Abstract
The incidence of the EGJA is rapidly increasing. The clinicopathological features have not yet been elucidated. The aim of this study was to analyze the differences in clinicopathological features and prognosis between patients with esophagogastric junctional adenocarcinoma (EGJA) and distal gastric adenocarcinoma (DGA).
In this retrospective study, 1230 patients who underwent gastrectomy between January 2006 and December 2010 in West China Hospital were enrolled. Patients were divided into 2 groups based on tumor location. Clinicopathological characteristics, postoperative complications, and survival outcomes were compared. Univariate and multivariate analysis were also used to evaluate the prognostic factors of DGA and EGJA.
Patients with gastric adenocarcinoma were divided into 2 study groups according to tumor location: 321 EGJA (26.1%) and 909 DGA (73.9%). Tumors with larger diameter, more advanced pT and pN stage were more common in EGJA. Significant differences were revealed in 3-year overall survival rate (3-YS) between 2 groups: EGJA (57.5%) and DGA (65.5%) (P = 0.001), and further analysis indicate that there was also significant difference on 3-YS between EGJA (76.9%) and DGA (84.2%) (P = 0.012) in stage II. From our multivariate analysis, we found that there were different independent prognostic indicators for DGA and EGJA.
The clinicopathological features of EGJA were strikingly different from DGA and patients with EGJA showed a worse prognosis when compared with DGA. The pT stage, pN stage, pM stage, tumor size, age, and radical degree were determined to be independent factors of prognosis for DGA, while only combined organ resection, pN stage, and pM stage were independent prognostic factors for EGJA.
INTRODUCTION
The global incidence of gastric cancer has decreased steadily, primarily due to a reduction in distal cancers.1 However, gastric cancer remains the fourth most common malignancy worldwide and there is higher incidence of the disease in Eastern Asian countries such as China, Korea, and Japan.1 By contrast, adenocarcinoma of esophagogastric junction or lower esophagus is one of the most rapidly increasing malignant diseases in West and seems to have different etiology from distal gastric cancer.2,3 The similar trend of EGJA was also reported by Japan, China, and Korea in recent years.4–6 EGJA is a tumor that has clinicopathological characteristics of both esophageal and gastric malignancies since the tumors occurring at the mucosa between the lower esophagus and upper third of stomach.7,8 Surgical resection with standard lymphadenectomy is a mainstay surgical treatment for patients with EGJA. An abdominal–transhiatal approach was also recommended by Sasako et al for Siewert type II and type III EGJA tumors in Japan.9 Most EGJAs are diagnosed with advanced stages, and the prognosis is worse than that of distal gastric adenocarcinoma (DGA).10 However, some researchers also found that the prognosis of patients with EGJA was no worse than that of patients with DGA in each equal TNM stage.11,12 It remains unclear whether the prognosis is due to different biologic characteristics or relative lower rate of detection. Some aspects of surgical therapy for EGJA, such as extent of resection and lymphadenectomy, remain controversial.10,11–15 However, with the introduction of the Siewert classification, which has a direct impact on the surgical treatment of these tumors, those discrepancies can be gradually diminished.16 The clinicopathological features of EGJA have not yet been elucidated. We are aware that only a few reports have emphasized on clinicopathological features and prognosis of EGJA in China.17,18 A standardize definition and knowledge of EGJA will facilitate future scientific research and academic exchanges.
Our previous study found that the proportion of EGJA among surgical patients was significantly increased in China from 1988 to 2012.19 Time trend of EGJA was antipodal to the DGA in China. On the basis of the previous retrospective gastric cancer registry in West China Hospital and the additional follow-up outcomes, we intended to analyze whether EGJA and DGA were also different in clinicopathological characteristics and prognosis. The aim of this study was to analyze differences in clinicopathological features, surgical treatment, and the prognosis between EGJA and DGA patients who underwent gastrectomy between January 2006 and December 2010 in West China Hospital.
METHODS
Patients
In this retrospective study, patients who underwent gastrectomy for gastric adenocarcinoma in Department of Gastrointestinal Surgery of West China Hospital, Sichuan University, China, from January, 2006 to December, 2010 were enrolled. In the present study, 1460 patients were admitted for the treatment of gastric cancer and 1350 patients (92.5%) underwent surgical resection. The exclusion criteria included the following: remnant gastric cancer (n = 20), synchronous gastric multicenter adenocarcinoma (n = 15), other malignancy of stomach (n = 21), gastric cancer involving the entire stomach (N = 56), and patients who had received neochemotherapy (n = 8). After exclusion, 1230 patients were remained and analyzed in our study. The location of the tumor was defined according to Japanese classification of gastric carcinoma: third English edition.20 Type of EGJA was according to Siewert classification.16 To avoid misclassification, each resected specimen was precisely measured and the distance between tumor center and esophagogastric junction was also recorded for EGJA in our hospital. Due to the low proportion of Siewert type I tumors and this subtype tumors were underwent transthoracic approach gastrectomy in the Department of Thoracic Surgery, type I tumors were not included in our study.21,22 We only analyzed Siewert type II and III tumors who underwent transabdominal surgical resection in the Department of Gastrointestinal Surgery in West China Hospital.
Surgical Treatment
All the patients in this study were underwent transabdominal total or subtotal gastrectomy according to the principles of Japanese Classification of Gastric Cancer.19 Transabdominal-hiatal total or proximal gastrectomy plus lymphadenectomy were performed in patients with EGJA, while distal gastrectomy was mainly performed in patients with DAG. D2/D2+ lymphadenectomy were routinely performed while D1/D1+ lymphadenectomy were selectively used in patients with early gastric adenocarcinoma. Intraoperative frozen section was a routinely procedure aiming to secure the resection margins without tumor cells. When patients with positive frozen resection margins intraoperatively, supplementary resection was performed when the remnant stomach was still resectable under the premise that without affecting anastomosis. For reconstruction, Billroth I and Billroth II were adopted for distal gastrectomy, Roux-en-Y anastomosis was accepted for total gastrectomy and some distal gastrectomy, while esophagus-gastric anastomosis (EGA) was accepted for proximal gastrectomy. For some tumors in advanced stage, combined organ resection would be performed to achieve a curative resection. For example, splenectomy was selectively performed in cases when advanced cancer located at posterior wall or greater curvature of stomach which invaded spleen and that had metastasis to lymph node at the splenic hilum or along the splenic artery.
Clinicopathological Data
Demographic variables (sex, age), surgical related parameters (radical degree, number of harvested lymph nodes, operation time), and survival outcomes and independent prognostic factors were compared. We also evaluated macroscopic type, histological differentiation grade, and pTNM stage. The classification of macroscopic type, histological differentiation grade, and postoperative TNM stages were based on Japanese classification of gastric carcinoma: 3rd English edition.20 The perioperative outcomes such as postoperative hospital stay (days) and postoperative complications and mortality were also analyzed between 2 groups.
Follow-Up
Overall survival was calculated from the time of surgery until death or the last follow-up contact. Follow-up assessments were performed every 3–6 months for the first 2 years, every 6–12 months for 3–5 years after surgery and then annually.23 The postoperative follow-up was carried out by regular out-patient visit and telephone interviews. Follow-up information was updated to January 1, 2014. Reasons for those patients lost follow-up were mainly because those patients refused out-patient visit or changed telephone number and address.
Statistical Analysis
Statistical analysis was performed using SPSS 19.0 (SPSS Inc, Chicago, IL). Continuous data were presented as the mean ± standard deviation (SD). The means of the 2 groups were assessed with one-way ANOVA test. Categorical data were compared by χ2 tests or Fisher exact test. Survival curves were derived from Kaplan–Meier estimates and the curves were compared by log-rank test. Prognostic factors were identified by univariate analysis, and further examined by multivariate analysis. The multivariate analysis was performed with the Cox proportion hazards model. Backward stepwise selection with a likelihood-ratio test was used for selecting variables for the Cox regression analysis. In our Cox proportional hazards model, p ≤ 0.05 was defined as the inclusion criteria and P > 0.1 as the exclusion criteria. The P value less than 0.05 was considered statistical significance. All the P values in our study were performed by two-sided test.
RESULTS
Patients
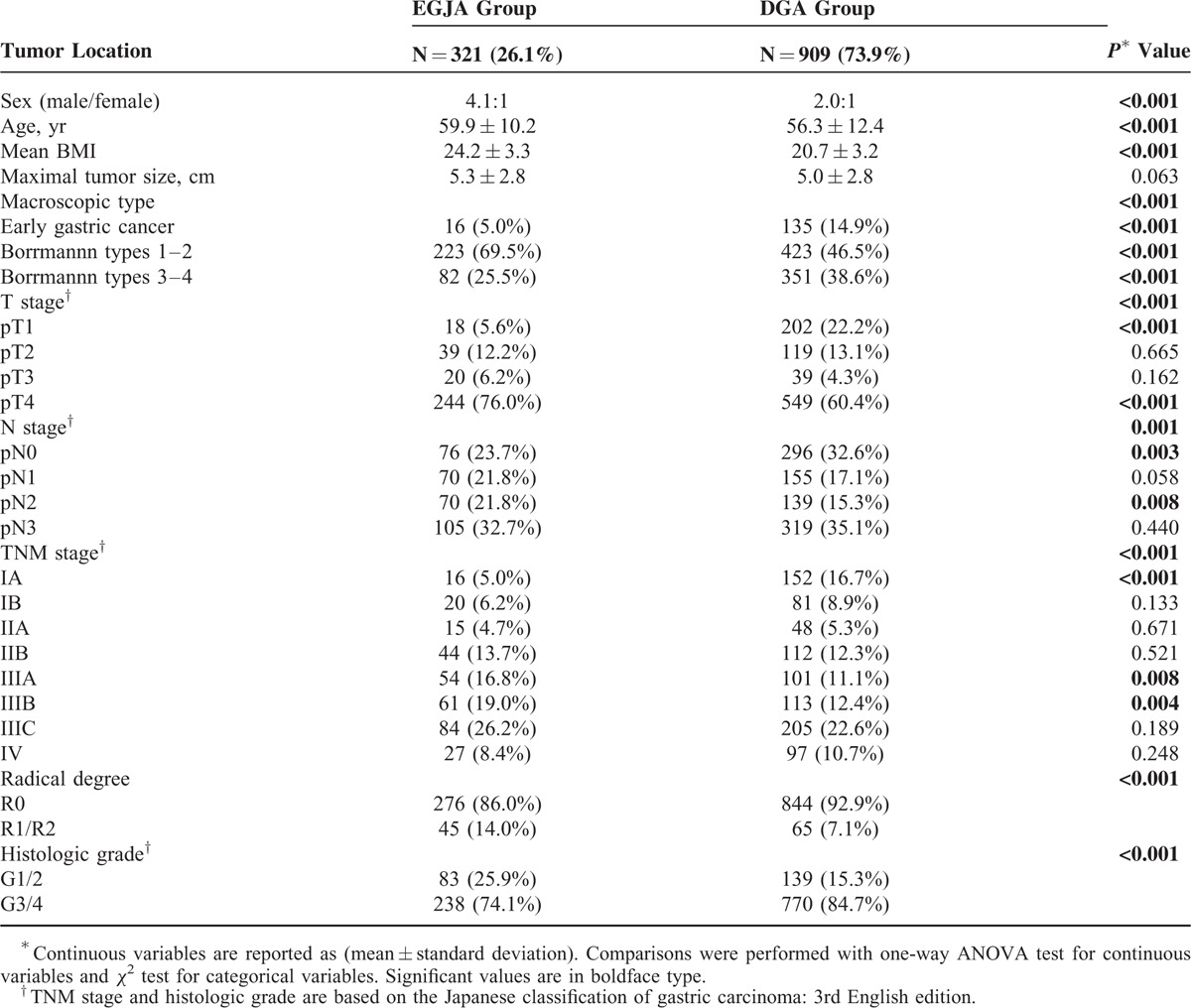
A total of 1230 patients were finally included in our study, 321 (26.1%) were allocated to the EGJA group, and 909 (73.9%) were in the DGA group. The majority of our patients were men in both groups, the average ages for patients were (59.9 ± 10.2) years and (56.3 ± 12.4) years, respectively, for EGJA and DAG (Table 1). Patients in the EGJA group also had a significantly higher body mass index (BMI) than that of the DGA group (24.3 ± 3.3 vs. 20.7 ± 3.2) (P < 0.001).
Table 1.
Clinicopathological Characteristics and Surgical Information of Adenocarcinoma by Tumor Location
Clinicopathological Characteristics
For the macroscopic type of gastric cancer, the proportion of Borrmann type 1 and type 2 was higher in patients with EGJA when compared with DGA (69.5% vs. 47.5%) (P < 0.001). The proportion of early gastric adenocarcinoma was significantly higher in the DGA group compared with EGJA group (22.2% vs. 5.6%) (P < 0.001) (Table 1). The proportion of lymph node metastasis was also higher in those with EGJA than DGA (76.3% vs. 67.4%) (P = 0.003). The distribution of TNM stage showed more advanced stage adenocarcinoma in EGJA than in DGA (P < 0.001). The rate of R0 resection was also lower in the EGJA group compared with the DGA group (86.0% vs. 92.8%) (P < 0.001) (Table 1).
Surgical Outcomes
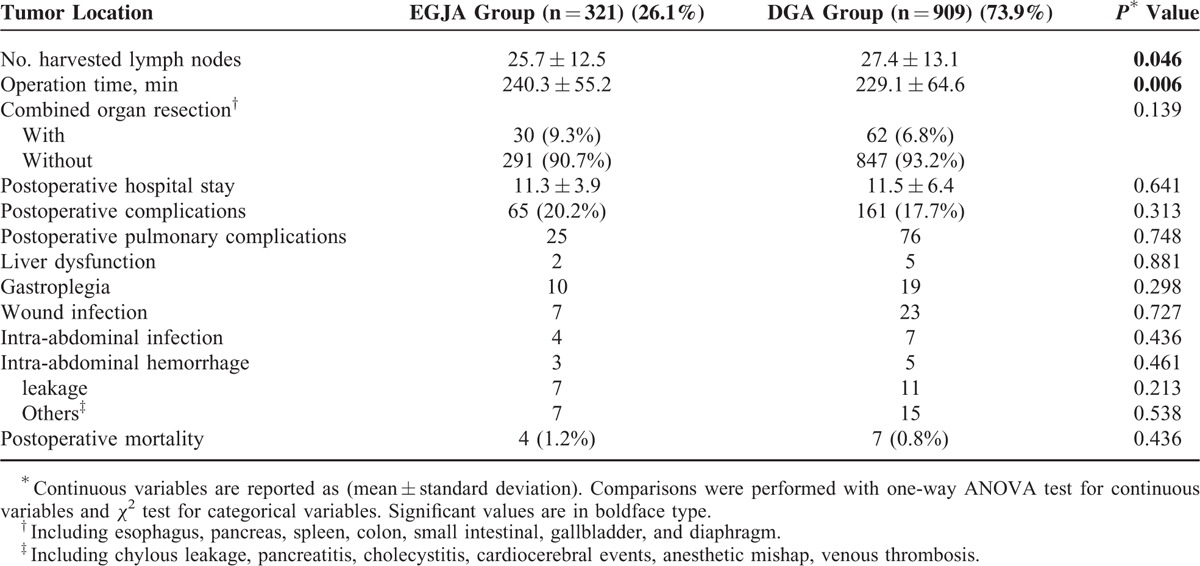
The surgical information was included in Table 2. The number of harvested lymph nodes was larger in the EGJA group (27.4 ± 13.1 vs. 25.7 ± 12.5) (P = 0.046). The overall rate of postoperative complications was 18.4% and no significant difference was observed between the EGJA and DGA groups (P = 0.313). In both groups the most frequent surgically related complications were wound infection (13.3%) and gastroplegia (12.8%). For mortality within postoperative 30 days: 4 patients in the EGJA group and 7 patients in the DGA group died of anastomotic leakage-related sepsis, postoperative respiratory failure, and cardio-cerebral vascular accidents (Table 2).
Table 2.
Information of Postoperative Surgical Outcomes Between EGJA and DGA Groups
Survival Outcomes
The median follow-up time was 59.4 (range 7.0–95.7) months. The overall 3-year survival rate was 57.5% in EGJA and 65.5% in DGA. Patients with EGJA had a significantly poor prognosis compared with those patients with DGA (P = 0.001) (Figure 1). The median survival time was 46 months for patients with EGJA, while this could not be applicable for DGA since the fatality rate was less than 50% by the end of follow-up. The 3-year survival rate was 61.7% in EGJA and 69.3% in DGA (P = 0.001) for patients with curative surgery (Figure 2). When conducting stratified analysis by TNM stage, we observed there was significant difference between 2 groups only in stage II (P = 0.012), while no significant difference in other stages (Figure 3). We also found that there was significant difference on survival outcomes between 2 groups for patients with pN0 tumors (P < 0.001) (Figure 4).
FIGURE 1.
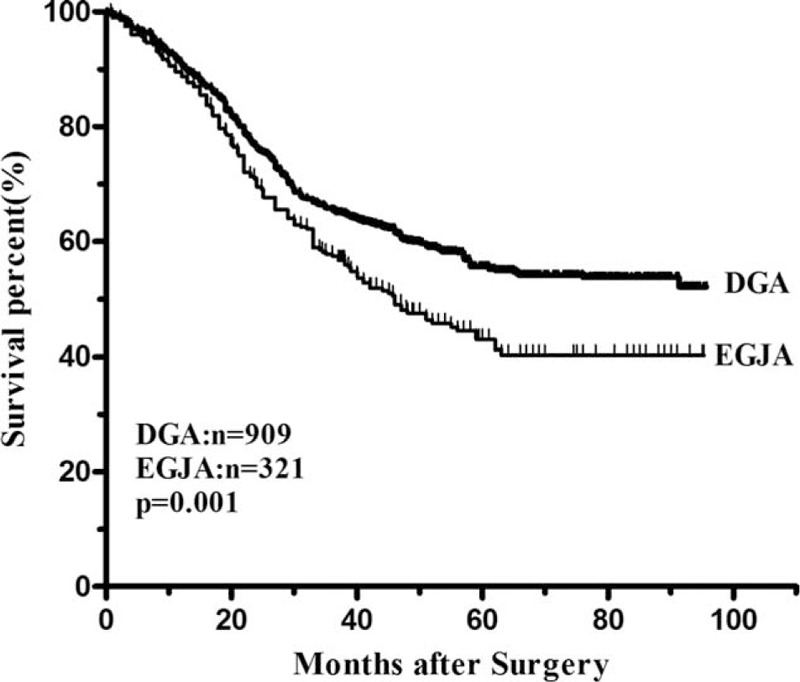
The overall survival curves of DGA and EGJA. The 3-year survival rate was significantly lower in the EGJA group than that in the DGA group (57.5% vs. 65.5%, P = 0.001).
FIGURE 2.
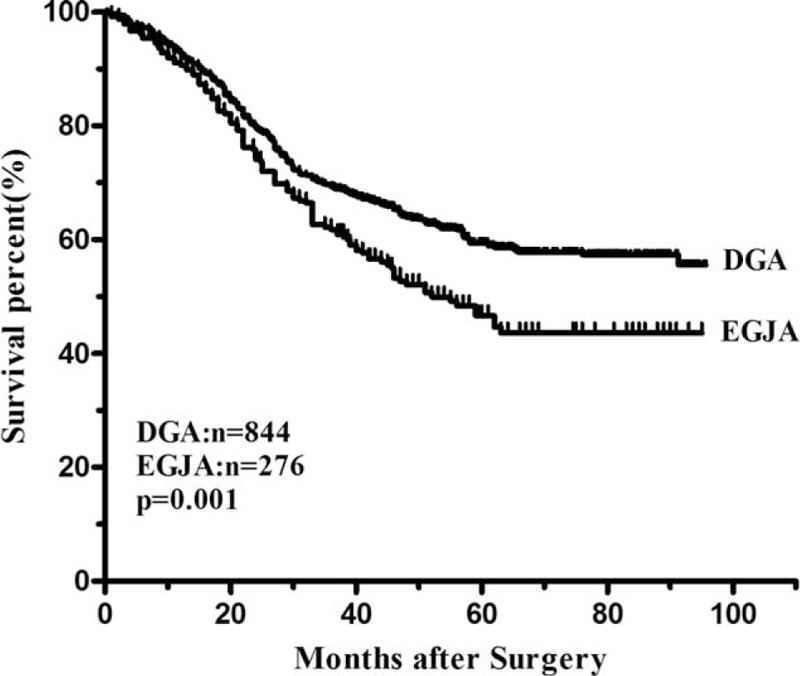
Survival curves of EGJA and DGA after R0 resection. The 3-year survival rate was significantly lower in the EGJA group than in the DGA group (61.7% vs. 69.3%, P = 0.001).
FIGURE 3.
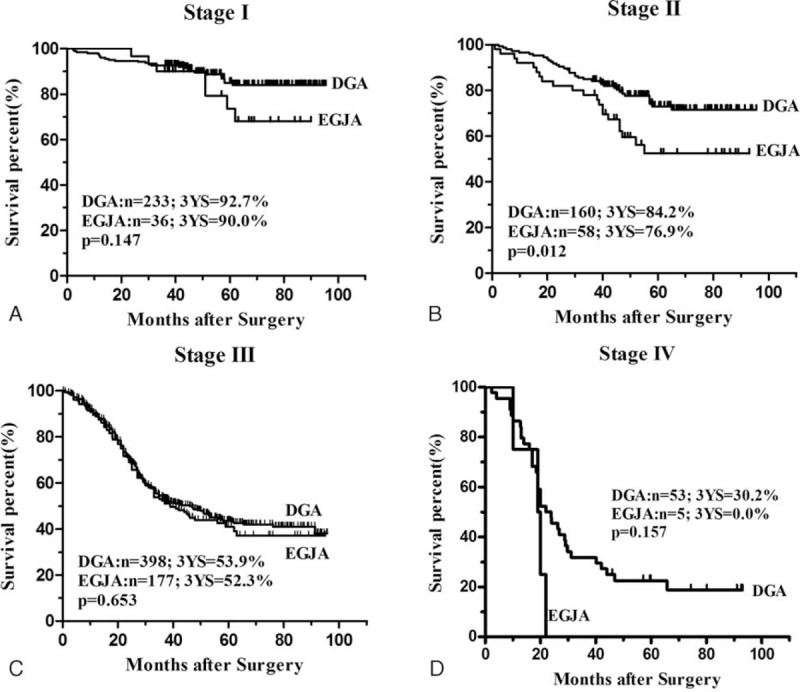
Survival curves of gastric adenocarcinoma after R0 resection in each stage based on the tumor stages: A, Patients with stage I tumors (n = 269). There was no significant difference on survival outcomes at this stage (P = 0.147). B, Patients with stage II tumors (n = 218). There was significant difference on survival outcomes at this stage (P = 0.012). C, Patients with stage III tumors (n = 575). There was no significant different on survival outcomes at this stage (P = 0.563). D, Patients with stage IV tumors (n = 58). There was no significant different on survival outcomes at this stage (P = 0.157).
FIGURE 4.
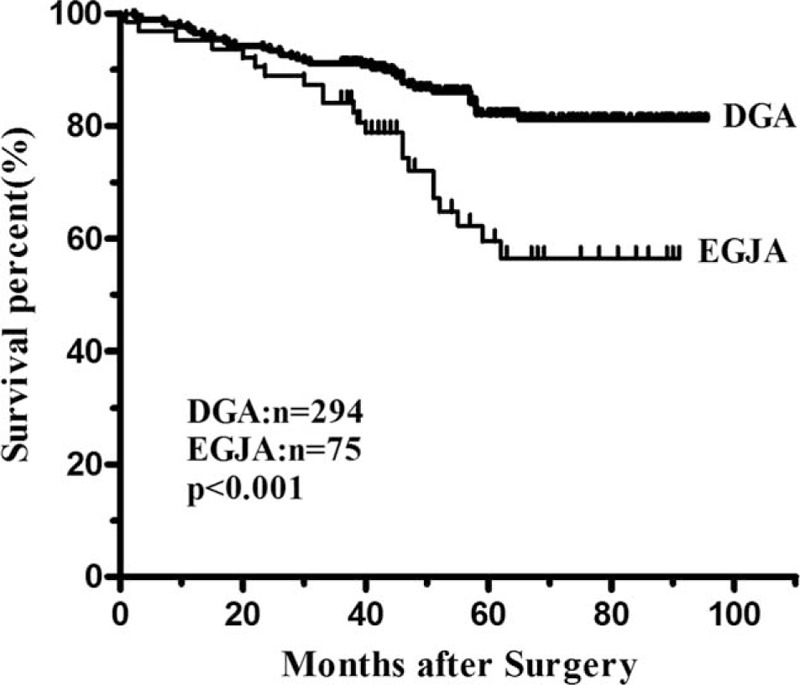
Survival curves of EGJA and DGA in the pN0 groups after R0 resection. The 3-year survival rate was significantly lower in the EGJA group than that in the DGA group (84.1% vs. 91.1%, P < 0.001).
Prognostic Factors
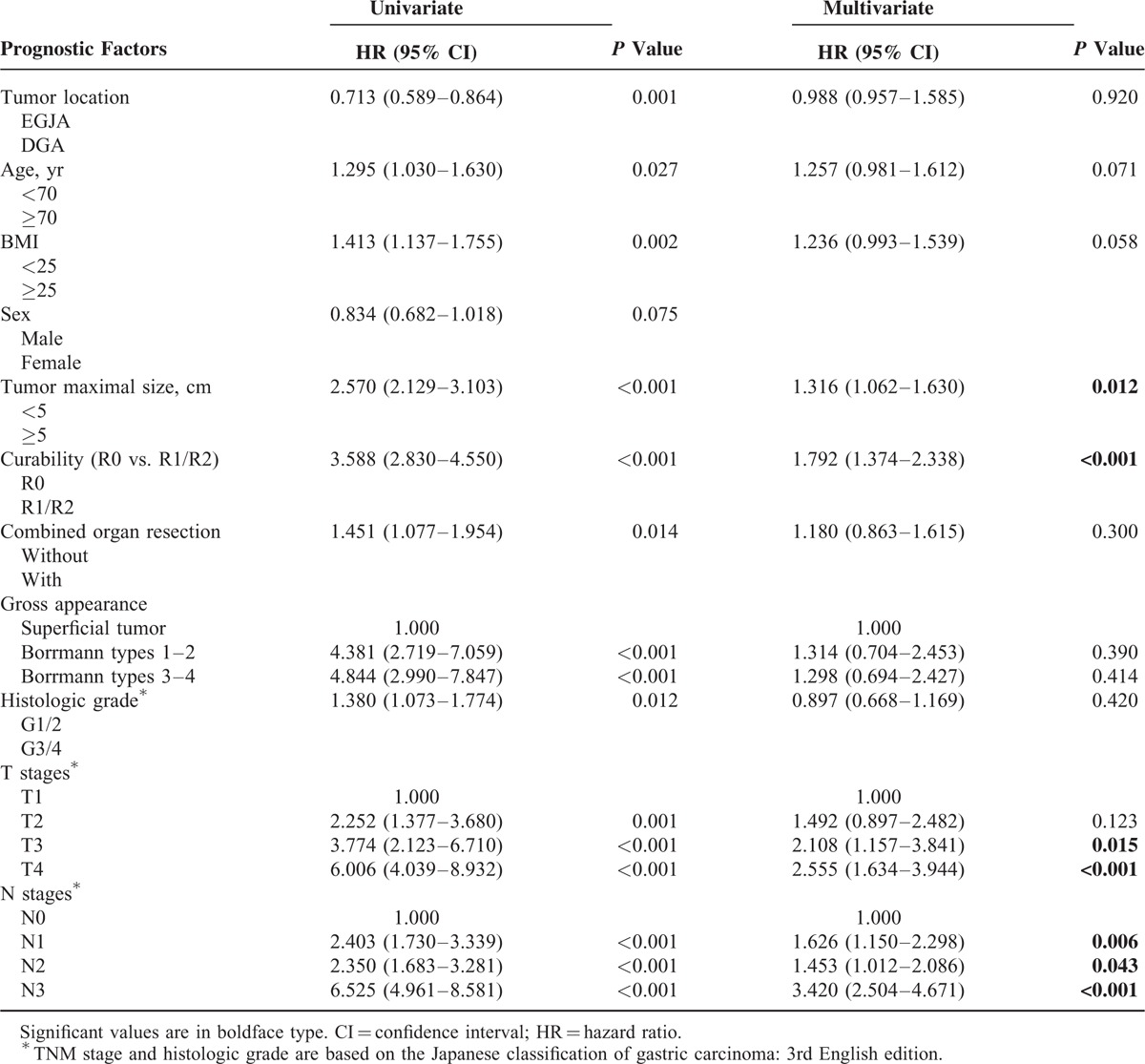
There were different prognostic factors between these 2 groups, multivariate analysis indicated that age (<70 vs. ≥70 years) (HR = 1.335, 95% CI 1.012–1.761, P = 0.041), tumor maximal size (HR = 1.534, 95% CI 1.186–1.985, P = 0.001), radical degree (HR = 1.335, 95% CI 1.030–2.144, P = 0.034) pT4 (HR = 2.291, 95% CI 1.413–3.716, P = 0.001), pN3 (HR = 4.071, 95% CI 2.789–5.941, P < 0.001), pM (HR = 1.450, 95% CI 1.047–2.029, P = 0.025) were independent prognostic factors for DGA, while only combined organ resection (HR = 1.716, 95% CI 1.053–2.797, P = 0.030), pN3 (HR = 3.429, 95% CI 2.098–5.604, P < 0.001), and pM (HR = 2.358, 95%CI 1.454–3.824, P = 0.001) were significant and independent prognostic indicators for EGJA (Table 3). Subsequent multivariate analysis also confirmed that tumor maximal size (HR = 1.316, 95% CI 1.062–1.630, P = 0.012), radical degree (HR = 1.792, 95% CI 1.374–2.338, P < 0.001), pT (HR = 2.555, 95% CI 1.634–3.944, P < 0.001), and pN (HR = 3.420, 95% CI 2.504–4.671, P < 0.001) were independent prognostic indicators for patients after resection for gastric adenocarcinoma (Table 4).
Table 3.
Different Prognostic Factors Between EGJA and DGA According to Cox Multivariate Analysis
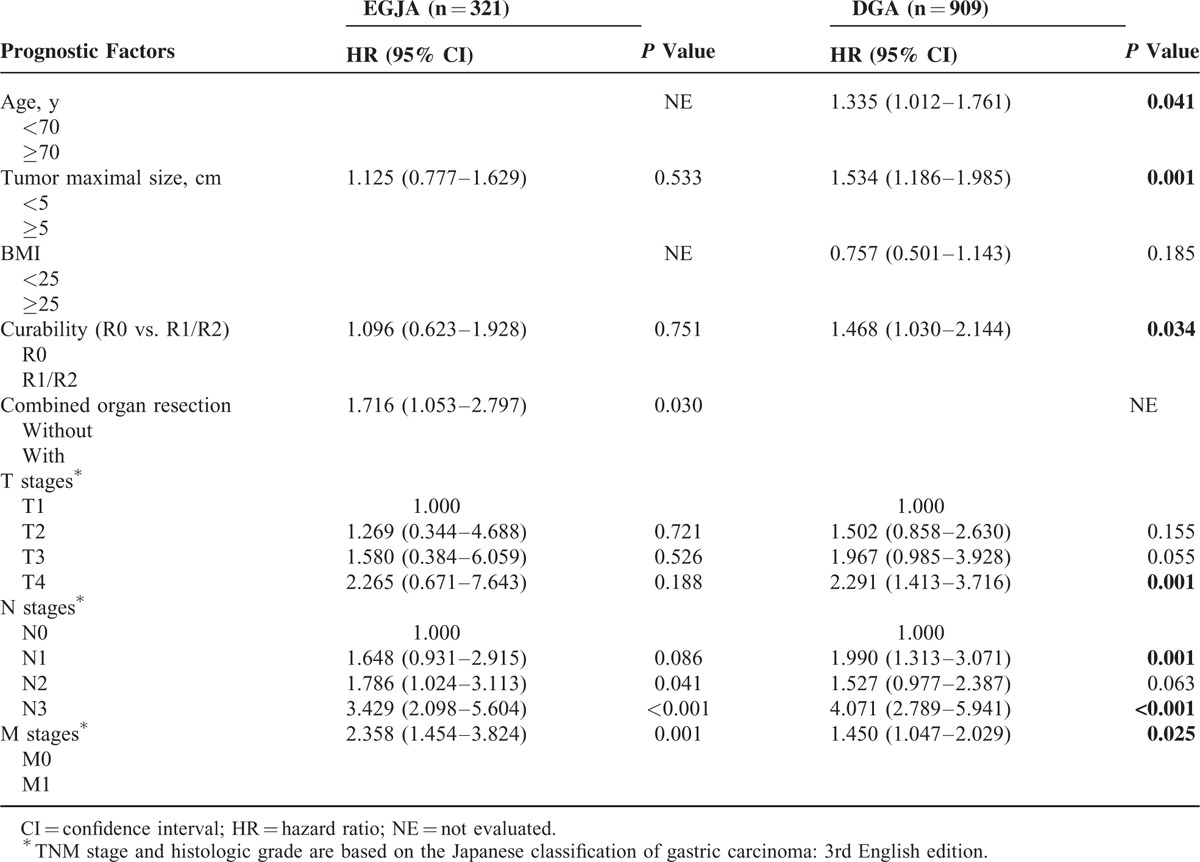
Table 4.
Prognostic Factors of Gastric Adenocarcinoma According to Cox Proportional Hazard Analysis
DISCUSSION
The incidence and mortality of gastric cancer has declined during the past 5 decades.1 However, the proportion of EGJA indicated an opposite trend to the whole gastric cancer. Many previous studies have elaborated an ascending trend of EGJA,3–5 which alert clinicians to put a premium on this lesion. Some previous researches have already presented the differences between EGJA and DGA too.7,8 In China, although the number of EGJA is still not dominant among the whole gastric cancers, the proportion of EGJA has sharply increased in recent years.5 According to our knowledge, most previous researchers have just depicted a relatively small number of surgically resected patients and the larger volume studies that came from China was still sparse. Our study is distinct because we enrolled a relative large number of consecutive Chinese EGJA patients during the study period and compared their clinicopathological features and survival outcomes with DGA. Due to the special location and structures of EGJA, this kind of tumor may have clinicopathological characteristics of both esophageal and gastric malignancies.7 The aim of this study was to identify key differences between EGJA and DGA to make us have a deeper knowledge of EGJA.
By analyzing our database of gastric adenocarcinoma from January 2006 to December 2010, we can see a significant higher prevalence of EGJA among surgical patients in our institution than reports from Western nations. Among 1230 patients, the proportions of EGJA and DGA were 26.1% and 73.9%, respectively. Our data demonstrated that EGJA was more common in older patients and higher BMI patients.
In line with many results of previous researches, EGJAs were associated with male sex and different characteristics. The different demographics of this people compared with DAG suggest that different processes are involved in the pathogenesis.24,25 We have also clearly indicated that the prognosis of patients with EGJA was worse than that of patients with DGA, even after curative resection, which was similar to some previous reports.8,10,26 More progressive tumors and lower rate of radical degree were revealed in EGJA when compared with DGA. Without a doubt, patients with EGJA also showed a worse prognosis when compared with DGA. However, tumor location was not a prognostic factor for gastric adenocarcinoma by multivariate analysis, only age, tumor size, curability, pT, and pN affected survival of gastric adenocarcinoma. However, since different distributions of radical degree and T-N-M stage between 2 groups, we could find that tumor location would be an independent prognostic factor after the T-N-M stage and radical degree were excluded in the analysis (HR = 0.815, 95% CI 0.671–0.990, P = 0.039) (data were not shown in the tables). We also find different prognostic factors for EGJA and DGA, unlike prognostic factor for DGA, combined organ resection may increase the hazard for EGJA. From our analysis, we also indicated that standard D2/D2+ lymphadenectomy was necessary for both DGA and EGJA. We think that many factors such as radical degree, pT stage, and tumor size may influence the prognosis that was not included in the Cox model due to relatively small volume for EGJA in our study.
After stratification analysis in patients who underwent curative surgery: stage I, stage III, and stage IV had no significant difference in survival outcomes between EGJA and DGA. This may be due to a great prognosis of tumor in early stage for all gastric cancer, regardless of where the tumor location is. In our study, survival discrepancies might be primarily due to higher proportion of advanced stages and lower rate of R0 resection in the EGJA group. If the 2 groups had a similar rate of advanced disease, the survival outcomes may also have been similar, since there was no difference on survival rate in each stage I, stage III, and stage IV. We also find patients with EGJA had a higher BMI; this may not be a negligent factor for the different outcomes. Even though we have not found that BMI was a prognostic factor for gastric cancer, patients with higher BMI would make the surgery more difficult which may lead to a lower rate of Ro resection and numbers of harvested lymph nodes. We have made a progress in the surgical technique for gastric cancer during the past 10 years, but different surgeons may have different operative volumes and experience. These factors may also create different outcomes of EGJA and DGA. Apart from these factors, a standard inferior mediastinal lymph node dissection was quite scant for EGJA patients due to technical difficulties from the transabdominal or transhiatal approaches.9 For patients without lymph nodes metastasis (pN0), EGJA also showed a worse prognosis when compared with DGA after curative resection in our study. This indicated that adequate lymph nodes dissection includes mediastinal lymph nodes, and an aggressive additional treatment after surgery was essential to improve survival of EGJA in pN0 patients, D2/D2+ should be recommended for EGJA in advanced stage.
One interesting finding of our study was that there was significantly worse prognosis after curative resection in stage II. We also observed that 50.5% (110/218) of patients were in stage T4aN0M0 among stage II. This indicated that EGJA and DGA may be 2 distinct tumors and tumor location was a prognostic factor for stage II. Because of the different anatomical structures between EGJA and DGA in stage II, the way of lymph nodes micrometastasis may be also different. We also consider that the difference on biological characteristics between DGA and EGJA would be more prominent in stage II. Tumors at different locations may have different responses to adjuvant chemotherapy, this may be another explanation for the difference. Tumors were deeper, with a higher rate of lymph node metastasis in the EGJA group which agrees with other groups.27 In accordance with the higher proportion of advanced stage, a lower radical degree rate was also observed in the EGJA group. Because of the special locations of cardia and fundus of stomach, EGJA especially Siewert type III tumors were not easy to detect under endoscopy compared with distal lesions. It is essential for physicians to reverse the endoscopic probe to find upper gastric lesions when performing endoscopic examinations. In addition, obvious typical symptoms associated with EGJA are insidious in early stage. Quite a lot of patients came to accept the endoscopy examination when the dysphagia appeared. As we all know, the lesion was comparatively large when patients felt dysphagia. For the anatomical aspect, the intraabdominal part of the esophagus, esophagogastric junction, and fundus are not totally covered by visceral peritoneum. These portions of the stomach are located extraperitoneally or retroperitoneally, which makes EGJA more prone to infiltrate the serosa and more inclined to peritoneal metastasis compared with DGA.28
The esophagogastric junction was a very special transitional area from squamous epithelium to glandular epithelium, which is rather different from the typical glandular epithelium of distal stomach. Different epithelial ingredients with different tumorigenesis might lead to discrepant prognosis for EGJA. The former research had found more proportion of undifferentiated type in gastric cardia cancer and leads to worse prognosis.29 However, in our study, we could see that a higher proportion of undifferentiated adenocarcinoma in the DGA group instead of EGJA group. Thus, the poor prognosis of EGJA may relate to various factors, such as stomach anatomy, different lymphatic metastasis path, and technical difficulties during surgery. McColl et al also proposed in that gastroesophageal reflux may partly lead to the development of intestinal metaplasia, neoplasm ad more undifferentiated tumor cells in EGJA.30 These indicated that biological discrepancies might be a dominant cause for the difference for EGJA and DGA. Thus, the EGJA differs from the distal gastric adenocarcinoma not only in anatomy but also in tumorigenesis and development mechanisms.31,32 Various prognostic factors for gastric adenocarcinoma have been discussed. In multivariate analysis of all patients, age, the radical degree, the T category, and the N category were independent prognostic factors for gastric adenocarcinoma. We can see diagnosis in earlier stages is a main factor to improve prognosis.
There was no statistically significant difference on postoperative hospital days and rate of postoperative complications between the 2 groups. We found more leakages after resection for EGJA than for DGA (2.2% vs. 1.2%) which is in accordance with other reports.28 The overall rate of postoperative complications was much lower in recent years.
Our study have some limitations: since this is a retrospective analysis comes from a single center in western China, the results of this study may not represent overall Chinese population well. There was some selection bias such as patients with Siewert I tumors were not included in this study. Because of different distributions of TNM stage and radical degree between DGA and EGJA, the Cox model analysis may have some biased estimate for survival outcomes in our study. Our outcomes may hint that EGJA and DGA may be 2 distinct tumors; however, we believed that more basic researches are needed to be further performed to find difference on biological characteristics between DGA and EGJA. Although some interesting results were generated in our study, we are still unable to answer all of the existing questions for the difference between EGJA and DGA.
In conclusion, compared with DAG, EGJA has distinct clinicopathological features and different prognosis. There were different prognostic factors for EGJA and DGA, and combined organ resection should not be recommended among EGJA patients. Our study indicated that Siewert types II and III may be a distinct disease entity, and these patients need different management strategies to those with DAG. Therefore, more vigorous additional treatment after surgery should be considered to improve survival of EGJA patients, and special attention is warranted for early detection and surveillance.
Acknowledgment
The authors thank the substantial work of Volunteer Team of Gastric Cancer Surgery (VOLTGA), West China Hospital, Sichuan University, China for the assistance on collecting the data.
Footnotes
Abbreviations: 3-YS = 3-year survival rate, BMI = body mass index, DGA = distal gastric adenocarcinoma, EGA = esophagus-gastric anastomosis, EJGA = esophagogastric junctional adenocarcinoma, G1 = well differentiated, G2 = moderate differentiated, G3 = lower differentiated, G4 = undifferentiated.
Domestic support from National Natural Science Foundation of China (Grant numbers: 81372344, 81301867) and New Century Excellent Talents in University Support Program, Ministry of Education of China (Grant number: 2012SCU-NCET-11-0343).
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010; 60:277–300. [DOI] [PubMed] [Google Scholar]
- 2.Devesa SS, Blot WJ, Fraumeni JF., Jr Changing patterns in the incidence of esophageal and gastric carcinoma in the United States. Cancer 1998; 83:2049–2053. [PubMed] [Google Scholar]
- 3.Stein HJ, Feith M, Siewert JR. Cancer of the esophagogastric junction. Surg Oncol 2000; 9:35–41. [DOI] [PubMed] [Google Scholar]
- 4.Blaser MJ, Saito D. Trends in reported adenocarcinomas of the oesophagus and gastric cardia in Japan. Eur J Gastroenterol Hepatol 2002; 14:107–113. [DOI] [PubMed] [Google Scholar]
- 5.Zhou Y, Zhang Z, Zhang Z, et al. A rising trend of gastric cardia cancer in Gansu Province of China. Cancer Lett 2008; 269:18–25. [DOI] [PubMed] [Google Scholar]
- 6.Ryu KW, Kim CS, Goo BH. Clinicopathologic characteristics of and prognosis for proximal gastric carcinomas. J Korean Surg Soc 2000; 59:223–228. [Google Scholar]
- 7.Pohl H, Welch HG. The role of over diagnosis and reclassification in the marked increase of esophageal adenocarcinoma incidence. J Nat Cancer Inst 2005; 97:142–146. [DOI] [PubMed] [Google Scholar]
- 8.Craanen ME, Dekker W, Blok P, et al. Time trends in gastric carcinoma: changing patterns of type and location. Am J Gastroenterol 1992; 87:572–579. [PubMed] [Google Scholar]
- 9.Sasako M, Sano T, Yamamoto S, et al. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol 2006; 7:644–651. [DOI] [PubMed] [Google Scholar]
- 10.Harrison LE, Karpeh MS, Brennan MF. Proximal gastric cancers resected via a transabdominal-only approach: results and comparisons to distal adenocarcinoma of the stomach. Ann Surg 1997; 225:678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim DY, Joo JK, Ryu SY, et al. Clinicopathological characteristics and prognosis of carcinoma of the gastric cardia. Dig Surg 2006; 23:313–318. [DOI] [PubMed] [Google Scholar]
- 12.Kunisaki C, Akiyama H, Nomura M, et al. Surgical outcomes for early gastric cancer in the upper third of the stomach. J Am Coll Surg 2005; 200:15–19. [DOI] [PubMed] [Google Scholar]
- 13.Stark SP, Romberg MS, Pierce GE, et al. Transhiatal versus transthoracic esophagectomy for adenocarcinoma of the distal esophagus and cardia. Am J Surg 1996; 171:478–482. [DOI] [PubMed] [Google Scholar]
- 14.Cushieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. Lancet 1996; 347:995–999. [DOI] [PubMed] [Google Scholar]
- 15.Bonenkamp JJ, Songun I, Hermans J, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet 1995; 345:745–748. [DOI] [PubMed] [Google Scholar]
- 16.Siewert JR, Stein HJ. Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 1998; 85:1457–1459. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Wang W, Cheng Y, et al. Adenocarcinomas of the esophagogastric junction: experiences at a single institution in China. World J Surg Oncol 2013; 11:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bai JG, Lv Y, Dang CX. Adenocarcinoma of the esophagogastric junction in China according to Siewert's classification. Jpn J Clin Oncol 2006; 36:364–367. [DOI] [PubMed] [Google Scholar]
- 19.Liu K, Yang K, Zhang WH, et al. Changes of esophagogastric junctional adenocarcinoma and gastroesophageal reflux disease among surgical patients during 1988–2012: a single-institution, high-volume experience in China. Ann Surg 2015; [Epub ahead of print]. DOI: 10.1097/SLA.0000000000001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011; 14:101–112. [DOI] [PubMed] [Google Scholar]
- 21.Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002; 347:1662–1669. [DOI] [PubMed] [Google Scholar]
- 22.Omloo JM, Lagarde SM, Hulscher JB, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the mid/distal esophagus: five-year survival of a randomized clinical trial. Ann Surg 2007; 246:992–1000. [DOI] [PubMed] [Google Scholar]
- 23.Ajani JA, Bentrem DJ, Besh SD, et al. Gastric cancer, version 2. J Natl Comprehensive Cancer Netw 2013; 11:531–546. [DOI] [PubMed] [Google Scholar]
- 24.Siewert JR, Stein HJ. Adenocarcinoma of the gastroesophageal junction: classification, pathology and extent of resection. Dis Esoph 1996; 9:173–182. [Google Scholar]
- 25.Siewert JR, Feith M, Werner M, et al. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg 2001; 232:353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruno L, Nesi G, Montinaro F, et al. Clinicopathologic findings and results of surgical treatment in cardiac adenocarcinoma. J Surg Oncol 2000; 74:33–35. [DOI] [PubMed] [Google Scholar]
- 27.Jang JH, Beron RI, Ahn HS, et al. Clinicopathological features of upper third gastric cancer during a 21-year period (single center analysis). J Gastric Cancer 2010; 10:212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piso P, Werner U, Lang H, et al. Proximal versus distal gastric carcinoma-what are the differences? Ann Surg Oncol 2000; 7:520–525. [DOI] [PubMed] [Google Scholar]
- 29.Maeda H, Okabayashi T, Nishimori I, et al. Clinicopathologic features of adenocarcinoma at the gastric cardia: is it different from distal cancer of the stomach? J Am Coll Surg 2008; 206:306–310. [DOI] [PubMed] [Google Scholar]
- 30.McColl KE. Cancer of the gastric cardia. Best Pract Res Clin Gastroenterol 2006; 20:687–696. [DOI] [PubMed] [Google Scholar]
- 31.Hamilton SR, Aaltonen LA. World Health Organization Classification of Tumours, Pathology and Genetics of Tumours of the Digestive System. 1st ed. Lyon, France: IARC Press; 2000, 281–292. [Google Scholar]
- 32.Ectors N, Driessen A, De Hertog G, et al. Is adenocarcinoma of the esophagogastric junction or cardia different from Barrett adenocarcinoma? Arch Pathol Lab Med 2005; 129:183–185. [DOI] [PubMed] [Google Scholar]


