Supplemental Digital Content is available in the text
Abstract
Nonalcoholic fatty liver disease (NAFLD) is a common liver disease that can progress to cirrhosis and liver failure. Anthocyanin, a member of the flavonoid family, has been shown to ameliorate NAFLD-associated pathologies in rodents.
The aim of this CONSORT-compliant pilot study is to evaluate the effects of anthocyanin supplementation on insulin resistance and liver injury biomarkers in patients with NAFLD.
A total of 74 subjects with NAFLD were divided into 2 groups in this double-blind, randomized study. Patients received either purified anthocyanin (320 mg/d) derived from bilberry and black currant or placebo for 12 weeks. Diet, physical activity, anthropometric parameters, glucose tolerance, and a set of biomarkers related to NAFLD were evaluated before and after intervention.
No significant differences were observed in nutrient intake, physical activity, anthropometric parameters, or plasma lipid profile between patients receiving anthocyanin or placebo. Compared to controls, the anthocyanin group exhibited significant decreases (P < 0.05, all comparisons) in plasma alanine aminotransferase (−19.1% vs 3.1%), cytokeratin-18 M30 fragment (−8.8% vs 5.6%) and myeloperoxidase (−75.0% vs −44.8%). Significant decreases from baseline in fasting blood glucose and homeostasis model assessment for insulin resistance were observed in the anthocyanin group; however, these differences were not significant relative to placebo controls. In addition, the oral glucose tolerance test indicated that anthocyanin supplementation significantly decreased the 2-hour loading glucose level compared to control (−18.7% vs −3.8%, P = 0.02).
A 12-week supplement of purified anthocyanin improved insulin resistance, indicators of liver injury, and clinical evolution in NAFLD patients. Further studies are warranted to determine the clinical applications of anthocyanin in NAFLD.
This trial was registered at clinicaltrials.gov as NCT01940263.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is currently the most prevalent chronic liver disease. NAFLD encompasses a wide spectrum of liver pathologies ranging from simple liver steatosis to nonalcoholic steatohepatitis (NASH), the latter of which is characterized by steatosis with features of cellular injury, including inflammation, and hepatocyte apoptosis.1 Simple steatosis was originally thought to be a benign condition; however, increasing evidence indicates that fatty livers are more vulnerable to factors that promote inflammation and fibrosis.2 In a long-term follow-up study in the United States, up to 5.4% of NAFLD cases ultimately progress to cirrhosis, liver failure, and hepatocellular carcinoma.3 Alarmingly, Chinese patients with NAFLD frequently exhibit significant hepatic necroinflammation and progressive liver fibrosis.4,5 NAFLD is now believed to be a major cause of liver-related morbidity and mortality; therefore, early treatment for NAFLD should be widely considered.
Insulin resistance is a key pathogenic factor in both the initiation and development of NAFLD. Several insulin sensitizers, including thiazolidinediones and metformin, have been tested in pilot studies and have been shown to significantly reduce liver inflammation and steatosis in NAFLD patients6,7; however, these compounds are associated with weight gain and raise concerns about cardiovascular safety.8 Thus, the side effects of insulin sensitizers are underestimated, particularly for simple cases of NAFLD in the absence of other complications.
Dietary and lifestyle changes are recommended as the primary treatment for NAFLD.9 Increased intakes of fruits and vegetables are likely to have important health benefits for NAFLD patients.10 In addition to vitamins, minerals, and fiber, fruits and vegetables contain high concentrations of bioactive compounds, such as carotenoids, tocopherols, and polyphenols. Among them, anthocyanin polyphenols are of particular interest since several epidemiological studies demonstrated their potential to reduce the risk of insulin resistance-related diseases, such as type 2 diabetes mellitus (T2DM) and cardiovascular disease.11–13 In a cross-sectional investigation of female adults in England, higher anthocyanin intake was associated with improvements in insulin resistance and inflammatory status.14 The beneficial effects of anthocyanins (either anthocyanin extracts from different plants or pure anthocyanin) have been demonstrated in several animal studies, with respect to oxidative stress, insulin resistance, hepatic steatosis, and inflammation, which are key features of NAFLD.15–17 Recent clinical trials have investigated the impact of consuming anthocyanins-rich foods on the development and progression of NAFLD. Consumption of purple sweet potato beverages was associated with favorable effects in lowering serum γ-glutamyl transferase and alanine aminotransferase (ALT) levels in healthy men with borderline hepatitis.18 Our previous study demonstrated that bayberry juice can protect against NAFLD in young adults by improving plasma antioxidant status as well as by inhibiting the inflammatory and apoptotic responses involved in this disease,19 but the effect of purified anthocyanin on NAFLD in humans has not been reported.
Given the potentially protective effects of anthocyanin on insulin resistance and liver injury and the need for effective therapies for NAFLD, the present study was designed to assess the effects of anthocyanin supplementation in NAFLD patients in a double-blinded placebo-controlled trial. Our objective was to evaluate the effect of anthocyanin purified from berries on selected biomarkers of liver injury. We also examined the effects of anthocyanin on body mass, blood pressure, lipid profile, and glucose homeostasis.
SUBJECTS AND METHODS
Subjects
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki, and was approved by the Ethics Committee of Sun Yat-Sen University prior to initiation of recruitment. Participants diagnosed with NAFLD ranging in age from 25 to 65 were recruited at the Shaoguan Railway Hospital, Shaoguan, China. A diagnosis of fatty liver was indicated based on ultrasound (Voluson E8, GE healthcare, Waukesha, WI) if 2 of the following criteria were met: increased hepatic echogenicity compared with cortical of the right kidney, blurring of liver vasculature, and deep attenuation of the ultrasonographic signal20; and plasma ALT >30 U/L in men and 19 U/L in women. Criteria were added after recruitment began, as a large fraction of recruited NAFLD patients had normal or even very low ALT levels that would reduce the likelihood of observing significant changes. Hepatic ultrasonography scanning was performed independently by 2 trained radiologists who were blinded to the participants’ details and to each other's diagnosis. Subjects were included if they were consistently classified as hepatic steatosis by both radiologists. Subjects with excessive alcohol consumption (ethanol >140 g/wk for men and >70 g/wk for women), cirrhosis, viral hepatitis, cardiovascular disease, cancer, and any consumption of nonsteroidal anti-inflammatory drugs, corticosteroids, or prescriptive medicines that affect liver function, lipid, or glucose metabolism were excluded.
Recruitment began on June 30, 2013, with the first participant identified on July 5, and ended with the last visit on October 30, 2013. A total of 116 subjects were screened, and 74 subjects (39 men and 35 women) were enrolled in the study. Written informed consent was obtained from each patient included in the study. All participants were compensated for their participation in the study. This trial was registered at clinicaltrials.gov as NCT01940263.
Study Design
This pilot study was designed according to the CONSORT 2010 statement.21 Participants were assigned in a 1:1 ratio into anthocyanin or placebo groups in a random and double-blinded manner. Randomization was performed using a computer-generated list of random numbers. Randomization was stratified by gender to ensure equal numbers of males and females in each group. Study nurses or clinical physicians enrolled participants. Scheduled participants were consecutively assigned by a medical technologist, who was unaware of enrolment status, to treatment codes that corresponded to labels on otherwise identical concealed containers. Participants, investigators, and outcome assessors were blinded to the treatment for the duration of the study. Treatment assignments were not revealed prior to data collection and analysis.
Anthocyanin and placebo capsules (Biolink AS, Sandnes, Norway) were identical in appearance and packaging. Anthocyanin capsules contained 80 mg of anthocyanins that were extracted from bilberry (blueberry) (Vaccinium myrtillus) and black currant (Ribesnigrum), as detailed previously.22 Placebo capsules consisted of maltodextrin and blue color. Two capsules were self-administered each morning and evening, yielding a daily intake of 320 mg anthocyanin. Our previous studies indicated beneficial effects of anthocyanin intervention on lipid profile and inflammatory status in dyslipidemic subjects when treated for 12–24 weeks22,23; consequently we carried out a 24-week intervention in this study. However, during the follow-up period, considering the high dropout rate and the coming Chinese New Year, it was difficult for subjects to remain on the prescribed diet during the Spring Festival, and the trial was terminated at the interim. The patients attended the clinic at weeks 4 and 8 for anthropometric assessments, and returned the remaining capsules and received the capsules for the next interval. Patient compliance was evaluated by counting the remaining capsules. Use of pharmacological agents, for example, metformin, thiazolidinediones, statins, and probiotics, or any medicines that may affect body weight, insulin resistance, lipid profile, or fatty liver was not allowed during the trial. Subjects were asked to maintain their normal lifestyle and not to use any anthocyanin-rich nutritional supplements, red wine, grapes, blueberry, etc, during this period. Adverse events were defined as injuries related to or caused by the study treatment. At each visit, patients were specifically asked about adverse events, and the physician checked for any association between treatment and adverse events.
Clinical and Laboratory Evaluation
Before and after the 12-week intervention, height, weight, waist, and hip circumference, and blood pressure were measured. Body mass index (BMI) was calculated as follows: body weight (kg)/[body height (m)]2. Waist circumference was measured at a level midway between the lower rib margin and iliac crest with the measuring tape encircling the body in a horizontal position. In addition, a short form (International Physical Activity Questionnaire [IPAQ]) and a 3-day dietary record were used to evaluate physical exercise and dietary intake.
Plasma glucose, lipid profile, and liver enzymes were tested using a fully automated biochemical analyzer (AU680, Beckman Coulter, Krefeld, Germany). Additional blood variables (hemoglobin, platelets, and white blood cells) were evaluated using an automated hematology analyzer (Sysmex 9000, Toa Medical Electronics, Kobe, Japan). Plasma insulin was determined by enzyme-linked immunosorbent assay (Human insulin ELISA kit, EMD Millipore Corporation, Billerica, MA). Insulin resistance was evaluated by homeostasis model assessment (HOMA-IR) and calculated as (fasting insulin [mU/L] × fasting glucose [mmol/L])/22.5.24 Plasma cytokeratin-18 fragment M30 (CK-18 M30) was measured using the M30 Apoptosense ELISA kit (PEVIVA, Bromma, Sweden) according to the manufacturer's protocols. Plasma myeloperoxidase (MPO) concentrations were measured using a commercially available ELISA kit (Hycult Biotec, Uden, The Netherlands). All plasma samples were analyzed in duplicate in the same run.
According to the willingness of participants, a 2-hour oral glucose tolerance tests (OGTT) was performed before and after intervention. Briefly, patients fasted for 10 hours, after which the first blood samples were drawn at 8:00 am to test fasting glucose. Patients were then given 75 g glucose dissolved in 300 mL water, and the second blood samples were drawn 2 hours after their first drink. The participants were instructed to avoid strenuous exercise, eat any food, or drink any beverage except water for 2 hours. Impaired glucose tolerance (IGT) was defined as 2-hour glucose >7.8 mmol/L, but <11.1 mmol/L, according to the Chinese Type 2 Diabetes Prevention Guide 2010.25
A noninvasive scoring system was used to assess the severity of liver injury in NAFLD patients. The score for each test was calculated according to the following formulas derived from the original studies: NAFLD fibrosis score (NFS) = −1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × aspartate aminotransferase (AST)/ALT ratio − 0.013 × platelet (×109/L) −0.66 × albumin (g/dL).26
Study Outcomes
The primary outcome measure was the difference in plasma ALT concentration. Secondary outcome measures included anthropometric measurements, fasting plasma levels of insulin, glucose, lipids, MPO, CK-18 M30, and NAFLD fibrosis score.
Statistical Analysis
Sample size calculations were performed using the nQuery Advisor 7.0 software (Statistical Solutions Ltd., Cork, Ireland) and were based on data from a preliminary study in Japan showing decreased ALT levels (from 51.3 to 42.8 U/L) in subjects consuming an anthocyanin-rich purple sweet potato beverage for 12 weeks.18 Assuming that a 15% decrease of ALT in the intervention group and a 5% increase of ALT in the control group would be detected, a sample size of 30 patients per group was necessary to detect a difference with 83% power at a 5% significance level using the 2-sided test. To account for dropouts, 74 participants were included in our study.
Data were analyzed according to the intention-to-treat principle. Patients missing laboratory measurements of the primary and secondary outcome measures were imputed using estimating-equation methods.27 SPSS 19.0 software (IBM-SPSS Inc, Chicago, IL) was used for statistical analysis of resulting data. The Kolmogorov–Smirnov test was performed to test normal distributions. Variables that were not normally distributed were logarithmically transformed to achieve normal distribution. Variables followed normal distributions and were described as means ± standard deviation. Data not exhibiting a normal distribution after transformation were expressed as medians with interquartile ranges. The percent change was calculated as follows: (value at 12 weeks – value at baseline)/value at baseline × 100. Percent changes expressed as medians and interquartile range for nonnormal distributions or the means and 95% confidence interval for normal distributions.
For primary or transformed data with a normal distribution, an independent Student t test was used to compare the differences at baseline between groups, and a paired Student t test was used to compare the differences before and after the intervention within groups. Otherwise, a Wilcoxon matched pairs signed rank test and a Mann–Whitney U test were used to compare characteristics in each group before and after intervention and differences between the 2 groups, respectively. Pearson correlation coefficients (r) were used to determine the association between the changes in the plasma CK-18 M30 concentrations and the changes in ALT in the 12-week study.
RESULTS
Patient Characteristics
A total of 74 volunteers were recruited. During the follow-up periods, 8 subjects dropped out by declining to return and 3 subjects were withdrawn by their physicians. In total, 63 participants (34 in the anthocyanin group and 29 in the control group) finished the whole procedure (Figure 1); however, all randomized patients were included in the intention-to-treat analysis. Among these subjects, an OGTT was administered to 33 patients (20 in the anthocyanin group, 13 in the control group). Based on the count of the recalled capsules at every visit, the level of compliance was very good, with capsule intake rates of 91.3% and 89.5% in the anthocyanin and placebo groups, respectively. None of the subjects experienced any serious adverse events resulting from the consumption of either the anthocyanin or placebo capsules throughout the trial period.
FIGURE 1.
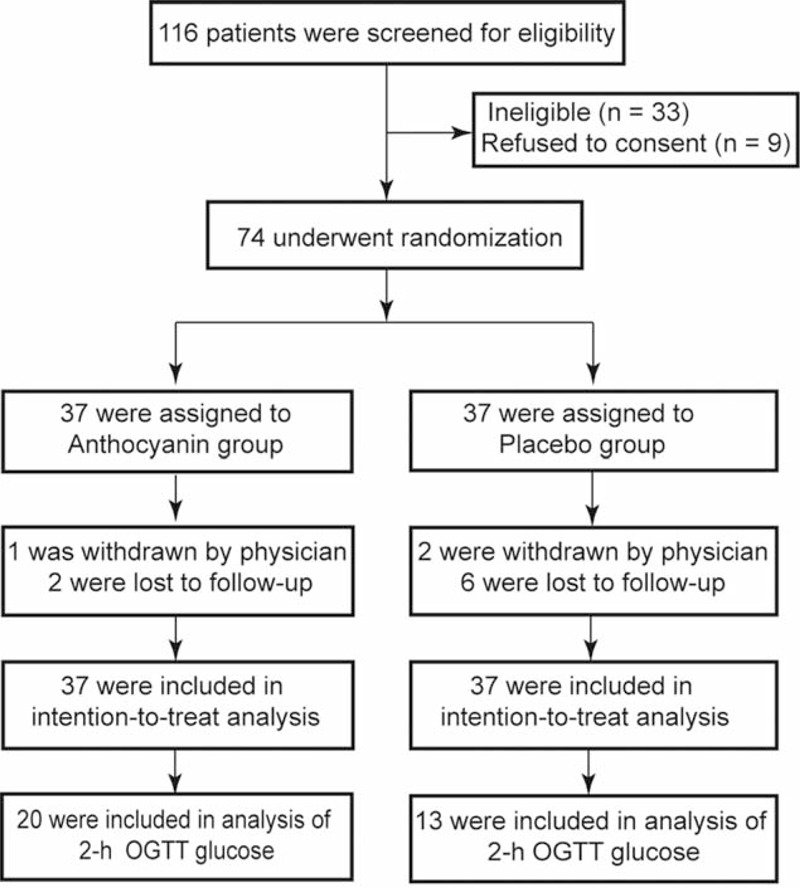
Consolidated standards of reporting trials flow diagram of the study participants.
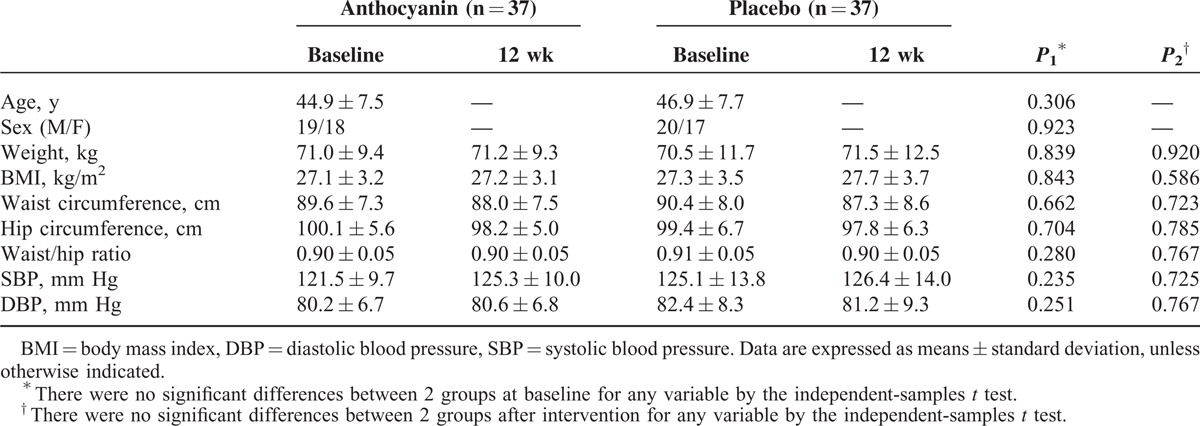
At baseline assessment, no significant differences in anthropometric characteristics were observed between the 2 groups. The 12-week intervention with purified anthocyanin had no impact on BMI, waist and hip circumference, or blood pressure in anthocyanin group compared to the placebo control group (Table 1).
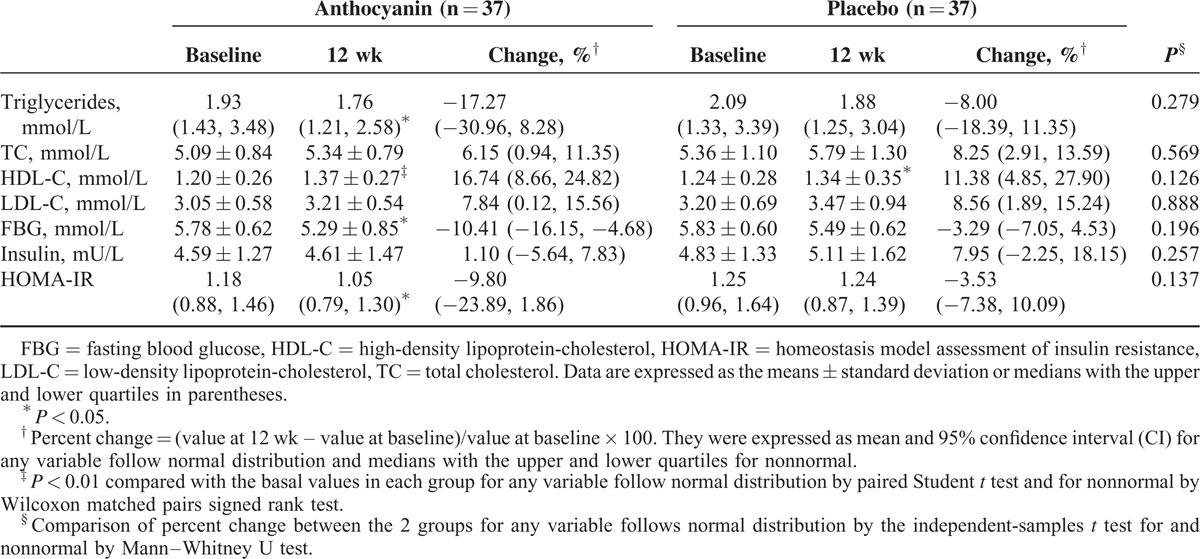
TABLE 1.
Anthropometric Characteristics Across Groups at Baseline and 12 wk
Based on the 3-day dietary record and the IPAQ, there were no significant differences in daily nutrient intake or physical activity between the 2 groups at baseline and after 12 weeks’ intervention (Supplementary Table 1, http://links.lww.com/MD/A272).
Primary Outcome
At baseline, there were no significant differences in plasma liver enzymes between the 2 groups (Table 2). After intervention, although no difference was observed between 2 groups after male-to-male or female-to-female comparisons, the total mean reduction of ALT levels in the anthocyanin group was greater than that of the placebo group (−19.1% vs −3.1%, P = 0.02). Compared to baseline, ALT levels were decreased significantly in both genders in the anthocyanin group; however, only females in the placebo group exhibited a significant drop in ALT levels (Figure 2). In addition, the mean value of both genders did not change significantly in the placebo group, which reflected a favorable effect of anthocyanin on chronic liver injury in the study participants.
TABLE 2.
Effects of Anthocyanin Supplementation on Biomarkers of Liver Injury
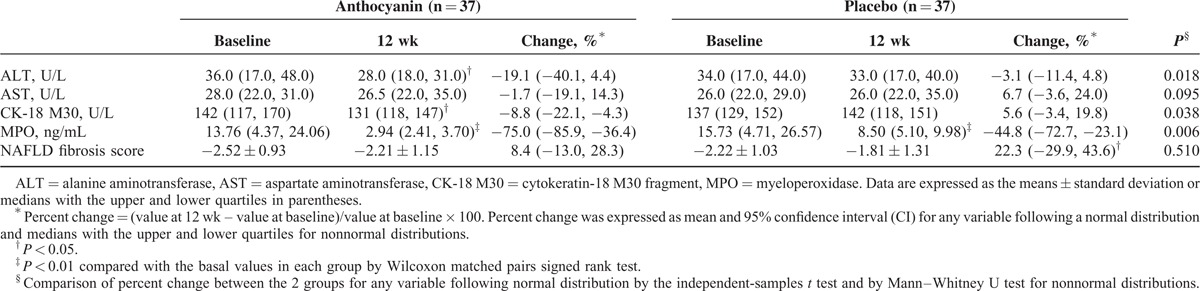
FIGURE 2.
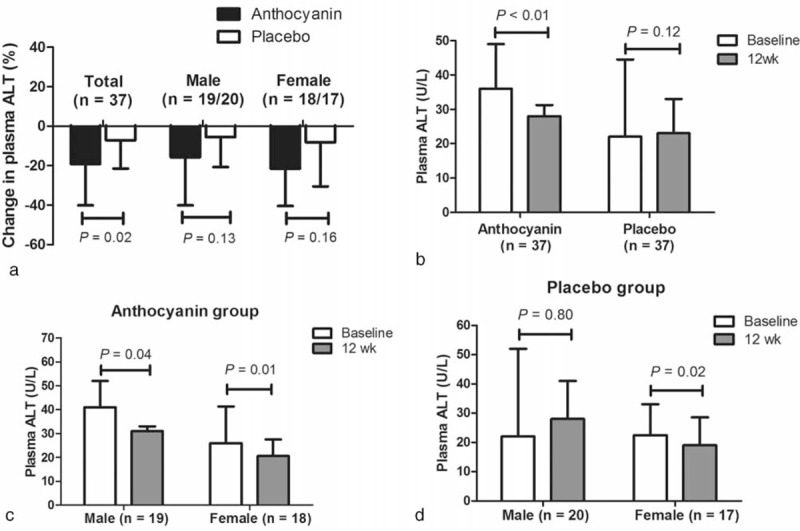
Effect of the 12-week anthocyanin intervention on ALT levels in NAFLD patients. The columns and error bars indicate medians with quartiles. After intervention, no differences were observed between the 2 groups across intragender comparisons; however, the total mean reduction of ALT levels in the anthocyanin group was greater than that in the placebo group (a), P compared difference between 2 groups by Mann–Whitney U test. Compared with baseline, there is a remarkable decline in ALT after a 12-week intervention in males, females, and total patients in the anthocyanin group (b, c), but only in females in the placebo group (b, d). P compared with the basal values in each group by Wilcoxon matched pairs signed rank test.
Secondary Outcomes
Lipid Profile and Insulin Resistance
As shown in Table 3, ingestion of anthocyanin for 12 weeks significantly decreased plasma triglyceride (TG) levels; however, the mean percentage changes were not significantly different from placebo controls. No effects on total plasma cholesterol or low-density lipoprotein-cholesterol were observed. Plasma levels of high-density lipoprotein-cholesterol increased moderately in both the groups, but this trend did not reach statistical significance across the 2 groups.
TABLE 3.
Effects of Anthocyanin Supplementation on Fasting Plasma Lipid Profile and Glucose and Insulin Concentrations
Fasting blood glucose and HOMA-IR decreased significantly from baseline in the anthocyanin group, but did not differ from the placebo values (Table 3). Insulin levels after 12 weeks was comparable to baseline in both anthocyanin and placebo groups. To examine insulin sensitivity and β-cell function, an oral glucose tolerance test was performed in 33 subjects, including 20 in anthocyanin group and 13 in the control group. Among these patients, 10 (50.0%) in the anthocyanin group and 6 (46.2%) in the control exhibited IGT. The anthropometric characteristics, dietary intake, physical activities, fasting blood glucose and insulin, and HOMA-IR were comparable between the 2 groups at baseline (Supplementary Table 2, http://links.lww.com/MD/A272). Anthocyanin supplementation significantly decreased the 2-hour OGTT glucose levels compared to the placebo control (−18.7% vs −3.8%, P = 0.02; Figure 3).
FIGURE 3.
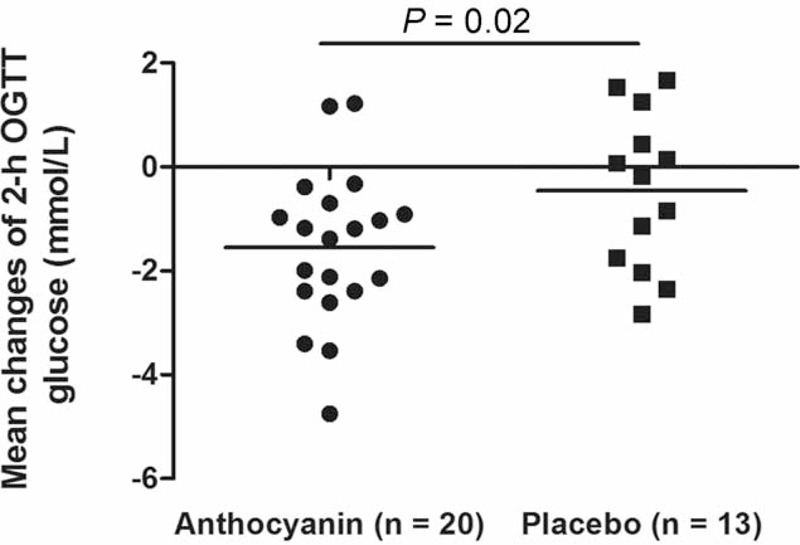
Changes in glucose concentration measured 2 h after a 75-g oral glucose load in the 2 treatments during the study period. P compared difference between 2 groups by independent-sample t test.
Other Liver Injury Biomarkers
Anthocyanin supplementation did not affect the release of AST, another important enzyme in the liver (Table 2). Plasma levels of CK-18 M30, a predictor of steatohepatitis,28 decreased by 8.8% after anthocyanin treatment and increased by 5.6% in the placebo group (P = 0.04). Inflammation and oxidative stress are involved in NAFLD progression, and MPO is linked to both inflammation and oxidative stress based on its location in leukocytes and role in catalyzing the formation of oxidizing agents.29 We next evaluated plasma levels of MPO and observed a significant difference in the effects of the 2 capsules on circulating MPO levels (−75.0% vs −44.8%, P < 0.01). Compared with baseline, the NAFLD fibrosis score increased by 22.3% in the placebo group (P < 0.05) but only 8.4% in the anthocyanin group (not significant), respectively. Furthermore, a positive correlation (r = 0.49, P < 0.01) between the plasma ALT and CK-18 M30 levels was observed in our study subjects (Figure 4).
FIGURE 4.
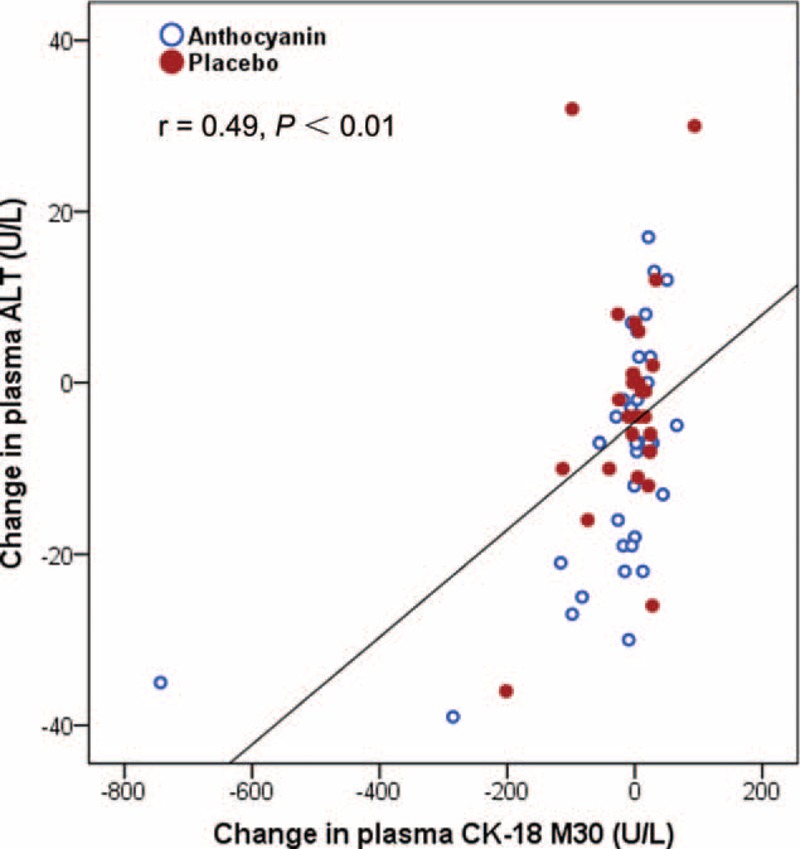
Correlation between changes in plasma ALT activities and the levels of CK-18 M30; n = 37 in each group. The data were evaluated by using Pearson correlation coefficients (r).
DISCUSSION
The results of this pilot study suggest that purified anthocyanin supplements can have favorable effects on insulin resistance and several plasma biomarkers of liver injury in patients with NAFLD. Although the patients in our study had relatively mild disease, they represent the spectrum of patients who are commonly seen in a primary care setting. The participants were able to maintain a constant body weight throughout the study (Supplementary Table 3, http://links.lww.com/MD/A272), excluding weight changes as a confounding factor that contributed to the improved features of NAFLD. Furthermore, participants were instructed not to alter their physical activity during the study. Therefore, in light of these findings, we suggest that an increase of dietary anthocyanin intake may help slow or reverse the clinical evolution of fatty liver disease, especially in early-stage NAFLD patients.
Previous studies of foods rich in anthocyanin have yielded positive findings on whole-body inflammatory status and insulin sensitivity in obese subjects.30,31 In the current study, a significant improvement or normalization of ALT, along with a marked improvement of glucose homeostasis, occurred in NAFLD patients after 12 weeks of anthocyanin treatment. Increasing evidence provided by cohort studies suggests that fatty liver may be a predictor of T2DM and cardiovascular disease,3 and our results are consistent with the observed association between higher anthocyanin intake and lower incidence of T2DM or cardiovascular disease.11,13 However, in contrast to the results in certain trials,22,31,32 we did not observe any effect of anthocyanin on plasma lipid profile. It is possible that the efficacy of anthocyanins on lipid profile responses depends, to some extent, on the population studied and the duration of the intervention.
Another important finding of this study is the marked decrease in plasma levels of caspase-cleaved CK-18 fragments in participants receiving anthocyanin treatment. CK-18 is the predominant keratin expressed in the liver and is a well-known substrate of caspases during apoptotic hepatocyte cell death.33 Other investigators have shown that CK-18 M30 has high overall accuracy in differentiating NAFLD from control subjects and moderate accuracy in differentiating NASH from simple steatosis.34 Our previous in vitro study showed that anthocyanin cyanidin 3-O-β-glucoside improved hepatocyte function and cell survival in response to high glucose stress.35 In this study, we observed a positive correlation between changes in plasma ALT activities and the levels of CK-18 M30 in anthocyanin-treated patients. These results suggest that CK-18 M30 has potential as a marker for predicting NAFLD progression.
Oxidative stress and inflammation are important mechanisms in the pathogenesis of NAFLD. MPO is a heme-containing peroxidase abundantly expressed in neutrophils and to a lesser extent in monocytes.29 Upon cell activation, MPO is released and MPO-derived oxidants have been associated with tissue damage in many diseases, particularly those characterized by acute or chronic inflammation.36 Increased numbers of MPO-expressing cells and accumulation of HOCl-modified and nitrated proteins in steatotic livers enhance progression of simple steatosis to steatohepatitis. In contrast, MPO deficiency attenuates the development of NASH and diminishes adipose tissue inflammation in mice on a high fat diet.37 A recent study demonstrated that plasma MPO was positively associated with insulin resistance and inflammation parameters in overweight subjects.38 In NASH patients, both hepatic MPO expression and plasma MPO levels were elevated compared with patients with similar BMI but without NASH.39 Our results suggest that anthocyanin supplementation reduced plasma MPO and showed a trend toward improvement in NAFLD fibrosis score. It is tempting to speculate that MPO may be a factor that directly links the hepatoprotective effects of anthocyanin to its well-documented anti-inflammatory and anti-oxidative properties.40
The majority of studies investigating anthocyanin intervention in humans have used foods containing multiple types of polyphenols. To the best of our knowledge, this is the first study comparing the effects of purified anthocyanin on blood-based biomarkers of NAFLD using a randomized double-blind design with placebo controls. Nevertheless, this study has some intrinsic limitations that must be acknowledged. First, as liver biopsies were not performed, NAFLD features such as steatosis and necroinflammation could not be evaluated. Second, our study was mildly underpowered by small sample size and a relatively short intervention period. We experienced a significant number of screen failures in the enrolment, and patient dropout rates were higher than anticipated, resulting in a lower number of evaluable patients than is estimated to be necessary to detect differences between groups, which may have led to a type II error. Finally, our patients had relatively low BMIs compared to those reported in Western studies; however, Asians generally develop visceral obesity and NAFLD at a lower BMI.5
In summary, our findings above linking the dietary anthocyanin supplement to improved characteristics of NAFLD are particularly important in light of the increasing incidence of NAFLD worldwide. Furthermore, this supplementation is an inexpensive, nontoxic therapy that results in overall health improvement, reducing the risk not only of chronic liver disease, but also T2DM and cardiovascular disease. Future large, prospective, well-designed, multicenter studies are needed to explore the durability of the benefits of anthocyanin.
Acknowledgments
The authors would like to express our gratitude to Dr Yichun Zhong (Shaoguan Railway Hospital) for his kind support of this work.
Footnotes
Abbreviations: ALT = alanine aminotransferase, BMI = body mass index, CK-18 M30 = cytokeratin-18 fragment M30, HOMA-IR = homeostasis model assessment of insulin resistance, MPO = myeloperoxidase, NAFLD = nonalcoholic fatty liver disease, NASH = nonalcoholic steatohepatitis, OGTT = oral glucose tolerance test, T2DM = type 2 diabetes mellitus.
This work was funded by grants from the National Basic Research Program (973 Program, 2012CB517506), National Natural Science Foundation (81372994, 81172655), and Guangdong Industry-University Research Foundation (2013B090600138).
All authors have read and approved the submitted version of the manuscript. Study concept: H-HG; data acquisition: P-WZ; data analysis and interpretation: F-XC, DL; drafting of the manuscript: P-WZ; critical revision of the manuscript for important intellectual content: W-HL; study supervision: H-HG.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology 2011; 54:1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fierbinteanu-Braticevici C, Negreanu L, Tarantino G. Is fatty liver always benign and should not consequently be treated? J Physiol Pharmacol 2013; 64:3–9. [PubMed] [Google Scholar]
- 3.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006; 44:865–873. [DOI] [PubMed] [Google Scholar]
- 4.Hui AY, Wong VW, Chan HL, et al. Histological progression of non-alcoholic fatty liver disease in Chinese patients. Aliment Pharmacol Ther 2005; 21:407–413. [DOI] [PubMed] [Google Scholar]
- 5.Wong VW, Chan HL, Hui AY, et al. Clinical and histological features of non-alcoholic fatty liver disease in Hong Kong Chinese. Aliment Pharmacol Ther 2004; 20:45–49. [DOI] [PubMed] [Google Scholar]
- 6.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010; 362:1675–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013; 145:574–582. [DOI] [PubMed] [Google Scholar]
- 8.Stein LL, Dong MH, Loomba R. Insulin sensitizers in nonalcoholic fatty liver disease and steatohepatitis: current status. Adv Ther 2009; 26:893–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finelli C, Tarantino G. Is there any consensus as to what diet or lifestyle approach is the right one for NAFLD patients? J Gastrointestin Liver Dis 2012; 21:293–302. [PubMed] [Google Scholar]
- 10.McCarthy EM, Rinella ME. The role of diet and nutrient composition in nonalcoholic fatty liver disease. J Acad Nutr Diet 2012; 112:401–409. [DOI] [PubMed] [Google Scholar]
- 11.Wedick NM, Pan A, Cassidy A, et al. Dietary flavonoid intakes and risk of type 2 diabetes in US men and women. Am J Clin Nutr 2012; 95:925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mink PJ, Scrafford CG, Barraj LM, et al. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr 2007; 85:895–909. [DOI] [PubMed] [Google Scholar]
- 13.Cassidy A, Mukamal KJ, Liu L, et al. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation 2013; 127:188–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jennings A, Welch AA, Spector T, et al. Intakes of anthocyanins and flavones are associated with biomarkers of insulin resistance and inflammation in women. J Nutr 2014; 144:202–208. [DOI] [PubMed] [Google Scholar]
- 15.Tang X, Shen T, Jiang X, et al. Purified anthocyanins from bilberry and black currant attenuate hepatic mitochondrial dysfunction and steatohepatitis in mice with methionine and choline deficiency. J Agric Food Chem 2015; 63:552–561. [DOI] [PubMed] [Google Scholar]
- 16.Morrison MC, Liang W, Mulder P, et al. Mirtoselect, an anthocyanin-rich bilberry extract, attenuates non-alcoholic steatohepatitis and associated fibrosis in ApoE ∗3Leiden mice. J Hepatol 2015; 62:1180–1186. [DOI] [PubMed] [Google Scholar]
- 17.Guo H, Xia M, Zou T, et al. Cyanidin 3-glucoside attenuates obesity-associated insulin resistance and hepatic steatosis in high-fat diet-fed and db/db mice via the transcription factor FoxO1. J Nutr Biochem 2012; 23:349–360. [DOI] [PubMed] [Google Scholar]
- 18.Suda I, Ishikawa F, Hatakeyama M, et al. Intake of purple sweet potato beverage affects on serum hepatic biomarker levels of healthy adult men with borderline hepatitis. Eur J Clin Nutr 2008; 62:60–67. [DOI] [PubMed] [Google Scholar]
- 19.Guo H, Zhong R, Liu Y, et al. Effects of bayberry juice on inflammatory and apoptotic markers in young adults with features of non-alcoholic fatty liver disease. Nutrition 2014; 30:198–203. [DOI] [PubMed] [Google Scholar]
- 20.Younossi ZM, Stepanova M, Negro F, et al. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012; 91:319–327. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Hopewell S, Schulz KF, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340:c869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qin Y, Xia M, Ma J, et al. Anthocyanin supplementation improves serum LDL- and HDL-cholesterol concentrations associated with the inhibition of cholesteryl ester transfer protein in dyslipidemic subjects. Am J Clin Nutr 2009; 90:485–492. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, Ling W, Guo H, et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: a randomized controlled trial. Nutr Metab Cardiovasc Dis 2013; 23:843–849. [DOI] [PubMed] [Google Scholar]
- 24.Lee IT, Chan YC, Lin CW, et al. Effect of cranberry extracts on lipid profiles in subjects with type 2 diabetes. Diabet Med 2008; 25:1473–1477. [DOI] [PubMed] [Google Scholar]
- 25.Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. J Am Med Assoc 2013; 310:948–959. [DOI] [PubMed] [Google Scholar]
- 26.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007; 45:846–854. [DOI] [PubMed] [Google Scholar]
- 27.Little RJ, D’Agostino R, Cohen ML, et al. The prevention and treatment of missing data in clinical trials. N Engl J Med 2012; 367:1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fitzpatrick E, Mitry RR, Quaglia A, et al. Serum levels of CK18 M30 and leptin are useful predictors of steatohepatitis and fibrosis in paediatric NAFLD. J Pediatr Gastroenterol Nutr 2010; 51:500–506. [DOI] [PubMed] [Google Scholar]
- 29.Schindhelm RK, van der Zwan LP, Teerlink T, et al. Myeloperoxidase: a useful biomarker for cardiovascular disease risk stratification? Clin Chem 2009; 55:1462–1470. [DOI] [PubMed] [Google Scholar]
- 30.Stull AJ, Cash KC, Johnson WD, et al. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr 2010; 140:1764–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wright OR, Netzel GA, Sakzewski AR. A randomized, double-blind, placebo-controlled trial of the effect of dried purple carrot on body mass, lipids, blood pressure, body composition, and inflammatory markers in overweight and obese adults: the QUENCH trial. Can J Physiol Pharmacol 2013; 91:480–488. [DOI] [PubMed] [Google Scholar]
- 32.Hassellund SS, Flaa A, Kjeldsen SE, et al. Effects of anthocyanins on cardiovascular risk factors and inflammation in pre-hypertensive men: a double-blind randomized placebo-controlled crossover study. J Hum Hypertens 2013; 27:100–106. [DOI] [PubMed] [Google Scholar]
- 33.Joka D, Wahl K, Moeller S, et al. Prospective biopsy-controlled evaluation of cell death biomarkers for prediction of liver fibrosis and nonalcoholic steatohepatitis. Hepatology 2012; 55:455–464. [DOI] [PubMed] [Google Scholar]
- 34.Kwok R, Tse YK, Wong GL, et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease: the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther 2014; 39:254–269. [DOI] [PubMed] [Google Scholar]
- 35.Jiang X, Tang X, Zhang P, et al. Cyanidin-3-O-beta-glucoside protects primary mouse hepatocytes against high glucose-induced apoptosis by modulating mitochondrial dysfunction and the PI3K/Akt pathway. Biochem Pharmacol 2014; 90:135–144. [DOI] [PubMed] [Google Scholar]
- 36.Nussbaum C, Klinke A, Adam M, et al. Myeloperoxidase: a leukocyte-derived protagonist of inflammation and cardiovascular disease. Antioxid Redox Signal 2013; 18:692–713. [DOI] [PubMed] [Google Scholar]
- 37.Rensen SS, Bieghs V, Xanthoulea S, et al. Neutrophil-derived myeloperoxidase aggravates non-alcoholic steatohepatitis in low-density lipoprotein receptor-deficient mice. PLoS One 2012; 7:e52411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez Garcia A, Rivera Rodriguez M, Gomez Alonso C, et al. Myeloperoxidase is associated with insulin resistance and inflammation in overweight subjects with first-degree relatives with type 2 diabetes mellitus. Diabetes Metab J 2015; 39:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rensen SS, Slaats Y, Nijhuis J, et al. Increased hepatic myeloperoxidase activity in obese subjects with nonalcoholic steatohepatitis. Am J Pathol 2009; 175:1473–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo H, Ling W. The update of anthocyanins on obesity and type 2 diabetes: experimental evidence and clinical perspectives. Rev Endocr Metab Disord 2015; 16:1–13. [DOI] [PubMed] [Google Scholar]


