Abstract
In this article, a retrospective analysis of 161 female patients with adolescent idiopathic scoliosis (AIS) is performed who underwent posterior correction and fusion using all-pedicle screw instrument.
The aim of this article is to find out preoperative factors that influence intraoperative blood loss (IOBL) in female patients with AIS.
The IOBL in posterior correction and fusion surgery for patients with idiopathic scoliosis greatly varies. The variables affecting the IOBL also greatly vary among different studies.
Medical records of all female patients with AIS who underwent posterior correction and fusion operations using the all-pedicle screw system in our hospital from January 2012 to January 2014 were reviewed. Patients with irregular menstruation, who underwent osteotomy, and using coagulants were excluded. Preoperative clinical data, including patient age, height, weight, Risser sign, day after last menstruation, major curve Cobb angle, fulcrum-bending Cobb angle, curve flexibility index, sagittal thoracic Cobb angle, sagittal lumbar Cobb angle, albumin, hemoglobin, platelet, activated partial thromboplastic time (APTT), prothrombin time, thrombin time, fibrinogen, fusion level, menstrual phase, and blood type, were collected. Data were further analyzed using multiple linear regression with forward elimination.
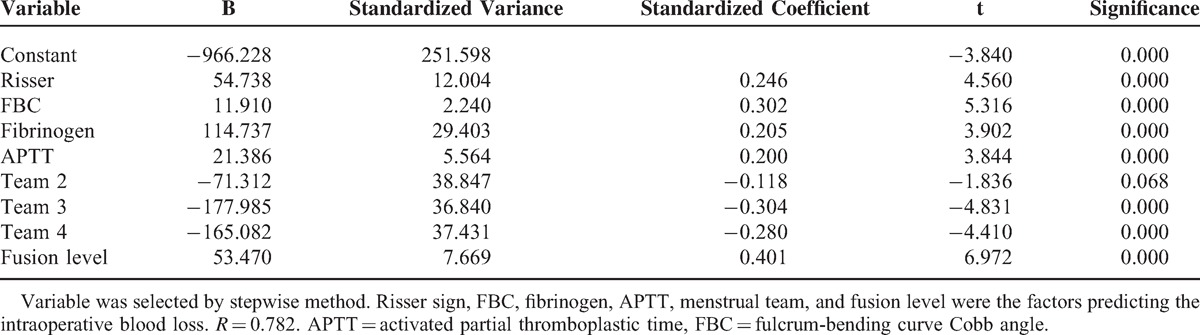
A total of 161 patients were included in this study. The mean IOBL was 933.98 ± 158.10 mL (500–2000 mL). Forward selection showed that fulcrum-bending Cobb angle, fusion level, Risser sign, APTT, fibrinogen, and menstrual phase were the preoperative factors that influenced the IOBL in female patients with AIS. Equation of IOBL was built by multiple linear regression: IOBL = −966.228 + 54.738 Risser sign + 18.910 fulcrum-bending Cobb angle + 114.737 fibrinogen + 21.386 APTT − 71.312 team 2 − 177.985 team 3 − 165.082 team 4 + 53.470 fusion level. R = 0.782.
Operation for patients with AIS was featured by large IOBL. Large fulcrum-bending Cobb angle, the number of level fused, higher Risser sign, high APTT, high preoperative blood fibrinogen concentration, and premenstrual phase predicted higher IOBL.
INTRODUCTION
Adolescent idiopathic scoliosis (AIS) is the most common form of scoliosis, which is defined as a 3-D deformity and consists of side curve >10°, deviation of sagittal spinal profile, and vertebrae rotation in the transverse plane, with a prevalence reported to be 2% to 3% in adolescent population.1 Posterior correction with multilevel spinal fusion using all-pedicle screw systems has been established as the primary approach for patients with AIS.2,3 Although multilevel spinal fusion using hook and/or pedicle screw constructs have shown several benefit in treating AIS,4 several intraoperative disadvantages and postoperative complications, including infection,5 blood loss,6 increased allogeneic blood transfusion (ALBT),7 screw loosening,8 proximal junctional kyphosis,9 adding-on phenomenon,10 and others,11 have been observed at either short or long follow-up time.
Among these complications, it was reported that intraoperative blood loss (IOBL) can be ranged from 750 to 1500 mL in posterior correction with multilevel spinal fusion surgery.6,12 Large IOBL has been proved to be associated with hypotension, anemia, coagulopathy, infection, allergic reactions, acute or delayed immune hemolytic reactions, iron overload, and graft-versus-host diseases.13
Therefore, it is essential to find out the predictors of IOBL, and take measures such as blood salvage appliance or hemostatics to reduce the IOBL based on these risk factors, thus to reduce the complications of serious hemorrhage. At the same time, we can also avoid side effect of hemostatics and the lavish of unnecessary blood saving appliance based on the predictors.
As is well known, female patients take the majority of patients with AIS. For patients with a major curve >30°, the female/male ratio could be as high as 10:1 and female patients were 70% to 90% of the patients with AIS who need surgical intervention.14,15 The ratio may become larger while comparing the sexual difference when the curve Cobb is >40°—when surgical intervention may be needed. Former studies have shown that the hemostatic factor, which may affect the IOBL, has variation in different phases during normal menstrual cycle.6,16 Also male and female patients with AIS have different IOBL during scoliosis surgery.6 Therefore, it is necessary to figure out the specific predictors for IOBL in female patients with AIS.
In this retrospective study, we collected preoperational clinical data of 161 female patients with AIS and analyzed them with multivariable regression, in order to find preoperative factors that might predict IOBL during posterior correction and fusion surgery for female patients with AIS.
MATERIALS AND METHODS
Patient Population
A total of 161 patients, who met the inclusion and exclusion criteria, and received the posterior correction and fusion operation using the all-pedicle screw system in our hospital from January 2012 to January 2014, were retrospectively reviewed. The inclusion criteria of patients were as follows: female patients with AIS; who underwent posterior correction and fusion surgery only; and the appliance used was all-pedicle screw system. Patients with other types of scoliosis, irregular menstruation, who underwent osteotomy, revision surgery, coagulative diseases that may affect blood coagulation, such as idiopathic thrombocytopenic purpura or hemophilia, and using coagulants were excluded from the study. The diagnosis of AIS was made by 2 experienced senior attending doctors independently following the description of Weinstein et al.17 Patients whose diagnosis was different were excluded from the study. This study was approved by the Institutional Review Board of our university, and all patients involved provided written informed consent for the study and surgery.
Data Collection
Data were collected in every patient, including the demographic data, radiographic measurements, and the data of preoperative blood laboratory tests. Curve flexibility index was calculated by the following equation:
 |
Menstrual group was decided by the following: (team 1: premenstrual group, 24-day menstruation; team 2: follicle group, 6–11 days; team 3: ovulatory group, 12–17 days; and team 4: luteal group, 18–23 days). Spinal thoracic sagittal and spinal lumbar sagittal groups were further decided according to the lumbar lordosis and thoracic curve, respectively. The summary of data collection is shown in Table 1.
TABLE 1.
Summary of Data Collection
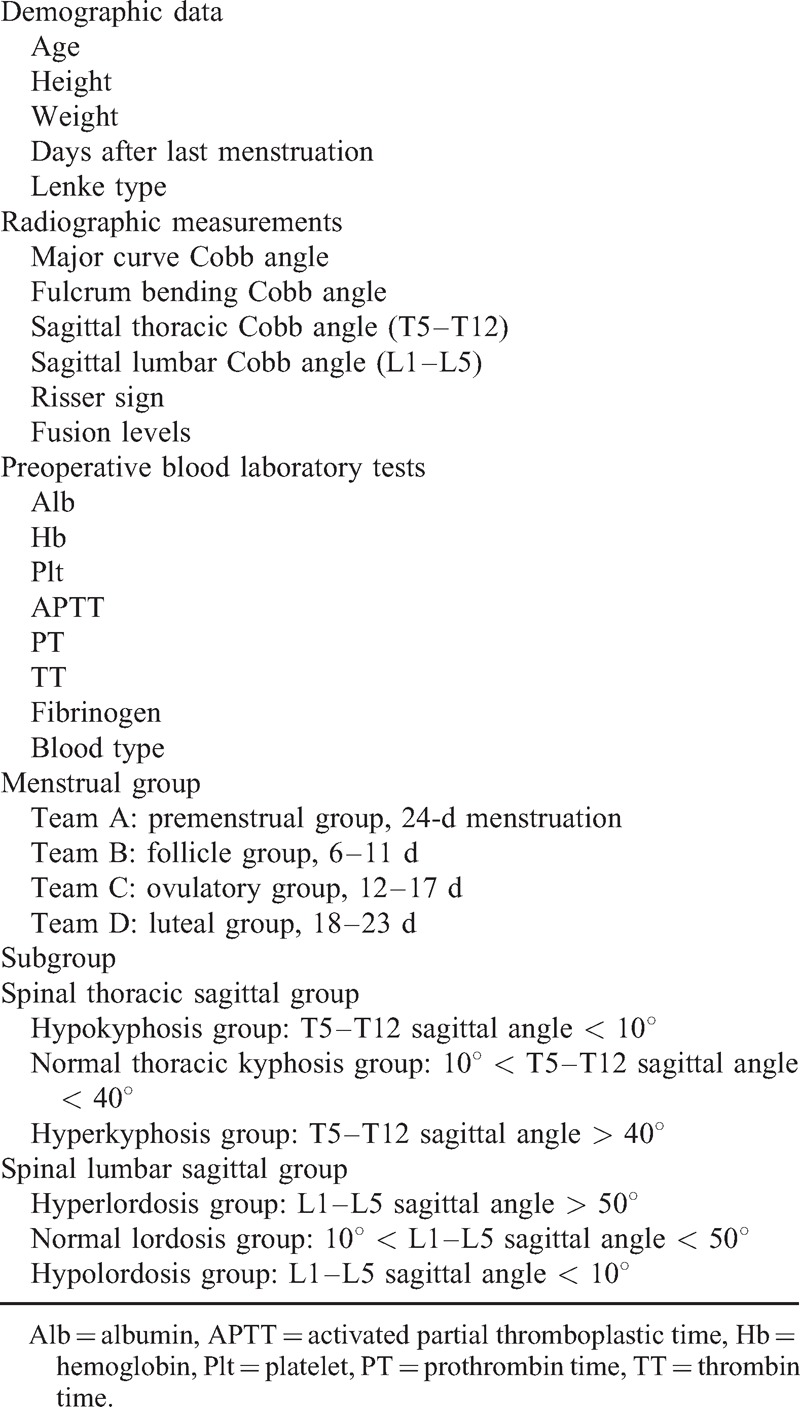
Variables such as menstrual group, scoliosis Lenke type, blood type, thoracic sagittal angle group, and lumbar sagittal angle group were changed into dummy variables.
Statistical Analysis
Data from different groups were analyzed using multiple linear regression model. Affecting factor was confined to 6. Forward method was used to select affecting factors. After affecting factors had been decided, enter method was used to build the multiple linear regression model. Statistical Package for Social Science software 20.0 (SPSS Inc, Chicago, IL) was used to perform the statistical analysis. P < 0.05 was selected as significant level. Graphs were drawn using GraphPad Prism 5.0 (GraphPad Software Inc, San Diego, CA).
RESULTS
A total of 161 patients were included in this study. Patients were divided into different groups according to the lumbar lordosis and thoracic curve. There were 121 patients with normal lumbar lordosis (10°–50°), 15 with hyperlordosis (>50°), and 25 with lumbar hypolordosis (<10°). Besides, there were 12 patients with thoracic hypokyphosis (>40°), 137 patients with normal thoracic curve (20°–40°), and 12 patients with thoracic hyperkyphosis (<20°). All data are presented in Tables 2 and 3.
TABLE 2.
General Characteristics of Female Patients With AIS
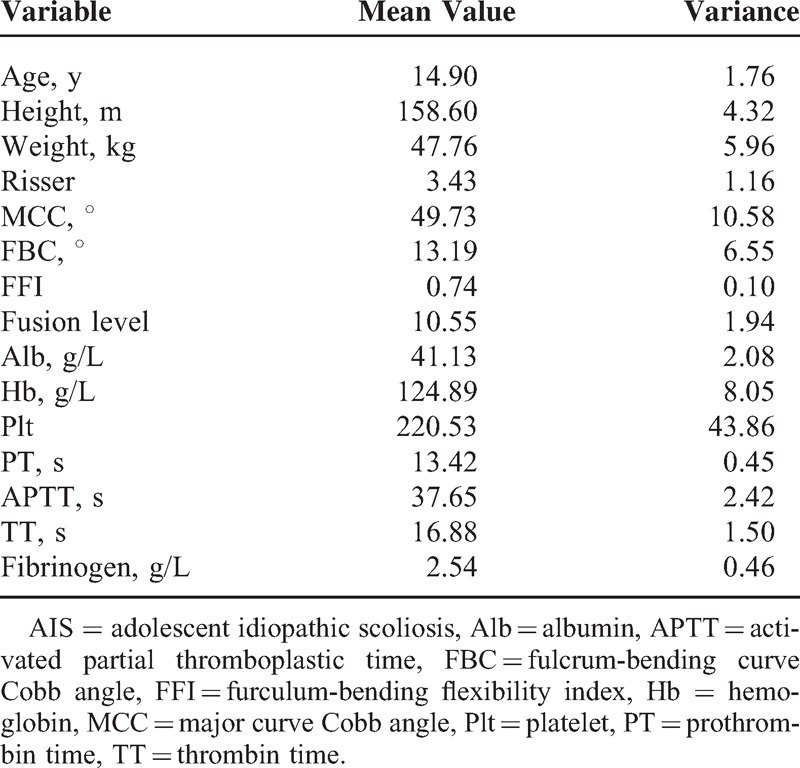
TABLE 3.
Characteristics of Dummy Variable

Forward variable selection showed that bending Cobb angle, fusion level, Risser sign, APTT, fibrinogen, and menstrual phase were the factors that influenced IOBL in female patients with AIS. Equation of IOBL was built by multiple linear regression: IOBL = −966.228 + 54.738∗Risser + 18.910∗fulcrum bending Cobb angle + 114.737∗fibrinogen + 21.386∗APTT - 71.312∗team 2 - 177.985∗team 3 - 165.082∗team 4 + 53.470∗fusion level. R = 0.782. The details are presented in Table 4.
TABLE 4.
Regression Result of Data Predicting Intraoperative Blood Loss
DISCUSSION
Blood loss, due to the surgical exposure of muscles, associated venous plexus, prolonged operative times, and significant bone bleeding, is a great concern in the procedure of AIS surgery. It is one of the major causes of morbidity18 and a risk factor of spinal fusion for AIS.19,20 Consequently, ALBT is required during the operation to prevent the complications of excessive blood loss. It was reported that blood transfusion is needed in 37% to 85% of patients in the posterior correction and fusion surgery for patients with AIS.21,22 However, a large number of complications, including allergic reactions, blood-borne infections,23,24 graft versus host disease,25 and others,24 have been observed in the procedure of allogeneic blood replacement. Besides, a heavy cost associated with transfusion is also a big burden for patients.26 Several studies6,27 have been conducted to explore the predictors of blood loss in the surgery. Ialenti et al6 conducted a retrospective study that included 340 patients with AIS and found sex, operative time, and preoperative kyphosis to be the most important predictors of increased blood loss in posterior spinal fusion for AIS. Hassan et al27 reported a 2-year review with 110 patients with scoliosis and confirmed that Cobb angles and number of fused segments are predictors of the IOBL. Meert et al28 found that fused level is an independent predictor of IOBL. In their study, they also found that some of the blood-saving appliances were actually not necessary, and therefore predicting the IOBL was important. However, when studying the predictors for IOBL, all of these studies did not separate the male and female patients.
In our study, menstrual phase, bending Cobb angle, fusion level, Risser sign, APTT, and fibrinogen were found to be significantly associated with IOBL by forward variable selection; however, other factors, including patient age, height, weight, major curve Cobb angle, curve flexibility index, sagittal thoracic Cobb angle, sagittal lumbar Cobb angle, albumin, hemoglobin, platelet (Plt), prothrombin time (PT)/thrombin time, and blood type, were not found to be significantly related to IOBL.
An innovator of our study was to independently study female patients and take menstrual phase into consideration. In our study, patients operated during 24-d menstruation had more IOBL than the other 3 groups. IOBL is significantly associated with the blood coagulation that involves several elements, including Plt, platelet–leukocyte aggregates, fibrinogen, and others.29 Several studies30,31 suggested that platelets played a significant role in blood coagulation, changing during the menstrual cycle. In the study by Rosin et al,29 platelet–leukocyte interaction by the determination of platelet–leukocyte aggregates, platelet P-selectin expression, and platelet fibrinogen receptor activation by platelet glycoprotein GPIIb/IIIa fibrinogen receptor binding were measured by flow cytometry in 20 healthy women during their menstrual cycle. They found that the number of platelet–granulocyte aggregates and platelet–monocyte aggregates was higher at ovulation compared to any other time-point of the menstrual cycle. Other blood coagulation factors were also thought to experience variation during normal menstrual cycle.16 The reason why the patients had more IOBL during the premenstruation phase might be attributed to the variation of estrogen. Several studies found that a sudden increase in body's estrogen leads to a hypercoagulable state.32,33 As the estrogen level in female body begin to fall on premenstrual phase, this may lead to a hypocoagulable state and cause more IOBL.
Fulcrum-bending Cobb angle and Risser sign show a positive correlation with the IOBL in our study. Yu et al34 reviewed 159 patients and divided them into 2 groups according to the blood loss. They compared the 2 groups and found the preoperative Cobb angle >50° to be a risk factor of large IOBL. Hassan et al35 reviewed a total of 110 patients with scoliosis during a period of 2 years and found that large Cobb angle increased transfusion requirement. From another cohort of 262 patients with AIS, the author reported that the number of levels fused, male sex, duration of surgery, use of pedicle screws, major Cobb angle, and age were predictors of IOBL.36 In our study, the fulcrum-bending Cobb angle instead of the preoperative Cobb angle was a predictor of IOBL. We speculate that this may be because of the flexible part of the curve that can be easily corrected while the rigid part (showing by the fulcrum-bending Cobb angle) is the more challenging and was positively correlated with surgery time and IOBL. Risser sign is also considered as a predictor in our study. Currently, there was no study that mentioned the predict effect of Risser sign. The reason that the Risser sign showed a predict effect may be that our patient population was adolescents experiencing rapid body development, and Risser sign was a comprehensive indicator that positively correlated with age, body weight, total blood volume, and skeleton maturation.
Fibrinogen is an essential protein that is directly involved in fibrin gel formation as the final step of a sequence of reactions triggered by a procoagulant stimulus,37 and APTT testing is integral to hemostasis testing. What was interesting in our study was that the increase in fibrinogen concentration actually led to more IOBL. Former studies showed that estrogen had an ability to increase blood fibrinogen concentration,38 and due to the estrogen variation during normal menstrual cycle, blood fibrinogen might be at its highest level in the premenstrual phase. Also, several studies reported that the blood fibrinogen was lowest after menstruation or during mid-follicle phase.39–41 Our study showed that premenstrual patients had more IOBL. We speculate that these might be the reasons that the fibrinogen was indicating more IOBL in our study. Abnormalities in APTT could lead to the deficiency of coagulation and great blood loss in the operation. Ialenti et al6 studied the effects of PT/APTT on IOBL and found no significant associations between PT/APTT and IOBL. However, fibrinogen and APTT were not analyzed in their study. Cederblad et al42 studied 30 normal women whose blood samples were taken on 6 occasions: days 1, 2, and 3 of menstruation; days 5–9 (follicular phase); days 12–16 (around ovulation); and days 19–23 (luteal phase). They found that the concentration of factor II–VII–X was lowest during menstruation. This result indicated that APTT might achieve its highest value before menstruation and related to the high IOBL in the premenstruation group in our study.
Several studies have been conducted to find whether number of fusion level was the predictor of blood loss in scoliosis surgery. In the study by Meert et al,28 number of vertebrae fused independently predicted a greater number of blood loss and allogeneic red cells transfused. Yoshihara and Yoneoka7 in their study thought that patients fused ≥9 levels were more likely to suffer from great IOBL and receive ALBT compared with those fused 4–8 levels. In our study, we also observed the same results, and in our formula, increased 1 unit of fusion level caused IOBL to increase by 53.470 units. Actually, the level of vertebral fused was the factor that has the highest predict value and is confirmed by most of the studies. This is reasonable because the prolonged fusion level need more explosion, pedicle screw implant, as well as prolonged operation time.
Despite a comprehensive analysis of the association between preoperative risk factors and IOBL, there are some limitations that should be addressed. First, IOBL was estimated by surgeons during the surgery and more precise methods should be used to calculate the blood loss. Second, this result was reported in single center, which might lead to publication bias. Therefore, more precise studies should be performed in multiple-center institutes.
CONCLUSION
Large fulcrum-bending Cobb angle, the number of level fused, higher Risser sign, high APTT, high preoperative blood fibrinogen concentration, premenstrual phase predicted higher IOBL, and a formula was developed to predict blood loss based on preoperative risk factors.
Footnotes
Abbreviations: AIS = adolescent idiopathic scoliosis, Alb = albumin, ALBT = allogeneic blood transfusion, APTT = activated partial thromboplastic time, IOBL = intraoperative blood loss, Plt = platelet, PT = prothrombin time, TT = thrombin time.
CL, MY, and Chao W are co-first authors.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Weinstein SL. Natural history. Spine 1999; 24:2592. [DOI] [PubMed] [Google Scholar]
- 2.Rose PS, Lenke LG, Bridwell KH, et al. Pedicle screw instrumentation for adult idiopathic scoliosis: an improvement over hook/hybrid fixation. Spine 2009; 34:852–857. [DOI] [PubMed] [Google Scholar]
- 3.Suk S-I, Kim J-H, Kim S-S, et al. Pedicle screw instrumentation in adolescent idiopathic scoliosis (AIS). Eur Spine J 2012; 21:13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lonner BS, Kondrachov D, Siddiqi F, et al. Thoracoscopic spinal fusion compared with posterior spinal fusion for the treatment of thoracic adolescent idiopathic scoliosis. J Bone Joint Surg Am 2006; 88:1022–1034. [DOI] [PubMed] [Google Scholar]
- 5.Alsiddiky A, Nisar KA, Alhuzaimi F, et al. Wound healing without drains in posterior spinal fusion in idiopathic scoliosis. J Coll Phys Surg Pak 2013; 23:558–561. [PubMed] [Google Scholar]
- 6.Ialenti MN, Lonner BS, Verma K, et al. Predicting operative blood loss during spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop 2013; 33:372–376. [DOI] [PubMed] [Google Scholar]
- 7.Yoshihara H, Yoneoka D. Predictors of allogeneic blood transfusion in spinal fusion for pediatric patients with idiopathic scoliosis in the United States, 2004-2009. Spine (Phila Pa 1976) 2014; 39:1860–1867. [DOI] [PubMed] [Google Scholar]
- 8.Abul-Kasim K, Ohlin A. Evaluation of implant loosening following segmental pedicle screw fixation in adolescent idiopathic scoliosis: a 2 year follow-up with low-dose CT. Scoliosis 2014; 9:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yagi M, King AB, Boachie-Adjei O. Incidence, risk factors, and natural course of proximal junctional kyphosis: surgical outcomes review of adult idiopathic scoliosis. Minimum 5 years of follow-up. Spine (Phila Pa 1976) 2012; 37:1479–1489. [DOI] [PubMed] [Google Scholar]
- 10.Cho RH, Yaszay B, Bartley CE, et al. Which Lenke 1A curves are at the greatest risk for adding-on... and why? Spine (Phila Pa 1976) 2012; 37:1384–1390. [DOI] [PubMed] [Google Scholar]
- 11.Lykissas MG, Crawford AH, Jain VV. Complications of surgical treatment of pediatric spinal deformities. Orthop Clin North Am 2013; 44:357–370. [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz G, Borkhuu B, Dhawale AA, et al. Comparative analysis of hook, hybrid, and pedicle screw instrumentation in the posterior treatment of adolescent idiopathic scoliosis,. J Pediatr Orthop 2012; 32:490–499. [DOI] [PubMed] [Google Scholar]
- 13.Carreon LY, Puno RM, Lenke LG, et al. Non-neurologic complications following surgery for adolescent idiopathic scoliosis. J Bone Joint Surgery Am 2007; 89:2427–2432. [DOI] [PubMed] [Google Scholar]
- 14.Raggio CL. Sexual dimorphism in adolescent idiopathic scoliosis. Orthop Clin North Am 2006; 37:555–558. [DOI] [PubMed] [Google Scholar]
- 15.Roberts DW, Savage JW, Schwartz DG, et al. Male-female differences in Scoliosis Research Society-30 scores in adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2011; 36:E53–E59. [DOI] [PubMed] [Google Scholar]
- 16.Knol HM, Kemperman RF, Kluin-Nelemans HC, et al. Haemostatic variables during normal menstrual cycle. Thromb Haemost 2012; 107:22–29. [DOI] [PubMed] [Google Scholar]
- 17.Weinstein SL, Dolan LA, Cheng JC, et al. Adolescent idiopathic scoliosis. Lancet 2008; 371:1527–1537. [DOI] [PubMed] [Google Scholar]
- 18.Akgul T, Dikici F, Ekinci M, et al. The efficacy of cell saver method in the surgical treatment of adolescent idiopathic scoliosis. Acta Orthop Traumatol Turc 2014; 48:303–306. [DOI] [PubMed] [Google Scholar]
- 19.Guigui P, Blamoutier A. Complications of surgical treatment of spinal deformities: a prospective multicentric study of 3311 patients. Revue Chir Orthop Reparatrice Appar Mot 2005; 91:314–327. [DOI] [PubMed] [Google Scholar]
- 20.Shapiro F, Sethna N. Blood loss in pediatric spine surgery. Eur Spine J 2004; 13 Suppl 1:S6–S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bess RS, Lenke LG, Bridwell KH, et al. Wasting of preoperatively donated autologous blood in the surgical treatment of adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2006; 31:2375–2380. [DOI] [PubMed] [Google Scholar]
- 22.Joseph SA, Jr, Berekashvili K, Mariller MM, et al. Blood conservation techniques in spinal deformity surgery: a retrospective review of patients refusing blood transfusion. Spine (Phila Pa 1976) 2008; 33:2310–2315. [DOI] [PubMed] [Google Scholar]
- 23.Kuklo TR, Owens BD, Polly DW., Jr Perioperative blood and blood product management for spinal deformity surgery. Spine J 2003; 3:388–393. [DOI] [PubMed] [Google Scholar]
- 24.Ridgeway S, Tai C, Alton P, et al. Pre-donated autologous blood transfusion in scoliosis surgery. J Bone Joint Surg Brit 2003; 85:1032–1036. [DOI] [PubMed] [Google Scholar]
- 25.Dodd RY. Current risk for transfusion transmitted infections. Curr Opin Hematol 2007; 14:671–676. [DOI] [PubMed] [Google Scholar]
- 26.Blanchette CM, Wang PF, Joshi AV, et al. Cost and utilization of blood transfusion associated with spinal surgeries in the United States. Eur Spine J 2007; 16:353–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hassan N, Halanski M, Wincek J, et al. Blood management in pediatric spinal deformity surgery: review of a 2-year experience. Transfusion 2011; 51:2133–2141. [DOI] [PubMed] [Google Scholar]
- 28.Meert KL, Kannan S, Mooney JF. Predictors of red cell transfusion in children and adolescents undergoing spinal fusion surgery. Spine (Phila Pa 1976) 2002; 27:2137–2142. [DOI] [PubMed] [Google Scholar]
- 29.Rosin C, Brunner M, Lehr S, et al. The formation of platelet-leukocyte aggregates varies during the menstrual cycle. Platelets 2006; 17:61–66. [DOI] [PubMed] [Google Scholar]
- 30.Freedman JE, Loscalzo J. Platelet-monocyte aggregates: bridging thrombosis and inflammation. Circulation 2002; 105:2130–2132. [DOI] [PubMed] [Google Scholar]
- 31.Peters MJ, Dixon G, Kotowicz KT, et al. Circulating platelet-neutrophil complexes represent a subpopulation of activated neutrophils primed for adhesion, phagocytosis and intracellular killing. Brit J Haematol 1999; 106:391–399. [DOI] [PubMed] [Google Scholar]
- 32.Ibrahimi E, Koni M. Effects of estrogens and progestagens on the primary variables of haemostasis. Int J Reprod Contracep Obstet Gynecol 2014; 3:31–33. [Google Scholar]
- 33.Van Rooijen M, Bremme K, Rosing J, et al. 69 rapid activation of haemostasis after hormonal emergency contraception. Thromb Res 2007; 119:S116. [PubMed] [Google Scholar]
- 34.Yu X, Xiao H, Wang R, et al. Prediction of massive blood loss in scoliosis surgery from preoperative variables. Spine 2013; 38:350–355. [DOI] [PubMed] [Google Scholar]
- 35.Hassan N, Halanski M, Wincek J, et al. Blood management in pediatric spinal deformity surgery: review of a 2-year experience. Transfusion 2011; 51:2133–2141. [DOI] [PubMed] [Google Scholar]
- 36.Ialenti MN, Lonner BS, Verma K, et al. Predicting operative blood loss during spinal fusion for adolescent idiopathic scoliosis. J Pediatr Orthop 2013; 33:372–376. [DOI] [PubMed] [Google Scholar]
- 37.Hoppe B. Fibrinogen and factor XIII at the intersection of coagulation, fibrinolysis and inflammation. Thromb Haemost 2014; 112:649–658. [DOI] [PubMed] [Google Scholar]
- 38.Swanepoel AC, Lindeque BG, Swart PJ, et al. Estrogen causes ultrastructural changes of fibrin networks during the menstrual cycle: a qualitative investigation. Microsc Res Tech 2014; 77:594–601. [DOI] [PubMed] [Google Scholar]
- 39.Kadir RA, Economides DL, Sabin CA, et al. Variations in coagulation factors in women: effects of age, ethnicity, menstrual cycle and combined oral contraceptive. ThrombHaemost 1999; 82:1456–1461. [PubMed] [Google Scholar]
- 40.Feuring M, Christ M, Roell A, et al. Alterations in platelet function during the ovarian cycle. Blood Coagul Fibrinolysis 2002; 13:443–447. [DOI] [PubMed] [Google Scholar]
- 41.Koh SC, Prasad R, Fong Y. Hemostatic status and fibrinolytic response potential at different phases of the menstrual cycle. Clin Appl Thromb Hemost 2005; 11:295–301. [DOI] [PubMed] [Google Scholar]
- 42.Cederblad G, Hahn L, Korsan-Bengtsen K, et al. Variations in blood coagulation, fibrinolysis, platelet function and various plasma proteins during the menstrual cycle. Pathophysiol Haemost Thromb 1977; 6:294–302. [DOI] [PubMed] [Google Scholar]


