Abstract
Colorectal neoplasm (CRN) and coronary heart disease (CHD) share common risk factors. We aimed to assess the risk for CRN in patients who are at high risk for developing CHD determined by measurements, which are independent from the risk factors for CRN.
This study was conducted on individuals who underwent total colonoscopic examination and were without history of CHD. Two-hundred thirty-five subjects (82 with CRN and 153 with normal colonoscopic findings) participated in the study. Colorectal carcinoma (CRC) was defined as the presence of adenocarcinoma. We measured carotid intima media thickness (CIMT), flow-mediated dilation (FMD), and calculated Framingham risk score (FRS) for all participants. An increased CIMT (≥1.0 mm), a decreased FMD (<10%), and a high FRS (>20%) were defined as high risks for developing CHD. The risk and the prevalence of CRN were analyzed in relation to the risk for developing CHD.
The ratio of the patients with overall-CRN and CRC was significantly higher in individuals who are at high risk for developing CHD compared with individuals who are at low risk for developing CHD by each 3 risk estimation method (P < 0.05 for all). An increased CIMT, a decreased FMD, and a high FRS score were significantly associated with the high risk for the presence of CRC (odds ratio [OR]: 6.018, OR: 3.699, and OR: 4.120, respectively). An increased CIMT, a decreased FMD, and an intermediate FRS were significantly associated with the risk for the presence of overall-CRN (OR: 3.607, OR: 1.866 and OR: 2.889, respectively).
The risk for CRN increases as the risk for developing CHD increases. It can be suggested that screening for CRN can be recommended for individuals who are at high risk for developing CHD.
INTRODUCTION
Colorectal carcinoma (CRC) is the second most commonly diagnosed cancer in women and the third most common in men, and the second leading cause of cancer-related death.1 Removal of premalignant adenomas can prevent the cancer and removal of localized cancer may prevent CRC-related death. Screening colonoscopy is the best way for prevention and early detection of the disease.
Risk estimation is important to identify individuals who have high risk for CRC; therefore, high-risk patients should be referred to screening for colorectal neoplasm (CRN) and interventions to reduce the burden of disease. For establishing a targeting screening strategy for CRC, it is essential to define patients who are at high risk for CRN, including CRC and its biological precursors.2 The current decision tool to determine the risk for CRC includes age, positive fecal occult blood test, history of inflammatory bowel disease, colonoscopic findings, family history for CRC, and genetic syndrome predisposing to CRC. Since screening programs have been applied, both the incidence and mortality rates of CRC have been declining. However, it is still ranking high among cancer-related burden.3 Thus, there may be other contributing factors for developing CRC.
Coronary heart disease (CHD) shares same risk factors with CRN such as smoking, age, male gender, obesity, and diabetes mellitus.4 It has also high incidence rates. Recently, investigators have suggested the presence of significant association between evident CHD and CRN.4–6 Chan et al5 have showed strong coexistence of CRN and overt CHD.4 Then, they have found that the prevalence of CRN was greater in patients with CHD in population undergoing coronary angiography. Yang et al6 have also found the prevalence of colorectal adenoma was greater in subjects with low-grade coronary atherosclerosis or significant CHD detected by coronary computed tomography angiography. Screening for CRN has been suggested in patients with overt CHD.4–6 But patients with overt CHD have increased risk for complications related with CHD and medications such as anti-platelet agents or anticoagulants during the course of colonoscopic evaluation. In contrast, patients who have risk factors for developing CHD, but not established CHD, are free from these limitations and screening of CRN may be more reliable in these individuals.5 But there has not been enough data about the prevalence and the risk for CRN in patients who are at high risk for developing CHD. Therefore, it is important to investigate the association between the presence of CRN and developing CHD.
Carotid intima media thickness (CIMT) and flow-mediated dilation (FMD) are noninvasive and easy reproducible techniques. Increased CIMT and decreased FMD have been shown to be associated with a high risk for CHD.7,8 Framingham risk score (FRS) has been developed to identify patients who are at high risk for CHD9 and can be used in primary care.
In the present study, we aimed to investigate the prevalence of and the risk for overall-CRN and CRC in individuals who are at high risk for developing CHD determined by measurements of CIMT, FMD, and FRS.
METHOD
Study Population
This cross-sectional study was conducted on individuals without CHD who underwent colonoscopic evaluation through appropriate indications between February 2014 and July 2014 at a single center in Turkey. This study was approved by institutional ethical board and was in accordance with the Helsinki Declaration. The subjects were given written information about the study and a standard questionnaire regarding their personal medical history, present medications, family history, and life style habits.
During the study period, a consecutive series of 637 subjects, 26 to 85 years of age, were evaluated. We excluded the subjects with the history of cardiovascular disease (n = 70), cerebrovascular disease (n = 35), and peripheral vascular disease (n = 22). We also excluded the subjects who had incomplete colonoscopic evaluation (n = 42), poor bowel preparation (n = 88), inflammatory bowel disease (n = 69), active gastrointestinal bleeding (n = 25), history of colorectal surgery (n = 9), CRC diagnosed beforehand (n = 4), and hereditary cancer syndrome (n = 1). After exclusion of inappropriate subjects, 272 subjects were eligible for the study. A total of 235 patients were accepted to participate into the study. Participation rate was 86.4%.
The location, number, and size of CRN were recorded according to the colonoscopy reports which were performed by the experienced colonoscopists. The participants were classified into 3 groups according to the colonoscopic findings and histological subtypes of polyps. Subjects with normal colonoscopic findings were defined as control group, subjects who have ≥1 adenomatous polyps with or without adenocarcinoma were included into overall-CRN group, and subjects who have adenocarcinoma were defined as CRC group. The participants were also classified according to their risk status for developing CHD. High risk for CHD was defined as having increased CIMT (≥1.0 mm), decreased FMD (<10%), high or intermediate FRS (10%–20% and >20%, respectively).
Measurements, Definitions, and Laboratory Assays
Anthropometric measurements, blood pressure, and laboratory tests were measured after a 12-hour fasting period on the day of the colonoscopy. Trained nurses measured the height and weight of participants. Blood pressure was measured after a 5-min rest with a standard mercury sphygmomanometer. The presence of hypertension was defined according to the 2013 hypertension guidelines of the European Society of Hypertension and the European Society of Cardiology,10 or the use of antihypertensive medication. Waist circumference was measured at the umbilicus level. Increased waist circumference was based on the definition of the Regional Office for the Western Pacific Region of World Health Organization criteria.11 The body mass index was calculated as kilogram per square meter. Diabetes mellitus was determined by American Diabetes Association guidelines in 2003.12 Metabolic syndrome was defined as having at least 3 of the criteria set by the Adult Treatment Panel III criteria, as updated by the American Heart Association.13
A venous blood sample was drawn from an ante-cubital vein. The levels of liver enzymes, lipids, glucose, and other biochemical markers were measured in the sera of subjects. The Homeostasis Model Assessment for Insulin Resistance (HOMA-IR), an index of insulin resistance (IR), was calculated with the serum insulin and glucose values of an individual.14 Presence of IR was defined as having HOMA-IR score ≥2.5.
The Risk Assessment for Estimating the 10-Year Risk of Coronary Heart Disease
The 10-year risk for developing CHD was estimated by using the Framingham/Adult Treatment Panel III Risk Score 2002, which has been validated as a predictive risk assessment tool for the development of CHD.9 The parameters, which are included in the FRS calculation method, are sex, age, total cholesterol level, high-density lipoprotein cholesterol level, systolic blood pressure, medication for hypertension, and cigarette smoking. The estimation for 10-year CHD risk was categorized as low risk (<10 %), intermediate risk (10%–20%), or high risk (>20%).15
Measurement of CIMT
The carotid arteries were evaluated using the same high-resolution B-mode system (Logiq P5, General Electric Company, Wauwatosa, USA) with a transducer frequency of 7.5 MHz. Measurements were made by readers blinded to all clinical information. The subjects were examined in the supine position with the head turned slightly to the left. The near and far wall of the right and left distal common carotid arteries was scanned in accordance with the Rotterdam Study ultrasound protocol.7 The IMT was measured only at a plaque-free site, along a 10-mm segment of the far wall of the common carotid arteries and measured as the distance between the lumen–intima interface and the media adventitia interface. For each side, at least 3 optimal longitudinal images were frozen in end-diastole by electrocardiogram R triggering. The average of the intima-media thickness of each of the 3 frozen images was calculated. For each individual, the general CIMT was determined as the average of measurements of both the left and right arteries. Cut-off value for increased CIMT was determined as ≥1.0 mm.16
Measurements of FMD and Flow of Brachial Artery
We measured FMD of the brachial artery according to the International Brachial Artery Reactivity Task Force guidelines17 using a novel ultrasound system equipped with an edge-tracking system for 2-dimentional imaging and a pulsed Doppler flow velocimeter for automatic measurement. The right brachial artery was scanned over a longitudinal section, 3 to 5 cm above from the right elbow and the arm was kept in the same position throughout the study. A pneumatic tourniquet placed around the distal forearm. first, the diameter of the brachial artery was recorded in the cubital region at rest. Subsequently, the cuff was inflated to 50 mmHg above from the systolic blood pressure of patient for 5 min and then increased flow was induced by sudden cuff deflation. The diameter of the artery was monitored continuously at the same point, and the maximum dilatation after deflation was recorded. The diameter of the brachial artery was measured from the anterior to the posterior interface between the media and adventitia (“m line”) at a fixed distance. All measurements were made at both end diastole and end systole to avoid possible errors resulting from variable arterial compliance. The change in diameter caused by FMD was expressed as the percentage relative to diameter in the initial resting scan. Cut-off value for decreased FMD was determined as <10%.8
Statistical Analysis
The statistical analysis was performed with The Statistical Package for the Social Sciences version 15.0 (SPSS Inc, Chicago, IL). The statistical results are presented as the mean ± standard deviation, percentages or median (minimum-maximum). We used One way ANOVA test for normally distributed data. Kruskal-Wallis test and Mann Whitney U test were used for non-normally distributed data. Kruskal–Wallis test and Mann–Whitney U test were used for nonnormally distributed data. Risk estimation and comparison of categorical data were made by the χ2 test and multinominal logistic regression analysis. Each odds ratio is presented together with its 95% confidence interval. P value <0.05 was considered statistically significant.
RESULTS
Baseline Characteristics of the Subjects
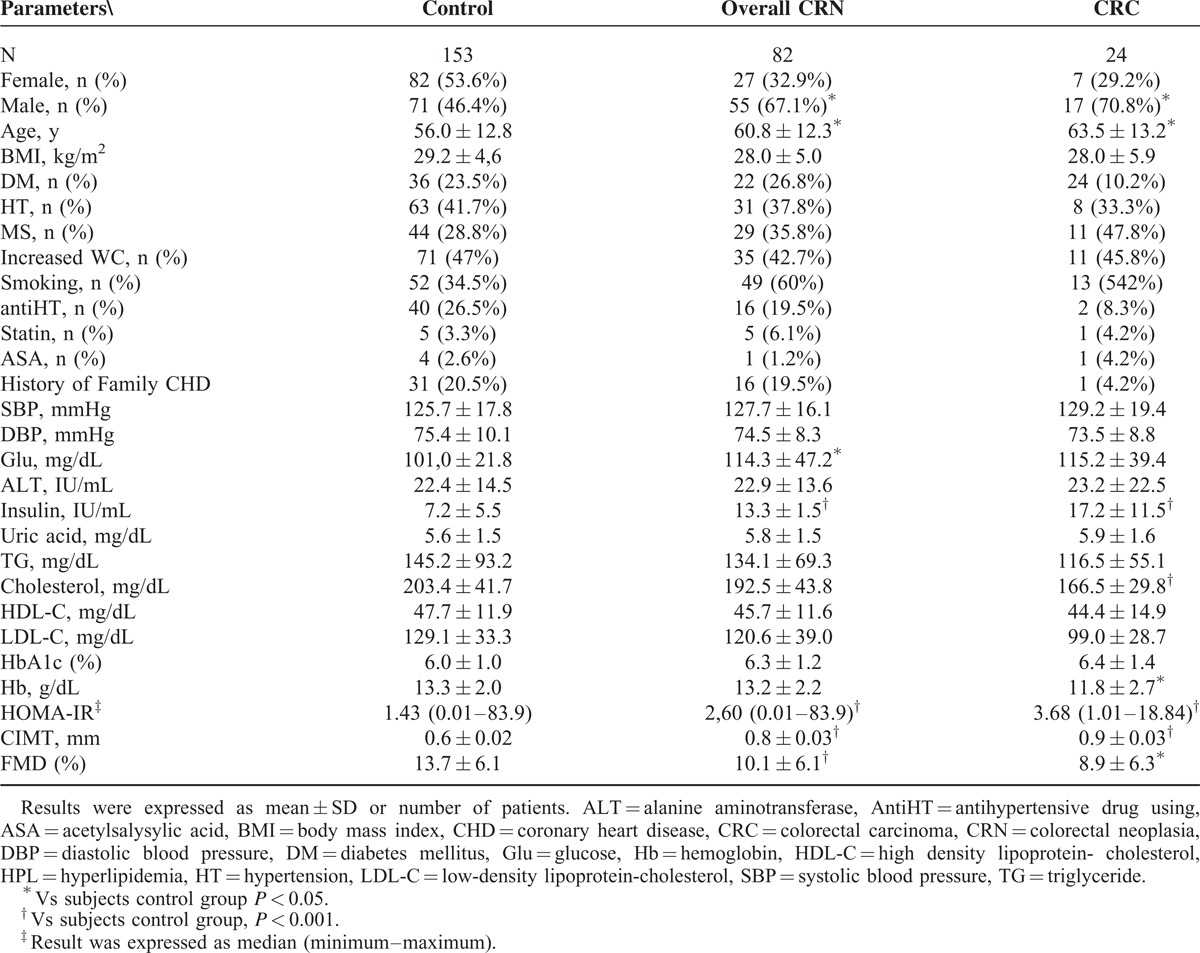
A total of 637 subjects were evaluated and 272 subjects were eligible after excluding 365 subjects according to exclusion criteria. Of these individuals, 235 subjects (86.4% participation rate) were included in the final analysis. The mean age of the enrolled subjects was 60.3 ± 12.6 years and there were 126 men (53%). A total of 82 subjects (35%) had ≥1 CRNs, of which 24 subjects (10%) had ≥1 CRCs. The subjects with CRN were more likely to be older than 50 years and to be male.
The baseline characteristics are summarized in Table 1. CRC and overall-CRN groups had significantly higher mean age than control group (P < 0.001). CRC group had significantly lower mean hemoglobin and mean total cholesterol levels than control group (P < 0.001 and P < 0.005, respectively). CRC and overall-CRN groups had higher HOMA-IR and insulin values than control group (P < 0.001). Overall-CRN group had significantly higher mean serum glucose level than control group (P < 0.005). CRC and overall-CRN groups had significantly higher mean CIMT values and significantly decreased FMD values (P < 0.001). There were no significant differences in other baseline characteristics between groups (Table 1).
TABLE 1.
Demographic and Clinical Characteristics of Groups
Prevalence of Colorectal Neoplasm in Relation to Risk for Developing Coronary Heart Disease
A total of 235 subjects were classified according to their risk status for developing CHD as follows: subjects who have normal CIMT (n = 176, 81.9%) and increased CIMT (n = 41, 19.9%); subjects who have normal FMD (n = 141, 65%) and decreased FMD (n = 76, 35%); subjects who have low FRS (n = 156, 66.4%), intermediate FRS (n = 56, 23.8%), and high FRS (n = 23, 9.8%). The prevalence of the overall-CRN was significantly higher in subjects with increased CIMT compared with those with normal CIMT (28.3% and 68.2%, P < 0.005). It was also significantly higher in subjects with decreased FMD and intermediate FRS compared with those with normal FMD and low FRS (26.2% and 52.3%, P < 0.05 and 26.3% and 39.1%, P < 0.05, respectively). The prevalence of the CRC was significantly higher in subjects with increased CIMT, decreased FMD, and high FRS compared with those with normal CIMT, FMD, and low FRS (6.3% and 31.7%, P < 0.05; 7.1% and 18.4%, P < 0.005; and 7.7% and 26.1%, P < 0.005, respectively). Figure shows that the proportions of overall-CRN and CRC increased with the increasing risk of CHD (Figure 1).
FIGURE 1.
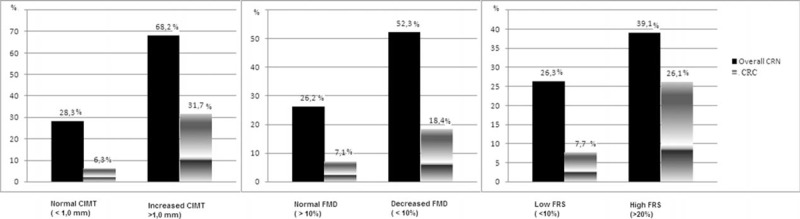
Prevalence of colorectal neoplasm with respect to developing risk for coronary heart disease.
Risk factors for Colorectal Neoplasm
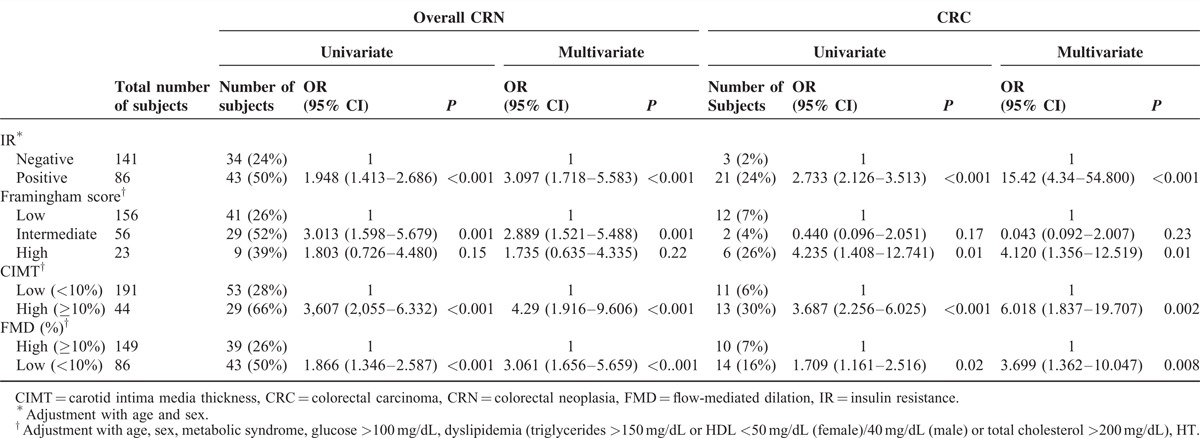
Table 2 shows the risk for overall-CRN and CRC according to the presence of IR, and risk predictors for CHD. The multivariate logistic regression analysis showed that the presence of IR, increased CIMT, decreased FMD, and high FRS were significantly associated with the high risk for the CRC (OR: 15.42, 95% CI: 4.34–54.800; OR: 6.018, 95% CI: 1.837–19.707; OR: 3.699, 95% CI: 1.362–10.047; and OR: 4.120, 95% CI: 1.356–12.519, respectively). The presence of IR, increased CIMT, decreased FMD, and intermediate FRS were significantly associated with the high risk for the overall-CRN in multivariate logistic regression analysis (OR: 3.097 95% CI: 1.718–5.583); OR: 4.29 95% CI: 1.916–9.606; OR: 3.061 95% CI: 1.656–5.659; OR: 2.889 95% CI: 1.521–5.488, respectively).
TABLE 2.
Risk of Overall and Advanced Colorectal Neoplasm
Association Between the Characteristics of Colorectal Neoplasm, Predictors of Coronary Heart Disease, and Insulin Resistance
Table 3 shows the risk for developing CHD and presence of IR according to presence of multiple (≥3) CRNs, large (≥1 cm) CRN, CRN with malignant or premalignant histology (high-grade dysplasia or invasive cancer), and any CRN located in the proximal colon. Multivariate logistic regression model revealed that presence of large CRN was associated with a high risk for increased CIMT and the presence of IR (OR: 2.12, 95% CI: 1.104–4.075 and OR: 1.792 95% CI: 1.154–2.783; respectively); presence of multiple CRNs was associated with a high risk for intermediate FRS (OR: 6.175, 95% CI: 1.887–20.203); CRN with malignant or premalignant histology was associated with high risk for increased CIMT, high FRS, and presence of IR (OR: 1.912, 95% CI: 1.108–3.298; OR: 5.333, 95% CI: 1.147–24.791; OR: 2.092, 95% CI: 1.480–2.959, respectively).
TABLE 3.
Characteristics of CRNs and Risk for CHD

DISCUSSION
This study showed a significantly increased risk for CRN in patients who are at high risk for CHD determined by measurements, which are independent from CRN risk factors.
In the literature, there is some evidence about significant association between the presence of CRN and overt CHD. Reicher-Reiss et al18 have observed an excess incidence of CRC in patients with overt CHD. Chan et al4,5 and Yang et al6 have showed an increased prevalence of CRN in patients with established CHD confirmed by coronary angiography or computed tomography. However, there has not been enough knowledge about the prevalence and the risk for CRN in patients who are at high risk for developing CHD. In the literature, only, Lee et al2 have investigated the prevalence of CRN in patients who are at high risk for CHD by considering FRS of patients and they have showed an increased prevalence of CRN in subjects with high FRS. However, this result has not been confirmed by any further studies until now.
Lee et al2 have suggested that patients who are at high risk for developing CHD might be referred to the screening program for CRN. Because the early detection of CRC and removal of the precursor lesions can be life-saving, the screening program is very important. To be cost-effective of a screening program of a disease, it must be common in the determined risk group. Because the calculation method of FRS includes age, sex, and smoking habit, which are also parameters of colorectal screening score suggested by Asia-Pacific Working Group of CRC,19 it is not surprising to find a high coexistence of these 2 diseases. These results need to be confirmed by measurements, which are independent from risk factors for CRN. In the present study, we determined the risk for CHD by measuring CIMT as an indicator of vascular structural alteration, and FMD as an indicator of endothelial function. These parameters are also predictors of the risk for CHD. We also calculated FRS to confirm our results. We found that the prevalence of overall-CRN and CRC increased as the risk for developing CHD increased. In a previous study,2 which showed the prevalence of CRN among patients who are at high risk for CHD determined by FRS, the overall prevalence of CRN and CRC were significantly higher in subjects with high risk for CHD compared with those with low risk for CHD (25.6% and 4.9%, 53.4% and 17.8%,, respectively). Their findings are similar to our results. Furthermore, the previous studies, which were conducted on patients with established CHD, have reported the similar proportions in accordance with our study.4–6
Our study also showed that an increased CIMT and a decreased FMD were associated with significantly high risk for overall-CRN and CRC. Although an intermediate FRS was associated with an increased risk for overall CRN, high FRS was associated with an increased risk for CRC in our study. These results also support the previous findings strongly.
In the study performed by Lee et al,2 they have found that multiple CRNs (≥3) and large CRN (≥1 cm) have been associated with high risk for CHD calculated by FRS. Similarly, we found that multiple CRNs, large CRN and malignant or premalignant histology were associated with ≥1 predictors of developing CHD.
Besides supporting the previous results, our study draws attention to the common pathways in the pathogenesis of both CRC and CHD. Both atherogenesis and carcinogenesis start on the healthy surface. With the effects of triggering factors, vascular endothelial cells and normal colonic mucosa show differences in terms of genetic mutations, proliferation, and inflammation in the course of time. CRC and CHD share similar risks factors, such as diabetes mellitus; hyperlipidemia; sedentary lifestyle; high-fat, low-fiber diet; obesity; and hypertension.20–25
IR plays important role in pathogenesis of both CRC and CHD; it also acts on the basis of most of risk factors for both diseases (obesity and diabetes mellitus). Insulin stimulates the growth of colorectal cells and increases bioactive insulin-like growth factor-1, which inhibits apoptosis and allows progression through the cell cycle. In the course of malignant progression from adenoma to carcinoma, insulin also plays a mitogenic role by means of ras mutations, which are important in colon carcinogenesis.26 In previous studies, CRC has been shown to be strongly associated with IR.27–30 In the present study, we found that patients with IR had significantly very high risk for overall-CRN and CRC.
IR is also activated in chronic inflammatory processes.33 Atherosclerosis and carcinogenesis are now well known as chronic inflammatory states.31 The development of CRC in patients with ulcerative colitis is an evidence for this concept. The tumor microenvironment contains a variety of leukocytes and inflammatory factors such as tumor necrosis factor-α and interleukine-6. In addition, tumor cells themselves produce tumor necrosis factor-α.32 Inflammation may be suspected for the simultaneous development of these 2 conditions. Toxic agents, high fat-containing diet, smoking, and obesity are known to induce chronic inflammation. Although there are so many similarities in the pathogenesis of both diseases, it cannot be ignored that these 2 diseases could occur simultaneously.
Our study had some limitations. It was a single center study, which had a relatively small sample size. Our study population included participants who were referred to screening colonoscopy because of they had the risk factors for CRN, especially advanced age. This may cause selection bias; further studies conducted on healthy subjects could clarify this issue. In our study, inflammatory markers, which may be related with the common mechanisms for both CRC and CHD, were not studied. Such markers may be included in upcoming studies to clarify the exact mechanism.
In conclusion, this study showed significant relationship between the presence of CRN and the risk for CHD by measuring independent factors from the risk factors for CRN. The patients who are at high risk for developing CHD were found to have an increased risk for the overall presence of CRN and CRC. According to our results and previous results, it can be suggested that relatively inexpensive screening tests such as fecal occult blood test, rectosigmoidoscopy can recommended for patients who are at high risk for developing CHD.
Footnotes
Abbreviations: CHD = coronary heart disease, CIMT = carotid intima media thickness, CRC = colorectal carcinoma, CRN = colorectal neoplasm, FMD = flow-mediated dilation, FRS = Framingham risk score, HOMA-IR = homeostasis model assessment for insulin resistance, IR = insulin resistance.
The authors report no conflicts of interest.
REFERENCES
- 1.US Cancer Statistics Working Group. United States Cancer Statistics: 1999–2005 Incidence and Mortality Website Report. Published 2009. [Google Scholar]
- 2.Young Lee J, Noh Hong S, Hwan Kim J, et al. Risk for coronary heart disease increases risk for colorectal neoplasm. Clin Gastroenterol Hepatol 2013; 11:695–702. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. Cancer J Clin 2014; 64:9–29. [DOI] [PubMed] [Google Scholar]
- 4.Chan AOO, Lam KF, Tong T, et al. Coexistence between colorectal cancer/adenoma andcoronaryarterydisease: resultsfrom 1382 patients. Aliment Pharmacol Ther 2006; 24:535–539. [DOI] [PubMed] [Google Scholar]
- 5.Chan AOO, Jim MH, Lam KF, et al. Prevalence of colorectal neoplasm among patients with newly diagnosed coronary artery disease. JAMA 2007; 298:1412–1419. [DOI] [PubMed] [Google Scholar]
- 6.Yang M, Cho J, Choi Y, et al. The association between coronary artery calcification and colorectal adenoma. Hepatogastroenterology 2012; 60:532–542. [DOI] [PubMed] [Google Scholar]
- 7.Bots ML, Hoes AW, Koudstaal PJ, et al. Common carotid intima-media thickness and risk of stroke and myocardial infarction the Rotterdam Study. Circulation 1997; 96:1432–1437. [DOI] [PubMed] [Google Scholar]
- 8.Neunteufl T, Katzenschlager R, Hassan A, et al. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis 1997; 129:111–118. [DOI] [PubMed] [Google Scholar]
- 9.National Cholesterol Education Program (NCEP) Expert Panelon Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002; 106:3143–3421. [PubMed] [Google Scholar]
- 10.Mancia G, Fagard R, Narkiewicz K, et al. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens 2013; 31:1925–1938. [DOI] [PubMed] [Google Scholar]
- 11.WHO/IASO/IOTF. The Asia-Pacific Perspective: Redefining Obesity and Its Treatment. Sydney: Health Communications Australia Pty Ltd, 2000. [Google Scholar]
- 12.American Diabetes Association (ADA) Clinical practice recommendations. Diabetes Care 2007; 30 (Suppl. 1):S42–S47. [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and b-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia 1985; 28:412–419. [DOI] [PubMed] [Google Scholar]
- 15.Ford ES, Giles WH, Mokdad AH. The distribution of 10-year risk for coronary heart disease among US adults: findings from the National Health and Nutrition Examination Survey III. J Am Coll Cardiol 2004; 43:1791–1796. [DOI] [PubMed] [Google Scholar]
- 16.Simon A, Megnien JL, Chironi G. The value of carotid intima-media thickness for predicting cardiovascular risk. Arterioscler Thromb Vasc Biol 2010; 30:182–185. [DOI] [PubMed] [Google Scholar]
- 17.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilatation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002; 39:257–265. [DOI] [PubMed] [Google Scholar]
- 18.Reicher-Reiss H, Jonas M, Goldbourt U, et al. Selectively increased risk of cancer in men with coronary heart disease. Am J Cardiol 2001; 87:459–462. [DOI] [PubMed] [Google Scholar]
- 19.Yeoh KG, Ho KY, Chiu HM, et al. The Asia-Pacific Colorectal Screening score: a validated tool that stratifies risk for colorectal advanced neoplasia in asymptomatic Asian subjects. Gut 2011; 60:1236–1241. [DOI] [PubMed] [Google Scholar]
- 20.Potter JD, Slattery ML, Bostick RM, et al. Colon cancer: a review of the epidemiology. Epidemiol Rev 1993; 15:499–545. [DOI] [PubMed] [Google Scholar]
- 21.Framingham Heart Study. Website. http://www.framinghamheartstudy.org. [Google Scholar]
- 22.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst 2005; 97:1679–1687. [DOI] [PubMed] [Google Scholar]
- 23.Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc 2008; 67:253–256. [DOI] [PubMed] [Google Scholar]
- 24.Giovannucci E, Ascherio A, Rimm EB, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 1995; 122:327–334. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci E, Rimm EB, Stampfer MJ, et al. A prospective study of cigarette smoking and risk of colorectal adenoma and colorectal cancer in U.S. men. J Natl Cancer Inst 1994; 86:183–191. [DOI] [PubMed] [Google Scholar]
- 26.Giovannucci E. Insulin and colon cancer. Cancer Causes Control 1995; 6:164–179. [DOI] [PubMed] [Google Scholar]
- 27.McKeown-Eyssen G. Epidemiology of colorectal cancer revisited: are serum triglycerides and/or plasma glucose associated with risk? Cancer Epidemiol Biomarkers Prev 1994; 3:687–695. [PubMed] [Google Scholar]
- 28.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat Rev Cancer 2004; 4:579–591. [DOI] [PubMed] [Google Scholar]
- 29.Bowers K, Albanes D, Limburg P, et al. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am J Epidemiol 2006; 164:652–664. [DOI] [PubMed] [Google Scholar]
- 30.Ahmed RL, Schmitz KH, Anderson KE, et al. The metabolic syndrome and risk of incident colorectal cancer. Cancer 2006; 107:28–36. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008; 454:436–444. [DOI] [PubMed] [Google Scholar]
- 32.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarker Prev 2009; 18:2569–2578. [DOI] [PubMed] [Google Scholar]
- 33.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest 2006; 116:1793–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]


