Supplemental Digital Content is available in the text
Abstract
Using nasopharyngeal carriage as a marker of vaccine impact, pneumococcal colonization and its relation to invasive disease were examined in children, their parents, and older adults in the United Kingdom following introduction of 7-valent pneumococcal conjugate vaccine (PCV7) and prior to 13-valent pneumococcal conjugate vaccine (PCV13).
A cross-sectional observational study was conducted, collecting nasopharyngeal swabs from children aged 25 to 55 months who had previously received 3 doses of PCV7, their parents, and adults aged ≥65 years. Pneumococcal serotyping was conducted according to World Health Organization guidelines with nontypeable isolates further analyzed by molecular serotyping. A national invasive disease surveillance program was conducted throughout the corresponding period.
Pneumococcus was isolated from 47% of children, 9% of parents, and 2.2% of older adults. For these groups, the percentage of serotypes covered by PCV7 were 1.5%, 0.0%, and 15.4%, with a further 20.1%, 44.4%, and 7.7% coverage added by those in PCV13. In each group, the percentage of disease due to serotypes covered by PCV7 were 1.0%, 7.4% and 5.1% with a further 65.3%, 42.1%, and 61.4% attributed to those in PCV13.
The prevalence of carriage is the highest in children, with direct vaccine impact exemplified by low carriage and disease prevalence of PCV7 serotypes in vaccinated children, whereas the indirect effects of herd protection are implied by similar observations in unvaccinated parents and older adults.
INTRODUCTION
Pneumococcal disease inflicts its greatest burden on children and the elderly. In children, it causes invasive diseases such as meningitis, pneumonia, and bacteremia, as well as mucosal diseases such as otitis media and sinusitis. Worldwide it is estimated to be responsible for 11% of all deaths in children <5 years of age, with a significant proportion of survivors going on to develop long-term sequelae.1,2 In adults aged ≥65 years, invasive pneumococcal disease (IPD), primarily in the form of pneumonia, is estimated to have an annual incidence in the range of 24 to 85/100,000.3 Older adults with community-acquired pneumonia have a mortality of 37.7%4 with a further 12% requiring long-term institutional care.5
Pneumococcus normally resides asymptomatically in the nasopharynx, rarely causing invasive disease. Studies have demonstrated that up to 50% of children acquire the organism by 6 months with carriage peaking at 3 years of age.6,7 Carriage rates throughout adulthood appear to progressively decline8,9; however, the data are relatively limited. Demographic factors such as crowding and smoking are believed to play a role in transmission,10 with increased rates of disease seen in institutional settings,11,12 and carriage in children who are exposed to other children either within the family or at daycare.10,13
Colonization with pneumococcus is an integral step to develop IPD, with the correlation between carriage and IPD clearly established and utilized to directly assess vaccine impact.14–16 In addition, carriage studies may also provide information on circulating antibiotic resistance, newly emergent serotypes, and enable predictions on the future impact of new vaccines.
There are nearly 100 known different serotypes of pneumococcus. Prior to the introduction of pneumococcal conjugate vaccination in the United Kingdom, 12 serotypes were responsible for 79% of IPD across all ages during 2004 to 2005.17 For children <5 years of age, approximately 70% of IPD was attributed to the serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F) covered by the 7-valent pneumococcal conjugate vaccine (PCV7) during 2004 to 2005.18 This narrow spectrum of disease-causing serotypes may reflect differences in both invasive disease potential and carriage frequency between serotypes.19
PCV7 was introduced into the UK immunization schedule in 2006, accompanied by a catch-up program immunizing all those <2 years of age. Since the introduction of PCV7, there has been >80% reduction in IPD caused by vaccine serotypes in children aged 2 to 4 years.20 The effect of herd protection has also been noted with indirect benefits evident in older age groups.21,22 At the same time, there has been vaccine serotype replacement, with increased carriage and IPD from non-PCV7 serotypes.21,23
A 13-valent pneumococcal conjugate vaccine (PCV13), containing the 7 serotypes found in PCV7 with the addition of a further 6 (1, 3, 5, 6A, 7F, and 19A), was incorporated into the UK immunization schedule, replacing PCV7 from April 2010. PCV13 contains the serotypes responsible for causing >70% of IPD in children <5 years of age from 2007 to 2008 in the United Kingdom.24
The transition from PCV7 to PCV13 in the UK immunization schedule provides a unique opportunity to examine the population effects on pneumococcal carriage and disease. Thus, this study aims to determine the nature of pneumococcal carriage in children, their parents, and older adults since the introduction of PCV7 and prior to wider coverage with PCV13. Additionally, the incidence of IPD and the relationship of demographic factors to carriage were also examined.
MATERIALS AND METHODS
Ethics
This study was performed in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Guidelines for Good Clinical Practice. Ethical approval was obtained from the National Research Ethics Service REC Numbers: 10/H0606/48 and 10/H0606/49.
Study Participants
A cross-sectional study was conducted, assessing pneumococcal carriage among healthy children aged 25 to 55 months, their parents, and adults aged ≥65 years from the Thames Valley region in the United Kingdom between November 2010 and September 2011. Study objectives were to determine, in a population under surveillance for IPD, the nasopharyngeal carriage rates of serotypes contained within PCV13 and those not contained within PCV13.
Children born between July 2006 and February 2009 were recruited along with a subset of their parents. Invitations to participate in this study were distributed via the child health computer department and/or through their daycare facility. Older adults were approached via their general practitioner, attendance at the University of the Third Age (an organization that aims to educate retired members of the community), or their influenza vaccination clinic.
Children who had previously received PCV13, incomplete immunization with PCV7, or parents/legal guardians unable or unwilling to give informed consent were excluded. In the parent and older adult groups, those who had previously received any pneumococcal polysaccharide conjugate vaccine or were unwilling or unable to give informed consent were excluded. Any potential participant, who was unwell with an acute respiratory illness, was febrile above 38°C on the day of the visit or preceding 24 hours or had a condition, which in the opinion of the investigator would put them at risk or compromise the integrity of the study, were also excluded.
Specimen Collection and Identification of Pneumococcal Isolates
Following assessment of eligibility and attainment of consent, the nasopharynx of each participant was swabbed according to the World Health Organization (WHO) guidelines.25 A single flexible aluminum shaft swab with rayon tip (Medical Wire & Equipment, Wiltshire, England) was passed through the anterior nares as far as the posterior pharynx and rotated 180° before removal. Swabs were cut into a tube of skim-milk-tryptone-glucose-glycerine transport medium, maintained at 2°C to 8°C, transported to the Nuffield Department of Clinical Medicine, University of Oxford, and cultured on blood agar within 8 hours of collection. Up to 3 morphologically distinct α-hemolytic colonies were selected for serotyping after overnight incubation at 37°C in 5% carbon dioxide. Pneumococcal isolates were identified by colony morphology. Serotyping was initially performed using the Quellung reaction with sera from Statens Serum Institut, Copenhagen, Denmark.
Samples that were nontypeable after 2 attempts via Quellung reaction were then analyzed by microarray-based molecular serotyping, detecting cps genes and genome content by arrayCGH (aCGH). Genomic DNA was extracted as previously described.26 Molecular serotyping was performed on DNA extracts using the BμG@S SP-CPS v1.4.0 microarray (St. Georges, University of London, London, United Kingdom). Briefly, DNA samples were fluorescently labelled and hybridized to the Agilent 8 × 15K format microarray (Agilent Technologies, Chershire, United Kingdom) according to manufacturer's instructions for the Agilent genomic DNA ULS labelling and Oligo aCGH hybridization reagent kits (Agilent Technologies, Chershire, United Kingdom). Data were statistically analyzed using a Bayesian hierarchical model27 to determine serotype, or combination of serotypes, present in the sample and assign a relative abundance to each serotype detected.
National IPD Data Collection
The numbers of IPD in each age group, for each serotype, were extracted from the national database for England and Wales maintained by Public Health England for the corresponding period of nasopharyngeal swab collection.28 Serotyping of IPD isolates was performed by Public Health England.
Sample Size and Statistical Analysis
A sample size of 600 children was selected to provide estimates with tight confidence intervals (CIs) that would allow future comparison to be made after commencement of the PCV13 program. Comparing serotype-specific pneumococcal carriage rates with those from studies conducted in 1999 to 20016 and 2003, this study would have approximately 90% power at the 5% significance level to find a difference in prevalence of 2% compared with 0%, or of 5% compared with 10%, or of 10% compared with 17%. No prior data were available for parents and older adults in this population and thus such projections could not be made.
Univariate logistic regression analyses were performed on data sets from all 3 age cohorts. Subjects with missing information for a variable were excluded from analysis for that variable only. CIs for proportions were calculated using the binomial exact method. Using the assumption that carriage rates in the study population are equivalent to those across the entire corresponding age group of England and Wales, odds ratios (ORs) were calculated as the odds of IPD for a particular serotype divided by the odds of carriage for the same serotype. A value of 0.5 was added prior to calculation of ORs to correct for 0 values. Analyses were conducted using R version 2.13.1 (Bell Laboratories, New Jersey, United States of America), Stata version 13, and GraphPad Prism 6 (GraphPad Software Inc, California, United States of America).
RESULTS
Following screening and informed consent, 600 subjects in the child age group were eligible for participation. Eight children were excluded prior to swabbing, whereas swabbing was subsequently refused in 17 subjects. The parent group consisted of 100 enrolled subjects who all completed the study. For the older adult group, 606 subjects were eligible following screening and informed consent. Six participants were excluded prior to swabbing, whereas 1 sample was received unlabelled and was also excluded. A total of 575 children, 100 parents, and 599 older adults were, therefore, enrolled into the study between November 2010 and September 2011 (Table 1). There was a significant bias toward female participants in the parent group.
TABLE 1.
Characteristics of Pneumococcal Carriage Study Subjects

Nasopharyngeal Carriage Across 3 Generations
Pneumococcal serotypes were identified by Quellung reaction on 247, 9, and 12 swabs from children, parents, and older adults, respectively. In the corresponding groups, microarray analysis allowed identification of pneumococci from a further 22, 0, and 1 sample/s. The majority of pneumococci (13/23) identified by microarray did not possess a cps gene and thus were classified as nontypeable. Overall, a total of 269/575 (46.8%) (95% CI 42.6–50.1), 9/100 (9.0%) (95% CI 4.2–16.4), and 13/599 (2.2%) (95% CI 1.2–3.7) swab cultures were positive for pneumococcus in the children, parent, and older adult groups, respectively (Figure 1A).
FIGURE 1.
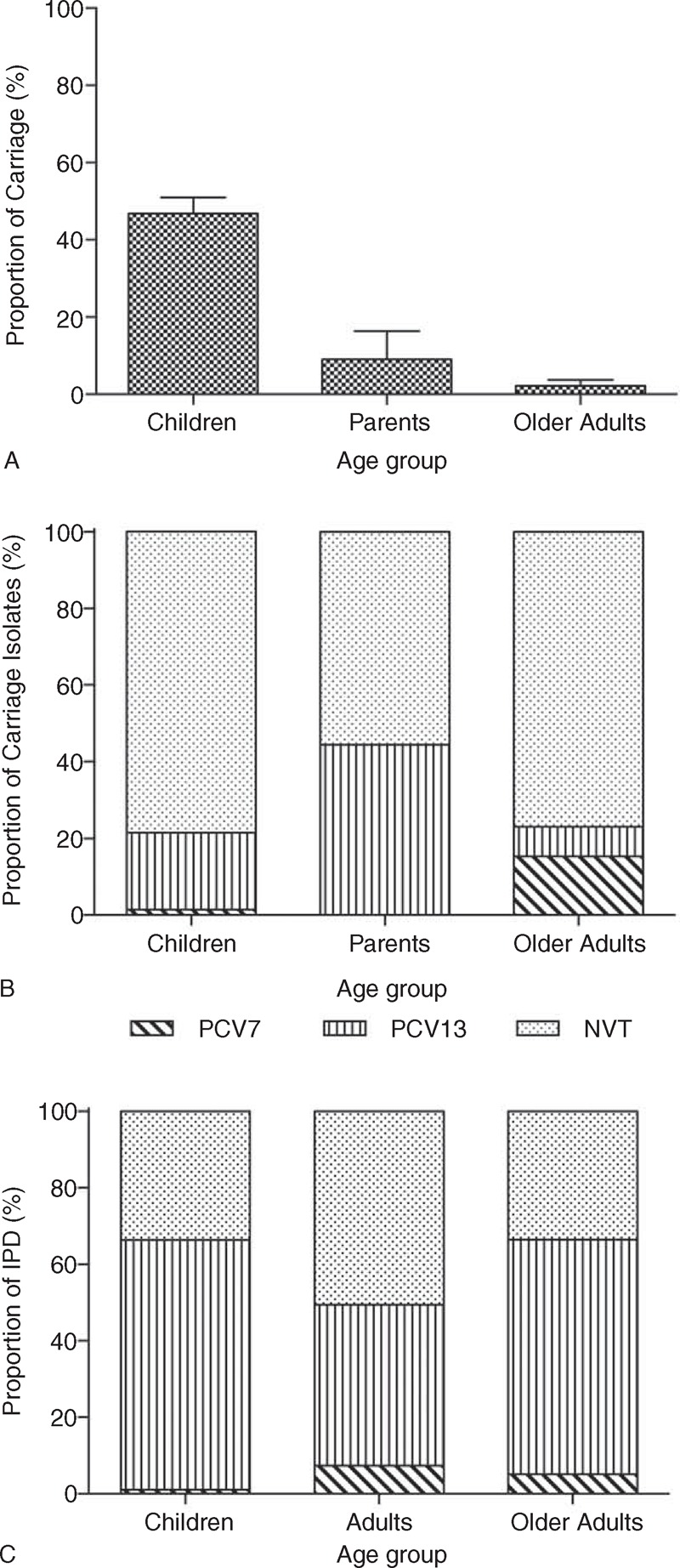
Total proportion of nasopharyngeal swabs from children (aged 25–55 months), their parents, and older adults (aged ≥65 years), collected between November 2010 and September 2011, which had pneumococcus identified (A). (Bars indicate 95% CI). These were then categorized according to whether the serotype was covered by the PCVs PCV7 and PCV13 or was a NVT (B). IPD isolates from across England and Wales for children, adults, and older adults, and across the corresponding time period, were serotyped by Public Health England and categorized according to whether the serotype was covered by the PCVs PCV7 and PCV13 or was a NVT (C). CI = confidence interval, IPD = invasive pneumococcal disease, NVT = nonvaccine type, PCV13 = 13-valent pneumococcal conjugate vaccine, PCV7 = 7-valent pneumococcal conjugate vaccine.
Nasopharyngeal Carriage and IPD due to Vaccine Serotypes
Pneumococcal serotypes were categorized according to whether they were included in PCV7 and PCV13 or were nonvaccine serotypes (NVTs) (Figure 1B). PCV7 serotypes accounted for 1.5%, 0.0%, and 15.4% of the total isolates in the children, parent, and older adult groups, respectively. In the corresponding groups, PCV13 serotypes accounted for an additional 20.1%, 44.4%, and 7.7% of the total isolates.
During the carriage study period, a total of 98, 1892, and 727 cases of IPD occurred across England and Wales, in children, adult, and older adult age groups, respectively.28 These IPD isolates were categorized according to whether the serotype was covered by PCV7 and PCV13 or was a NVT (Figure 1C). PCV7 serotypes accounted for 1.0%, 7.4%, and 5.1% of isolates from children, adult, and older adult groups, respectively. In the same groups, PCV13 serotypes accounted for an additional 65.3%, 42.1%, and 61.4% of the total isolates.
Serotype-Specific Nasopharyngeal Carriage
From the 269 children who had at least 1 serotype of pneumococcus isolated, 5 (1.9%) had 2 serotypes identified (4 of these were by microarray). In this group, a total of 35 different serotypes were identified (Figure 2A). Within the serotypes covered by PCV7, 6B (0.7%) was the most frequently isolated. Of the serotypes covered by PCV13, 3 (6.6%) and 19A (9.9%) predominated. Most frequently identified NVTs were 6C (9.1%), 11A (8.8%), and 23B (8.8%). Analysis of demographic data showed a trend toward an association between carriage and daycare attendance (OR 1.68, 95% CI 0.96–2.96, P = 0.07) (Table 2).
FIGURE 2.
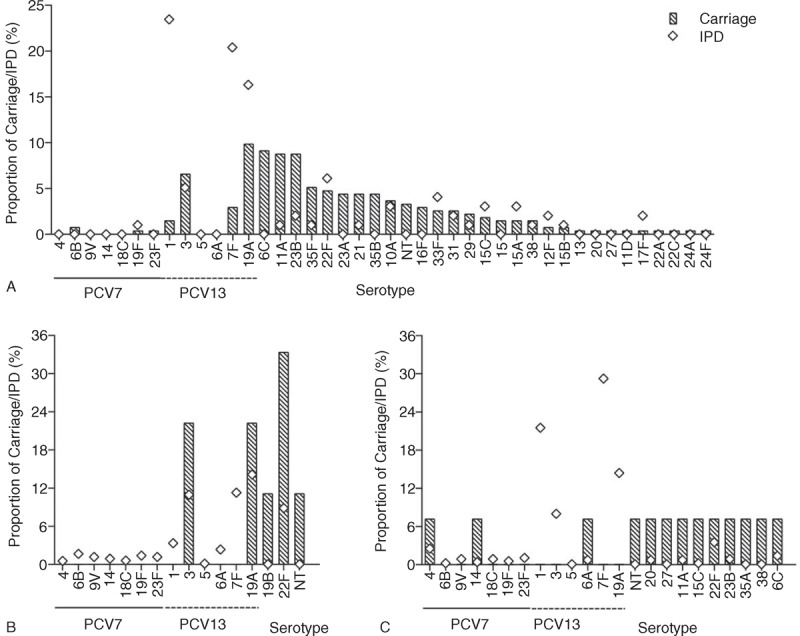
The proportion of nasopharyngeal pneumococcal carriage by each identified serotype in PCV7-vaccinated children aged 25 to 55 months (A), their parents (B), and older adults (C) collected between November 2010 and September 2011 (bars), and the proportion of IPD in England and Wales, identified by Public Health England, over the corresponding time period (diamonds). IPD = invasive pneumococcal disease, NT = nontypeable, PCV7 = 7-valent pneumococcal conjugate vaccine.
TABLE 2.
Pneumococcal Carriage in Children and its Relationship to Demographic Factors

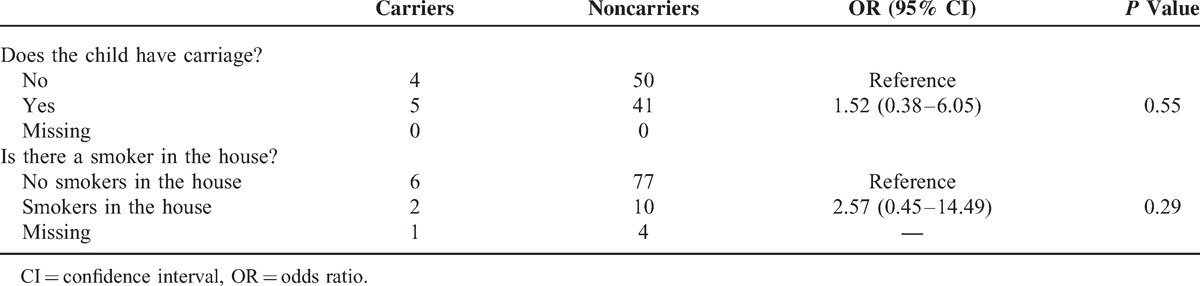
From the 9 isolates obtained from parents, 4 distinct serotypes were identified, with 22F (33.3%) the most frequently found (Figure 2B). No isolates were from serotypes covered by PCV7. Of the additional serotypes covered by PCV13, 3 (22.2%) and 19A (22.2%) were isolated. Of the colonized parents, 5 (55.6%) had children who also had pneumococcus isolated; however, no parent had the same serotype as their child. There was no significant association between a parents being a carrier if their child was a carrier (P = 0.55) (Table 3).
TABLE 3.
Pneumococcal Carriage in Parents and its Relation to Smoking Status
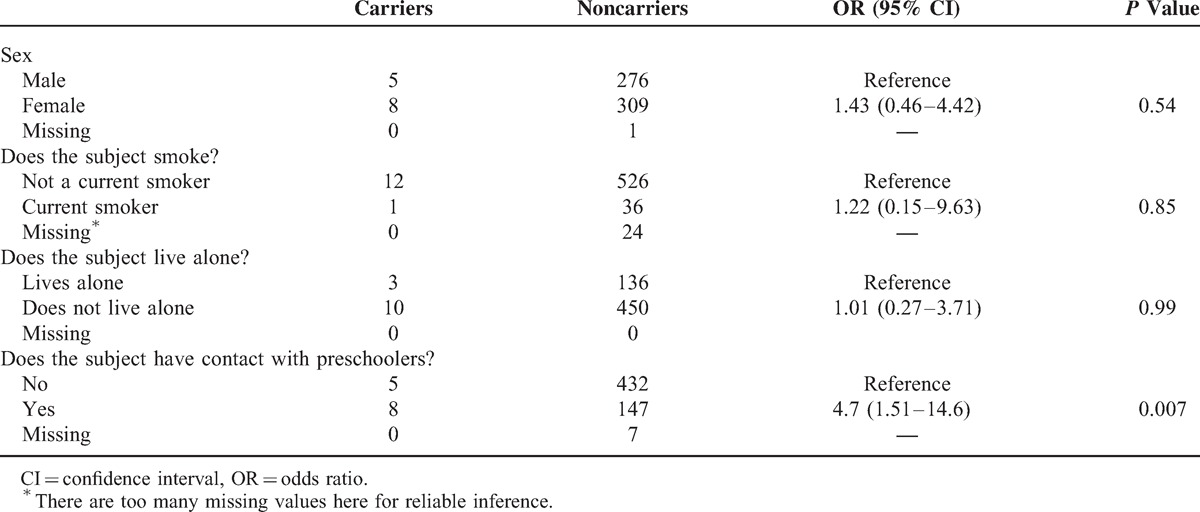
In the older adult participants, pneumococcus was isolated from 13/599 (2.2%), with 12 distinct serotypes and 1 identified as nontypeable (Figure 2C). All serotypes identified were found on a single occasion. Serotypes 4 and 14 were the only isolates identified that are covered by PCV7. Of the additional serotypes covered by PCV13, only 6A was identified. Demographic data analysis demonstrated a significant relationship between the odds of carriage and contact with preschoolers (OR 4.7, 95% CI 1.51–14.6, P = 0.007) (Table 4).
TABLE 4.
Demographic Factors Affecting Carriage in Older Adults
Serotype-Specific IPD
From IPD samples across all the age groups, a total of 57 different pneumococcal serotypes were identified. For each age group, there were 20, 47, and 43 different serotypes identified in children, adults, and older adults, respectively. In all age groups, the 3 serotypes with the highest proportion of IPD are included in PCV13 but not PCV7. These were serotypes 1 (23.5%), 7F (20.4%), and 19A (16.3%) in the child group, 19A (14.1%), 7F (11.2%), and 3 (10.9%) in the adult group, and 7F (24.4%), 1 (17.9%), and 19A (12.0%) in the older adult group (Figure 2). The largest discordances, between carriage and disease, were seen with serotypes 1 and 7F for all groups. The ORs of IPD to carriage were calculated for each of the serotypes covered by PCV13 in the child group and related to carriage frequency (Figure 3). These data demonstrate that both serotypes 1 (OR 21.4, 95% CI 7.2–63.7) and 7F (OR 8.8, 95% CI 3.7–20.8) have the highest odds of IPD relative to carriage. The serotype-specific OR relative to carriage frequency of NVTs for children demonstrates the spread of different IPD potentials (Supplementary Figure 1, http://links.lww.com/MD/A122). Similar calculations in the parent and older adult groups are not demonstrated due to the low carriage and disease frequencies of vaccine serotypes observed.
FIGURE 3.
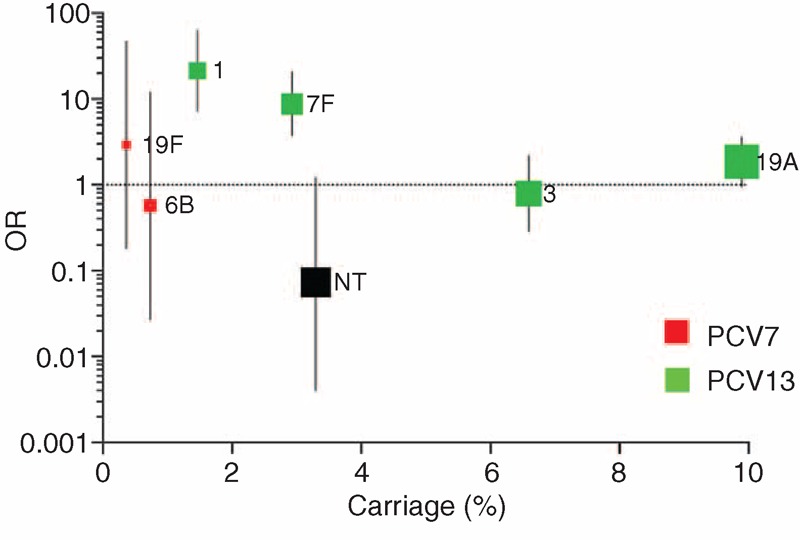
The invasive disease potential expressed as an odds ratio with 95% CI for serotypes covered by PCV13 were calculated and represented against the proportion of nasopharyngeal carriage in the preschool children age group. The size of the marker is relative to the ranking of the serotype's OR weighting. Serotypes 4, 5, 6A, 9V, 14, and 18C were not isolated from the nasopharynx, whereas serotype 23F did not cause disease in any children and hence is not represented. CI = confidence interval, OR = odds ratio, PCV13 = 13-valent pneumococcal conjugate vaccine.
DISCUSSION
This is the first study to demonstrate serotype-specific carriage of pneumococcus within 3 generations of the same community after introduction of PCV7 into the routine immunization schedule. These data demonstrate that preschool-aged children form the primary reservoir of pneumococcal carriage in the community, and transmission to older age groups is facilitated by interaction with them. The direct impact of PCV7 on carriage and IPD is demonstrated by the low rates observed in vaccinated children, with indirect effects implied by the low prevalence seen in the unvaccinated parents and older adults. The high proportion of carriage and disease attributed to the additional serotypes covered by PCV13 suggest that introduction of the higher valency vaccine into this population will result in a significant decrease in IPD and carriage.
The high overall carriage rates seen in the child group are consistent with previous studies conducted in the United Kingdom and other industrialized countries.7,29,30 Comparatively lower carriage rates were seen in the parent, and even more so, in the older adult groups. This finding is consistent with current, albeit limited literature in adults from industrialized countries, which ranges from 5.4% to 8%,30,31 and a study in older adults showing a 0.3% carriage prevalence.8 In addition, a recent study has demonstrated oropharyngeal sampling compared with nasopharyngeal sampling to have a higher yield for detecting pneumococcus in adults.32 Progressive acquisition of immunity due to environmental exposure to pneumococcus may explain the age-related decline in the rate of colonization, with serotype-specific antibody responses having been shown to increase with age.32 However, there appears to be discordance between IPD, carriage rates, and antibody levels in older adults.32,33 An explanation for this is lower functional antibody levels in older adults, such as those observed after pneumococcal polysaccharide vaccination.34
Serotypes covered by PCV7 appear to be carried in low proportions across all age groups, with the caveat that the proportions in the older adult group are difficult to interpret given the low overall rate of carriage. Similarly, the proportion of IPD due to serotypes covered by PCV7 is low in all age groups and in line with the carriage rates. Carriage studies performed in children from the United Kingdom prior to introduction of PCV7 in 2006 demonstrated higher carriage rates of the serotypes covered by PCV7.6,30,35,36 The study by Hussain et al30 in 2005 demonstrated that 52% of pneumococcal carriage isolates from children aged 3 to 4 years were covered by PCV7 serotypes. Similarly, it was shown that 56% of pneumococcal positive swabs from infants <6 months of age were serotypes covered by PCV7.6 These studies are in marked contrast to the 1.5% of samples from children in the current study that are included in PCV7. This striking difference in carriage of PCV7 serotypes pre- and postvaccine introduction is in keeping with the findings (from 31.9 to 3.6%) by Flasche et al37 in 2011.
Surveillance of IPD cases in children and adults from England and Wales has demonstrated a discernible decline in disease due to vaccine serotypes as PCV7 has been introduced.24 By contrast, there has been an increase in IPD due to the NVTs following PCV7 introduction.35 This is consistent with the serotype replacement described in other settings, where PCVs have been utilized.36 Development of herd protection, post-PCV7 introduction into a community immunization program, has been demonstrated in other settings by the reduction in carriage and IPD due to the serotypes covered by the vaccine in unvaccinated adults.22,23 It is believed that this process occurs by reducing transmission of the serotypes covered by the vaccine from vaccinated children who form the reservoir of carriage within the community. Thus, the findings of reduced carriage and IPD due to the vaccine-covered serotypes in unvaccinated adults in this study are consistent with this hypothesis of herd protection.
Different serotypes appear to have differing characteristics in their ability to colonize a host and initiate disease. Prior studies have shown serotypes 1, 5, and 7F to have a high invasive disease potential, but these are relatively infrequently isolated from carriers.37,38 In contrast, serotypes 6B, 19F, and 23F have lower attack rates but are more frequently carried.15,16,38 Similarly, in this study, serotypes 1 and 7F appeared to have the greatest discordance between carriage and IPD in all age groups. Serotype 19A was also responsible for high proportions of IPD; however, it was also frequently carried in both the preschool children and parent groups, which is in keeping with its previously described intermediate attack rate.16 Serotypes 1, 7F, and 19A form part of the additional coverage offered by PCV13 over PCV7, and thus the IPD data anticipate a further significant reduction in disease following PCV13 introduction.
A wide variety of demographic factors have been implicated in increasing the risk of pneumococcal carriage, including smoke exposure, housing density, and recent respiratory infection.9 Smoking or exposure to smokers within the household could not clearly be shown to be a risk factor in this study, due to the low numbers of smokers in the cohort. Notably those studies that have shown a relation to smoke exposure and carriage are in nonindustrialized settings,9,10 where there are high rates of exposure to smoke from open fires and less awareness about the health effects of cigarette smoke exposure. Settings where there is increased mixing of young children such as daycare centers and preschools or higher density of people living at home have been shown to be risk factors for carriage.9,13,39 There was only a trend toward daycare attendance being a risk for carriage in this study, although the study may be underpowered as a result of the low proportion of children (14%) who did not attended daycare in the cohort. The observation in this study, that older adults are at significantly increased odds of being a carrier if they had contact with preschool children, is consistent with a prior study in Alaskan adults who were found to be more likely to carry pneumococcus if they lived with a child <5 years of age,22 and lends weight to the hypothesis that interaction with the carriage reservoir is responsible for transmission.
Limitations of the study are the sample size, female predominance in the parent group, serological identification technique, and cross-sectional study design. Low rates of carriage in the parent and older adult groups indicate that future studies in these age ranges would benefit from larger sample sizes. There was a significant overrepresentation of women in the parent group; however, there was no indication of a sex effect in either the children or older adult age groups. Identification of pneumococcal serotypes by the WHO-defined methodology is less sensitive and more labor-intensive than DNA detection-based modalities, which are now more readily available and provide a higher resolution of information. Despite these limitations, the results of this study clearly demonstrate the following: low carriage prevalence of vaccine serotypes in unvaccinated adults, older adults, and vaccinated children, with lower frequency of pneumococcal carriage in older age groups; a significant relationship between carriage in older adults and contact with preschool-aged children.
The strengths of this study are the large number of adults and children from whom swabs were collected, the comparison with IPD data collected across the same time period, and the potential future use of these data for comparison in the post-PCV13 era.
A further reduction in IPD cases is expected following PCV13 introduction, as the bulk of IPD post-PCV7 in this study population is due to the additional serotypes covered by the higher valency vaccine. Emergence of replacement serotypes has only had a small impact on IPD, with the prevalence of emergent serotypes readily detectable by continued scrutiny of the carriage reservoir.
The Strategic Advisor Group of Experts on immunization have recommended, on the basis of current data, that ongoing surveillance for serotype replacement takes place.40 Carriage studies are warranted to provide information on the characteristics of circulating pneumococci in a population, as a surrogate of the potential impact of vaccines, and to inform clinical decision-making and future vaccine development.
Acknowledgments
The authors express their appreciation to Janice Strand, Sue Palmer Hill, and Stephen Zingwe. The authors gratefully acknowledge all the participants and their parents for participating in the study. Data from this study were presented at the 9th International Symposium on Pneumococci and Pneumococcal Disease, March 9 to 13, 2014. All requests for study data should be sent to rama.kandasamy@paediatrics.ox.ac.uk.
Footnotes
Abbreviations: CI = confidence interval, IPD = invasive pneumococcal disease, NVT = nonvaccine type, PCV7 = 7-valent pneumococcal conjugate vaccine, PCV13 = 13-valent pneumococcal conjugate vaccine, WHO = World Health Organization.
This work was supported by an investigator-initiated grant provided by Pfizer Limited. AJP and MDS conducted clinical trials on behalf of Oxford University, which are sponsored by manufacturers of PCVs, but did not receive any personal payments from them. The University of Oxford receives unrestricted educational grants for courses and conferences organized by AJP from vaccine manufacturers. MDS and RL have received financial assistance from vaccine manufacturers to attend conferences.
Pfizer Limited did not have any role in study development, conduction, analysis, and manuscript preparation. MDS has participated in advisory boards for vaccine manufacturers, but receives no personal payments for this work.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.O’Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 2009; 374:893–902. [DOI] [PubMed] [Google Scholar]
- 2.Koedel U, Scheld WM, Pfister HW. Pathogenesis and pathophysiology of pneumococcal meningitis. Lancet Infect Dis 2002; 2:721–736. [DOI] [PubMed] [Google Scholar]
- 3.WHO Publication. Pneumococcal vaccines WHO position paper—2012—recommendations. Vaccine 2012; 30:4717–4718. [DOI] [PubMed] [Google Scholar]
- 4.Mufson MA, Stanek RJ. Bacteremic pneumococcal pneumonia in one American City: a 20-year longitudinal study, 1978–1997. Am J Med 1999; 107 (1A):34S–43S. [DOI] [PubMed] [Google Scholar]
- 5.Koivula I, Sten M, Makela PH. Prognosis after community-acquired pneumonia in the elderly: a population-based 12-year follow-up study. Arch Intern Med 1999; 159:1550–1555. [DOI] [PubMed] [Google Scholar]
- 6.Sleeman KL, Daniels L, Gardiner M, et al. Acquisition of Streptococcus pneumoniae and nonspecific morbidity in infants and their families: a cohort study. Pediatr Infect Dis J 2005; 24:121–127. [DOI] [PubMed] [Google Scholar]
- 7.Bogaert D, Sluijter M, Toom NL, et al. Dynamics of pneumococcal colonization in healthy Dutch children. Microbiology 2006; 152 (pt 2):377–385. [DOI] [PubMed] [Google Scholar]
- 8.Ridda I, Macintyre CR, Lindley R, et al. Lack of pneumococcal carriage in the hospitalised elderly. Vaccine 2010; 28:3902–3904. [DOI] [PubMed] [Google Scholar]
- 9.Mackenzie GA, Leach AJ, Carapetis JR, et al. Epidemiology of nasopharyngeal carriage of respiratory bacterial pathogens in children and adults: cross-sectional surveys in a population with high rates of pneumococcal disease. BMC infectious diseases 2010; 10:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turner P, Turner C, Jankhot A, et al. A longitudinal study of Streptococcus pneumoniae carriage in a cohort of infants and their mothers on the Thailand-Myanmar border. PLoS One 2012; 7:e38271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nuorti JP, Butler JC, Crutcher JM, et al. An outbreak of multidrug-resistant pneumococcal pneumonia and bacteremia among unvaccinated nursing home residents. N Engl J Med 1998; 338:1861–1868. [DOI] [PubMed] [Google Scholar]
- 12.Slack M, Krahe D. Cluster of Serotype 5 Invasive Pneumococcal Disease in a Religious Community in North London. Presented at 6th International Symposium on Pneumococci and Pneumococcal Diseases June 8 to 12, 2008, Reykjavik, Iceland, (poster presentation) 2008. [Google Scholar]
- 13.Principi N, Marchisio P, Schito GC, et al. Risk factors for carriage of respiratory pathogens in the nasopharynx of healthy children. Ascanius Project Collaborative Group. Pediatr Infect Dis J 1999; 18:517–523. [DOI] [PubMed] [Google Scholar]
- 14.Davis SM, Deloria-Knoll M, Kassa HT, et al. Impact of pneumococcal conjugate vaccines on nasopharyngeal carriage and invasive disease among unvaccinated people: review of evidence on indirect effects. Vaccine 2013; 32:133–145. [DOI] [PubMed] [Google Scholar]
- 15.Brueggemann AB, Peto TE, Crook DW, et al. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J Infect Dis 2004; 190:1203–1211. [DOI] [PubMed] [Google Scholar]
- 16.Nurhonen M, Cheng AC, Auranen K. Pneumococcal transmission and disease in silico: a microsimulation model of the indirect effects of vaccination. PLoS One 2013; 8:e56079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pneumococcal serotype distribution for samples referred for serotyping epidemiological years (July-June): 2000/1–2005/6. 2009. http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/Pneumococcal/EpidemiologicalDataPneumococcal/BackgroundEpidemiologyPneumococcal/IPDTables/pneumoPneumococcalSerotypeDistribution Accessed on 21st May 2013. [Google Scholar]
- 18.Percentage of total number of cases of invasive pneumococcal disease (IPD) serotypes in the 7-valent vaccine for under 5 year olds and all ages for epidemiological year: July 2002—June 2006. 2008. http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/Pneumococcal/EpidemiologicalDataPneumococcal/BackgroundEpidemiologyPneumococcal/IPDGraphs/pneumoPercentageTotalNumberIPDUnder5s0206 Accessed on 21st May 2013. [Google Scholar]
- 19.Sjostrom K, Spindler C, Ortqvist A, et al. Clonal and capsular types decide whether pneumococci will act as a primary or opportunistic pathogen. Clin Infect Dis 2006; 42:451–459. [DOI] [PubMed] [Google Scholar]
- 20.Cumulative weekly number of reports of Invasive Pneumococcal Disease due to any of the seven serotypes in Prevenar 7: children aged 2 to 4 years in England and Wales by epidemiological year: July–June (2005–to date). 2013. http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/Pneumococcal/EpidemiologicalDataPneumococcal/CurrentEpidemiologyPneumococcal/InPrevenar7/pneumo02Cummulativeweekly24inPrevenar7 Accessed on 21st May 2013. [Google Scholar]
- 21.Miller E, Andrews NJ, Waight PA, et al. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis 2011; 11:760–768. [DOI] [PubMed] [Google Scholar]
- 22.Hammitt LL, Bruden DL, Butler JC, et al. Indirect effect of conjugate vaccine on adult carriage of Streptococcus pneumoniae: an explanation of trends in invasive pneumococcal disease. J Infect Dis 2006; 193:1487–1494. [DOI] [PubMed] [Google Scholar]
- 23.Lexau CA, Lynfield R, Danila R, et al. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 2005; 294:2043–2051. [DOI] [PubMed] [Google Scholar]
- 24.Kaye P, Malkani R, Martin S, et al. Invasive Pneumococcal Disease (IPD) in England & Wales after 7-valent conjugate vaccine (PCV7); potential impact of 10 and 13-valent vaccines. 2009. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1245581527892 Accessed on 21st May 2013. [Google Scholar]
- 25.O’Brien KL, Nohynek H. Report from a WHO Working Group: standard method for detecting upper respiratory carriage of Streptococcus pneumoniae. Pediatr Infect Dis J 2003; 22:e1–e11. [DOI] [PubMed] [Google Scholar]
- 26.Turner P, Hinds J, Turner C, et al. Improved detection of nasopharyngeal cocolonization by multiple pneumococcal serotypes by use of latex agglutination or molecular serotyping by microarray. J Clin Microbiol 2011; 49:1784–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newton R, Hinds J, Wernisch L. Empirical Bayesian models for analysing molecular serotyping microarrays. BMC Bioinformatics 2011; 12:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trotter CL, Waight P, Andrews NJ, et al. Epidemiology of invasive pneumococcal disease in the pre-conjugate vaccine era: England and Wales, 1996–2006. J Infect 2010; 60:200–208. [DOI] [PubMed] [Google Scholar]
- 29.Bogaert D, van Belkum A, Sluijter M, et al. Colonisation by Streptococcus pneumoniae and Staphylococcus aureus in healthy children. Lancet 2004; 363:1871–1872. [DOI] [PubMed] [Google Scholar]
- 30.Hussain M, Melegaro A, Pebody RG, et al. A longitudinal household study of Streptococcus pneumoniae nasopharyngeal carriage in a UK setting. Epidemiol Infect 2005; 133:891–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jounio U, Juvonen R, Bloigu A, et al. Pneumococcal carriage is more common in asthmatic than in non-asthmatic young men. Clin Respir J 2010; 4:222–229. [DOI] [PubMed] [Google Scholar]
- 32.Balmer P, Borrow R, Findlow J, et al. Age-stratified prevalences of pneumococcal-serotype-specific immunoglobulin G in England and their relationship to the serotype-specific incidence of invasive pneumococcal disease prior to the introduction of the pneumococcal 7-valent conjugate vaccine. Clin Vaccine Immunol 2007; 14:1442–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Invasive pneumococcal disease incidence rate per 100,000 population by age grouping England and Wales, 1996–2005. 2008. http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/Pneumococcal/EpidemiologicalDataPneumococcal/BackgroundEpidemiologyPneumococcal/IPDGraphs/pneumoIPDIncidenceRate/ Accessed on 21st May 2013. [Google Scholar]
- 34.Devaster JM, Leroux-Roels I, Leroux-Roels G, et al. Inferior humoral response in elderly versus young adults to the 23-valent polysaccharide vaccine. In: Program and abstract book of the 5th International Symposium on Pneumococci and Pneumococcal Diseases; April 2 to 6, 2006; Alice Springs, Australia; p244. [Google Scholar]
- 35.Cumulative weekly number of reports of Invasive Pneumococcal Disease due to any of the serotypes NOT IN Prevenar13™: Children aged 2 to 4 years in England and Wales by epidemiological year: July–June (2005–to date). 2013. http://www.hpa.org.uk/Topics/InfectiousDiseases/InfectionsAZ/Pneumococcal/EpidemiologicalDataPneumococcal/CurrentEpidemiologyPneumococcal/NotInPrevenar13/pneumo05Cummulativeweekly24NOTINPrevenar13vacc/ Accessed on 21st May 2013. [Google Scholar]
- 36.Hsu KK, Shea KM, Stevenson AE, et al. Underlying conditions in children with invasive pneumococcal disease in the conjugate vaccine era. Pediatr Infect Dis J 2011; 30:251–253. [DOI] [PubMed] [Google Scholar]
- 37.Flasche S, Van Hoek AJ, Sheasby E, et al. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS medicine 2011; 8:e1001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sleeman KL, Griffiths D, Shackley F, et al. Capsular serotype-specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J Infect Dis 2006; 194:682–688. [DOI] [PubMed] [Google Scholar]
- 39.Huang SS, Hinrichsen VL, Stevenson AE, et al. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics 2009; 124:e1–e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meeting of the Strategic advisory group of experts on immunization, November 2011—conclusions and recommendations. Wkly Epidemiol Rec 2012; 87:1–16. http://www.who.Int/werhttp://www.who.Int/wer. Accessed on 20th October 2013. [PubMed] [Google Scholar]


