIRD does not provide detailed personal information, including information pertaining to smoking status, body mass index, and physical activity, all of which may be confounding factors. Second, the NHIRD does not record OP classification, route of exposure, or exposure periods, which might be another limitation. Third, we used ICD-9 codes to define the outcomes of interest, particularly regarding DVT, which may have led to misclassification. However, in Taiwan, because universal health insurance has been implemented and doctors are reimbursed by administrative specialists, a peer review system was established to reduce the rate of false positives.
In conclusion, this nationwide study of 9223 patients with OP intoxication over approximately 51,954 (DVT) and 51,991 (PTE) follow-up person-years shows that patients with OP intoxication have a 1.55-fold increased risk of DVT compared with the general population. The risks of DVT were significant and reached 2.12-fold of the HR of OP poisoning patients with any comorbidity compared with those without OP poisoning and comorbidities. These findings emphasize the importance of clinical awareness and additional holistic care for preventing the development of DVT and PTE to minimize morbidity and mortality from these potentially lethal compounds.
Footnotes
Abbreviations: AChE = acetylcholinesterase, CI = confidence interval, CRP = C-reactive protein, CVA = cerebral vascular disease, DVT = deep vein thrombosis, HR = hazard ratio, ICD-9-CM = International Classification of Diseases, Ninth revision, Clinical Modification, NHI = National Health Insurance, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institutes, OP = organophosphate, PTE = pulmonary thromboembolism, ROS = reactive oxygen species.
This study was supported by the National Science Council, Executive Yuan, Taiwan, R.O.C. (NSC-101–2320-B-039–007-MY3), China Medical University Hospital, Taichung, Taiwan (DMR-103–049), partially supported by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212–113002), Health and Welfare surcharge for the sale of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW103-TD-B-111–03, Taiwan), and China Medical University under the Aim for the Top University Plan of the Ministry of Education, Taiwan.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Banks CN, Lein PJ. A review of experimental evidence linking neurotoxic organophosphorus compounds and inflammation. Neurotoxicology 2012; 33:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lionetto MG, Caricato R, Calisi A, et al. Acetylcholinesterase as a biomarker in environmental and occupational medicine: new insights and future perspectives. Biomed Res Int 2013; 2013:321213.doi: 10.1155/2013/321213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang CC, Deng JF. Intermediate syndrome following organophosphate insecticide poisoning. J Chin Med Assoc 2007; 70:467–472. [DOI] [PubMed] [Google Scholar]
- 4.Mishra V, Srivastava N. Organophosphate pesticides-induced changes in the redox status of rat tissues and protective effects of antioxidant vitamins. Environ Toxicol 2013; doi: 10.1002/tox.21924. [DOI] [PubMed] [Google Scholar]
- 5.Abdollahi M, Karami-Mohajeri S. A comprehensive review on experimental and clinical findings in intermediate syndrome caused by organophosphate poisoning. Toxicol Appl Pharmacol 2012; 258:309–314. [DOI] [PubMed] [Google Scholar]
- 6.Heit JA, Silverstein MD, Mohr DN, et al. Predictors of survival after deep vein thrombosis and pulmonaryembolism: a population-based, cohort study. Arch Intern Med 1999; 159:445–453. [DOI] [PubMed] [Google Scholar]
- 7.Bagot CN, Arya R. Virchow and his triad: a question of attribution. Br J Haematol 2008; 143:180–190. [DOI] [PubMed] [Google Scholar]
- 8.Zoller B, Li X, Sundquist J, et al. Risk of pulmonaryembolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet 2012; 379:244–249. [DOI] [PubMed] [Google Scholar]
- 9.Bardin PG, van Eeden SF, Moolman JA, et al. Organophosphate and carbamate poisoning. Arch Intern Med 1994; 154:1433–1441. [PubMed] [Google Scholar]
- 10.Zunec S, Kopjar N, Zeljezić D, et al. In vivo evaluation of cholinesterase activity, oxidative stress markers, cyto- and genotoxicity of K048 oxime–a promising antidote against organophosphate poisoning. Basic Clin Pharmacol Toxicol 2014; 114:344–351. [DOI] [PubMed] [Google Scholar]
- 11.Crinnion WJ. Environmental medicine, part 4: pesticides - biologically persistent and ubiquitous toxins. Altern Med Rev 2000; 5:432–447. [PubMed] [Google Scholar]
- 12.Kmiecik B, Skotny A, Batycka M, et al. Influence of oxidative stress on tissue regeneration. Polim Med 2013; 43:191–197. [PubMed] [Google Scholar]
- 13.Samad F, Ruf W. Inflammation, obesity, and thrombosis. Blood 2013; 122:3415–3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eisenhardt SU, Thiele JR, Bannasch H, et al. C-reactive protein: how conformational changes influence inflammatory properties. Cell Cycle 2009; 8:3885–3892. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Lee YH, Park YH, et al. The difference in C-reactive protein value between initial and 24 hours follow-up (D-CRP) data as a predictor of mortality in organophosphate poisoned patients. ClinToxicol (Phila) 2013; 51:29–34. [DOI] [PubMed] [Google Scholar]


