Abstract
This article evaluates the association of hepatic, renal, and inflammatory biomarkers with changes in systolic (SBP) and diastolic (DBP) blood pressure (BP) during healthy pregnancies.
A prospective cohort study with 225 healthy pregnant women was conducted in Rio de Janeiro, Brazil. SBP and DBP were evaluated throughout pregnancy (5th–13th, 20th–26th, and 30th–36th gestational weeks) and were the outcomes. The following biomarkers were measured at the first trimester and analyzed according to tertiles of the sample distribution and were considered the main independent predictors: alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), uric acid (UA), creatinine (Cr), and C-reactive protein (CRP) concentrations. The statistical analysis included 3 stages of modeling with the longitudinal linear mixed-effects procedures: Model 1 was adjusted for gestational age and quadratic gestational age; Model 2 included interactions between the biomarkers and gestational age; and Model 3 was adjusted for self-reported skin color, education, parity, early-pregnancy body mass index (BMI) (under/normal <25; overweight/obese ≥25 kg/m2), smoking habit, and leisure-time physical activity. Additional models were performed for CRP and UA with the inclusion of interaction terms between the biomarkers and BMI.
Women classified in the third tertile of the ALP (≥61.1 U/L; βSBP = 3.474; 95% confidence interval [CI]: 0.955–5.992; βDBP = 3.291; 95% CI: 1.098–5.485), ALT (≥14.3 U/L; βSBP = 2.232; 95% CI: 0.221–4.242; βDBP = 2.355; 95% CI: 0.721–3.989), and Cr values (≥48.6 μmol/L; βDBP = 1.927; 95% CI: 0.347–3.508) presented higher BP levels during pregnancy compared to those in the first and second tertiles. Women in the highest tertile of the ALP concentration distribution presented a lower rate of change in SBP and DBP during pregnancy (interaction term with gestational age βSBP = −0.004; 95% CI: −0.007 to −0.001; P = 0.02; βDBP = −0.003; 95% CI: −0.006 to −0.001; P = 0.01). Higher UA concentrations were associated with higher SBP levels only in overweight/obese women (β = 3.878; 95% CI: 0.687–7.068), whereas higher CRP concentrations (≥2.6 mg/L) were associated with higher DBP in under/normal weight women (β =2.252; 95% CI: 0.267–4.236).
ALP, ALT, and Cr concentrations were positively associated with BP levels, whereas ALP was associated with a lower rate of change in BP. The associations of UA and CRP with BP differ according to the early-pregnancy BMI.
INTRODUCTION
Pregnancy induces significant adjustments in the cardiovascular system to provide an adequate blood supply for fetal development.1 Some studies have shown that the blood pressure (BP) changes significantly during a healthy pregnancy, that is, the levels decrease from the first to the second trimester followed by an increase until delivery.1–5 Factors such as maternal age, parity, and body mass index (BMI) have already been reported to be associated with BP levels during normal and complicated pregnancies.3,5 The lack of a drop in BP during mid-pregnancy may be a maladaptive process, whereas women who develop hypertensive disorders of pregnancy (HDP) may present progressive increases in BP levels throughout pregnancy.4
Although the pathophysiology of HDP is still incompletely understood, endothelial dysfunction, characterized by an imbalance between endothelial-mediated vasodilatation and constriction due to decreased nitric oxide bioavailability, may participate in the pathogenesis of HDP.6,7 Previous studies have shown that inflammatory markers8 and hepatic9 and renal function10 are associated with HDP. Some of these biomarkers, such as C-reactive protein (CRP), alkaline phosphatase (ALP), and uric acid (UA), have been reported to be associated with HDP. However, there is still scarce information regarding the association of these biomarkers with the changes in BP during pregnancy, and the majority of these studies have employed a cross-sectional design.10,11 CRP is a systemic inflammatory marker synthesized in the liver and may play a role in eliciting the inflammatory response observed in preeclampsia (PE).8,11 A longitudinal study reported that the first-trimester CRP concentrations were positively associated with the systolic blood pressure (SBP) and diastolic blood pressure (DBP) levels during pregnancy, although the associations were attenuated after adjustment for maternal BMI.12 Along the same lines, studies have indicated that UA concentrations may predict the progression to PE, whereas preeclamptic women presented higher serum concentrations of this biomarker when compared with normotensive women.13,14 Indeed, higher concentrations of UA have also been shown to be associated with inflammation, oxidative stress, and endothelial dysfunction.14,15 It has also been reported that renal function is highly correlated with SBP and DBP in women who develop HDP and that the combination of urinary creatinine (Cr) and SBP in early pregnancy can be considered a predictor of PE.16,17
Thus, considering the importance of adaptive BP changes during normal pregnancy, the scarce information concerning the effect of maternal biological markers on prospective BP changes during pregnancy and the potential use of this biomarkers on the prevention of HDP, the aim of this study was to evaluate the association of first-trimester hepatic, renal, and inflammatory markers with SBP and DBP levels and rate of changes during healthy pregnancies.
METHODS
Design and Study Population
This study comprised a prospective cohort of pregnant women who received prenatal care at a public health care center in the city of Rio de Janeiro, Brazil. The women were monitored 3 times during pregnancy at the 5th–13th (study baseline), 20th–26th, and 30th–36th gestational weeks. The recruitment occurred between November 2009 and October 2011. Women were invited to participate if they met the following eligibility criteria: were between 5 and 13 weeks pregnant at the time of recruitment; between 20 and 40 years of age; free from any chronic diseases (except obesity); and free from infectious diseases. A total of 299 pregnant women agreed to participate and were recruited for this study. We excluded women who presented twin pregnancies (n = 4), were diagnosed with an infectious (n = 9) or chronic noncommunicable disease (n = 12), presented missing baseline data for BP (n = 20), had a miscarriage (n = 25), or a stillbirth (n = 4). Following these exclusions, the baseline sample comprised 225 healthy pregnant women. We further evaluated 192 women at the second trimester and 194 at the third trimester (13.8% of follow-up losses).
Blood Pressure Measurements
The SBP and DBP were evaluated four times in the first trimester on 2 distinct days with an interval of 11.0 days (95% CI: 9.2–12.8) and twice during the second and third trimesters with a 30-minute interval between measurements. The mean SBP and DBP values were used. BPs were measured with an automated oscillometric BP monitoring system (Omron HEM-742, São Paulo, Brazil), which had been previously validated for the Brazilian adult population.18 However, to the best of our knowledge, this device has not been validated for pregnant women. A suitably sized cuff to the brachial circumference was used and the BP was measured after at least a 5-minutes rest, with the women sitting, according to current recommendations.19
Blood Samples
Blood samples were collected at the study baseline after a 12-hour fast and were immediately centrifuged at 5000 rpm for 5 minutes. The serum and plasma were separated and stored at −80°C until the analyses were performed. The serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), and ALP concentrations (U/L) were measured via a spectrophotometric method using commercial kits (DiaSys Diagnostic Systems GmbH, Holzheim, Germany) with sensitivities of 4, 2, and 2 U/L, respectively. The serum concentrations (μmol/L) of UA and Cr were also detected using a spectrophotometric method and commercial kits (DiaSys Diagnostic Systems GmbH) with sensitivities of 4.2 and 8.8 μmol/L, respectively. Serum concentrations of CRP were measured with a high sensitivity (mg/L) via the immunoturbidimetric method using an ultrasensitive commercial kit (DiaSys Diagnostic Systems GmbH) with a sensitivity of 0.05 mg/L.20
Covariates Assessment
We evaluated the following baseline socioeconomic, demographic, lifestyle, and reproductive variables with a structured questionnaire: maternal age (years), education (years of schooling), self-reported skin color (black or mixed/white), marital status (married/stable partnership or single), monthly per-capita family income (US dollars), smoking habit (no/yes), alcohol consumption (no/yes), prepregnancy leisure-time physical activity (LTPA: no/yes), and parity (0/≥1 parturition).
The anthropometric measurements were standardized and collected by trained interviewers.21 The height was measured in duplicate at baseline using a portable stadiometer (Seca Ltd., Hamburg, Germany). Weight was measured in the 3 pregnancy visits with an electronic scale (Filizzola PL 150, Filizzola Ltd., Brazil) with a 150-kg capacity and 0.1-kg variation. Early-pregnancy BMI was calculated using weight and height measured at baseline. The gestational age (weeks) was estimated using the first ultrasound performed prior to 26 gestational weeks. Nineteen women did not have this information and the reported date of the last menstrual period was used.
Statistical Analyses
The women's baseline characteristics were presented as means and standard deviations or as proportions depending on the variable distribution. The SBP and DPB levels at each pregnancy trimester were described using the means and 95% confidence intervals (CIs).
The hepatic, renal, and inflammatory biomarkers were categorized into tertiles of the sample distribution, as this allowed us to identify a trend among tertiles. The differences on the SBP and DBP means among tertiles for each trimester were compared using ANOVA and the Bonferroni post hoc test.
Longitudinal mixed-effects (LMEs) regression models were used to evaluate the association of the first trimester biomarkers with the changes in SBP and DBP during pregnancy. The gestational age and the quadratic gestational age were included in all the LME models to fit the quadratic function of the association of BP with time. LME models assess the interindividual and intraindividual changes, consider that the repeated measures of the same individual are correlated, and allow the evaluation of the data with unbalanced time intervals.22,23
Gestational age (weeks) was included in the LME models as both random and fixed effects to adjust for the overall and the individual variations in the BP levels throughout time. All other covariates were analyzed as fixed-effects only. The model was fitted including an intercept–slope correlation in the random effects assuming an unstructured covariance matrix.
We fitted 3 LME models using the first-trimester biomarkers’ tertiles as the factor variable (independent variable). Considering the sample size constrains and the similarities of SBP and DBP mean values between some tertiles, we opted to present the results combining the first and second against the third tertile of each biomarker at each trimester of pregnancy. However, the CRP concentrations were categorized differently, and the analysis considered the women in the first versus those in the second and third tertiles because the associations of the CRP concentrations with the BP levels were similar for the 2 highest tertiles. Model 1 included gestational age and quadratic gestational age. Model 2 included the interaction term (between each factor variable and quadratic gestational age) and gestational age. Finally, Model 3 was fully adjusted for maternal age, self-reported skin color, education, parity, early-pregnancy BMI, smoking habit, and prepregnancy LTPA. We fitted models separately for SBP and DBP.
We analyzed the interaction of each factor variable with early-pregnancy maternal BMI in a model adjusted for gestational age and quadratic gestational age, in order to evaluate the BMI effect modification in the relation between the biomarkers and BP. For those biomarkers that presented a statistically significant interaction with the BMI, that is, UA and CRP, an additional evaluation of their association with BP levels was performed, stratifying by BMI categories (under/normal weight [BMI < 25 kg/m2] or overweight/obese [BMI ≥ 25 kg/m2]). We did not include interaction terms with gestational age in these models.
Scatter plots were elaborated to illustrate the distribution of the SBP and DBP levels and their predicted rates of change throughout pregnancy according to the first-trimester biomarker tertiles. The longitudinal prediction of the BP changes was fitted using a crude LME regression model. The prediction was performed for the 5th to the 39th week of gestation.
The statistical analyses were performed using Stata Data Analysis and Statistical Software version 12.0 (Stata Corp., College Station, TX). Values were considered to be statistically significant when P < 0.05.
Ethics
The study was approved by both the Research Ethics Committees of the Municipal Secretariat of Health and Civil Defense of the State of Rio de Janeiro (Protocol number: 0012.0.249.000-09, approved on August 13th, 2009) and of the Maternity Hospital (Protocol number: 0023.0.361.000-08). All participants signed a 2-way term of consent, which was obtained freely and spontaneously, after all necessary clarifications had been provided.
RESULTS
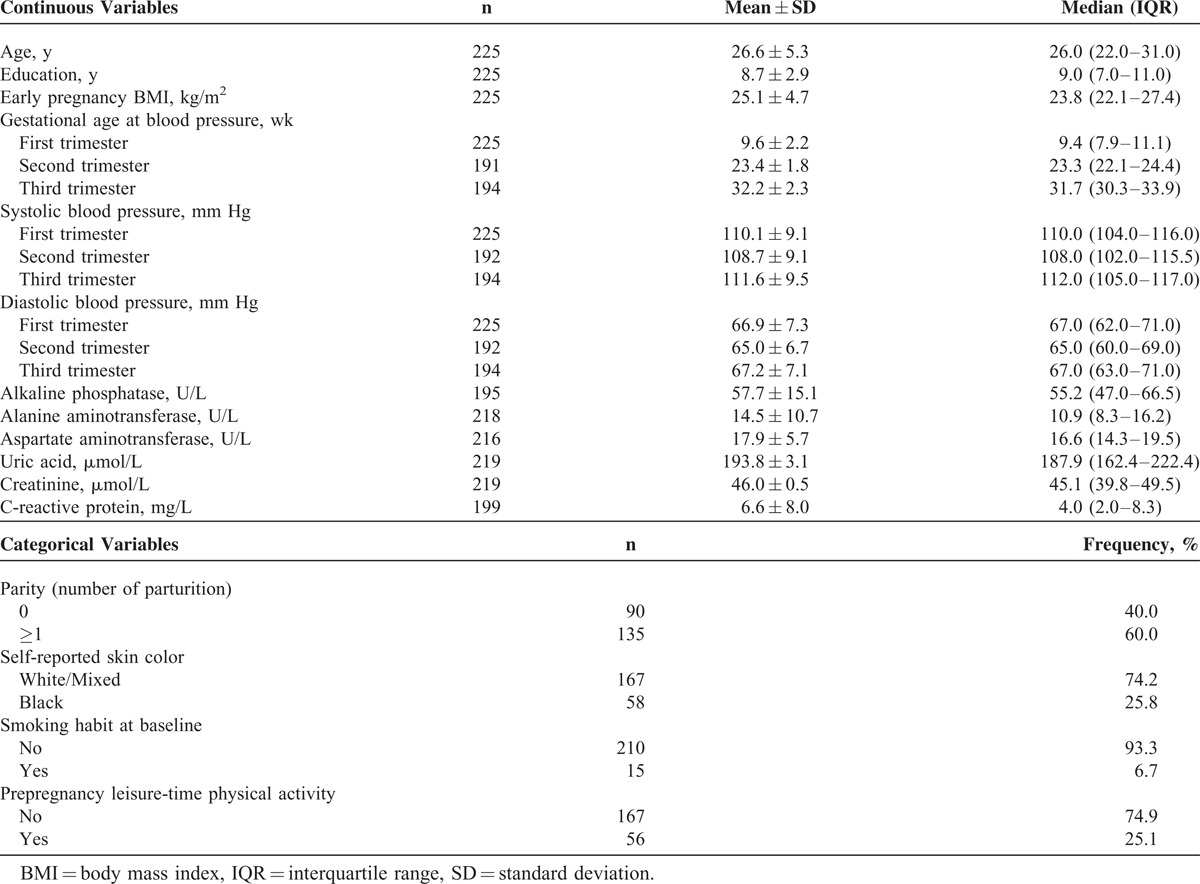
The pregnant women presented a mean age of 26.6 ± 5.3 years and had 8.7 ± 2.9 years of education; 40.0% (n = 90) of the women were nulliparous, and 60% had ≥1 parturitions. The mean early-pregnancy BMI was 25.1 ± 4.7 kg/m2 (41.3% of the women were classified with pregestational excessive weight; 28.4% overweight and 12.9% obese—results not shown in tables). The women had a mean concentration of 57.7 ± 15.1 U/L of ALP, 14.5 ± 10.7 U/L of ALT, 17.9 ± 5.7 U/L of AST, 193.8 ± 3.1 μmol/L of UA, 46.0 ± 0.5 μmol/L of Cr, and 6.6 ± 8.1 mg/L of CRP at the first trimester. Overall, SBP and DBP decreased from the first to the second trimester and then increased in the third trimester (Table 1).
TABLE 1.
Characteristics of Pregnant Women Followed at a Public Health Center in Rio de Janeiro, Brazil, 2009–2012
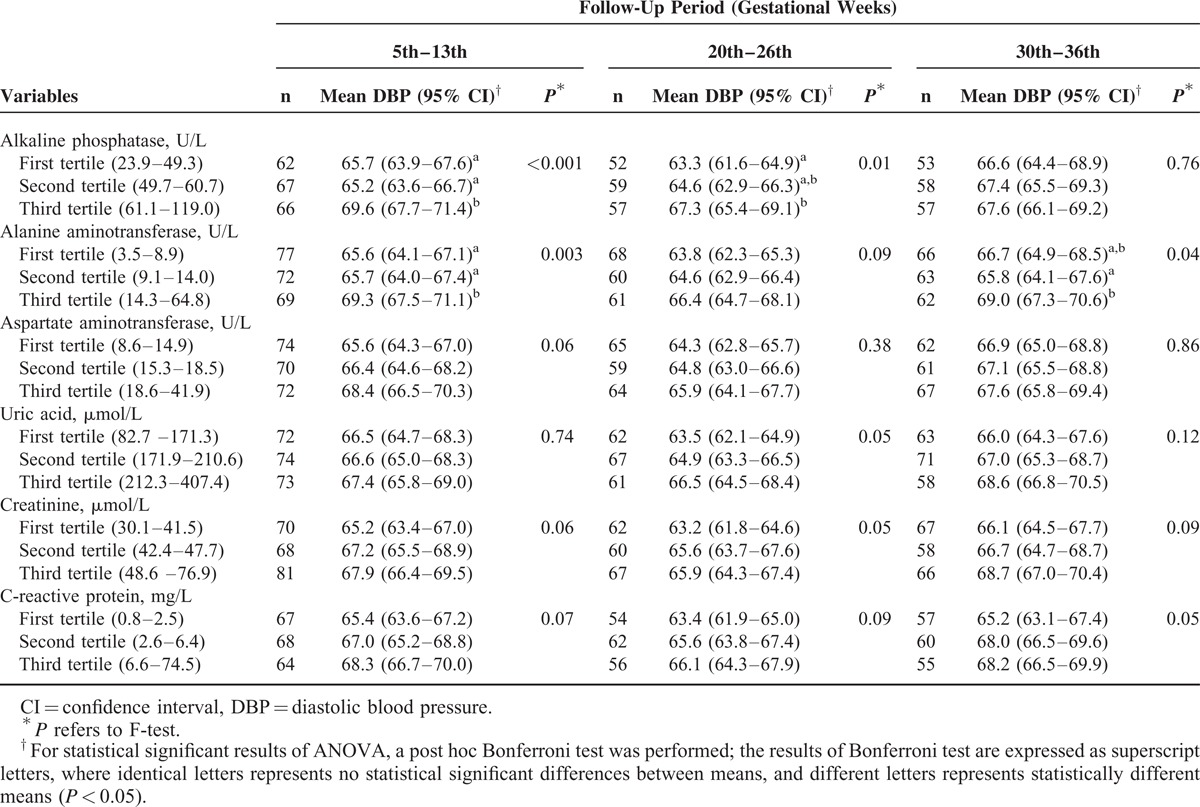
Women classified in the third tertile of the sample distribution of the hepatic and renal biomarkers tended to present higher mean values of SBP and DBP during pregnancy compared with those in the first and second tertiles, except for CRP, which tended to have higher values at the second and third trimester compared with the first one. These differences were statistically significant for SBP at the first trimester for ALP and ALT; at the second trimester for UA and CRP; and at the third trimester for ALT and UA. For DBP, the statically significance occurred for ALP and ALT at the first trimester; for ALP at the second trimester; and for ALT at the third trimester (Tables 2 and 3).
TABLE 2.
Mean SBP Levels During Pregnancy According to First-Trimester Tertiles of Hepatic, Renal, and Inflammatory Biomarkers, in Women Followed at a Public Health Center in Rio de Janeiro, Brazil, 2009 to 2012
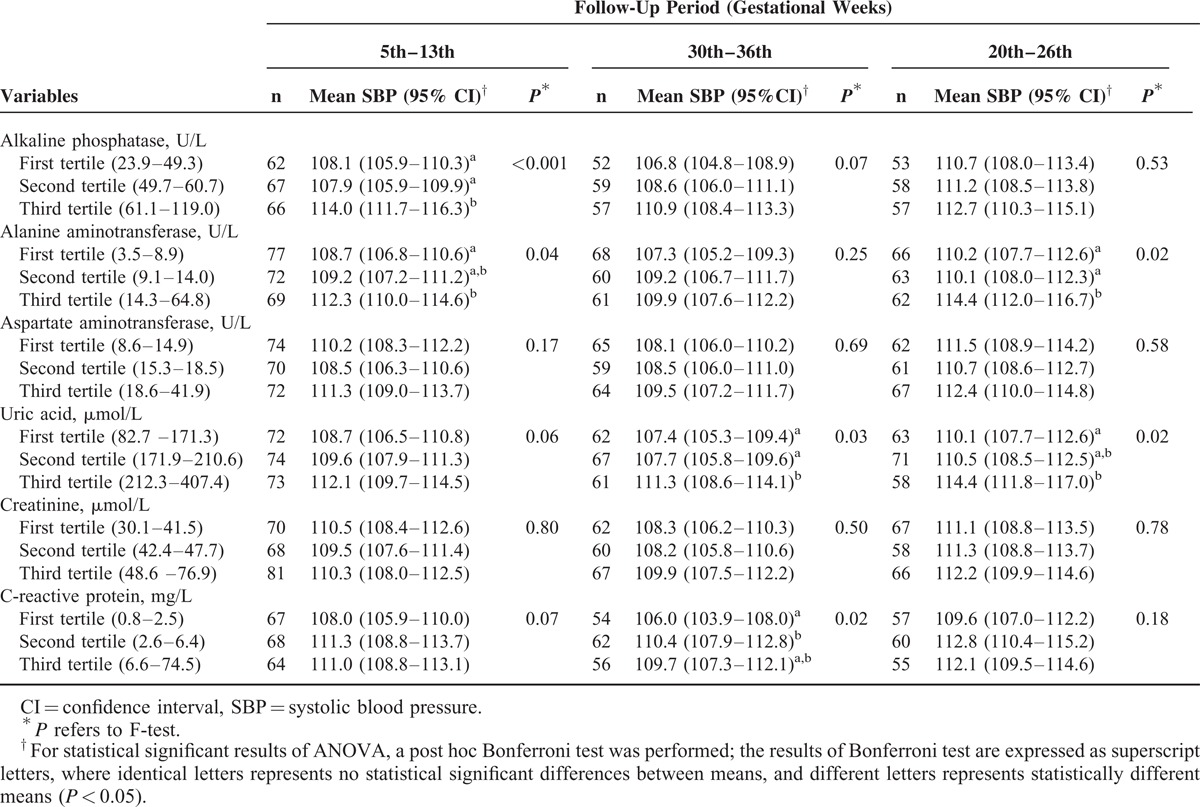
TABLE 3.
Mean DBP Levels During Pregnancy According to First-Trimester Tertiles of Hepatic, Renal, and Inflammatory Biomarkers, in Women Followed at a Public Health Center in Rio de Janeiro, Brazil, 2009 to 2012
Women classified in the third tertile of the ALP and ALT concentrations presented higher SBP (βALP = 3.474; 95% CI: 0.955–5.992; P = 0.01; βALT = 2.232; 95% CI: 0.221–4.242; P = 0.03) and DBP (βALP = 3.291; 95% CI: 1.098–5.485; P = 0.003; βALT = 2.355; 95% CI: 0.721–3.989; P = 0.01) during pregnancy compared with those in the first and second tertiles in the fully adjusted model (Model 3). The Cr concentrations were only positively associated with the DBP levels. The first-trimester AST concentrations were not associated with BP during pregnancy. The positive association of the concentrations of UA with SBP and CRP with both SBP and DBP were statistically significant only in the crude analysis (Model 1). These associations were attenuated after further adjustments and became nonsignificant (Models 2 and 3) (Tables 4 and 5 and Figure 1).
TABLE 4.
Longitudinal Changes of SBP During Pregnancy According to First-Trimester Tertiles of Hepatic, Renal, and Inflammatory Biomarkers, in Women Followed at a Public Health Center in Rio de Janeiro, Brazil, 2009 to 2012
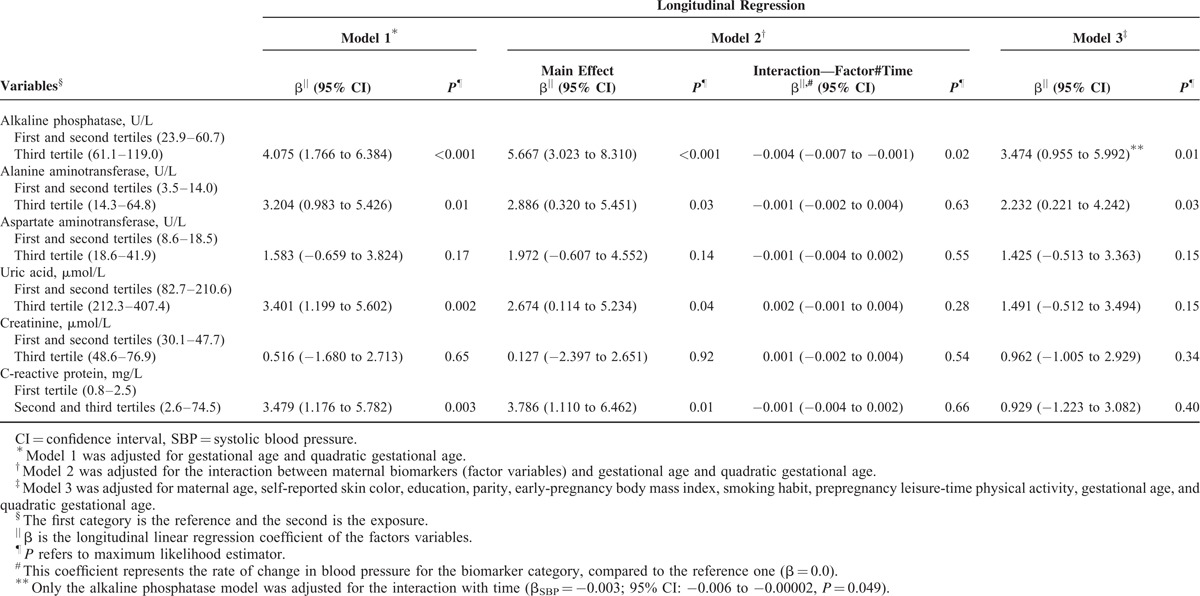
TABLE 5.
Longitudinal Changes of DBP During Pregnancy According to First-Trimester Tertiles of Hepatic, Renal, and Inflammatory Biomarkers, in Women Followed at a Public Health Center in Rio de Janeiro, Brazil, 2009 to 2012
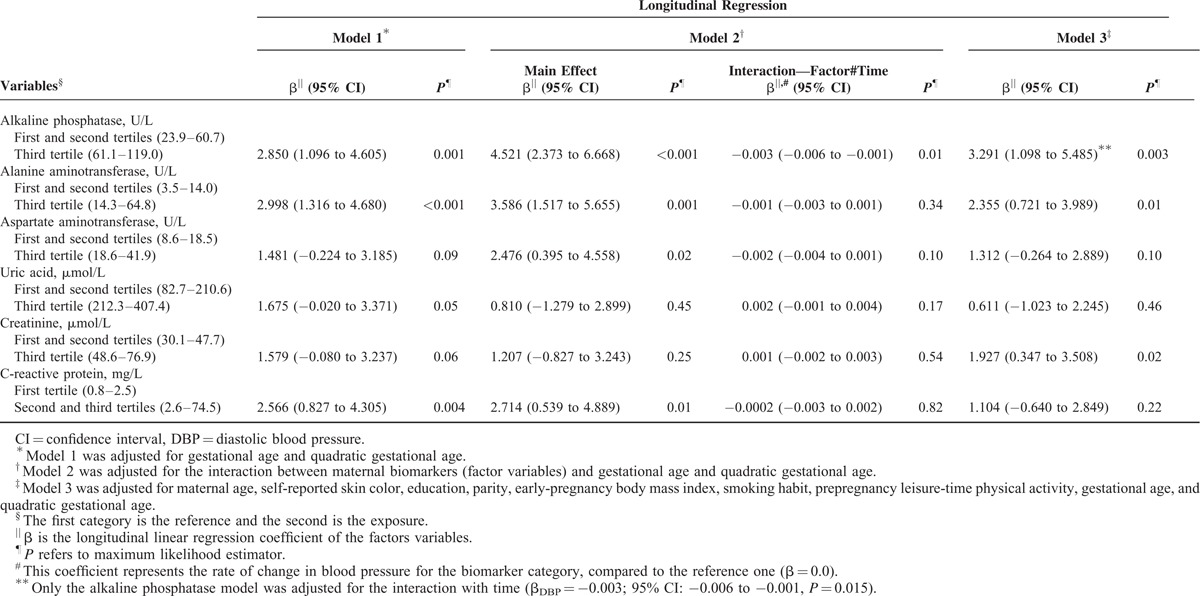
FIGURE 1.
Blood pressure changes during pregnancy according to maternal first-trimester biomarkers, in women followed at a public health center in Rio de Janeiro, Brazil, 2009 to 2012. (A) Alkaline phosphatase (ALP). (B) Alanine aminotransferase (ALT). (C) Creatinine (Cr). Left panels: systolic blood pressure; right panels: diastolic blood pressure. Note: Fitted values were predicted using a longitudinal linear regression model for each tertile of the first-trimester biomarkers.
Early-pregnancy BMI was considered the main confounder responsible for diminishing the associations between UA, CRP, and BP. Results on the association of the UA and CRP concentrations with the BP levels, stratifying for early-pregnancy BMI, revealed that women in the highest tertile of the UA concentrations presented increased SBP (β = 3.878; 95% CI: 0.687–7.068; P = 0.02) in the overweight/obese subgroup, but not in the under/normal weight BMI subgroup, compared with those in the lower tertiles. For CRP, the association only occurred with DBP in the under/normal weight group, in which women with CRP concentrations ≥2.6 mg/L presented higher DBP levels throughout pregnancy than those with a concentration of CRP ≤2.5 mg/L (β = 2.252; 95% CI: 0.267–4.236; P = 0.03) (Model 3) (Table 6 and Figure 2).
TABLE 6.
Blood Pressure Changes During Pregnancy According to Tertiles of C-Reactive Protein and Uric Acid Stratified for BMI at the First Trimester, in Women Followed at a Public Health Center in Rio de Janeiro, Brazil, 2009 to 2012
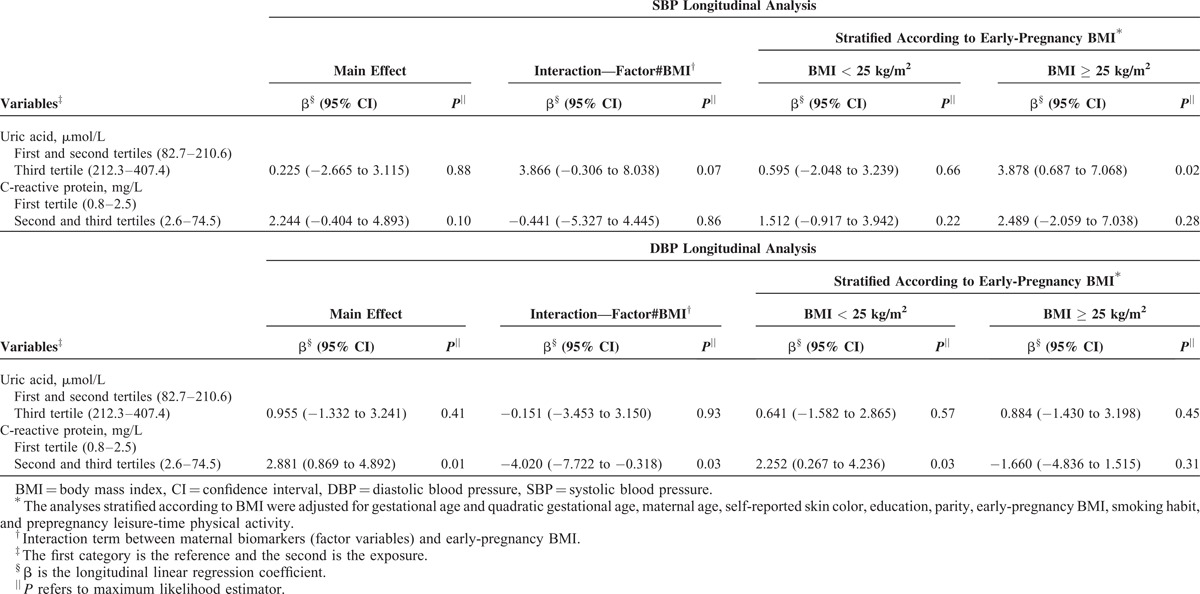
FIGURE 2.
Blood pressure changes during pregnancy according to tertiles of C-reactive protein and uric acid stratified for body mass index (BMI) at the first trimester, in women followed at a public health center in Rio de Janeiro, Brazil, 2009 to 2012. (A) Uric acid tertiles in BMI < 25 kg/m2. (B) Uric acid tertiles in BMI ≥ 25 kg/m2. (C) C-reactive protein in tertiles in BMI < 25 kg/m2. (D) C-reactive protein in tertiles in BMI ≥ 25 kg/m2. Note: Fitted values were predicted using a longitudinal linear regression model for each tertile of the first-trimester biomarkers.
The only significant interaction term between a biomarker and the gestational time was for ALP (βSBP = −0.004; 95% CI: −0.007 to −0.001; P = 0.02; βDBP = −0.003; 95% CI: −0.006 to −0.001; P = 0.01), which indicates that the women in the highest tertile of the ALP concentration distribution presented higher BP levels but had a significantly lower rate of change in SBP and DBP during pregnancy compared with those in the first and second tertiles. The gestational time variables (linear and quadratic) were significant in all the analyses (P < 0.001) (Tables 4 and 5 and Figure 1).
DISCUSSION
This study presents 4 main findings regarding BP levels and rate of changes during pregnancy. Initially, we observed that women with first-trimester serum concentrations of ALP and ALT in the highest tertile of the sample distribution presented higher SBP and DBP levels in the first trimester compared with women in the first and second tertiles. Second, serum Cr concentrations were positively associated only with DBP, even after adjustment for potential confounders, such as early-pregnancy BMI, maternal age, self-reported skin color, parity, smoking habit, and prepregnancy LTPA. Third, women in the highest tertile of the sample distribution of ALP presented lower rates of BP changes during pregnancy. Finally, we observed that the associations of the UA and CRP concentrations with BP levels during pregnancy were influenced by the maternal BMI, that is, the concentration of CRP was associated with a higher DBP only in under/normal weight women, whereas the concentration of UA was associated with higher SBP only in overweight/obese women.
As far as we know, this is the first study that has evaluated the association of hepatic, renal, and inflammatory biomarkers with prospective BP changes during healthy pregnancies. However, some potential limitations should be discussed. The first limitation is the lack of information concerning the serum concentrations of biological markers at the second and third trimesters. Second, in contrast to some prospective studies that had several BP measurements,3,4 we obtained only 3 BP measurements during pregnancy. Nevertheless, our measurements assessed the 3 trimesters of pregnancy and were sufficient to detect the well-described pattern of a mid-pregnancy BP drop and late-pregnancy BP increase.2 A third issue is that 31 women (13.8% losses on follow-up) did not have all the BP measurements during pregnancy. However, this limitation was counterbalanced by the statistical technique used for the data analyses. The LME procedure allowed the use of the information from all 225 women who had at least one BP measurement during pregnancy. Finally, the lack of validation studies of the used oscillometric automated device for pregnant women may also be considered a possible limitation.
Pregnant women classified in the third tertile of the ALP and ALT of the sample distributions presented higher levels of SBP and DBP in the first trimester compared with those in the first and second tertiles. We did not find studies that associated these biomarkers with longitudinal measures of BP during pregnancy, but the association between abnormalities of liver function and HDP is well known.24,25 Mei-Dan et al26 reported ALT concentrations at up to 20 weeks of gestation, as a predictive marker for both mild and severe PE. Mangal et al27 observed higher concentrations of serum and placental ALP for pregnancy-induced hypertensive mothers compared with normotensive ones. Both study results corroborate with our findings. ALP in pregnancy may be derived from placenta, liver, or bone; however, as we had not evaluated ALP isoenzymes, we cannot ascertain whether the associations between elevated ALP with higher BP levels and lower BP changes may reflect subclinical liver (eg, nonalcoholic fatty liver disease), bone (eg, vitamin D deficiency), or placental dysfunction.
In our study, healthy pregnant women classified in the third tertile of the sample distribution of the serum Cr concentrations presented higher DBP levels, but no statistical significant association was observed with SBP levels and SBP and DBP changes. We did not find studies that evaluated this association during healthy pregnancies. All the aspects of kidney physiology are affected by gestation. During this period, the glomerular filtration rate increases from 40% to 50% and often leads to lower serum Cr concentrations.28 However, a recent study conducted by Son et al29 found that preeclamptic women presented higher serum Cr concentrations (72.5 μmol/L) compared to the healthy pregnant controls (51.3 μmol/L).
The concentration of UA is an important biochemical marker of renal dysfunction and has been associated with the development of hypertension.30 We observed that, in the unadjusted analysis, women with UA concentrations >212.3 μmol/L (3.6 mg/dL) presented increased SBP and DBP levels during pregnancy, but this association was no longer significant after the adjustment for confounders. In agreement with our results, Martell-Claros et al31 evaluated 283 pregnant women in a prospective cohort during pregnancy and showed that women with UA concentrations >187.4 μmol/L (3.15 mg/dL) in the first trimester presented an increased risk of gestational hypertension after adjustment for the first-trimester SBP. However, the authors did not adjust their analysis for important confounders, such as maternal BMI. Indeed, the early-pregnancy BMI was the covariate that most attenuated the association between UA and BP levels in our analysis. When the analysis was stratified for BMI, we observed that this association remained statistically significant only in the overweight/obese subgroup. Hence, we suppose that the association between UA concentrations and BP levels during pregnancy is mainly influenced by the maternal overweight/obesity status.
The CRP concentrations were associated with SBP and DBP levels during pregnancy in the crude analysis, but we found no association at all after adjustment for important confounders, such as early-pregnancy BMI. A recent systematic review and meta-analysis also reported that the evaluation of serum CRP in early pregnancy might predict the development of PE. However, the authors verified an effect of BMI on this association and recognized the need for further studies to evaluate this association with an adjustment or stratification for prepregnancy BMI.32 Taking this recommendation into consideration, we further investigated this association and stratified our sample for early-pregnancy maternal BMI. We found that the CRP concentrations were positively associated with higher DBP levels in both the crude and the adjusted models, but only in under/normal weight women. No association was observed in the overweight/obese subgroup.
Two longitudinal studies that used different analyses found similar results.11,12 Qiu et al11 evaluated 60 women who developed PE and 506 who remained normotensive throughout pregnancy and observed that, in the overall sample, the association between CRP concentrations and PE did not remain statistically significant after adjusting for prepregnancy BMI. The authors also stratified their analysis for BMI and observed that lean women (prepregnancy BMI <25 kg/m2) with CRP concentrations ≥4.9 mg/L presented a 2.5-fold increased risk of PE (95% CI: 1.1–5.5) compared with those with a concentration of CRP <4.9 mg/L. No association was observed among the overweight women. A prospective population-based cohort study conducted by de Jonge et al.12 evaluated 5816 pregnant women (334 with HDP) and found that higher concentrations of CRP (≥20.0 mg/L) were associated with higher SBP and DBP levels during pregnancy and with an increased risk of pregnancy-induced hypertension. However, the associations were no longer observed after an adjustment for the BMI. These previous findings combined with our results indicate that in overweight/obese women, most of the effect of inflammation on BP levels may be mediated by maternal weight; however, in under/normal weight women, this association is not explained solely by maternal weight and may involve different pathways.
In summary, this prospective study with healthy women provides new evidence of factors associated with BP levels and changes during pregnancy. Women with high serum ALP and ALT concentrations in the first trimester of pregnancy presented a significant and positive association with SBP and DBP levels. Furthermore, ALP was associated with BP rate of change during pregnancy. Serum Cr concentrations were associated only with DBP levels. We further observed that the concentrations of CRP were positively associated with DBP in under/normal weight women, whereas UA was associated with SBP only in overweight/obese women. These results support the hypothesis that hepatic, renal, and inflammatory biomarkers are involved with BP during pregnancy, even in normotensive women. Likewise, further studies evaluating the factors associated with the serum concentrations of these markers during pregnancy would be of great relevance, especially with women with higher risk of developing HDP, as the measurement of these biomarkers could be considered in the screening of women with this condition.
Acknowledgments
The authors would like to thank the Carlos Chagas Filho Research Foundation from the State of Rio de Janeiro (FAPERJ) for providing funds for this study.
Footnotes
Abbreviations: ALP = alkaline phosphatase, ALT = alanine aminotransferase, AST = aspartate aminotransferase, BMI = body mass index, BP = blood pressure, BRAIN = Basic Research and Advanced Investigations in Neurosciences, CI = confidence interval, Cr = creatinine, CRP = C-reactive protein, DBP = diastolic blood pressure, HDP = hypertensive disorders of pregnancy, IQR = interquartile range, LMEs = longitudinal mixed effects, LTPA = leisure-time physical activity, PE = preeclampsia, SBP = systolic blood pressure, SD = standard deviation, UA = uric acid.
This study was funded by Carlos Chagas Filho Research Foundation from the State of Rio de Janeiro (FAPERJ). GK and GFS are researchers fellows from the National Council for Scientific and Technological Development (CNPq). IE has received scholarship from the CNPq. FR received scholarship from the National School of Public Health. RHM, CB, and AAFV have received scholarship from the Brazilian Coordination Body for the Training of University Level Personnel (CAPES). NSL has received scholarship from FAPERJ.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Savu O, Jurcuţ R, Giuşcă S, et al. Morphological and functional adaptation of the maternal heart during pregnancy. Circ Cardiovasc Imaging 2012; 5:289–297. [DOI] [PubMed] [Google Scholar]
- 2.Farias DR, Franco-Sena AB, Rebelo F, et al. Total cholesterol and leptin concentrations are associated with prospective changes in systemic blood pressure in healthy pregnant women. J Hypertens 2014; 32:127–134. [DOI] [PubMed] [Google Scholar]
- 3.Macdonald-Wallis C, Tilling K, Fraser A, et al. Established preeclampsia risk factors are related to patterns of blood pressure change in normal term pregnancy: findings from the Avon Longitudinal Study of Parents and Children. J Hypertens 2011; 29:1703–1711. [DOI] [PubMed] [Google Scholar]
- 4.Grindheim G, Estensen M-E, Langesaeter E, et al. Changes in blood pressure during healthy pregnancy: a longitudinal cohort study. J Hypertens 2012; 30:342–350. [DOI] [PubMed] [Google Scholar]
- 5.Ochsenbein-Kölble N, Roos M, Gasser T, et al. Cross sectional study of automated blood pressure measurements throughout pregnancy. Bjog Int J Obstet Gynaecol 2004; 111:319–325. [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Scholl TO. Maternal biomarkers of endothelial dysfunction and preterm delivery. Plos One 2014; 9:e85716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agarwal A, Aponte-Mellado A, Premkumar BJ, et al. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol Rbe 2012; 10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paternoster DM, Fantinato S, Stella A, et al. C-reactive protein in hypertensive disorders in pregnancy. Clin Appl Thromb Hemost 2006; 12:330–337. [DOI] [PubMed] [Google Scholar]
- 9.Knapen MF, Mulder TP, Bisseling JG, et al. Plasma glutathione S-transferase alpha 1-1: a more sensitive marker for hepatocellular damage than serum alanine aminotransferase in hypertensive disorders of pregnancy. Am J Obstet Gynecol 1998; 178:161–165. [DOI] [PubMed] [Google Scholar]
- 10.Von Versen-Hoeynck FM, Hubel CA, Gallaher MJ, et al. Plasma levels of inflammatory markers neopterin, sialic acid, and C-reactive protein in pregnancy and preeclampsia. Am J Hypertens 2009; 22:687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu C, Luthy DA, Zhang C, et al. A prospective study of maternal serum C-reactive protein concentrations and risk of preeclampsia. Am J Hypertens 2004; 17:154–160. [DOI] [PubMed] [Google Scholar]
- 12.De Jonge LL, Steegers EAP, Ernst GDS, et al. C-reactive protein levels, blood pressure and the risks of gestational hypertensive complications: the Generation R Study. J Hypertens 2011; 29:2413–2421. [DOI] [PubMed] [Google Scholar]
- 13.Bellomo G, Venanzi S, Saronio P, et al. Prognostic significance of serum uric acid in women with gestational hypertension. Hypertension 2011; 58:704–708. [DOI] [PubMed] [Google Scholar]
- 14.Tsukimori K, Yoshitomi T, Morokuma S, et al. Serum uric acid levels correlate with plasma hydrogen peroxide and protein carbonyl levels in preeclampsia. Am J Hypertens 2008; 21:1343–1346. [DOI] [PubMed] [Google Scholar]
- 15.Bainbridge SA, Roberts JM. Uric acid as a pathogenic factor in preeclampsia. Placenta 2008; 29 suppl A:S67–S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuromoto K, Watanabe M, Adachi K, et al. Increases in urinary creatinine and blood pressure during early pregnancy in pre-eclampsia. Ann Clin Biochem 2010; 47:336–342. [DOI] [PubMed] [Google Scholar]
- 17.Seow K-M, Tang M-H, Chuang J, et al. The correlation between renal function and systolic or diastolic blood pressure in severe preeclamptic women. Hypertens Pregnancy 2005; 24:247–257. [DOI] [PubMed] [Google Scholar]
- 18.Coleman A, Freeman P, Steel S, et al. Validation of the Omron MX3 Plus oscillometric blood pressure monitoring device according to the European Society of Hypertension international protocol. Blood Press Monit 2005; 10:165–168. [DOI] [PubMed] [Google Scholar]
- 19.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans: an AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. J Clin Hypertens Greenwich Conn 2005; 7:102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson D, Milford-Ward A, Whicher JT. The value of acute phase protein measurements in clinical practice. Ann Clin Biochem 1992; 29 (Pt 2):123–131. [DOI] [PubMed] [Google Scholar]
- 21.Gordon CC, Chumlea WC, Roche AF. Stature, recumbent length, and weight. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Books; 1988. 3–8. [Google Scholar]
- 22.Singer JD. Applied longitudinal data analysis: modeling change and event occurrence. Oxford: Oxford University Press; 2003. [Google Scholar]
- 23.Twisk JWR, de Vente W. The analysis of randomised controlled trial data with more than one follow-up measurement. A comparison between different approaches. Eur J Epidemiol 2008; 23:655–660. [DOI] [PubMed] [Google Scholar]
- 24.Than NN, Neuberger J. Liver abnormalities in pregnancy. Best Pract Res Clin Gastroenterol 2013; 27:565–575. [DOI] [PubMed] [Google Scholar]
- 25.Bacq Y. Liver diseases unique to pregnancy: a 2010 update. Clin Res Hepatol Gastroenterol 2011; 35:182–193. [DOI] [PubMed] [Google Scholar]
- 26.Mei-Dan E, Wiznitzer A, Sergienko R, et al. Prediction of preeclampsia: liver function tests during the first 20 gestational weeks. J Matern-Fetal Neonatal Med 2013; 26:250–253. [DOI] [PubMed] [Google Scholar]
- 27.Mangal A, Gaur U, Jain A, et al. Alkaline phosphatase and placental alkaline phosphatase activity in serum of normal and pregnancy induced hypertensive mothers. J Int Med Sci Acad 2007; 20:117–120. [Google Scholar]
- 28.Cheung KL, Lafayette RA. Renal physiology of pregnancy. Adv Chronic Kidney Dis 2013; 20:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Son GH, Kwon JY, Lee S, et al. Comparison of serum and urinary nephrin levels between normal pregnancies and severe preeclampsia. Eur J Obstet Gynecol Reprod Biol 2013; 166:139–144. [DOI] [PubMed] [Google Scholar]
- 30.Feig DI. Serum uric acid and the risk of hypertension and chronic kidney disease. Curr Opin Rheumatol 2014; 26:176–185. [DOI] [PubMed] [Google Scholar]
- 31.Martell-Claros N, Blanco-Kelly F, Abad-Cardiel M, et al. Early predictors of gestational hypertension in a low-risk cohort. Results of a pilot study. J Hypertens 2013; 31:2380–2385. [DOI] [PubMed] [Google Scholar]
- 32.Rebelo F, Schlüssel MM, Vaz JS, et al. C-reactive protein and later preeclampsia: systematic review and meta-analysis taking into account the weight status. J Hypertens 2013; 31:16–26. [DOI] [PubMed] [Google Scholar]


