Abstract
The purpose of the report was to evaluate the role of fluorine-18 fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) in staging gastric cancer comparing it with contrast enhancement computed tomography (CECT).
This retrospective study included 45 patients who underwent performed whole body CECT and 18F-FDG PET/CT before any treatment. We calculated CECT and 18F-FDG PET/CT sensitivity, specificity, accuracy, positive and negative predictive values (PPV and NPV) for gastric, lymphnode, and distant localizations; furthermore, we compared the 2 techniques by McNemar test. The role of 18F-FDG PET/CT semiquantitative parameters in relation to histotype, grading, and site of gastric lesions were evaluated by ANOVA test.
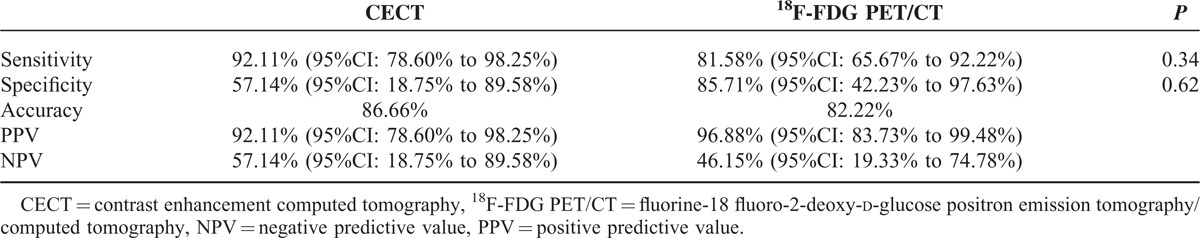
Sensitivity, specificity, accuracy, PPV and NPV of CECT, and 18F-FDG PET/CT for gastric lesion were, respectively, 92.11%, 57.14%, 86.66%, 92.11%, 57.14% and 81.58%, 85.71%, 82.22%, 96.88%, 46.15%. No differences were identified between the 2 techniques about sensitivity and specificity. No statistical differences were observed between PET parameters and histotype, grading, and site of gastric lesion. The results of CECT and 18F-FDG PET/CT about lymphnode involvement were 70.83%, 61.90%, 66.66%, 68%, 65% and 58.33%, 95.24%, 75.55%, 93.33%, 66.67%. The results of CECT and 18F-FDG PET/CT about distant metastases were 80%, 62.86%, 66.66%, 38.10%, 91.67% and 60%, 88.57%, 82.22%, 60%, 88.57%. 18FDG PET/CT specificity was significantly higher both for lymphnode and distant metastases.
The 18F-FDG PET/CT is a useful tool for the evaluation of gastric carcinoma to detect primary lesion, lymphnode, and distant metastases using 1 single image whole-body technique. Integration of CECT with 18F-FDG PET/CT permits a more valid staging in these patients.
INTRODUCTION
Gastric cancer is the 4th most common cancer worldwide.1 Each year, 1 million patients (pts) are diagnosed, accounting for 12% of all cancer deaths.2
Although curative surgery remains the mainstay of gastric cancer treatment, surgical morbidity from gastrectomy is significant.3
Patients’ selection prior to surgery is essential to avoid the morbidity and mortality of unnecessary surgery in those who will not benefit from the intervention but can be submitted to chemotherapy and radiotherapy.4
Diagnosis is mainly performed by endoscopy associated with biopsy. Accurate staging of the disease including the local invasion extent, lymphnode involvement, and distant metastases is important for patients management and surgical planning.3
At the state of art, gastric carcinoma is staged with endoscopic ultrasonography, contrast enhancement computed tomography (CECT), and occasionally laparoscopy.5–7
Conventionally, morphology-based imaging tools are helpful for preoperative staging, but they have been found incomplete due to their technical limitations.8,9
In recent decades fluorine-18 fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography (18F-FDG PET/CT) has proven to be useful in the diagnosis and evaluation of malignancies by providing metabolic information and apporting advantages in staging, therapeutic evaluation, and recurrence surveillance.10,11 Several clinical guidelines, including those of the National Comprehensive Cancer Network and The European Society for Medical Oncology, suggest that 18F-FDG PET/CT imaging may improve staging.12,13 Although these recommendations, its value in staging gastric carcinomas is still controversial.
The purpose of this study was to evaluate the role of 18F-FDG PET/CT in staging gastric carcinoma comparing it with CECT. Furthermore, we investigated if morphological and functional parameters might play a role in staging gastric carcinomas.
METHODS
Patients
Five hundred and seventy-two 18F-FDG PET/CT were performed from March 2007 to March 2013 for gastric carcinoma evaluation. Fifty-seven of them were performed for staging, 301 for restaging and 214 in follow-up.
The 57 18F-FDG PET/CT performed with staging intent corresponded to 57 pts; all of them had been pathologically diagnosed with gastric carcinoma by endoscopic biopsy.
This retrospective study included only 45 pts who underwent whole-body CECT and 18F-FDG PET/CT before any treatment and within 30 days between them.
The CECT scan was performed on average 18 days before the 18FDG-PET/CT scan (range 2–30 days).
Pathological staging was evaluated according to the 7th edition of the American Joint Committee on Cancer Staging guidelines, and histologic types were classified according to the World Health Organization classifications.14
18F-FDG PET/CT and CECT results were compared with the gold standard, established as histological examination in 29 pts who were submitted to surgery, and as very close clinical-instrumental follow-up (specific and/or not specific symptoms, US, CECT, and Magnetic Resonance) in remnant 16 pts.
All patients had already given their consent for the use of their data for clinical research.
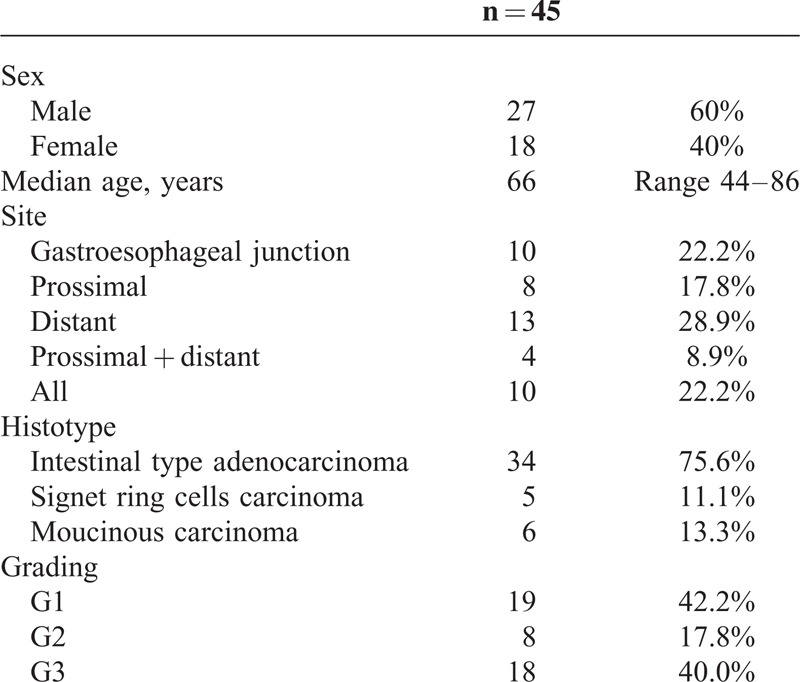
Patients’ clinical and pathological characteristics are described in Table 1.
TABLE 1.
Patients’ Clinical and Pathological Characteristics
CECT Technique
CT examinations were performed with equipment MDCT with 16 layers (TSX-101°, Aquilion 16, Toshiba Medical Systems, Tokyo, Japan), using the following acquisition parameters: slice thickness 1 mm, pitch 1.75; increment 0.6 mm, rotation time 0.5 seconds, kV/mAs 120/250. All examinations included contrast enhancer administration. Water was orally administered immediately prior to CT scanning to obtain gastric distension.
18F-FDG PET/CT Technique
Images were acquired with a Discovery LSA PET/CT device (GE Healthcare, Waukesha, WI) that integrates a PET (advance n × I) with 16-slice CT scanner (light speed plus). All patients, before 18F-FDG administrations fasted for at least 8 hours and had a capillary blood glucose of <160 mg/mL. The image acquisition was obtained 50 minutes after the intravenous injection of 4.6 MBq/kg of 18F-FDG.
Patients were hydrated by drinking 500 mL of water and urinated as needed. The CT scan was carried out from the external acoustic meatus to the root of the thigh with patients lying on their back with hands above their head. The CT acquisition parameters were 340 mA (auto), 120 kV, slice thickness 3.75 mm, tube rotation time 0.8 milliseconds, and collimation field of view of 50 cm. The CT images were reconstructed with a filtered back projection. The CT data were used for the attenuation correction of PET scanning, which was performed immediately after the acquisition of CT images. The CT scans were performed without administration of contrast enhancer. The PET acquisition was obtained in caudal-cranial direction; PET was reconstructed with a matrix of 128 × 128, ordered subset expectation maximum iterative reconstruction algorithm (2 iterations, 28 subsets), 8 mm Gaussian filter, and 50 cm field of view.
CECT and 18F-FDG PET/CT Interpretations
CECT and 18F-FDG PET/CT blindly and independently respectively by a radiologist and a nuclear physician with at least 8 years of experience were evaluated. Both were unaware of the patients medical history.
CECT was considered positive for gastric malignancy in case of description of a polipoid mass with or without ulceration or of a focal thickening of the wall with irregular mucosal6,15; positive for lymphnode involvement if there was at least one lymphnode enlargement in the abdomen; positive for distant metastases if there was at least one lesion in sites different from stomach and lymphnodes.
18F-FDG PET/CT was considered positive for gastric malignancy in case of any increased 18F-FDG uptake exceeding that of the adjacent normal gastric wall; positive for lymphnode involvement for any increased 18F-FDG uptake in at least 1 lymphnode; positive for distant metastases for at least 1 area of increased 18F-FDG uptake in sites different from stomach and lymphnodes. Gastric distension obtained by drinking 500 mL of water before images acquisition was used to reduce false positive.
Volume of interest (VOI) was drawn semiautomatically on the high 18F-FDG uptake area, with boundaries drawn large enough to incorporate each target lesion in the 3 axes of PET images.
Semiquantitative analysis was performed calculating max and mean standardized uptake values (SUVmax and SUVmean), using the maximum and mean activity values within each VOI with the highest radioactivity concentration, normalized to the injected dose, and patient's body weight.
In order to collect the metabolic tumor volume (MTV) and total lesion glycolysis (TLG) a fixed threshold value of 40% of the SUVmax uptake was used to determine tumor margins automatically, according to the previously published method of Larson et al16 and Lee.17
SUVmax and SUVmean were collected in all 45 pts, while MTV and TLG were evaluated in the 32/45 pts in whom 18FDG PET/CT resulted positive for the gastric lesion.
Statistical Analysis
We calculated CECT and 18F-FDG PET/CT sensitivity, specificity, accuracy, positive predictive value (PPV) and negative predictive value (NPV) for gastric, lymphnode, and distant localizations; furthermore, we compared the performance of the 2 techniques by McNemar test.
The role of 18F-FDG PET/CT semiquantitative parameters in relation to histotype, grading, and site of gastric lesions were evaluated by ANOVA test.
Linear regression was performed to evaluate SUVmax and SUVmean in relation to lesion size.
A P value < 0.05 was considered statistically significant. The analyses were performed using MedCalc software version 14.12.0 (MedCalc Software bvba, Ostend, Belgium).
RESULTS
Gastric Lesions
CECT resulted positive for gastric localizations in 38/45 pts (84.4%) and negative in 7/45 pts (15.6%). 18F-FDG PET/CT resulted positive for gastric localization in 32/45 pts (71.1%) and negative in 13/45 pts (28.9%). An example of patient with 18F-FDG PET/CT positive for gastric localization is showed in Figure 1.
FIGURE 1.
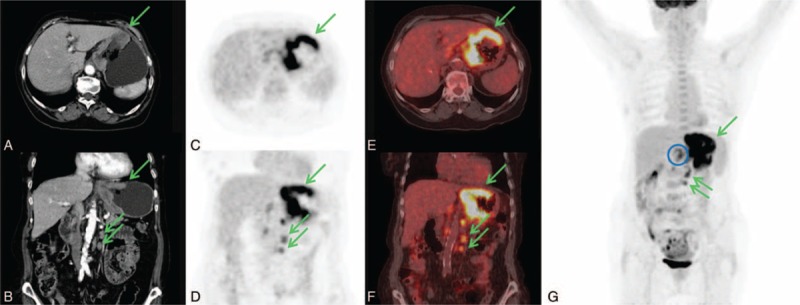
An 83-years-old woman with diagnosis of signet ring cell carcinoma obtained by cardias biopsy. CECT axial and coronal images (A, D) showed regular and diffuse thickening larger than 10 cm in the upper part of the stomach and in left paraortic lymphnodes (green arrows). 18F-FDG PET/CT axial and coronal PET and fused images (B, C, E, F) showed the gastric lesion (SUVmax 13.3) and the left paraortic lymphnodes (SUVmax 7.2) (green arrows). Furthermore, 18F-FDG PET/CT detected celiac lymphnodes involvement (SUVmax 5.1) as is better showed in MIP image (blue circle). CECT = contrast enhancement computed tomography, 18F-FDG PET/CT = fluorine-18 fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography, SUV = standardized uptake value.
Results concerning sensitivity, specificity, accuracy, PPV and NPV, and McNemar analysis are reported in Table 2.
TABLE 2.
CECT and 18F-FDG PET/CT Gastric Lesions Diagnostic Performance
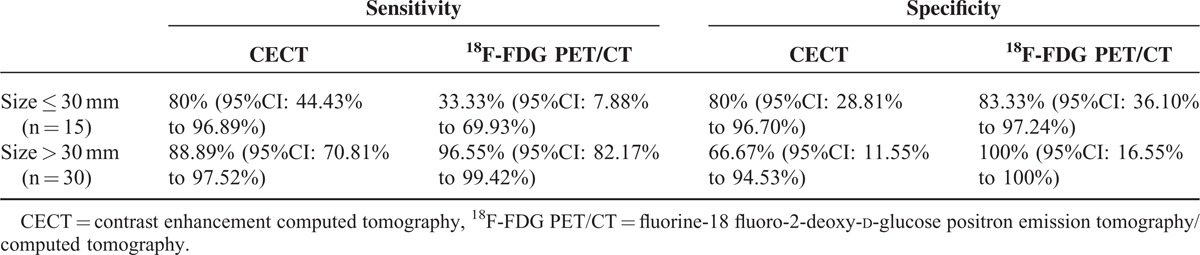
The mean value of the maximum diameter was 52.59 mm (range 4–≥100). Considering 30 mm, as a threshold value for maximum diameter we divided patients in 2 groups: size ≤30 mm and >30 mm.18 Results about CECT and 18F-FDG PET/CT sensitivity and specificity in the 2 groups are reported in Table 3.
TABLE 3.
CECT and 18F-FDG PET/CT Results in the Groups of Patients Divided Considering 30 mm as Size Threshold
SUVmax and SUVmean resulted positively related to lesion dimension (SUVmax = 3.53 + 0.11 × lesion size, F = 8.91, P = 0.005; SUVmean = 1.99 + 0.06 × lesion size, F = 7.07, P = 0.01)
The results about PET semiquantitative parameters are reported in Table 4. No statistical differences were observed between PET parameters and histotype, grading, and site of gastric lesion.
TABLE 4.
Semiquantitative Parameters Results
Lymphnodes Involvement
CECT resulted positive for abdominal lymphnode involvement in 25/45 pts (55.6%) and negative in 20/45 pts (44.4%).
18FDG PET/CT resulted positive for abdominal lymphnode involvement in 15/45 pts (33.3%) and negative in 30/45 pts (66.7%).
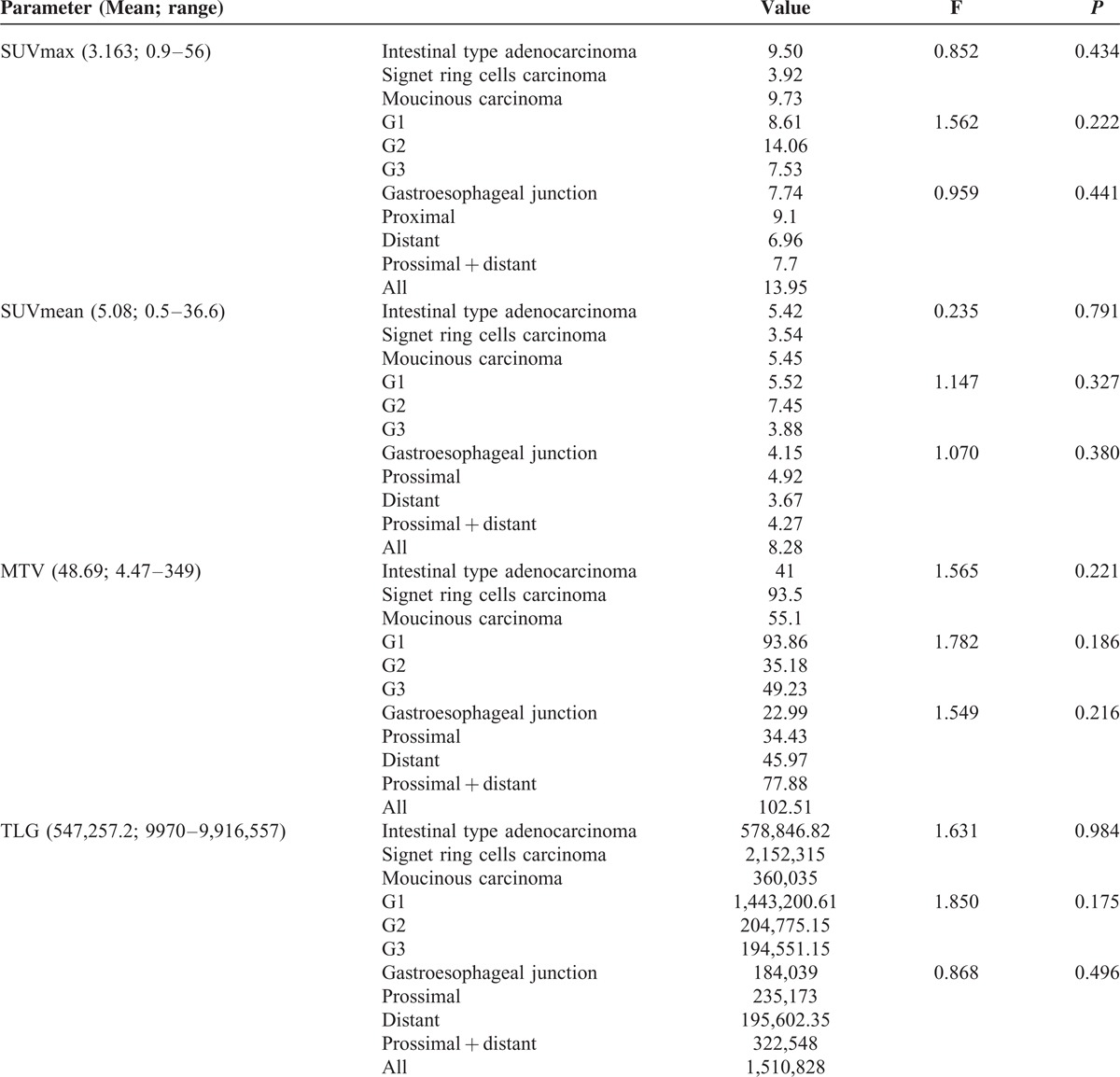
Results concerning sensitivity, specificity, accuracy, PPV and NPV, and McNemar analysis are reported in Table 5; it was markable that 18FDG PET/CT specificity was significantly higher than CECT.
TABLE 5.
CECT and 18F-FDG PET/CT Lymphnode Involvement Diagnostic Performance
Distant Metastases
CECT resulted positive for distant metastases in 20/45 pts (44.4%) and negative in 25/45 pts (55.6%). 18FDG PET/CT resulted positive for distant metastases in 10/45 pts (22.2%) and negative in 35/45 pts (77.8%). An example of a patient in whom distant metastases observed at CECT resulted negative at 18F-FDG PET/CT is reported in Figure 2.
FIGURE 2.
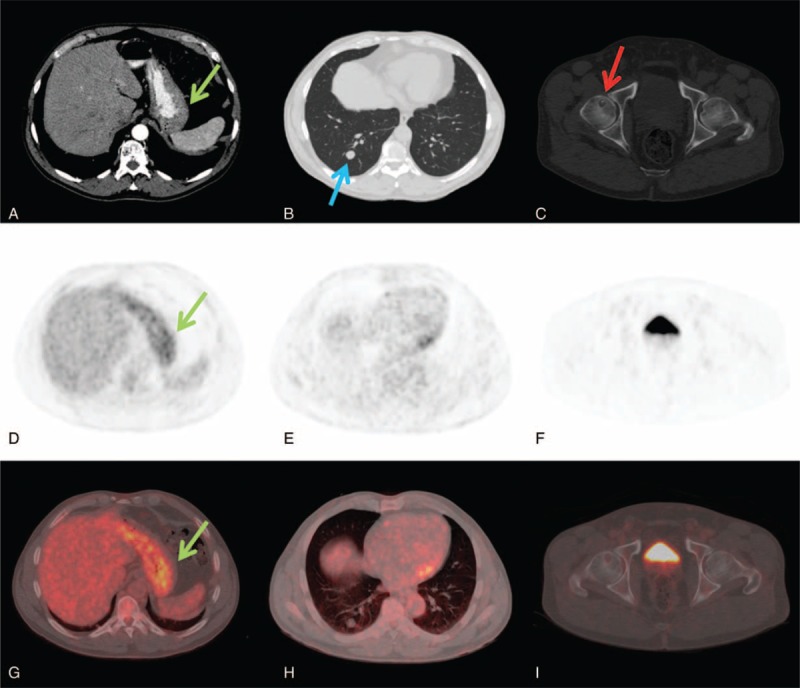
A 54-years-old man with intestinal type gastric carcinoma. CECT axial images showed localized gastric wall thickening (A, green arrow), a right lung nodule of 15 mm suspected for metastases (B, blue arrow) and a osteolytic lesion in the right femoral head doubtful for herniation pit (C, red arrow). 18F-FDG PET/CT axial PET and fused images confirmed the gastric lesion with SUVmax of 4.8 (D, G) but did not showed any 18F-FDG uptake in the lung nodule (E, H) and in the right femoral head (F, I). CECT = contrast enhancement computed tomography, 18F-FDG PET/CT = fluorine-18 fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography, SUV = standardized uptake value.
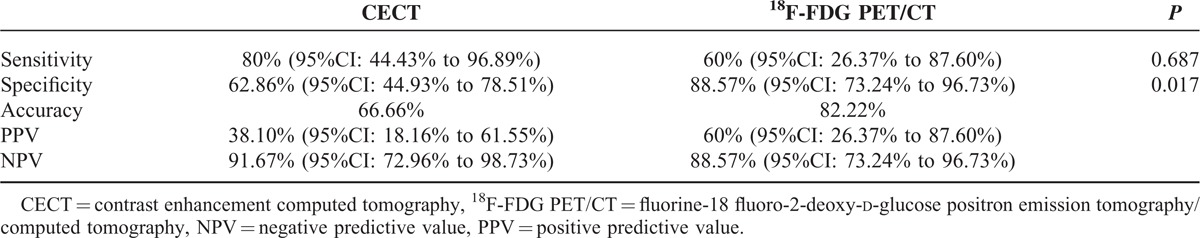
Results concerning sensitivity, specificity, accuracy, PPV and NPV, and McNemar analysis are reported in Table 6; it was markable that 18FDG PET/CT specificity was significantly higher than CECT.
TABLE 6.
CECT and 18F-FDG PET/CT Distant Metastases Diagnostic Performance
DISCUSSION
Gastric Lesions
Literature reports a good performance of CECT in gastric lesions evaluation, with diagnostic accuracy that varies from 77% to 89%19; also in our population the performance of CECT was as much valid (accuracy of 86.6%).
Until now, it has been reported that 18F-FDG PET/CT has 21% to 100% of sensitivity and 78% to 100% of specificity for detecting gastric tumors.20–23 Our results of sensitivity (81.58%) and specificity (85.71%) are among the best ones and showed a good performance of both techniques, although 18FDG PET/CT specificity was slightly higher than CECT (57.14%) the difference was not statistically significant.
It is necessary to consider the technical and histopathological factors affecting the visibility of primary tumors on 18F-FDG PET/CT. 18F-FDG is not a tumor-specific tracer so many benign lesions in the stomach, such as gastritis, leiomyoma, and polyps can have moderate to intense 18F-FDG uptakes and obscure the primary tumor.3 Moreover, empty stomach states further reduces the specificity of the technique as low as 50% due to the high incidence of normal gastric wall uptakes.24 To decrease the physiological uptake, many methods have been experimented, including the ingestion of food, but it has been demonstrated that simple distention of the stomach using water improves the diagnostic performance of 18F-FDG PET/CT in detecting and localizing primary tumors25,26; in our experience drinking 500 mL of water before images acquisition was useful for a sufficient gastric distension.
The principal factors that can influence gastric lesions’ detectability are tumor size, histological type, and localization.3
Tumor size is the major factor; Mukai et al showed that 18F-FDG PET/CT had a sensitivity of 76.7% for the detection of gastric carcinomas >30 mm but only 16.8% for those less than 30 mm.18 Because late-stage tumors are usually larger in size with deeper invasion, advanced gastric carcinomas tend to yield a higher sensitivity in 18F-FDG PET/CT imaging (from 93% to 98%) than early gastric carcinomas (from 26% to 63%).27 According to literature, we choosed 30 mm as a threshold and observed a markable reduction in sensitivity and negligible in specificity respect to overall patients results if size ≤30 mm (33.33% vs 81.58%; 83.33 vs 85.71%), while there were negligible increase both in sensitivity and specificity if size >30 mm (96.55% vs 81.58%; 100% vs 85.71%). Obviously 18F-FDG PET/CT had a very good performance in larger lesion, but also in small lesions its specificity was high.
Most studies reported that 18F-FDG PET/CT has significantly lower sensitivities in detecting mucinous and signet ring cells carcinomas than the intestinal-type adenocarcinoma.4,18,20,28
The lower 18F-FDG uptake in these hystotypes may depend by the low-density diffuse infiltration of adenocarcinoma cells, the existence of extracellular or intracellular metabolically inert mucus content and the low expression level of glucose transporter 1.20,28
We did not perform a comparison among histotypes because our population included 75.5% of intestinal type carcinomas. However in our population only 1/6 mucinous carcinoma and 1/5 signet ring cells carcinoma (also with 5 mm diameter) resulted false negative at 18F-FDG PET/CT.
Regarding the differentiation, lower 18F-FDG uptake may be observed in poorly differentiated histotypes, which is likely due to the low concentration of cancer cells in primary lesions.29 In our population only 2/18 G3 pts resulted false negative at 18F-FDG PET/CT and one of them was the 5 mm signet ring cells carcinoma.
About the tumor's localization, 18F-FDG PET/CT detection of gastroesophageal junction carcinomas was reported to be more sensitive than other stomach parts, probably due to the higher incidence of intestinal types in this site, but some researchers reported that 18F-FDG PET/CT had a similar detectability for gastric carcinomas independently by site.20,30
In our population only 1/13 pts with gastric lesion localized in the distant part of the stomach resulted false negative; anyway we did not perform a comparison among localizations because of the variability of size and histotypes.
PET semiquantitative parameters have been investigated for their role in 18F-FDG PET/CT interpretations. The commonly accepted parameters are SUVmax and SUVmean, while MTV and TLG are not accepted for clinical use. Some authors refer these results are affected by many factors including those influencing gastric lesion detection; others did not find any differences in their values respect to histological type and localization.4,11,31,32
In our study we analyzed SUVmax, SUVmean, MTV, and TLG of gastric lesions, and we found a positive relation between SUVmax and lesion size, and SUVmean and lesion size; otherwise, no differences in none of the PET parameter were identified among the influencing factors histotype, grading, and localization (Table 3).
Lymphnode Involvement
The presence of lymphnode metastases is one of the most important prognostic factors in gastric carcinoma, and a correct staging is fundamental to define the necessity and the extension of the lymphodenectomy.26
To date, CECT effectiveness in predicting lymphnode metastases has not been satisfactory using any criteria, and there is still no worldwide consensus. Although there is a clear correlation between the lymphnode size and metastases, microscopic metastases in normal-size lymphnodes and lymphnode enlargement resulting from reactive or inflammatory changes are common in gastric cancer patients.33
The overall accuracy, sensitivity, and specificity of lymphnode staging by CECT reported in literature varies from 69% to 92%, 78% to 92%, and 73.9%, respectively, and they are strictly dependent on the size of lymphnodes.19
One meta-analysis reported that the sensitivity and specificity of 18F-FDG PET/CT in staging lymphnode involvement ranged between 85.7% and 97.0%. Other individual studies reported that 18F-FDG PET/CT was less sensitive but more specific compared with commonly used CECT.22,34 The reasons for the low sensitivity of 18F-FDG PET/CT are the histological type of the primary tumor and the size of metastatic lymphnodes that could be smaller than 3 mm.22,35 In spite of the low sensitivity, 18F-FDG PET/CT usually showed a higher specificity than most other imaging modalities, including CECT because 18F-FDG PET/CT diagnose lymphnode metastases using glucose metabolism rather than the size change.3 A limit reported for 18F-FDG PET/CT is the detection of perigastric lymphnodes involvement difficult to discriminate because of the radioactive volume effect generated by the nearby primary tumor.36
Our population's results were lower than literature for CECT such as 18F-FDG PET/CT sensitivity, but they confirmed the higher specificity of 18F-FDG PET/CT than CECT (95.24% vs 61.90%, P = 0.039) probably due to the lymphnodes small size and the primitive histological subtype. Localization in perigastric lymphnodes cannot be considered as an influencing factor in our population because they were detected only in 6 pts, 5 of them correctly interpreted.
Distant Metastases
The most frequent sites of distant metastases include liver, lungs, bones, adrenal glands, and peritoneum.
CECT is actually the conventional tool for detecting distant metastases also from gastric cancer, but it may present limits especially for areas such as peritoneum.3
Even if Pan et al37 reported more than 96.6% accuracy of CECT in distant metastases detection in 350 pts with gastric cancer, a following meta-analysis by Wang and Chen38 reported a large difference in CECT sensitivities between hepatic metastases and peritoneal carcinomatosis (74% vs 33%).
18F-FDG PET/CT showed a good performance in detecting solid organ metastases with a sensitivity of 95.2% and a specificity of 100%, even if it resulted less effective in the detection of bone metastases.29,39
Some studies reported 18F-FDG PET/CT lower sensitivity compared with CECT for the diagnosis of peritoneal seeding, nevertheless 18F-FDG PET/CT could still be useful for detecting peritoneal metastases, especially when the CECT results are equivocal, avoiding unnecessary laparotomy in a considerable portion of patients. Even Lim et al showed its better specificity than CECT (99% vs 92%).4,36,40,41
Results of our population were quite different from literature; we found lower values for CECT accuracy (66.6%), higher for CECT sensitivity (80%), and lower for 18F-FDG PET/CT sensitivity (60%). The difference between 18F-FDG PET/CT and CECT specificities resulted statistically significant (88.57 vs 62.86%, P = 0.017) for distant metastases.
The explanation of these differences is the metastases sites observed in our population in only 10/45 pts: liver was involved in 6 pts, lungs in 2 pts, bones in 3 pts, and adrenal gland and peritoneum in 1 pts, respectively.
The low specificity of CECT can be explained mostly with the high rate of lung nodules misinterpreted (7/45 pts).
There were some limitations in our study such as the retrospective nature that implies selection bias and the small number of the sample, even if in line with literature.
Furthermore, histological confirmation of lymphnode and distant metastases should be obtained if feasible but biopsy of each lesion is not ethically recommended, so clinical-instrumental follow-up has also been considered the gold standard.
CONCLUSION
18F-FDG PET/CT is a useful tool for the evaluation of gastric carcinoma; it can detect primary lesion, lymphnode, and distant metastases using 1 single image whole-body technique.
Our result of higher specificity of 18F-FDG PET/CT for lymphnode and distant metastases detection may suggest the important role of this technique in changing the extent of lymphadenectomy or reducing futile laparotomies.
We believe that integration of conventional imaging such as CECT with 18F-FDG PET/CT permits a more valid whole-body staging in patients with gastric carcinomas.
Footnotes
Abbreviations: CECT = contrast enhancement computed tomography, 18F-FDG PET/CT = fluorine-18 fluoro-2-deoxy-d-glucose positron emission tomography/computed tomography, MTV = metabolic tumor volume, NPV = negative predictive value, PPV = positive predictive value, Pts = patients, SUV = standardized uptake value, TLG = total lesion glycolysis, VOI = volume of interest.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011; 61:69–90. [DOI] [PubMed] [Google Scholar]
- 2.Krejs GJ. Gastric cancer: epidemiology and risk factors. Dig Dis 2010; 28:600–603. [DOI] [PubMed] [Google Scholar]
- 3.Wu CX, Zhu ZH. Diagnosis and evaluation of gastric cancer by positron emission tomography. World J Gastroenterol 2014; 20:4574–4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smyth E, Schoder H, Strong VE, et al. A prospective evaluation of the utility of 2-deoxy-2-[18F]fluoro-d-glucose positron emission tomography and computed tomography in staging locally advanced gastric cancer. Cancer 2012; 15:5481–5488. [DOI] [PubMed] [Google Scholar]
- 5.Coupe NA, Karikios D, Chong S, et al. Metabolic information on staging FDG-PET-CT as a prognostic tool in evaluation of 97 patients with gastric cancer. Ann Nucl Med 2014; 28:128–135. [DOI] [PubMed] [Google Scholar]
- 6.Moschetta M, Stabile Ianora AA, Anglani A, et al. Preoperative T staging of gastric carcinoma obtained by MDCT vessel probe reconstructions and correlations with histological findings. Eur Radiol 2010; 20:138–145. [DOI] [PubMed] [Google Scholar]
- 7.Moschetta M, Scardapane A, Telegrafo M, et al. Differential diagnosis between benign and malignant ulcers: 320-row CT virtual gastroscopy. Abdom Imaging 2012; 37:1066–1073. [DOI] [PubMed] [Google Scholar]
- 8.Kiff RS, Taylor BA. Comparison of computed tomography, endosonography, and intraoperative assessment in TN staging of gastric carcinoma. Gut 1994; 35:287–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lorusso F, Fonio P, Scardapane A. Gatrointestinal imaging with multidetector CT and MRI. Recenti Prog Med 2012; 103:493–499. [DOI] [PubMed] [Google Scholar]
- 10.Niccoli-Asabella A, Altini C, Notaristefano A, et al. A retrospective study comparing contrast-enhanced computed tomography with 18F-FDG-PET/CT in the early follow-up of patients with retroperitoneal sarcomas. Nucl Med Comm 2013; 34:32–39. [DOI] [PubMed] [Google Scholar]
- 11.Altini C, Niccoli Asabella A, De Luca R, et al. Comparison of 18F-FDG PET/CT methods of analysis for predicting response to neoadjuvant chemoradiation therapy in patients with locally advanced low rectal cancer. Abdom Imaging 2014; (in press). [DOI] [PubMed] [Google Scholar]
- 12.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Gastric Cancer. 2013. Available from: URL: http://www.nccn.org/professionals/physician_gls/pdf/. DOI: 10.1007/s00261-014-0277-8. [Google Scholar]
- 13.Waddell T, Verheij M, Allum W, et al. Gastric cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24:vi57–vi63. [DOI] [PubMed] [Google Scholar]
- 14.Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th edNew York: Wiley-Blackwell; 2009. [Google Scholar]
- 15.Gore RM, Levine MS. Trattato di Radiologia Gastrointestinale. Roma: VerduciEditore; 2002. [Google Scholar]
- 16.Larson SM, Erdi Y, Akhurst T, et al. Tumor treatment response based on visual and quantitative changes in global tumor glycolysis using PET-FDG imaging. The visual response score and the change in total lesion glycolysis. Clin Positron Imaging 1999; 2:159–171. [DOI] [PubMed] [Google Scholar]
- 17.Lee JA. Segmentation of positron emission tomography images: some recommendations for target delineation in radiation oncology. Radiother Oncol 2010; 96:302–307. [DOI] [PubMed] [Google Scholar]
- 18.Mukai K, Ishida Y, Okajima K, et al. Usefulness of preoperative FDG-PET for detection of gastric cancer. Gastric Cancer 2006; 9:192–196. [DOI] [PubMed] [Google Scholar]
- 19.Hallinan JT, Venkatesh SK. Gastric carcinoma: imaging diagnosis, staging and assessment of treatment response. Cancer Imaging 2013; 13:212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stahl A, Ott K, Weber WA, et al. FDG PET imaging of locally advanced gastric carcinomas: correlation with endoscopic and histopathological findings. Eur J Nucl Med Mol Imaging 2003; 30:288–295. [DOI] [PubMed] [Google Scholar]
- 21.Yun M, Lim JS, Noh SH, et al. Lymph node staging of gastric cancer using (18)F-FDG PET: a comparison study with CT. J Nucl Med 2005; 46:1582–1588. [PubMed] [Google Scholar]
- 22.Kim SK, Kang KW, Lee JS, et al. Assessment of lymph node metastases using 18F-FDG PET in patients with advanced gastric cancer. Eur J Nucl Med Mol Imaging 2006; 33:148–155. [DOI] [PubMed] [Google Scholar]
- 23.Kim EY, Lee WJ, Choi D, et al. The value of PET/CT for preoperative staging of advanced gastric cancer: comparison with contrast-enhanced CT. Eur J Radiol 2011; 79:183–188. [DOI] [PubMed] [Google Scholar]
- 24.Kamimura K, Nagamachi S, Wakamatsu H, et al. Role of gastric distention with additional water in differentiating locally advanced gastric carcinomas from physiological uptake in the stomach on 18F-fluoro-2-deoxy-D-glucose PET. Nucl Med Commun 2009; 30:431–439. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Z, Li F, Zhuang H. Gastric distension by ingesting food is useful in the evaluation of primary gastric cancer by FDG PET. Clin Nucl Med 2007; 32:106–109. [DOI] [PubMed] [Google Scholar]
- 26.Yun M. Imaging of gastric cancer metabolism using 18 F-FDG PET/CT. J Gastric Cancer 2014; 14:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dassen AE, Lips DJ, Hoekstra CJ, et al. FDGPET has no definite role in preoperative imaging in gastric cancer. Eur J Surg Oncol 2009; 35:449–455. [DOI] [PubMed] [Google Scholar]
- 28.Ott K, Fink U, Becker K, et al. Prediction of response to preoperative chemotherapy in gastric carcinoma by metabolic imaging: results of a prospective trial. J Clin Oncol 2003; 21:4604–4610. [DOI] [PubMed] [Google Scholar]
- 29.Yoshioka T, Yamaguchi K, Kubota K, et al. Evaluation of 18F-FDG PET in patients with advanced, metastatic, or recurrent gastric cancer. J Nucl Med 2003; 44:690–699. [PubMed] [Google Scholar]
- 30.Wu AJ, Goodman KA. Positron emission tomography imaging for gastroesophageal junction tumors. Semin Radiat Oncol 2013; 23:10–15. [DOI] [PubMed] [Google Scholar]
- 31.Namikawa T, Okabayshi T, Nogami M, et al. Assessment of 18F-florodeoxyglucose positron emission tomography combined with computed tomography in the preoperative management of patients with gastric cancer. Int J Clin Oncol 2013; 19:649–655. [DOI] [PubMed] [Google Scholar]
- 32.Kim J, Lim ST, Na CJ, et al. Pretreatment F-18 FDG PET/CT parameters to evaluate progression-free survival in gastric cancer. Nucl Med Mol Imaging 2014; 48:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi J, Joo I, Lee JM. State-of-the-art preoperative staging of gastric cancer by MDCT and magnetic resonance imaging. World J Gastroenterol 2014; 20:4546–4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ha TK, Choi YY, Song SY, et al. F18-fluorodeoxyglucose-positron emission tomography and computed tomography is not accurate in preoperative staging of gastric cancer. J Korean Surg Soc 2011; 81:104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mönig SP, Zirbes TK, Schröder W, et al. Staging of gastric cancer: correlation of lymph node size and metastatic infiltration. AJR Am J Roentgenol 1999; 173:365–367. [DOI] [PubMed] [Google Scholar]
- 36.Chen J, Cheong JH, Yun MJ, et al. Improvement in preoperative staging of gastric adenocarcinoma with positron emission tomography. Cancer 2005; 103:2383–2390. [DOI] [PubMed] [Google Scholar]
- 37.Pan Z, Zhang H, Yan C, et al. Determining gastric cancer respectability by dynamic MDCT. Eur Radiol 2010; 20:613–620. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Chen JQ. Imaging in assessing hepatic and peritoneal metastases of gastric cancer: a systematic review. BMC Gastroenterol 2011; 11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung HW, Lee EJ, Cho YH, et al. High FDG uptake in PET/CT predicts worse prognosis in patients with metastatic gastric adenocarcinoma. J Cancer Res Clin Oncol 2010; 136:1929–1935. [DOI] [PubMed] [Google Scholar]
- 40.Rubini G, Altini C, Notaristefano A, et al. Peritoneal carcinomatosis from ovarian cancer: role of 18F-FDG-PET/CT and CA125. Recenti Prog Med 2012; 103:510–514. [DOI] [PubMed] [Google Scholar]
- 41.Lim JS, Kim MJ, Yun MJ, et al. Comparison of CT and 18F-FDG pet for detecting peritoneal metastases on the preoperative evaluation for gastric carcinoma. Korean J Radiol 2006; 7:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]


