Supplemental Digital Content is available in the text
Abstract
Left ventricular diastolic dysfunction (LVDD) is common among patients undergoing peritoneal dialysis (PD). We examined the relationship between LVDD, major adverse cardiovascular events (MACE), and mortality in PD patients. A total of 149 patients undergoing PD with preserved left ventricular systolic function were included and followed for 3.5 years. LVDD was diagnosed (according to the European Society of Cardiology guidelines) by conventional and tissue Doppler echocardiography. Serum high-sensitivity C-reactive protein (hsCRP) was measured. The location and volume of adipose tissue were assessed by computed tomography (CT) at the level of the fourth lumbar vertebra. Subjects with LVDD had higher levels of hsCRP, and more visceral and peritoneal fat than controls. The relationship between adjusted visceral adipose tissue and LVDD became nonsignificant when hsCRP and baseline demographic data were introduced into the logistic regression model (odds ratio = 1.52, P = 0.07). Subsequent hierarchical multivariate Cox regression analysis showed that LVDD was one of the most powerful determinants of MACE and mortality after adjusting for all confounding factors (hazard ratio [HR]: 1.71, 95% confidence interval [CI]: 1.43–3.51, P = 0.02 and HR: 2.25, 95% CI: 1.45–2.91, P = 0.04, respectively). Systemic inflammation (hsCRP) was also significantly associated with MACE and mortality (HR: 2.03, P = 0.03 and HR: 2.16, P = 0.04, respectively). LVDD is associated with systemic inflammation and increased visceral fat in patients undergoing PD. LVDD is also a sensitive, independent indicator of future MACE and mortality in PD patients.
INTRODUCTION
Patients with end-stage renal disease (ESRD) undergoing either hemodialysis or peritoneal dialysis (PD) more often have the problems of hypertension, vascular stiffness due to physiological response. Cardiovascular disease (CVD) is also the leading cause of mortality in patients with ESRD.1 According to our recent study, higher inflammation status in PD patients could double the risk of left ventricular diastolic dysfunction (LVDD).2 Nevertheless, patients with PD suffer disproportionately from fluid overload and systemic inflammation.3,4 All of the aforementioned factors contribute to the high prevalence of LVDD in patients undergoing PD.3,5
In PD patients, body fat distribution is modified and plays a pivotal role in the development and progression of both diastolic and systolic heart failure (HF).6 In our previous studies, we found that systemic adiposity might influence the development of LVDD by increasing inflammation.6,7 However, there is little information about the prognostic value of LVDD and the possible complex interplay between LVDD, adipose tissue distribution, and inflammation in PD patients. We thus hypothesized that systemic adiposity might lead to increased inflammation that, in turn, results in the development of LVDD and poorer prognosis (Supplemental Figure 1, http://links.lww.com/MD/A262).
In this prospective study, we followed a cohort of PD patients to learn whether the development of LVDD in PD patients may be an independent factor predictive of long-term mortality and morbidity. In addition, we used hierarchical regression models to examine the interplay between adipose tissue distribution, inflammation, and LVDD, as well as their involvement in the long-term prognosis of PD patients (Supplemental Figure 1, http://links.lww.com/MD/A262).
MATERIALS AND METHODS
Study Participants and Study Design
We enrolled 242 Taiwanese PD patients undergone PD with conventional PD solution. All the patients had received PD for >6 months at the clinic of the National Taiwan University Hospital, Taipei, Taiwan, from July 2007 to March 2009. The baseline characteristics and laboratory data of these patients have been previously reported elsewhere.8 The same group of patients was followed till death or study termination. PD adequacy was measured by calculating peritoneal Kt/V (where K is the peritoneal clearance of urea, t is the dialysis time, and V is the volume of distribution of urea, which is approximately equal to the patient's total body water). Residual renal function (RRF) was measured with a 24-hour urine collection to calculate renal Kt/V. All subjects underwent echocardiography and abdominal computed tomography (CT) scanning to determine left ventricular diastolic function and visceral adiposity, respectively. Participants were classified as having LVDD according to our previous studies.9–11 The designed patient flow diagram was depicted in Supplemental Figure 2, http://links.lww.com/MD/A262. One hundred forty-nine PD patients were included in the final analysis.
Written informed consent was obtained from every participating patient. The study was approved by the Institutional Review Board of the National Taiwan University Hospital (NTUH-REC No. 200808062R).
Detail methods about measurements of biochemical data, echocardiographic, and determination of adipose tissue distribution by CT were stated in our previous article.8 All blood samples were processed in a blinded manner, and none of the examiners was aware of the patients’ clinical characteristics. Biochemical data and plasma C-reactive protein (CRP) were all measured. Echocardiographic data including structure, flow, and tissue Doppler imaging were collected according to the American Society of Echocardiography guideline using an iE33 xMATRIX echocardiography system, Philips Healthcare P.O. Box 10.000, 5680 DA Best, The Netherlands as in our previous article.8 Adiposity imaging of each subject was performed using a 16-section multidetector CT scanner and various contents of adipose tissue amounts were examined and recorded.8
Assessments of Primary and Secondary Endpoints
After patients had received the examination mentioned earlier including an abdominal CT and echocardiography, they were monitored for hospitalization, CVD, and mortality. Cardiac disease, cerebrovascular disease, and severe ischemic events were categorized as CVD. The primary endpoint was all-cause mortality, and the secondary endpoint was the occurrence of a major adverse cardiovascular event (MACE), which included hospitalization due to HF, myocardial infarction (MI), recurrent coronary artery disease (CAD), stroke, peripheral artery occlusive disease, and arrhythmia. All patients were followed from study entry until the occurrence of primary/secondary endpoints, transplantations (censorship), or December 31, 2012 (censorship).
Statistical Analysis
All statistical analyses were performed with SPSS 16.0 for Windows XP (SPSS Inc. Chicago, IL). Between-group baseline characteristics and echocardiographic findings were compared using a Student unpaired t test for continuous data and a χ2 test for categorical data. Survival was defined as the duration between enrollment and the first occurrence of an event or censorship. Kaplan–Meier curves were plotted to determine survival differences between patients with and without LVDD. Hazard ratios (HRs), 95% confidence intervals (95% CI), and P values were reported for comparisons between the 2 groups. Hierarchical logistic regression models were used to determine the risk of LVDD after adjusting for clinically apparent risk factors influencing survival of PD patients (eg, age, hypertension, diabetes mellitus [DM], and/or dyslipidemia) and/or factors associated with the development of LVDD (eg, fat components and inflammation). Hierarchical regression is a statistical method of exploring the relationships among, and testing hypotheses about, a dependent variable and several independent variables. In hierarchical regression, the independent variables are entered into the analysis in a sequence of blocks. By adding more predictors in each step of regression analysis, we are able to test the accuracy for hypothesis of a complex relationship or an assumed pathway.12 Using Cox regression and entering factors and covariates into the model in a hierarchical manner, we attempted to determine the relationship between systemic adiposity, inflammation (high-sensitivity CRP, hsCRP), and the intermediate outcome (ie, LVDD) using a 3-model approach that adjusted for baseline patient risk factors (eg, age, DM, hypertension, and dyslipidemia); and the interplay between systemic adiposity, inflammation (hsCRP), and the presence of LVDD, and the association of LVDD with survival and MACE, as part of the main analysis that consisted of 4 steps. To examine the association with primary and secondary endpoints, we performed 4 hierarchical Cox regression models. We performed correlation analysis or χ2 test for baseline characteristics, laboratory results, echocardiographic data, and systemic adiposity with outcomes (Supplemental Table 1A and B, http://links.lww.com/MD/A262). Significant factors (P < 0.05) associated with outcomes along with traditional risk factors were included in further regression models. In model 1, age, DM, hypertension, RRF, body mass index, hemoglobin, prior MI, and LDL levels were included using the Cox regression approach. In model 2, we further evaluated the potential effects of systemic adiposity on mortality or MACE. In model 3, we added inflammation into the analysis. Finally, we evaluated the independent role of LV diastolic function for both endpoints. We then separated the patients into 2 groups (ie, with and without LVDD). Between-group differences in survival were evaluated by a log-rank test. All statistical tests were 2-sided and a P value of <0.05 was considered to be statistically significant. For the power of the current study, we used 2-sample average method of the DSS research power calculator to estimate the required sample size (http://www.dssresearch.com/KnowledgeCenter/toolkitcalculators/samplesizecalculators.aspx). Based on a previous study (33), if PD patients with LVDD function had an HR of 3.2 (95% CI: 1.2–8.8,), we estimated that 50 patients in either group would be needed to achieve 80% statistical power (given α = 0.05). If a power calculation was done based on our patients (number = 149), we can calculate that the power is 0.92.
RESULTS
Demographic and Echocardiographic Characteristics of PD Patients
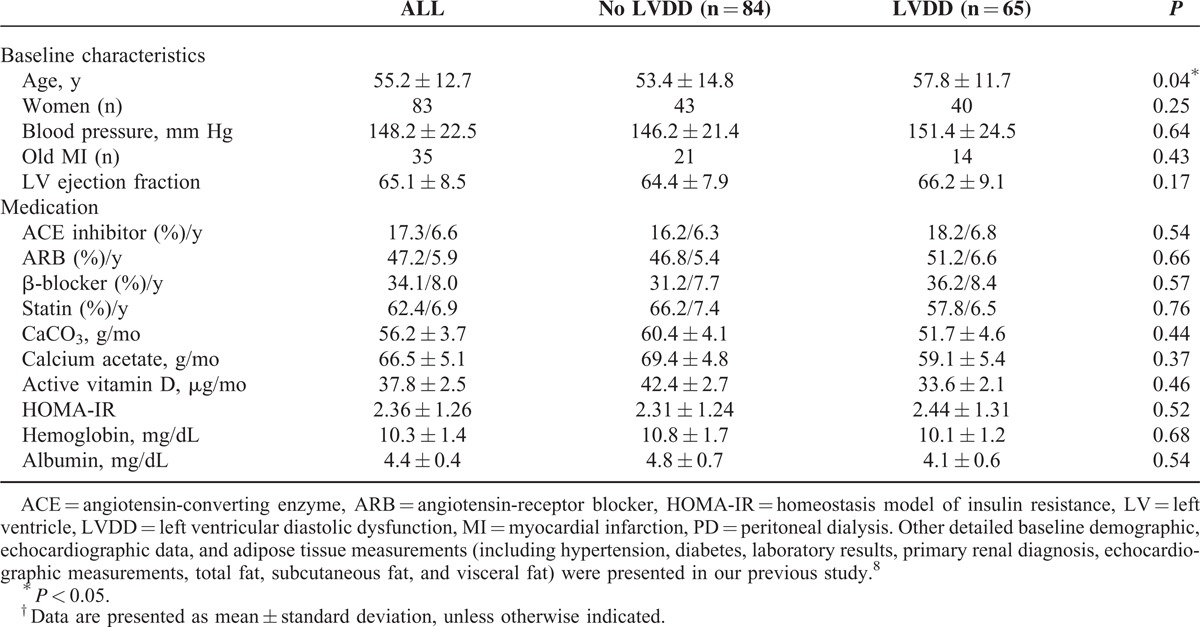
Of the 149 PD patients consecutively enrolled into this study, 65 were found to have LVDD. The baseline characteristics in general and the presence or absence of LVDD are summarized in Table 1. According to our previous published data,8 patients with LVDD were predominantly older and more likely to have hypertension and hyperlipidemia. PD patients with LVDD also had significantly higher hsCRP levels. Our PD patients had prolonged mitral inflow A ratio, a decreased peak annular early diastolic velocity of the lateral mitral annulus in tissue Doppler imaging (e′).8 In terms of adipose tissue distribution, patients with LVDD had significantly larger fat depots (measured in square centimeter), including visceral fat, peritoneal fat, and retroperitoneal fat.8
TABLE 1.
Baseline Patient Demographics of the 149 Patients Undergoing Peritoneal Dialysis†
Risk of Mortality and MACE in PD Patients
All patients (149) were carefully followed until the end of the study. There were 2 patients in the LVDD group and another 2 subjects in the non-LVDD group who received kidney transplantation. The overall mortality for PD patients was 18 patients in the LVDD (28%) group and 8 patients in the non-LVDD group (9%), whereas the incidence of MACE was 10 patients in the non-LVDD group (12%) and 22 patients in the LVDD group (33%). The 1 and 3-year cumulative all-cause mortality rates were 3% and 6%, respectively, in the non-LVDD group as compared with 4% and 11%, respectively, in the LVDD group. The 1 and 3-year cumulative MACE rates were 3% and 9%, respectively, in the non-LVDD group as compared with 16% and 24%, respectively, in the LVDD group. For overall MACE, the LVDD group experienced 1 stroke, 8 MIs, 10 HF hospitalizations, and 5 CAD revascularizations whereas the non-LVDD group had no strokes, 3 MIs, 6 HF hospitalizations, and 4 CAD revascularizations. There were 6 noncardiac and 12 cardiac deaths in the LVDD group, and 3 noncardiac and 5 cardiac deaths in the non-LVDD group. The mean follow-up period for the whole group of patients was 3.8 years. These results are summarized in Table 2.
TABLE 2.
Risk of Mortality and Major Adverse Cardiovascular Events of the 149 Patients Undergoing Peritoneal Dialysis†

Correlates of LVDD in PD Patients
We have used logistic regression models to determine the correlates of LVDD in our previous cross-section analysis8; it was found that visceral fat and peritoneal fat were associated with LVDD after adjusting for age, DM, hypertension, and dyslipidemia. However, after adjusting for the systemic inflammation (ie, hsCRP), systemic inflammation was significantly associated with LVDD whereas visceral or peritoneal fat content were no longer to be related to LVDD.8
Prognostic Predictors in PD Patients
To examine the association with primary and secondary endpoints, we examined the association by univariate analysis first. HRs for important factors obtained from univariate Cox regression analysis were presented in Supplemental Table 2A and B, http://links.lww.com/MD/A262. We then performed 4 hierarchical Cox regression models. In model 1, none of the clinical characteristics was associated with MACE or mortality (Tables 3 and 4). In model 2, visceral fat/subcutaneous fat (V/S) was independently associated with MACE and mortality after adjusting for age, DM, hypertension, RRF, hemoglobin, prior MI, and LDL (HR: 1.70; 95% CI: 1.35–2.41; P = 0.04 for MACE and HR: 1.84; 95% CI: 1.22–2.91, P = 0.04 for mortality) (Tables 3 and 4, model 2). In model 3, which incorporated baseline risk factors, adjusted visceral adiposity, and systemic inflammation (hsCPR) was significantly associated with both MACE and survival (adjusted HR: 2.31; 95% CI: 1.47–3.42; P = 0.03 and adjusted HR: 2.37; 95% CI: 1.18–3.41; P = 0.03, respectively), whereas V/S was no longer related to the measured outcomes (Tables 3 and 4, model 3). Last, in model 4, when LVDD was incorporated, LVDD was associated with both MACE and mortality (HR: 1.71; 95% CI: 1.43–3.51; P = 0.02 and HR: 2.25; 95% CI: 1.45–2.91; P = 0.04, respectively) (Tables 3 and 4, model 4). The Kaplan–Meier plots for mortality and MACE of PD patients, according to the presence and absence of LVDD, are presented in Figures 1 and 2, respectively. We also excluded the patients with old MI and repeated the regression analysis. The major findings remained similar after the analysis (Supplemental Table 3A and B, http://links.lww.com/MD/A262).
TABLE 3.
HRs for Factors Obtained From Multivariate Cox Regression Analysis of Time to Major Adverse Cardiovascular Events in 149 Patients Undergoing Peritoneal Dialysis
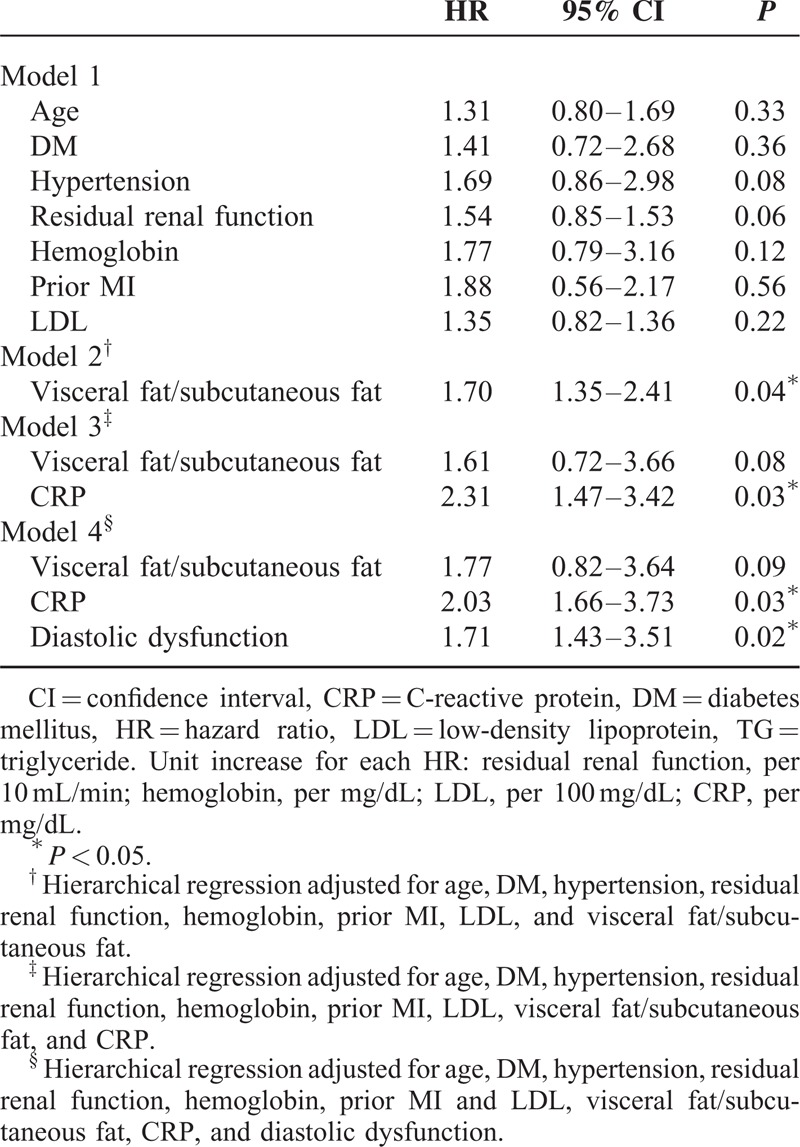
TABLE 4.
HRs for Factors Obtained From Multivariate Cox Regression Analysis of Survival or Time to Mortality in 149 Patients Undergoing Peritoneal Dialysis
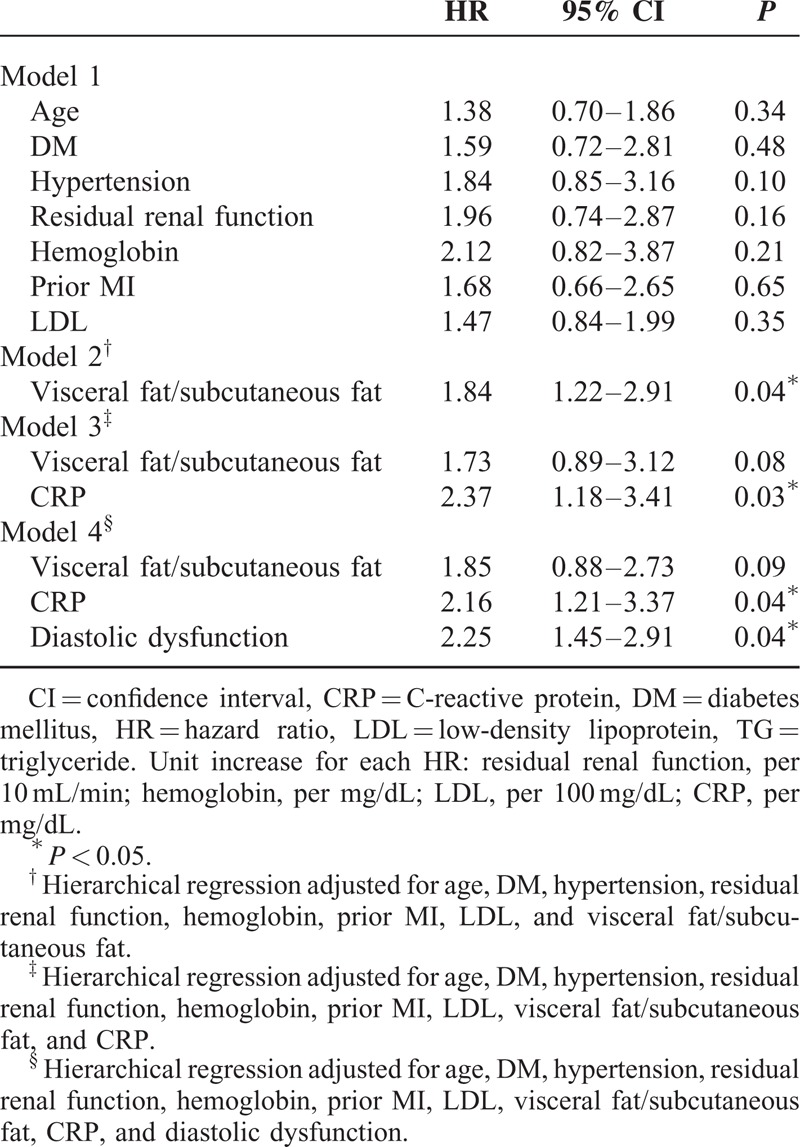
FIGURE 1.
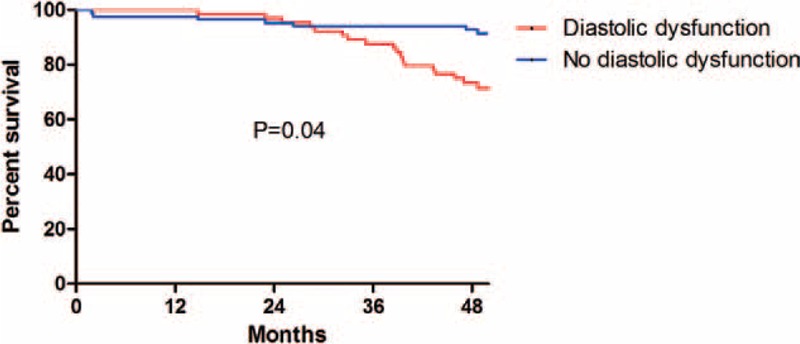
Kaplan–Meier plots of survival for patients undergoing peritoneal dialysis with or without left ventricular diastolic dysfunction. According to Kaplan–Meier analysis, there was a significant difference between the 2 groups (diastolic dysfunction vs no diastolic dysfunction). Left ventricular diastolic dysfunction was a significant risk factor for mortality (P = 0.04).
FIGURE 2.
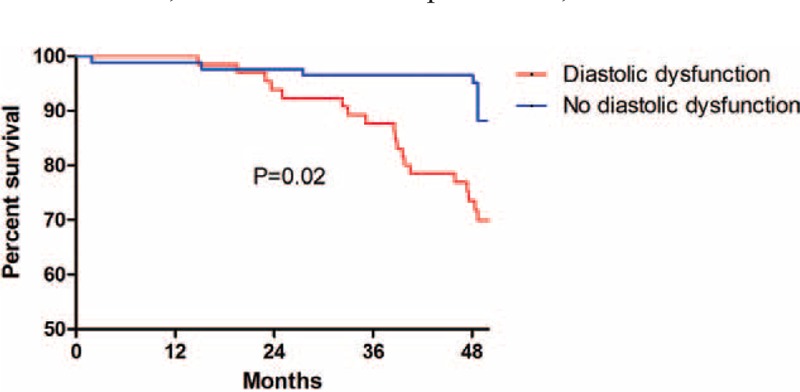
Kaplan–Meier plots of major adverse cardiovascular event-free survival for patients undergoing peritoneal dialysis with or without left ventricular diastolic dysfunction. According to Kaplan–Meier analysis, there was a significant difference between the 2 groups (diastolic dysfunction vs no diastolic dysfunction). Left ventricular diastolic dysfunction was a significant risk factor for major adverse cardiovascular events (P = 0.02).
DISCUSSION
Our current study is the extension study from our previous article.8 The findings from previous cross-sectional correlational study suggested that adipose tissue contribute to the development of inflammation that, in turn, lead to LVDD especially in PD patients. Visceral fat did not influence LVDD directly. The aim of our current study is to evaluate whether the sequential influence of adipose tissue, inflammation, and LVDD could extend to solid outcomes in this distinct population. Accordingly, we longitudinally followed the same group of patients for around 3.8 years and conclude that LVDD is an independent risk factor for overall mortality and cardiovascular events in PD patients. We demonstrated that the relationship between visceral fat and LVDD is partially attenuated when adjustment is made for inflammation. In addition, LVDD and systemic inflammation were observed to be 2 independent risk factors for mortality and MACE in PD patients. To the best of our knowledge, we are the first group to investigate the prognostic role of LVDD in PD patients after correcting for systemic inflammation, comorbidities, and even adiposity, as determined by CT.
LVDD may influence the prognosis of PD patients for several potential reasons. First, although we controlled for some common comorbidities, systemic inflammation, and even adiposity, we found that unadjusted confounding factors may also influence the results. For example, the presence of significant CAD is known to be associated with LVDD,14 and CVD is a leading cause of morbidity and mortality in ESRD patients.15–17 In addition, LVDD has also been proposed to be an independent marker of mortality in different groups of patients and usually precedes LV systolic dysfunction, which may, in turn, lead to worse outcomes.18
In the present study, we first performed multivariate logistic regression analysis including several fat components. The results revealed that only V/S correlated significantly with LVDD. In fact, recent reviews suggested a greater impact of visceral adipose tissue and central obesity, rather than other fat components on the inflammatory process.19 Matsuzawa20 concluded that visceral fat accumulation, rather than other adipose tissue, has been shown to cause impaired glucose metabolism, lipid disorders, and hypertension mainly through the attenuation of plasma adiponectin.20 However, we demonstrated that the amount of visceral fat did not influence the prognosis of PD patients after adjusting for confounding factors including systemic inflammation. Nevertheless, systemic inflammation had a major influence on both morbidity and mortality. Excess adipose tissue is associated with insulin resistance, a proinflammatory status, and a higher risk for CAD, all of which are contributory to worse outcomes.21,22 A recent report concluded that increased visceral fat at the initiation of PD is not an independent predictor of poor survival.23 In our study, we demonstrated that an increase in visceral fat is associated with a higher level of inflammation, which, in turn, may be associated with the development of LVDD and a worse prognosis. Based on the findings of our present and previous studies,6,8 we may suggest that the effects of visceral fat on worsening prognosis are at least partially through increasing inflammation, and thereby, the development of LVDD.
There are several possible molecular mechanisms underlying the complex relationships among central obesity, inflammation, and LVDD.24 First, PD patients usually had increase amount of systemic adiposity during the initiation of the treatment, possible due to changes of uncoupling protein that could influence the lipid metabolism.25 In addition, visceral fat could release growth factors to a higher extent than other systemic adipose tissue.26 Structural study also shows that some visceral adipocytes can protrude from the mesothelial surface and may get into direct contact with dialysate.27 Therefore, dialysate may easily contact the parietal adipose tissue. The repeated exposure to dialysate could lead to continuous change in peritoneal physiology during PD that could be associated with the activation of peritoneal adipocytes and higher inflammation status.
In addition, patients on PD have increased adiposity amount owing to greater absorption of glucose from the dialysate. Increase in adiposity is reported to be a source of inflammatory cytokines or adipokines, for example, leptin, which is increased in PD patients.28 Leptin is also reported to have strong interaction with proinflammatory cytokines including interleukin-1 and tissue necrosis factor-α.29 Finally, there are studies about persistent release of proinflammatory mediators in PD patients with maintenance or after an episode of peritonitis.30 Long-term PD is often associated with structural alterations of the peritoneal membrane that are closely related to chronic systemic inflammatory responses. On one hand, chronic inflammation remains an important cause of morbidity in patients with end-stage renal failure, and, on the other hand, we found that inflammatory cytokines had a direct effect on sarcoplasmic reticulum calcium ATPase (SERCA2) at the transcription level and through downregulation of SERCA2 gene expression, inflammatory cytokines may cause cardiac diastolic dysfunction by decreasing diastolic calcium reuptake.31 Therefore, with higher chronic systemic inflammatory responses, PD patients are prone to higher chances of development of LVDD as well as worse prognosis.
Several studies focused on LVDD with mortality and cardiovascular morbidity in the ESRD population.32 Zaslavsky et al13 recruited 68 hemodialysis patients with or without diabetes for a 4.25-year follow-up, and concluded that the presence of moderate or severe degrees of LVDD is a significant prognostic factor for all-cause mortality in diabetic patients starting hemodialysis. Another study evaluated the association between LVDD (using E/e′ and left atrium volume index), RRF, and adverse cardiovascular outcomes. The authors found that LVDD may be a significant predictor of rapid decline in RRF and adverse cardiac outcomes in patients starting PD.33 Apart from previous studies, we collected detailed demographic data, biochemical data, and performed precise evaluation for LVDD and systemic adiposity in a larger PD cohort. Furthermore, we delineated the complex association between inflammation, systemic adiposity, LVDD, and outcomes.
It should be noted that this study had several limitations. First, we did not assess the severity of LVDD via echocardiography, which may have identified a “dose-dependent” correlation with prognosis. Furthermore, we selected Taiwanese patients undergoing PD with a stationary dialysis period of ≥6 months. Although we followed all of the patients carefully and controlled for most available risk factors, the generalizability in emergent dialysis patient or in other populations still should be determined in a future study. Regarding the possible pathways that LVDD may influence prognosis of PD patients, other associations and possible pathways may be demonstrated if we had more cases. The results from the hierarchical regression analysis are consistent with the hypothesis of the sequential biological pathway. However, because of the cross-sectional design in the initial evaluation of adiposity, inflammation, and diastolic dysfunction, we are conservative in stating a “causal” relationship. In addition, the sample size was relatively small with also limited events rates during 3.8 years of follow-up. Although we adjusted for confounding factors by several Cox regression models, it is difficult to tease out the relative contributions of multiple explanatory covariates when one has a small sample size and an even smaller number of outcome events. Therefore, the study had limited power to explore the true clinical effect of many of the listed measures, which warrants further evaluation in larger cohort or randomized control studies.
To the best of our knowledge, the current study was the first to show that LVDD is an independent, sensitive marker of MACE and mortality in PD patients. Therefore, the examination of patients undergoing PD should include an echocardiography with tissue Doppler imaging study, and if LVDD is diagnosed, a careful follow-up and better management of risk factors for LVDD is warranted. In addition, the impact of visceral fat on worse outcomes in patients undergoing PD is partially attenuated when adjustment is made for inflammatory markers and LVDD. We also delineated the complex relationships among central obesity, inflammation, LVDD, and prognosis in PD patients using hierarchical regression analysis.
Footnotes
Abbreviations: CAD = coronary artery disease, CT = computed tomography, DM = diabetes mellitus, ESRD = end-stage renal disease, HF = heart failure, LVDD = left ventricle diastolic dysfunction, MACE = major cardiovascular disease, MI = myocardial infarction, PD = peritoneal dialysis, RRF = residual renal function.
C-KW and J-KL have contributed equally to this article.
This work was, in part, supported by grants from the National Science Council of the Republic of China (103-2314-B-002-145) and the TVGH-NTUH Joint Research Program (VN104–01).
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32 (5 suppl 3):S112–S119. [DOI] [PubMed] [Google Scholar]
- 2.Lee JK, Lin HH, Tsai CT, et al. Differential association of proinflammatory cytokines with left ventricular diastolic dysfunction in subjects with and without continuous ambulatory peritoneal dialysis. Nutr Metab Cardiovasc Dis 2012; 22:974–980. [DOI] [PubMed] [Google Scholar]
- 3.Bajraktari G, Berbatovci-Ukimeraj M, Hajdari A, et al. Predictors of increased left ventricular filling pressure in dialysis patients with preserved left ventricular ejection fraction. Croat Med J 2009; 50:543–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stenvinkel P. Inflammation in end-stage renal disease: the hidden enemy. Nephrology (Carlton) 2006; 11:36–41. [DOI] [PubMed] [Google Scholar]
- 5.London GM. Cardiovascular disease in chronic renal failure: pathophysiologic aspects. Semin Dial 2003; 16:85–94. [DOI] [PubMed] [Google Scholar]
- 6.Wu CK, Huang YT, Lin HH, et al. Dissecting the mechanisms of left ventricular diastolic dysfunction and inflammation in peritoneal dialysis patients. PLoS One 2013; 8:e62722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu CK, Yang CY, Lin JW, et al. The relationship among central obesity, systemic inflammation, and left ventricular diastolic dysfunction as determined by structural equation modeling. Obesity (Silver Spring) 2012; 20:730–737. [DOI] [PubMed] [Google Scholar]
- 8.Lin HH, Lee JK, Yang CY, et al. Accumulation of epicardial fat rather than visceral fat is an independent risk factor for left ventricular diastolic dysfunction in patients undergoing peritoneal dialysis. Cardiovasc Diabetol 2013; 12:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu CK, Luo JL, Tsai CT, et al. Demonstrating the pharmacogenetic effects of angiotensin-converting enzyme inhibitors on long-term prognosis of diastolic heart failure. Pharmacogenomics J 2010; 10:46–53. [DOI] [PubMed] [Google Scholar]
- 10.Wu CK, Tsai CT, Chang YC, et al. Genetic polymorphisms of the angiotensin II type 1 receptor gene and diastolic heart failure. J Hypertens 2009; 27:502–507. [DOI] [PubMed] [Google Scholar]
- 11.Wu CK, Tsai CT, Hwang JJ, et al. Renin-angiotensin system gene polymorphisms and diastolic heart failure. Eur J Clin Invest 2008; 38:789–797. [DOI] [PubMed] [Google Scholar]
- 12.Greenland S. Hierarchical regression for epidemiologic analyses of multiple exposures. Environ Health Perspect 1994; 102 (suppl 8):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaslavsky LM, Pinotti AF, Gross JL. Diastolic dysfunction and mortality in diabetic patients on hemodialysis: a 4.25-year controlled prospective study. J Diabetes Complicat 2005; 19:194–200. [DOI] [PubMed] [Google Scholar]
- 14.Vlasseros I, Katsi V, Vyssoulis G, et al. Aggravation of left ventricular diastolic dysfunction in hypertensives with coronary artery disease. Hypertens Res 2013; 36:885–888. [DOI] [PubMed] [Google Scholar]
- 15.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 16.McCullough PA. Why is chronic kidney disease the “spoiler” for cardiovascular outcomes? J Am Coll Cardiol 2003; 41:725–728. [DOI] [PubMed] [Google Scholar]
- 17.Steinmetz OM, Panzer U, Stahl RA, et al. Statin therapy in patients with chronic kidney disease: to use or not to use. Eur J Clin Invest 2006; 36:519–527. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Del-Arbol L, Achecar L, Serradilla R, et al. Diastolic dysfunction is a predictor of poor outcomes in patients with cirrhosis, portal hypertension, and a normal creatinine. Hepatology 2013; 58:1732–1741. [DOI] [PubMed] [Google Scholar]
- 19.de Heredia FP, Gomez-Martinez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc 2012; 71:332–338. [DOI] [PubMed] [Google Scholar]
- 20.Matsuzawa Y. The role of fat topology in the risk of disease. Int J Obes (Lond) 2008; 32 (suppl 7):S83–S92. [DOI] [PubMed] [Google Scholar]
- 21.Sanches FM, Avesani CM, Kamimura MA, et al. Waist circumference and visceral fat in CKD: a cross-sectional study. Am J Kidney Dis 2008; 52:66–73. [DOI] [PubMed] [Google Scholar]
- 22.Yamauchi T, Kuno T, Takada H, et al. The impact of visceral fat on multiple risk factors and carotid atherosclerosis in chronic haemodialysis patients. Nephrol Dial Transplant 2003; 18:1842–1847. [DOI] [PubMed] [Google Scholar]
- 23.Choi SJ, Kim EJ, Park MY, et al. Does body fat mass define survival in patients starting peritoneal dialysis? Perit Dial Int 2014; 34:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai KN, Leung JC. Peritoneal adipocytes and their role in inflammation during peritoneal dialysis. Mediators Inflamm 2010; 2010:495416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nordfors L, Heimburger O, Lonnqvist F, et al. Fat tissue accumulation during peritoneal dialysis is associated with a polymorphism in uncoupling protein 2. Kidney Int 2000; 57:1713–1719. [DOI] [PubMed] [Google Scholar]
- 26.Fain JN, Madan AK, Hiler ML, et al. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology 2004; 145:2273–2282. [DOI] [PubMed] [Google Scholar]
- 27.Di Paolo N, Sacchi G. Atlas of peritoneal histology. Perit Dial Int 2000; 20 (suppl 3):S5–S96. [PubMed] [Google Scholar]
- 28.Kim DJ, Oh DJ, Kim B, et al. The effect of continuous ambulatory peritoneal dialysis on change in serum leptin. Perit Dial Int 1999; 19 (suppl 2):S172–S175. [PubMed] [Google Scholar]
- 29.Wolf G, Chen S, Han DC, et al. Leptin and renal disease. Am J Kidney Dis 2002; 39:1–11. [DOI] [PubMed] [Google Scholar]
- 30.Pecoits-Filho R, Stenvinkel P, Wang AY, et al. Chronic inflammation in peritoneal dialysis: the search for the holy grail? Perit Dial Int 2004; 24:327–339. [PubMed] [Google Scholar]
- 31.Wu CK, Lee JK, Chiang FT, et al. Plasma levels of tumor necrosis factor-alpha and interleukin-6 are associated with diastolic heart failure through downregulation of sarcoplasmic reticulum Ca2+ ATPase. Crit Care Med 2011; 39:984–992. [DOI] [PubMed] [Google Scholar]
- 32.Farshid A, Pathak R, Shadbolt B, et al. Diastolic function is a strong predictor of mortality in patients with chronic kidney disease. BMC Nephrol 2013; 14:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JK, Kim SG, Kim MG, et al. Left ventricular diastolic dysfunction as a predictor of rapid decline of residual renal function in patients with peritoneal dialysis. J Am Soc Echocardiogr 2012; 25:411–420. [DOI] [PubMed] [Google Scholar]


