Abstract
Postoperative radiotherapy is critical for reducing local relapse for advanced rectal carcinoma but has many side effects. Our study compared the dose distribution of target volumes, protection of normal organs at risk (OAR), and monitor unit (MU) for 3 radiotherapy techniques (3-dimensional conformal radiation therapy [3D-CRT], intensity-modulated radiation therapy [IMRT], and RapidArc (Varian Medical Systems, Inc., Palo Alto, CA, USA)). The results advocate for the clinical application of RapidArc technique in the future.
Thirty postoperative patients with rectal cancer were enrolled. The 3 radiotherapy plans mentioned above were designed for each patient. The target volume coverage indicators included average dose, conformity index (CI), and homogeneity index (HI) of planning tumor volume (PTV). OAR included the bladder, small intestine, colon, and bilateral proximal femurs. The 30 patients were divided into 3 groups (10 cases in each group) for postoperative radiotherapy with the 3D-CRT, IMRT, or RapidArc technique, respectively.
Both the IMRT and RapidArc plans have a significantly higher average PTV dose and better CI and HI (P < 0.01) than 3D-CRT. IMRT and RapidArc result in significantly lower doses of irradiation for all the OAR examined. Both the IMRT and RapidArc plans have a significantly lower V40 of the bladder, small intestine, and colon than 3D-CRT (P < 0.01). The IMRT and RapidArc plans can also reduce the maximum dose (Dmax) for the left proximal femur, V30, and V40 of bilateral proximal femurs compared with 3D-CRT (P < 0.01). Compared with IMRT, RapidArc can further reduce the Dmax of the small intestine, the Dmax and V30 of the bilateral proximal femurs, and the V40 of the right proximal femur (P < 0.01). RapidArc reduces MU remarkably compared with IMRT (P < 0.01). Regarding acute side effects, IMRT and RapidArc can greatly reduce the incidence of grade 3 radiation-induced cystitis and grade 2 enteritis.
Both IMRT and RapidArc are better than 3D-CRT regarding PTV coverage and OAR protection. Furthermore, RapidArc is superior to IMRT regarding protection of the small intestine and bilateral proximal femurs and requires a reduced treatment time. RapidArc could be widely applied for postoperative radiotherapy for patients with ΙΙ–ΙΙΙ stage rectal cancer.
INTRODUCTION
Rectal carcinoma is still one of the most common malignancies. In recent years, improvements in diagnosis, staging, and multimodality treatments have provided both local control and survival benefits.1,2 The reduction of local relapse has largely resulted from the introduction of the total mesorectal excision approach and improvements in the radiotherapy technique and method.2–5 The contribution of radiotherapy to local control greatly depends on the dose coverage of the target volume. Prolonged survival will require higher demands for favorable quality of life. The dose for normal organs at risk (OAR) directly results in irradiation-related early or late toxicities. Furthermore, how to improve comfort during radiotherapy and reduce displacement resulting from organ movement is critical for the accuracy of radiotherapy.
RapidArc is an advanced image-guided intensity-modulated radiation therapy (IMRT) developed in 2008. As a new technique, its clinical scope of application needs to be further defined. Our study was designed to analyze the differences between the 3-dimensional conformal radiation therapy (3D-CRT), IMRT, and RapidArc plans to provide the best radiotherapy options for postoperative rectal patients.
MATERIALS AND METHODS
Patient Clinical Data
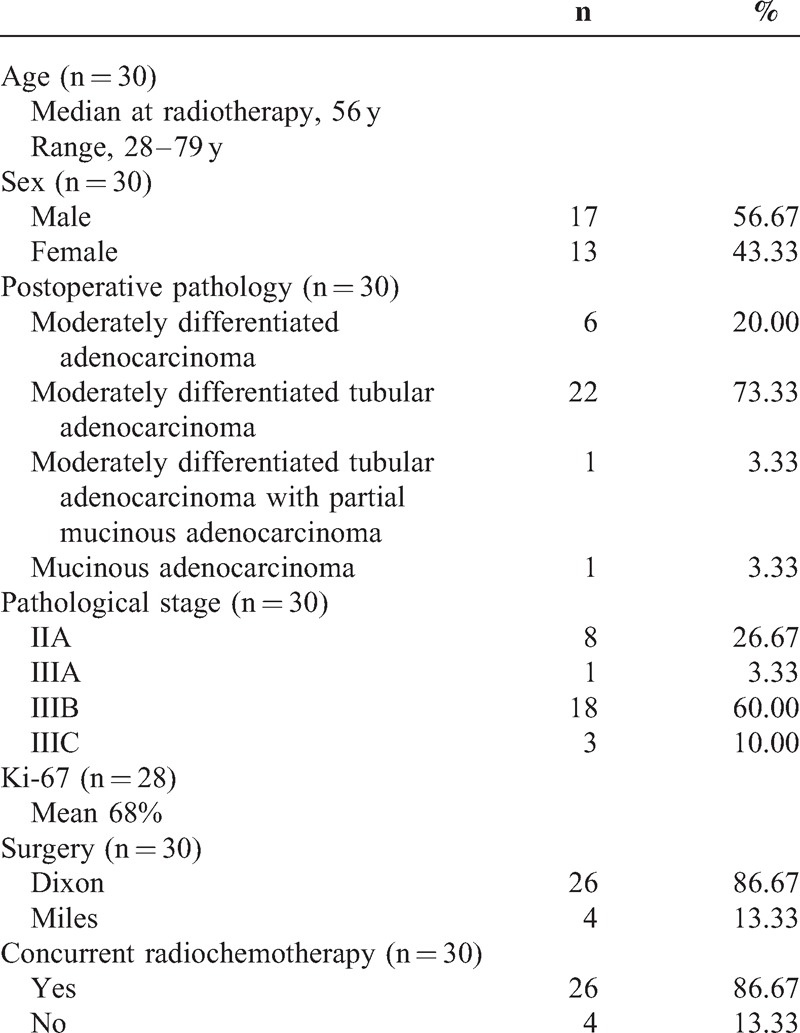
The 30 patients (men, 17; women, 13) who were admitted to our department from November 2011 to January 2014 were enrolled. This study was approved by the ethics committee of The First Hospital of Jilin University. The final diagnosis was confirmed by postoperative pathology as rectal adenocarcinoma. The median age was 56 (ranging from 28 to 79). The pathological stage was II–III according to the National Comprehensive Cancer Network Clinical Practice Guidelines in Rectal Cancer.6 The concrete postoperative pathology was as follows: moderately differentiated tubular adenocarcinoma (22 cases), moderately differentiated adenocarcinoma (6 cases), moderately differentiated tubular adenocarcinoma with partial mucinous adenocarcinoma (1 case), and mucinous adenocarcinoma (1 case). The mean Ki-67 index was 68% (30%–90%). All the patients underwent surgery (Dixon, 26 cases; Miles, 4 cases), postoperative radiotherapy, and adjuvant chemotherapy. During radiotherapy, 26 patients performed concurrent chemotherapy with Capecitabine or Tegafur, and 4 patients received irradiation alone (Table 1). The 30 patients were divided into 3 groups (10 cases in each group) for postoperative radiotherapy with the 3D-CRT, IMRT, or RapidArc technique, respectively.
TABLE 1.
Clinicopathological Features of Enrolled Patients
Patient Setup and Simulation
All the patients were required to empty the bladder and rectum 1 hour before simulation. The urine was retained. Half an hour before simulation, the patients were administered oral diatrizoate to contrast the small intestine. A thermoplastic shell was used to fix the patients in a supine position. We performed a CT scan with contrast at 5 mm intervals from the upper edge of L1 to 5 cm below the ischial tuberosity.
Target Volume Declination
The clinical tumor volume (CTV) included the tumor bed and the related high-risk lymphatic drainage area. The planning tumor volume (PTV) was expanded 1.0 cm in the craniocaudal dimension and 0.5 cm in the bilateral, superior, and inferior dimensions of the CTV.
Declination of OAR
The bladder, small intestine, colon, and bilateral proximal femurs were declined as OAR by radiotherapy oncologists according to the Male/Female Pelvic Normal Tissue RTOG (Radiation Therapy Oncology Group) Consensus Contouring Guidelines.7 The colon and small intestine were not contoured 1 cm above the PTV.
Radiotherapy Plan Design
The prescribed dose for PTV was 50 Gy/2 Gy/25 f. We designed 3 different radiotherapy plans for each patient: a 3-field 3D-CRT plan, a 7-field IMRT plan, and a 2-arc volumetric-modulated arc therapy plan. All the plans were designed by the Eclipse 10.0 plan system (Varian Medical Systems, Palo Alto, CA). We chose the x-ray energy, and adjusted beam weights and wedge plate angles on the basis of different target volume contours. The 7-field IMRT plans were designed inversely and coplanarly with a 6 MV x-ray. The gantry angles were 180°, 130°, 80°, 30°, 330°, 280°, and 230°. The 2-arc volumetric-modulated arc therapy plan was performed with 181°–179° clockwise and 179°–181° counterclockwise rotation with a 6 MV x-ray. The collimator angle was 20° or 340°, respectively.
Statistical Analysis
SPSS 15.0 statistical software (SPSS, Inc., Chicago, IL, USA) was used for data analysis. The average dose, conformity index (CI), homogeneity index (HI), maximum dose (Dmax), V30, V40, V50, and monitor unit (MU) were expressed as x¯ ± s. Multiple groups of samples were compared with an ANOVA (analysis of variance).
RESULTS
Patient Side Effects and Outcomes
According to the RTOG Acute Radiation Injury Grading Standards,8 in the 3D-CRT group, 2 patients had grade 3 radiation-induced cystitis (20%), and 6 patients (60%) had grade 2 radiation-induced enteritis. In the IMRT and RapidArc groups, the patients developed only grade 1 radiation-induced cystitis and enteritis.
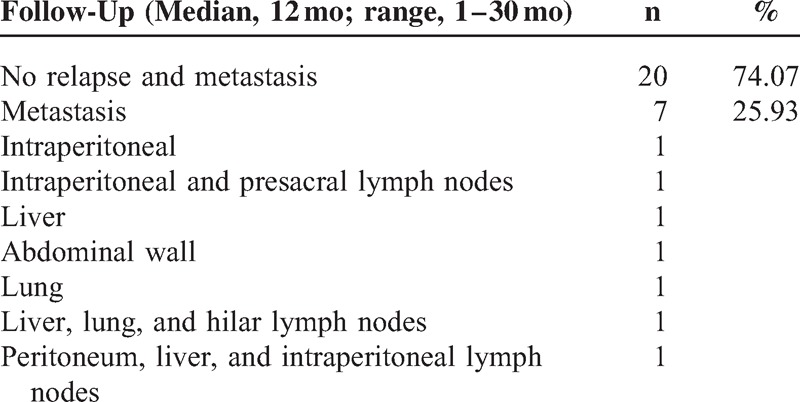
The 27 patients received careful follow-up (median, 12 months; range, 1–30 months). Three patients failed to follow-up; 20 patients were alive without any sign of relapse and metastasis; 7 patients had metastases. The metastatic sites are described in Table 2.
TABLE 2.
Patient Outcomes (n = 27)
Dose Distribution of Target Volume
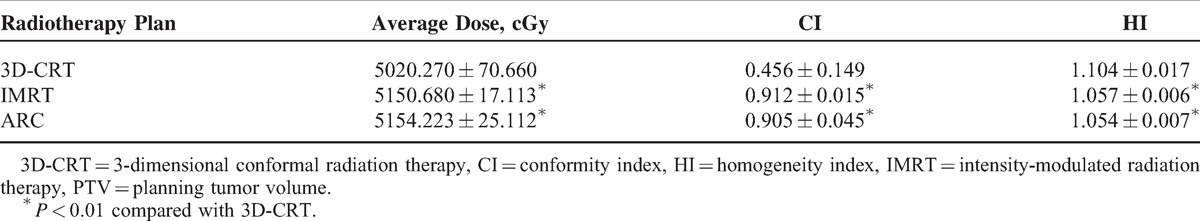
As Table 3 demonstrates, both the IMRT and RapidArc plans deliver a significantly higher average dose for PTV and have a better CI and HI (P < 0.01) than 3D-CRT. However, there was no significant difference between RapidArc and IMRT regarding these aspects.
TABLE 3.
Comparison of Average PTV Dose and CI and HI (n = 30)
OAR Sparing
IMRT and RapidArc resulted in a significantly lower dose of irradiation for all the OAR examined (Tables 4 and 5).
TABLE 4.
Irradiated Dose and Volume for Bladder, Small Intestine, and Colon (n = 30)
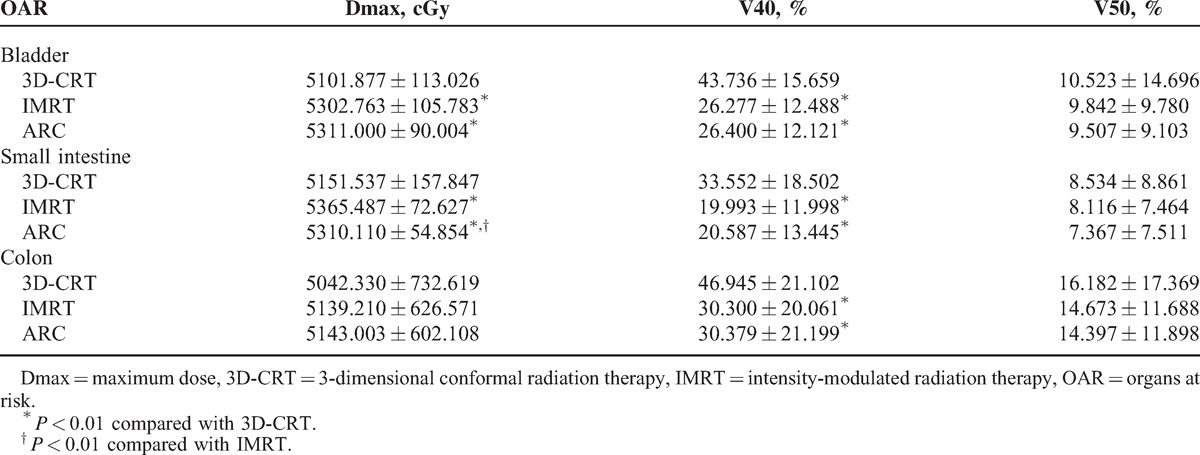
TABLE 5.
Irradiated Dose and Volume of Proximal Femurs (n = 30)
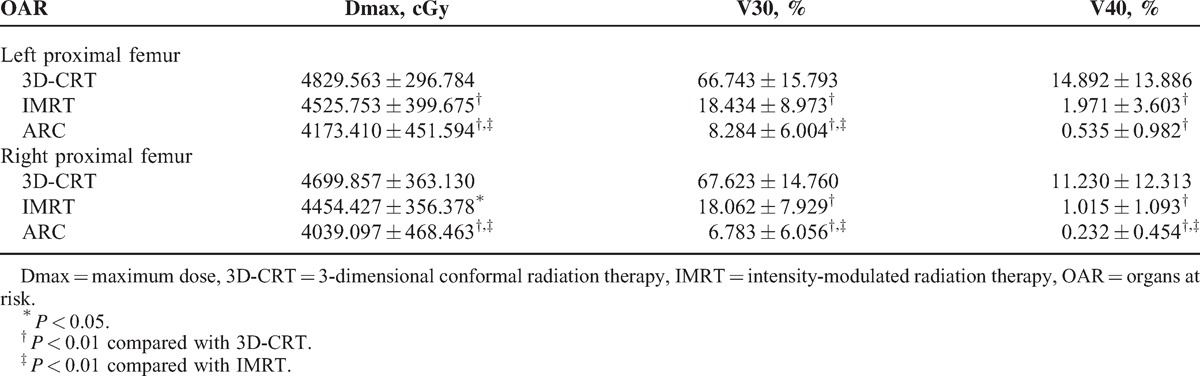
For the V40 of bladder, small intestine, and colon, both the IMRT and RapidArc plans were significantly lower than 3D-CRT (P < 0.01) (Table 4). The IMRT and RapidArc plans can also reduce the Dmax of the left proximal femur and the V30 and V40 of the bilateral proximal femurs compared with 3D-CRT (P < 0.01) (Table 5). Compared with IMRT, RapidArc can further reduce the Dmax of the small intestine (P < 0.01) (Table 4), the Dmax and V30 of the bilateral proximal femurs, and the V40 of the right proximal femur (P < 0.01) (Table 5).
Expected Delivery Time
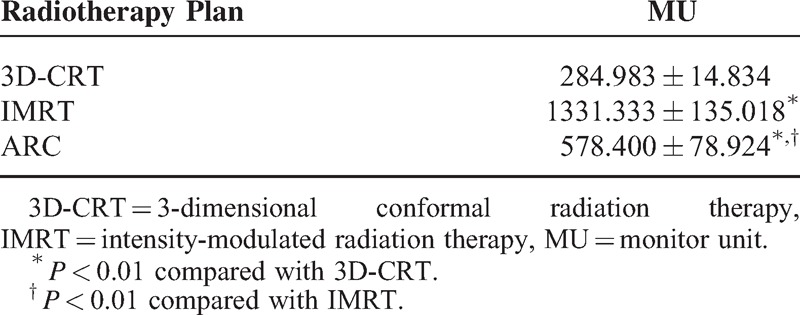
Regarding the MU, RapidArc was remarkably lower than IMRT (P < 0.01) (Table 6).
TABLE 6.
Monitor Unit Comparison (n = 30)
DISCUSSION
Rectal carcinoma is one of the most common malignancies and leading causes of death worldwide. In China, recently, rectal carcinoma has a higher incidence and affects more young individuals. Early diagnosis and an improvement of treatment modalities have contributed to a decrease of mortality and prolonged survival.9 Radiotherapy is crucial to local control. Compared with surgery alone, the addition of radiotherapy reduces local relapse by 37% to 46%.10,11 The benefits of neoadjuvant or adjuvant radiotherapy have been verified by many reports.10,12,13 The addition of chemotherapy greatly increases the risk of treatment-related toxicity.14 Advanced radiotherapy techniques can reduce the irradiation of OAR15 reducing the intensity of chemoradiotherapy.16,17 With improvements in survival, more treatment-related adverse events will occur. Thus, improving the patients’ quality of life requires more attention.
The report by Komori et al18 revealed that multiple complications, including gut inflammation and edema, could occur after postoperative irradiation for locally advanced stage III rectal carcinoma. Late-onset complications were frequent, hard to resolve, and seriously impaired the patients’ quality of life. More importantly, approximately 25% of late-onset complications occurred >10 years after pelvic radiotherapy. Brown et al19 also demonstrated that pelvic radiotherapy could impair long-term rectal function, which is directly related to the irradiated volume and dose for the bowel. Therefore, Kim et al10 performed a thorough follow-up of late radiotherapy toxicity for patients with rectal carcinoma. Radiotherapy induced more anastomosis-related complications and nonbowel-related complications, including pelvic wall changes, compared with the nonradiotherapy counterpart. With time, the incidence increased gradually. Late complications were often caused by irreversible damage and seldom responded to treatment.10 Therefore, the prevention of severe radiation-induced side effects is critical. Our analysis demonstrated that both IMRT and RapidArc reduced the V40 of the bladder, small intestine, and colon significantly compared with 3D-CRT (P < 0.01), which is consistent with the observed lower incidence of acute radiation-induced cystitis and enteritis. Wolff et al20 compared protons, 3D-CRT, IMRT, and RapidArc for locally advanced rectal carcinoma in 2011. However, the authors did not analyze the dose differences for the proximal femurs. In our study, the IMRT and RapidArc plans reduced the Dmax of the left proximal femur and the V30 and V40 of the bilateral proximal femurs compared with 3D-CRT (P < 0.01). Compared with IMRT, RapidArc can further reduce the Dmax of small intestine, the Dmax and V30 of the bilateral proximal femurs, and the V40 of the right proximal femur (P < 0.01). The dosimetric advantage of RapidArc will reduce patient suffering from acute and late radiation-related side effects.
During RapidArc delivery, the dose rate, multileaf collimator field, and gantry rotation speed are simultaneously adjusted.21 The most prominent advantage of RapidArc compared with conventional IMRT is that RapidArc can greatly reduce the treatment time. First, RapidArc can eliminate displacement due to organ motion offering accurate delivery. Second, a short treatment period can improve patient comfort, especially for rectal patients requiring urine retention during irradiation. Lastly, a low MU can extend the use of the linac (linear accelerator).
CONCLUSION
RapidArc can result in better dose coverage for tumor volume and the protection of normal tissues. RapidArc is superior to IMRT regarding the protection of the small intestine and bilateral proximal femurs and also requires reduced treatment time, which facilitates accurate delivery and accelerator protection. In the future, RapidArc should be widely applied for postoperative radiotherapy for patients with II–III stage rectal cancer.
Footnotes
Abbreviations: 3D-CRT = 3-dimensional conformal radiation therapy, CI = conformity index, CTV = clinical tumor volume, Dmax = maximum dose, HI = homogeneity index, IMRT = intensity-modulated radiation therapy, MU = monitor unit, OAR = organs at risk, PTV = planning target volume.
ML and BL contributed equally to this article.
The present study was supported by the Science and Technology Department of Jilin Province (grant no:3D512J233428).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Tudyka V, Blomqvist L, Beets-Tan RG, et al. EURECCA consensus conference highlights about colon & rectal cancer multidisciplinary management: the radiology experts review. Eur J Surg Oncol 2014; 40:469–475. [DOI] [PubMed] [Google Scholar]
- 2.Abbas MA, Chang GJ, Read TE, et al. Optimizing rectal cancer management: analysis of current evidence. Dis Colon Rectum 2014; 57:252–259. [DOI] [PubMed] [Google Scholar]
- 3.Maurer CA, Renzulli P, Kull C, et al. The impact of the introduction of total mesorectal excision on local recurrence rate and survival in rectal cancer: long-term results. Ann Surg Oncol 2011; 18:1899–1906. [DOI] [PubMed] [Google Scholar]
- 4.Alberda WJ, Verhoef C, Nuyttens JJ, et al. Intraoperative radiation therapy reduces local recurrence rates in patients with microscopically involved circumferential resection margins after resection of locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2014; 88:1032–1040. [DOI] [PubMed] [Google Scholar]
- 5.Ippolito E, Mertens I, Haustermans K, et al. IGRT in rectal cancer. Acta Oncol 2008; 47:1317–1324. [DOI] [PubMed] [Google Scholar]
- 6.Engstrom PF, Arnoletti JP, Benson AB, et al. NCCN Clinical Practice Guidelines in Oncology: rectal cancer. J Natl Compr Canc Netw 2009; 7:838–881. [DOI] [PubMed] [Google Scholar]
- 7.Gay HA, Barthold HJ, O’Meara E, et al. Pelvic normal tissue contouring guidelines for radiation therapy: a Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys 2012; 83:e353–e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trotti A, Byhardt R, Stetz J, et al. Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys 2000; 47:13–47. [DOI] [PubMed] [Google Scholar]
- 9.Benson ABT, III, Bekaii-Saab T, Chan E, et al. NCCN clinical practice guidelines in oncology (NCCN guidelines): rectal cancer. J Natl Compr Canc Netw 2012; 10:1528–1564.23221790 [Google Scholar]
- 10.Kim SH, Kim JH, Jung SH. Late complications after proctectomy in rectal cancer patients who underwent radiotherapy. World J Surg 2014; 38:2471–2476. [DOI] [PubMed] [Google Scholar]
- 11.Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: a systematic overview of 8507 patients from 22 randomised trials. Lancet 2001; 358:1291–1304. [DOI] [PubMed] [Google Scholar]
- 12.Myerson RJ, Tan B, Hunt S, et al. Five fractions of radiation therapy followed by 4 cycles of FOLFOX chemotherapy as preoperative treatment for rectal cancer. Int J Radiat Oncol Biol Phys 2014; 88:829–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pieterse AH, Stiggelbout AM, Baas-Thijssen MC, et al. Benefit from preoperative radiotherapy in rectal cancer treatment: disease-free patients’ and oncologists’ preferences. Br J Cancer 2007; 97:717–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fiorica F, Cartei F, Licata A, et al. Can chemotherapy concomitantly delivered with radiotherapy improve survival of patients with resectable rectal cancer? A meta-analysis of literature data. Cancer Treat Rev 2010; 36:539–549. [DOI] [PubMed] [Google Scholar]
- 15.Samuelian JM, Callister MD, Ashman JB, et al. Reduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2012; 82:1981–1987. [DOI] [PubMed] [Google Scholar]
- 16.Karasawa K. The role of radiation therapy for rectal cancer: recent trend [article in Japanese]. Nihon Rinsho 2014; 72:127–133. [PubMed] [Google Scholar]
- 17.Meyer J, Czito B, Yin FF, et al. Advanced radiation therapy technologies in the treatment of rectal and anal cancer: intensity-modulated photon therapy and proton therapy. Clin Colorectal Cancer 2007; 6:348–356. [DOI] [PubMed] [Google Scholar]
- 18.Komori K, Kimura K, Kinoshita T, et al. Complications associated with postoperative adjuvant radiation therapy for advanced rectal cancer. Int Surg 2014; 99:100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brown LC, Atherton PJ, Neben-Wittich MA, et al. Assessment of long-term rectal function in patients who received pelvic radiotherapy: a pooled North Central Cancer Treatment Group Trial analysis, N09C1. Support Care Cancer 2013; 21:2869–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolff HA, Wagner DM, Conradi LC, et al. Irradiation with protons for the individualized treatment of patients with locally advanced rectal cancer: a planning study with clinical implications. Radiother Oncol 2012; 102:30–37. [DOI] [PubMed] [Google Scholar]
- 21.Bedford JL, Warrington AP. Commissioning of volumetric modulated arc therapy (VMAT). Int J Radiat Oncol Biol Phys 2009; 73:537–545. [DOI] [PubMed] [Google Scholar]


