Abstract
Distinguishing between benign and malignant pancreatic cysts remains a clinical challenge. The aim of this study was to investigate the influence of body mass index (BMI) and preoperative clinical and cyst features, as described by the International Consensus Guidelines, on malignancy in patients with pancreatic mucinous cystic neoplasms (PMCNs).
A retrospective cohort study was performed on patients with PMCNs who underwent surgical resection between January 1994 and June 2014. Preoperative BMI, clinical demographic data, cystic features, tumor markers, and surgical pathology results were analyzed. Predictors of malignancy were determined by univariate and multivariate analysis using logistic regression.
One hundred sixty-four cases of PMCNs, including 106 intraductal papillary mucinous neoplasms (IPMNs) and 58 mucinous cystic neoplasms (MCNs), were analyzed. On univariate analysis, older age (P = 0.008), male sex (P = 0.007), high-risk stigmata (P = 0.007), diabetes mellitus (DM; P = 0.008), and BMI >25 (P < 0.001) were associated with malignancy. Multivariate analysis found that BMI >25 (odds ratio, 3.99; 95% confidence interval: 1.60–10) was an independent predictor of malignancy. In subgroup analysis, BMI >25 was an independent predictor of malignancy in IPMNs but not in MCNs.
Overweight patients with IPMNs have a higher risk of malignancy and should be followed closely or undergo resection. The operative strategy for PMCNs should consider cyst-related and patient-related risk factors.
INTRODUCTION
Pancreatic cystic neoplasms (PCNs) are being diagnosed with increasing frequency due to the increased use of higher quality cross-sectional imaging and the aging of the population.1 PCNs, which include serous cystic neoplasms (SCNs), mucinous cystic neoplasms (MCNs), intraductal papillary mucinous neoplasms (IPMNs), solid pseudopapillary neoplasms (SPNs), and various other cystic neoplasms, are a heterogeneous group of tumors with distinct biological features and a wide range of malignant potential, which depends on the histologic type.2 Pancreatic mucinous cystic neoplasms (PMCNs), including IPMN and MCN, can trigger significant anxiety for patients and their physicians, as these cysts are considered to be premalignant lesions.3,4 Appropriately timed surgical resection can reduce the mortality from pancreatic cancer. However, surgical resection for PCN is associated with significant rates of morbidity and mortality.5–7 Moreover, not all cysts have a similar risk of malignancy. Selecting patients with PCNs who should undergo surgical resection remains problematic in the absence of confirmed malignancy.
The 2012 International Consensus Guidelines for the management of PMCNs recommended surgical resection without further testing in patients whose cysts have suspected branch duct IPMN (BD-IPMN) with high-risk stigmata.8 Cysts with worrisome features require further evaluation, and consideration of surgery is recommended in patients with such cysts because of the elevated risk of malignancy.8 However, the guidelines for surgical resection of PMCNs cannot be applied to all PMCNs.9 It is sometimes challenging to differentiate unifocal BD-IPMNs from MCNs on preoperative imaging studies. Many studies have used patient features and cystic features as the primary predictors for evaluating the malignant potential, with inconsistent results; as these predictors have been used to create consensus guidelines, the current guidelines may not be satisfactory.10–13 Further identification of additional factors and evaluating the additive role of multiple risk factors are required to preoperatively predict the malignant potential of PMCNs. Excess body weight is a risk factor for several types of human cancers, including colon, breast, esophageal, kidney, and pancreatic cancers.14–18 Overweight and obesity are associated with the risk of pancreatic cancer, as well as worse overall survival.15,19,20 A previous study has shown that, in Western countries, obesity is associated with a greater frequency of malignant transformation of BD-IPMNs.21 The impact of body mass index (BMI) on the malignant progression of PMCNs has not previously been fully investigated in Asia. The purpose of this study was to determine whether overweight is associated with a greater frequency of malignant PMCNs in patients undergoing resection in Taiwan. The second aim of the study was to analyze the effect of the revised international guidelines by evaluating the influence of cystic features on the risk of malignancy in PMCNs.
METHODS
Patient Selection
The study population was drawn from patients undergoing surgery for PCNs between January 1994 and June 2014 at National Taiwan University Hospital. A total of 164 consecutive patients with pathologically proven PMCNs, including IPMNs and MCNs, formed the study group.
Data Collection
A retrospective review of clinical and pathologic information was performed. Information collected included age at the time of surgery, sex, smoking status, presence of symptoms (abdominal pain, jaundice, weight loss, and history of pancreatitis), presence of diabetes, anthropometric characteristics, preoperative carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) levels, imaging studies and pathology reports. BMI was calculated at the time of surgery. Radiographic variables analyzed included the type of imaging performed (computed tomography or magnetic resonance imaging), cyst size (recorded as the maximum dimension measured on cross-sectional imaging), and the presence of mural nodules, lymphadenopathy, and dilation of the pancreatic duct. Each case was retrospectively reviewed for high-risk stigmata and worrisome features, according to the revised International Consensus Guidelines.8 Recorded high-risk stigmata included obstructive jaundice in a patient with a cystic lesion on the head of the pancreas, an enhancing solid component within the cyst, or the main pancreatic duct (MPD) that was larger than 10 mm in size. Recorded worrisome features included thickened/enhancing cyst walls, a main duct size of 5 to 9 mm, the presence of a non-enhancing mural nodule, and an abrupt change in the caliber of the pancreatic duct with distal pancreatic atrophy. In accordance with the revised guidelines, a cyst size of 3 cm or larger was not considered to be worrisome on its own; however, the predictive value of cyst size for malignancy was analyzed. The study was approved by the institutional review board of the National Taiwan University Hospital.
Interpretation of Pathological Diagnoses
All pathologic specimens were reviewed by a single experienced pathologist (Y.M. Jeng) to confirm the diagnosis of IPMN or MCN according to the World Health Organization (WHO) criteria. The degree of dysplasia was categorized as low-, intermediate-, or high-grade, according to the fourth edition of the WHO classification system. The IPMN were also classified into branch-duct IPMN (BD-IPMN), main-duct IPMN (MD-IPMN), or mixed IPMN; histological subtype classifications, ie, gastric, intestinal, pancreatobiliary, or oncocytic, were based on gross and microscopic histological findings. In this study, malignant IPMN and MCN were defined as invasive carcinomas. The PMCNs were classified as PMCNs (including all IPMNs and MCNs), PMCNs with the presence of a cyst on imaging (including all PMCNs except for MD-IPMNs), IPMNs, and MCNs. Predictors of malignancy were investigated for each subgroup.
Statistical Analysis
To compare the between-group demographic data, we used Student unpaired t test for normally distributed continuous variables and the Mann–Whitney U test for non-normally distributed variables. We used the χ2 test for categorical data and Fisher exact test when cell counts were fewer than 5. Univariate analysis was performed to identify independent risk factors that were correlated with the presence of malignancy. We used multiple stepwise logistic regression analysis to identify significant independent risk factors that were correlated with the presence of malignancy in the univariate analysis. We also adjusted for age at study enrollment, sex, and biomedical characteristics to prevent confounding. We estimated the strength of the associations by calculating the odds ratio (OR). The optimal cutoff points for discriminating between malignant and benign tumors were sought by constructing receiver operating characteristic (ROC) curves, which were generated by calculating the sensitivities and specificities for BMI and CA 19-9 at several predetermined cutoff points. All tests were 2-tailed with the statistical significance level set at P < 0.05, and all analyses were performed with SPSS version 17.0 software (IBM, Armonk, New York, NY).
RESULTS
Patient Characteristics
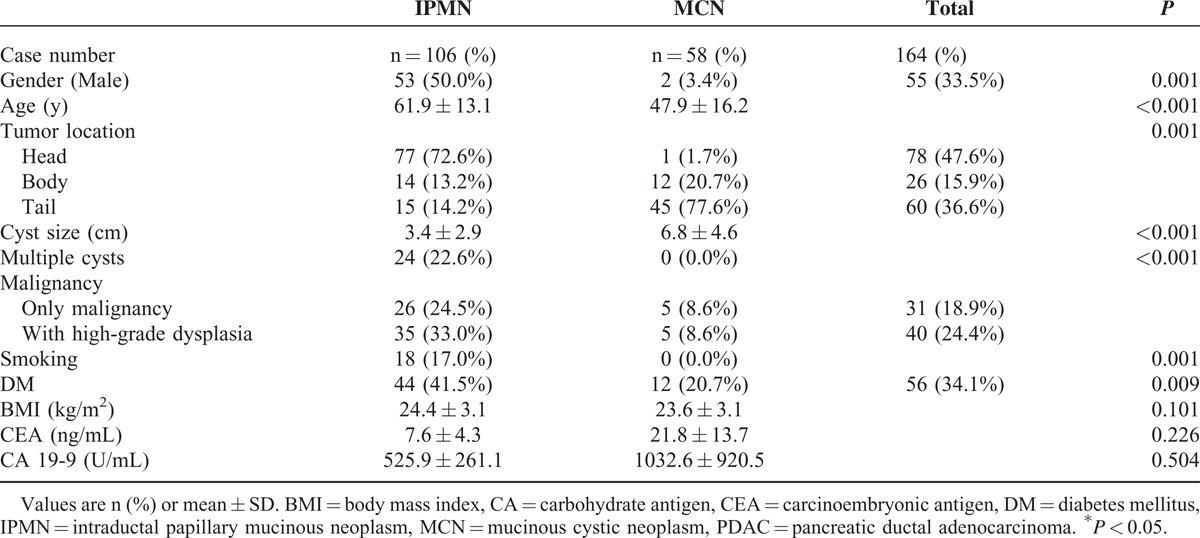
A total of 164 patients with PMCNs, including 106 IPMNs and 58 MCNs, underwent surgical resection, and their complete records were reviewed. The patients’ preoperative demographic and clinical characteristics are shown in Table 1 and differed significantly between patients with IPMNs and those with MCNs, including sex, age, tumor location, cyst size, the number of cysts, and the presence of diabetes mellitus (DM). There were 31 (18.9%) cases of carcinoma (26 in the IPMN group and 5 in the MCN group). The frequency of malignancy in patients with IPMNs and MCNs was 24.5% and 8.6% (P = 0.013), respectively. BMI, serum CEA, and CA 19-9 level did not differ significantly between patients with IPMNs and those with MCNs.
TABLE 1.
Clinical and Pathological Characteristics in 164 Patients With Resected Pancreatic Mucinous Cystic Neoplasms
Benign and Malignant PMCNs
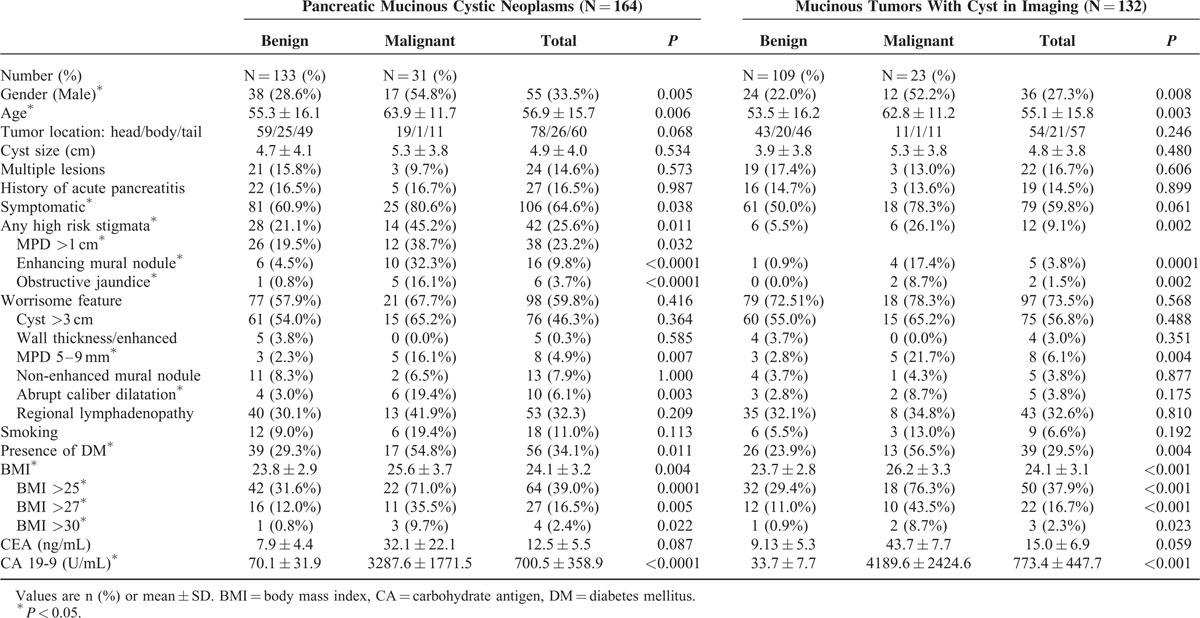
Table 2 shows a comparison of clinical features between the malignant and benign mucinous tumors. Patients with malignant mucinous tumors were older and more symptomatic than those with benign tumors. Of the 164 PMCNs, 42 (25.6%) tumors had high-risk stigmata, including 14 (45.2%) malignant tumors and 28 (21.1%) benign tumors (P = 0.011). High-risk stigmata were more frequent in malignant tumors, including the presence of a main pancreatic duct that was >1 cm (38.7% vs. 19.5%, P = 0.032), enhancing mural nodules (32.3% vs. 4.5%, P < 0.001) and obstructive jaundice (16.1% vs. 0.8%, P < 0.001). Of the 164 PMCNs, 98 (59.8%) tumors had worrisome features, including cysts >3 cm, wall thickness/enhancement, MPD 5 to 9 mm, and a non-enhancing mural nodule. Patients with malignant tumors were more likely to have an MPD that was >5 to 9 mm, abrupt dilatation of the MPD, and DM. Preoperative BMI was higher in patients with malignant PMCNs than in patients with benign PMCNs (25.6 ± 3.7 vs. 23.8 ± 2.9, P = 0.004). The serum level of CA 19-9 was higher in patients with malignant tumors than in those with benign tumors (3287.6 ± 1771.5 vs. 70.1 ± 31.9, P = 0.0001). The cyst size and serum CEA levels did not differ significantly between malignant and benign mucinous tumors.
TABLE 2.
Clinical and Imaging Characteristics of Malignant and Benign Pancreatic Mucinous Cystic Neoplasms (N = 164) and Mucinous Tumors With Cyst in Imaging (N = 132)
Subgroup Analysis
Characteristics of Mucinous Tumors With Cysts on Imaging (All PMCNs Excluding Main-Duct IPMNs)
There were 132 mucinous tumors with cysts on imaging, including 66 BD-IPMNs, 8 mixed-type IPMNs, and 58 MCNs. We analyzed this subgroup because these patients are commonly encountered in clinical practice; however, it is not possible to confirm the diagnosis preoperatively, as these cystic lesions have similar imaging features. Table 2 displays a comparison of clinical features between the malignant and benign mucinous cystic tumors in this group. There were 23 malignant and 109 benign tumors. Patients with malignant cystic tumors were older and had more high-risk stigmata than patients with benign tumors, including the presence of enhancing mural nodules. The only worrisome feature that differed between patients with malignant versus benign tumors was the MPD between 5 and 9 mm, which was more common in patients with malignant tumors (21.7% vs. 2.8%, P = 0.004). In patients with malignant tumors, DM was more common, and the preoperative BMI was higher. The proportion of patients who were overweight was higher among patients with malignant tumors than in patients with benign tumors (76.3% and 29.4%, respectively; P < 0.001). The serum level of CA 19-9 was higher in patients with malignant tumors. The cyst size, tumor location, number of cysts, history of acute pancreatitis, symptoms, smoking history, and serum CEA level did not differ significantly between patients with malignant tumors and those with benign tumors. ROC curve analysis showed that the optimal CA 19-9 cutoff for differentiating malignant tumors from benign tumors was 55.9 U/mL; the sensitivity was 61.9%, the specificity was 89.7%, and the area under the curve was 0.911 (95% CI: 0.704–0.917).
Characteristics of IPMNs and MCNs
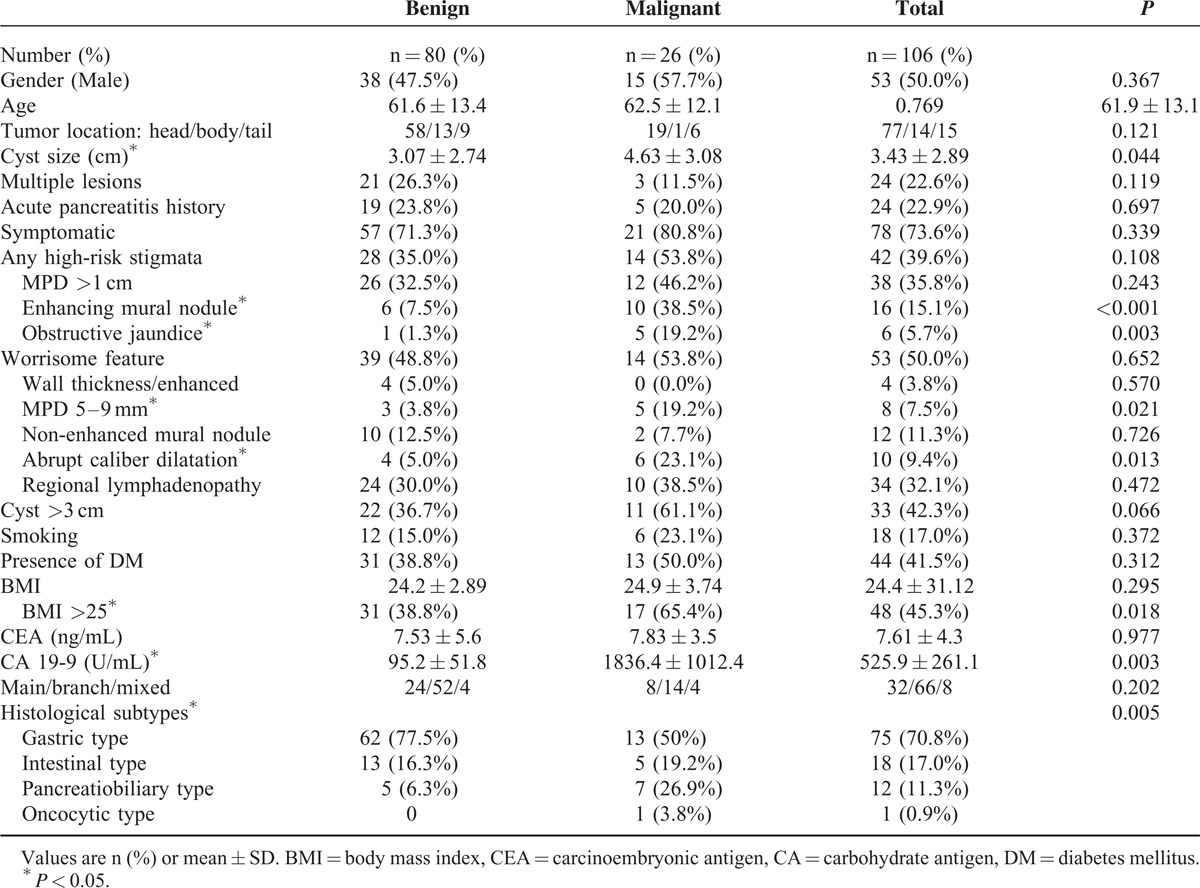
Of the 106 IPMNs, there were 75 (70.8%) of the gastric subtype, 18 (17.0%) of the intestinal subtype, 12 of the (11.3%) pancreatobiliary subtype, and 1 (0.9%) of the oncocytic subtype. There were 26 malignant IPMCs. A comparison of the clinical features of malignant and benign IPMNs is shown in Table 3. Malignant IPMNs had larger cysts and a higher rate of associated enhancing mural nodules, obstructive jaundice, an MPD between 5 and 9 mm and abrupt dilatation of the MPD. Patients with malignant IPMNs were more likely to have a BMI >25 and had higher serum CA 19-9 levels than patients with benign IPMNs. There were no statistically significant differences between malignant and benign IPMNs in terms of sex, age, tumor location, presence of symptoms, rates of DM, and serum CEA level.
TABLE 3.
Clinical and Imaging Characteristics in 106 Intraductal Papillary Mucinous Neoplasms
Of the 58 MCNs, 5 were malignant and 53 were benign. Patients with malignant MCNs were older than those with benign MCNs (71.0 ± 6.0 vs. 45.8 ± 15.2 y, respectively; P = 0.001). All 5 patients with malignant MCNs were symptomatic. Patients with malignant MCNs had higher BMI (28.6 ± 1.7 vs. 23.1 ± 2.7, P < 0.001) and higher serum CEA (143.4 ± 120.1 vs. 8.52 ± 7.27, P = 0.003), and CA 19-9 levels (10253 ± 9189.9 vs. 30.0 ± 7.9, P = 0.001) compared with patients with benign MCNs. Patients with malignant MCNs were more likely to have a BMI >25 (11/53 among patients with benign MCNs vs. 5/5 among patients with malignant MCNs, P = 0.001). All 5 patients with malignant MCNs were overweight (BMI >25). There were no statistically significant differences in cyst size between malignant and benign MCNs.
Preoperative Predictors of Malignancy
Pancreatic Mucinous Cystic Neoplasms
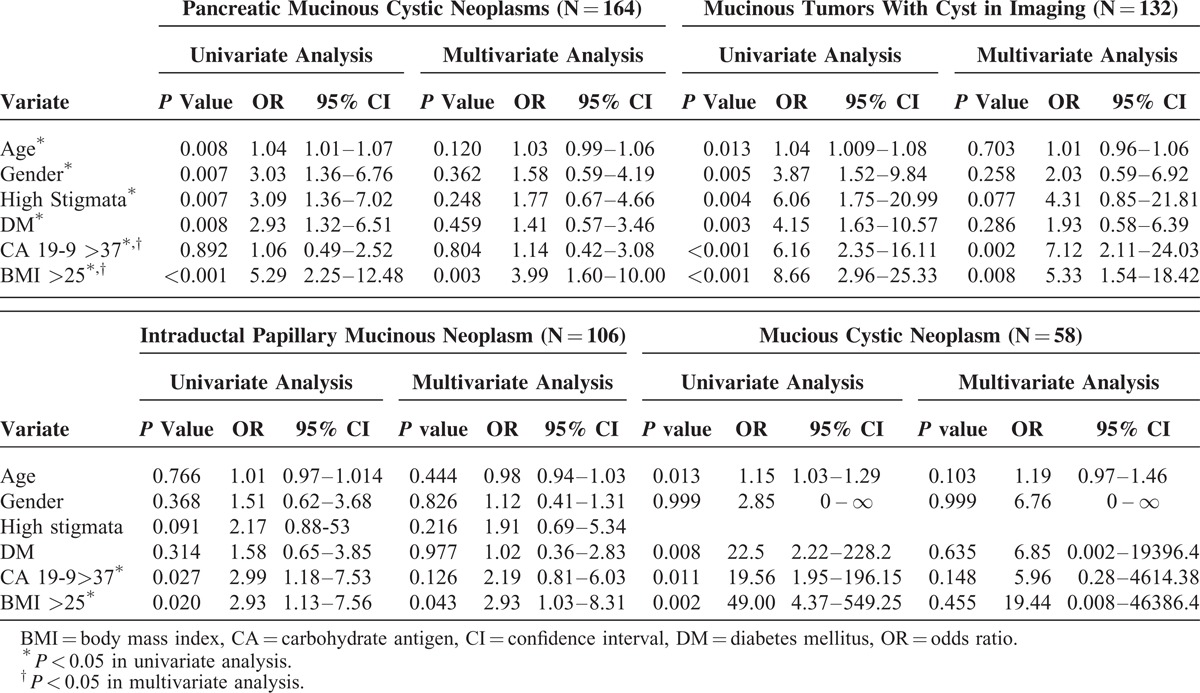
In the univariate analysis, age, sex, presence of high-risk stigmata, DM, and BMI >25 were associated with a higher risk of malignancy (Table 4). In the multivariate analysis, only BMI >25 was an independent preoperative predictor for malignancy (OR 3.99, 95% CI: 1.59–10.004, P = 0.003).
TABLE 4.
Univariate and Multivariate Analysis of Preoperative Clinical Characteristics in Predicting Malignancy in all Mucinous Tumors (N = 164), Mucinous Tumors With Cyst in Imaging (N = 132), Intraductal Papillary Mucinous Neoplasm (N = 106), and Mucious Cystic Neoplasm (N = 58)
Mucinous Tumors With Cysts on Imaging
In the univariate analysis, age, sex, the presence of high-risk stigmata, a history of DM, CA 19-9 >37 U/mL, and a BMI >25 were associated with a higher risk of malignancy (Table 4). In the multivariate analysis, both CA 19-9 >37 U/mL and BMI>25 were independent preoperative predictors for malignancy (OR 7.12, 95% CI: 2.11–24.03, P = 0.002 and OR 5.33, 95% CI: 1.54 -18.42, P = 0.008, respectively).
Intraductal Papillary Mucinous Neoplasms
In the univariate analysis, CA 19-9 >37 U/mL and BMI >25 were associated with a higher risk of malignancy in patients with IPMNs. In the multivariate analysis, BMI >25 was an independent preoperative predictor of malignancy in IPMNs (OR 2.93, 95% CI: 1.03–8.31, P = 0.043, respectively) (Table 4).
Mucinous Cystic Neoplasms
In the univariate analysis, CA 19-9 >37 U/mL and BMI >25 were associated with a higher risk of malignancy in MCNs (OR 19.56, 95% CI: 1.95–196.15, P = 0.011 and OR 49, 95% CI: 4.37–549.25, P = 0.002, respectively); however, these differences were not significant in the multivariate analysis (Table 4).
Diagnostic Accuracy of Predictors of Malignancy
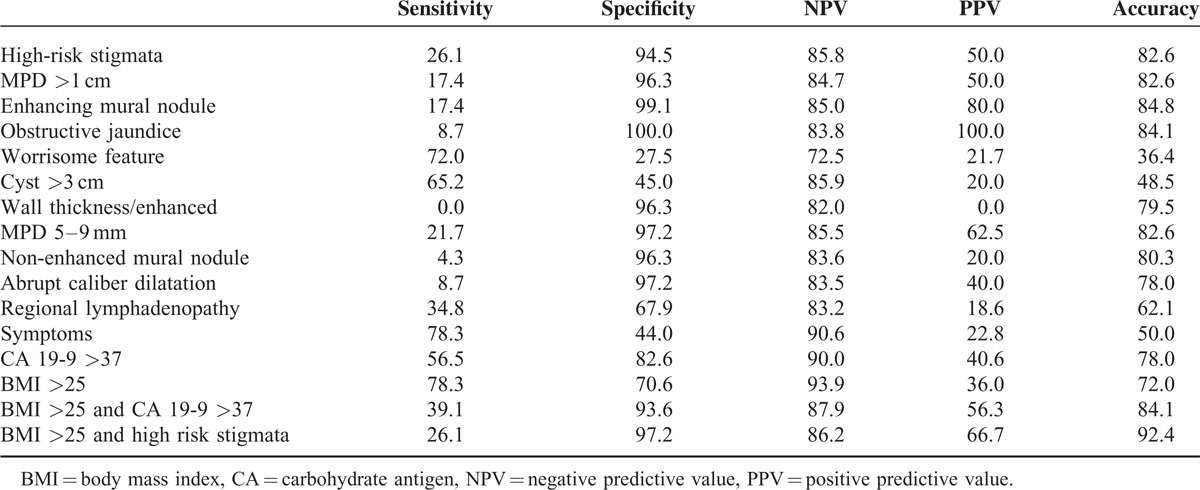
Table 5 shows the diagnostic value of predictors of malignancy according to the 2012 revised International Consensus Guidelines, BMI and CA 19-9 level. We combined mucinous tumors with cysts (BD-IPMN and MCN) for the analysis owing to their marked similarity on preoperative imaging studies. The presence of an enhancing mural nodule had the highest diagnostic accuracy (84.8%), with a sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of 17.4, 99.1, 80, and 85%, respectively. A combination of serum CA 19-9 level and BMI >25 increased the diagnostic accuracy for predicting malignancy from 72–78% to 84.1%. The combination of a BMI >25 and the presence of high-risk stigmata had the highest diagnostic accuracy (92.4%) for predicting malignancy.
TABLE 5.
Diagnostic Performance of Preoperative Clinical and Cystic Characteristics in 132 Resected Mucinous Tumors with Cyst in Imaging
DISCUSSION
Selecting patients with PCN for surgical resection remains a major clinical challenge in the absence of confirmed malignancy. Preoperative evaluation of PCNs primarily relies on imaging findings; however, imaging alone has a high rate of misdiagnosis.22 An improved understanding of the risk factors for cyst-related malignancy based on pre-operative cyst features and patient characteristics could assist in making management decisions. Previous studies have shown that older age, male sex, and the presence of diabetes are significant predictors of malignant cysts.23,24 Sturm et al analyzed 274 patients with BD-IPMNs and demonstrated that obesity is associated with a greater frequency of malignancy among patients with BD-IPMN in Western countries.21 Overweight and obesity are well-established risk factors for pancreatic adenocarcinoma and many other types of cancer.17,25 Pooled analyses and meta-analyses have confirmed an increased risk (20–50%) of pancreatic cancer in obese individuals.26,27 There are multiple mechanisms linking obesity to the promotion of gastrointestinal carcinogenesis, and increasing evidence suggests that obesity might also increase adenoma growth.28–30 In the present study, the univariate analysis showed that older age, male sex, the presence of DM, a BMI >25, the CA 19-9 level and the presence of high-risk stigmata were significant predictors of malignancy of PMCNs. However, only a BMI >25 was a significant predictor of malignancy of PMCNs in the multivariate analysis. Our results demonstrated that overweight is associated with a 3.99-fold increased risk of malignancy in PMCNs. In the subgroup analysis, the PMCNs were classified as mucinous tumors with cysts on imaging, IPMNs, and MCNs. The reason to analyze mucinous tumors with cyst on imaging as a subgroup is because we tried to find pre-operative predictor of malignancy in mucinous tumors. BD-IPMNs and MCNs are mucinous tumors with cysts on imaging and commonly encountered in clinical practice. It is challenging to differentiate unifocal BD-IPMNs from MCNs on preoperative imaging studies and not possible to confirm the diagnosis preoperatively, as these cystic lesions have similar imaging features. The univariate analysis showed that overweight was also associated with the risk of malignancy in mucinous tumors with cysts on imaging, IPMNs and MCNs. In the multivariate analysis, BMI >25 was also an independent preoperative predictor of malignancy of mucinous tumors with cysts on imaging and IPMNs. In addition, the combination of a BMI >25 and high-risk stigmata had the highest accuracy (92%) in predicting malignancy in PMCNs. BMI is a useful parameter for risk stratification in patients with PMCNs and should be considered in management decisions for patients with PMCNs, especially in IPMNs. However, BMI >25 was not an independent preoperative predictor of malignancy of MCNs in the multivariate analysis. The influence of BMI on malignancy in patients with MCNs needs further investigation.
The 2012 revised International Consensus Guidelines for the management of IPMNs suggested that these lesions should be resected in symptomatic patients, as well as those with high-risk stigmata or worrisome features. The frequency of malignancy of IPMN and MCN in our study was 24.5% and 8.6%, respectively, which are within the range reported in a prior surgical series. In our study, we confirmed that the cyst features described in the International Consensus Guidelines, including high-risk stigmata and some of the worrisome features, are associated with an increased risk of malignancy. Among the imaging findings in cases with high-risk stigmata and worrisome features, enhancing mural nodules had the highest accuracy for preoperatively predicting the malignancy of PMCNs, followed by MPD dilation and the presence of a non-enhancing mural nodule (Table 5). Multiple cystic lesions were not significantly associated with malignancy. These findings are consistent with a recent meta-analysis that investigated the use of imaging findings to distinguish benign from malignant BD-IPMNs.31 However, variables that have been found to be associated with malignancy in previous studies, as well as the indications for resection in the consensus guideline, were not significant in our multivariate analysis. When considering the clinical significance of each variable as a predictor of malignancy in the present study, only BMI >25 was found to be predictive of malignancy after multiple regression analysis. In particular, recent meta-analyses have yielded contradictory results regarding whether a cyst diameter of larger than 3 cm is a predictor of malignancy.31,32 In our study, cyst size was not significantly associated with risk of malignancy in PMCNs with cysts on imaging (combined IPMNs and MCNs) or in the analysis of the IPMNs and MCNs subgroups. Resection of PMCNs based on cyst size alone is therefore no longer appropriate.
CA 19-9 is an established tumor marker for pancreatic malignancy. The postoperative level of CA 19-9 has been reported to be useful in predicting survival after curative surgery for pancreatic adenocarcinoma.33 One recent article reported that preoperative serum CA 19-9 could predict the presence of malignancy in pancreatic cystic neoplasm patients, with a 67% sensitivity and 78% specificity.34 Increased CA 19-9 levels have been reported to be a predictor of malignancy in IPMNs.35,36 In our study, the preoperative CA 19-9 level could predict malignancy with 56.5% sensitivity, 82.6% specificity, and 78% accuracy in patients with PMCNs. In addition, serum CA 19-9 level combined with a BMI >25 increased the predictive accuracy to 84.1% in the present study, demonstrating that patient-related risk factors, such as CA 19-9, should be complementary factors that are taken into consideration with cystic features when deciding whether surgical intervention for PMCNs should be performed.
The retrospective nature of this study has some limitations. First, our analysis evaluated patients who underwent surgical resections, and thus there may have been selection bias between obese and non-obese patients. Second, we do not have complete data on lifestyle risk factors such as physical activity, which could confound our interpretation of the results. Third, the study was limited by its relatively small sample size for the analysis of each subgroup. Clearly, a large prospective cohort of PMCN patients, including multiple ethnic groups, should be studied over the long term to answer unsolved questions in the management of the increasing number of patients with PMCNs.
In summary, our data demonstrate that overweight is an independent preoperative predictor of malignancy in patients with IPMNs. As the prevalence of obesity continues to increase, the effects of obesity on the management of pancreatic cystic neoplasm must be elucidated and studied in the future. In addition, overweight is a modifiable risk factor, and weight control could prevent the progression of PMCNs to pancreatic cancer. More intensive surveillance or aggressive treatment may be required in overweight patients with PMCNs.
Footnotes
Abbreviations: BMI = body mass index, IPMN = intraductal papillary mucinous neoplasm, MCN = mucinous cystic neoplasm, PMCN = pancreatic mucinous cystic neoplasm.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Weinberg BM, Spiegel BM, Tomlinson JS, et al. Asymptomatic pancreatic cystic neoplasms: maximizing survival and quality of life using Markov-based clinical nomograms. Gastroenterology 2010; 138:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med 2004; 351:1218–1226. [DOI] [PubMed] [Google Scholar]
- 3.Hruban RH, Maitra A, Kern SE, et al. Precursors to pancreatic cancer. Gastroenterol Clin North Am 2007; 36:831–849.vi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salvia R, Fernandez-del Castillo C, Bassi C, et al. Main-duct intraductal papillary mucinous neoplasms of the pancreas: clinical predictors of malignancy and long-term survival following resection. Ann Surg 2004; 239:678–685.discussion 685–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardacre JM, McGee MF, Stellato TA, et al. An aggressive surgical approach is warranted in the management of cystic pancreatic neoplasms. Am J Surg 2007; 193:374–378.discussion 378–379. [DOI] [PubMed] [Google Scholar]
- 6.Allen PJ, D’Angelica M, Gonen M, et al. A selective approach to the resection of cystic lesions of the pancreas: results from 539 consecutive patients. Ann Surg 2006; 244:572–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka M. Controversies in the management of pancreatic IPMN. Nat Rev Gastroenterol Hepatol 2011; 8:56–60. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka M, Fernandez-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012; 12:183–197. [DOI] [PubMed] [Google Scholar]
- 9.Sawhney MS, Al-Bashir S, Cury MS, et al. International Consensus Guidelines for Surgical Resection of Mucinous Neoplasms Cannot Be Applied to All Cystic Lesions of the Pancreas. Clin Gastroenterol Hepatol 2009. [DOI] [PubMed] [Google Scholar]
- 10.Wong J, Weber J, Centeno BA, et al. High-grade dysplasia and adenocarcinoma are frequent in side-branch intraductal papillary mucinous neoplasm measuring less than 3 cm on endoscopic ultrasound. J Gastrointestinal Surg 2013; 17:78–84.discussion p 84–75. [DOI] [PubMed] [Google Scholar]
- 11.Fritz S, Klauss M, Bergmann F, et al. Small (Sendai negative) branch-duct IPMNs: not harmless. Ann Surg 2012; 256:313–320. [DOI] [PubMed] [Google Scholar]
- 12.Sahora K, Mino-Kenudson M, Brugge W, et al. Branch duct intraductal papillary mucinous neoplasms: does cyst size change the tip of the scale? A critical analysis of the revised international consensus guidelines in a large single-institutional series. Ann Surg 2013; 258:466–475. [DOI] [PubMed] [Google Scholar]
- 13.Sawhney MS, Al-Bashir S, Cury MS, et al. International consensus guidelines for surgical resection of mucinous neoplasms cannot be applied to all cystic lesions of the pancreas. Clin Gastroenterol Hepatol 2009; 7:1373–1376. [DOI] [PubMed] [Google Scholar]
- 14.Patel AV, Rodriguez C, Bernstein L, et al. Obesity, recreational physical activity, and risk of pancreatic cancer in a large U.S. cohort. Cancer Epidemiol Biomarkers Prev 2005; 14:459–466. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Morris JS, Liu J, et al. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA 2009; 301:2553–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calle EE, Thun MJ. Obesity and cancer. Oncogene 2004; 23:6365–6378. [DOI] [PubMed] [Google Scholar]
- 17.Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 2008; 371:569–578. [DOI] [PubMed] [Google Scholar]
- 18.Tao W, Lagergren J. Clinical management of obese patients with cancer. Nature reviews. Clin Oncol 2013; 10:519–533. [DOI] [PubMed] [Google Scholar]
- 19.Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology 2007; 132:2208–2225. [DOI] [PubMed] [Google Scholar]
- 20.Fleming JB, Gonzalez RJ, Petzel MQ, et al. Influence of obesity on cancer-related outcomes after pancreatectomy to treat pancreatic adenocarcinoma. Arch Surg 2009; 144:216–221. [DOI] [PubMed] [Google Scholar]
- 21.Sturm EC, Roch AM, Shaffer KM, et al. Obesity increases malignant risk in patients with branch-duct intraductal papillary mucinous neoplasm. Surgery 2013; 154:803–808.discussion 808–809. [DOI] [PubMed] [Google Scholar]
- 22.Visser BC, Yeh BM, Qayyum A, et al. Characterization of cystic pancreatic masses: relative accuracy of CT and MRI. Am J Roentgenol 2007; 189:648–656. [DOI] [PubMed] [Google Scholar]
- 23.Huang ES, Turner BG, Fernandez-Del-Castillo C, et al. Pancreatic cystic lesions: clinical predictors of malignancy in patients undergoing surgery. Aliment Pharmacol Ther 2010; 31:285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu BU, Sampath K, Berberian CE, et al. Prediction of malignancy in cystic neoplasms of the pancreas: a population-based cohort study. Am J Gastroenterol 2014; 109:121–129.quiz 130. [DOI] [PubMed] [Google Scholar]
- 25.Michaud DS, Giovannucci E, Willett WC, et al. Physical activity, obesity, height, and the risk of pancreatic cancer. JAMA 2001; 286:921–929. [DOI] [PubMed] [Google Scholar]
- 26.Arslan AA, Helzlsouer KJ, Kooperberg C, et al. Anthropometric measures, body mass index, and pancreatic cancer: a pooled analysis from the Pancreatic Cancer Cohort Consortium (PanScan). Arch Intern Med 2010; 170:791–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsson SC, Orsini N, Wolk A. Body mass index and pancreatic cancer risk: a meta-analysis of prospective studies. Int J Cancer 2007; 120:1993–1998. [DOI] [PubMed] [Google Scholar]
- 28.Aleman JO, Eusebi LH, Ricciardiello L, et al. Mechanisms of obesity-induced gastrointestinal neoplasia. Gastroenterology 2014; 146:357–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Betes M, Munoz-Navas MA, Duque JM, et al. Use of colonoscopy as a primary screening test for colorectal cancer in average risk people. Am J Gastroenterol 2003; 98:2648–2654. [DOI] [PubMed] [Google Scholar]
- 30.Siddiqui A, Pena Sahdala HN, Nazario HE, et al. Obesity is associated with an increased prevalence of advanced adenomatous colon polyps in a male veteran population. Dig Dis Sci 2009; 54:1560–1564. [DOI] [PubMed] [Google Scholar]
- 31.Kim KW, Park SH, Pyo J, et al. Imaging features to distinguish malignant and benign branch-duct type intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Ann Surg 2014; 259:72–81. [DOI] [PubMed] [Google Scholar]
- 32.Anand N, Sampath K, Wu BU. Cyst features and risk of malignancy in intraductal papillary mucinous neoplasms of the pancreas: a meta-analysis. Clin Gastroenterol Hepatol 2013; 11:913–921.quiz e959–e960. [DOI] [PubMed] [Google Scholar]
- 33.Berger AC, Garcia M, Jr, Hoffman JP, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: a prospective validation by RTOG 9704. J Clin Oncol 2008; 26:5918–5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goh BK, Tan YM, Thng CH, et al. How useful are clinical, biochemical, and cross-sectional imaging features in predicting potentially malignant or malignant cystic lesions of the pancreas? Results from a single institution experience with 220 surgically treated patients. J Am Coll Surg 2008; 206:17–27. [DOI] [PubMed] [Google Scholar]
- 35.Fritz S, Hackert T, Hinz U, et al. Role of serum carbohydrate antigen 19-9 and carcinoembryonic antigen in distinguishing between benign and invasive intraductal papillary mucinous neoplasm of the pancreas. Br J Surg 2011; 98:104–110. [DOI] [PubMed] [Google Scholar]
- 36.Shin SH, Han DJ, Park KT, et al. Validating a simple scoring system to predict malignancy and invasiveness of intraductal papillary mucinous neoplasms of the pancreas. World J Surg 2010; 34:776–783. [DOI] [PubMed] [Google Scholar]


