Supplemental Digital Content is available in the text
Abstract
Sorafenib has been recommended as first- or second-line treatment for metastatic renal cell carcinoma (mRCC) by several guidelines. The objective of this study is to evaluate the efficacy of sorafenib monotherapy in Chinese patients with mRCC and determine the prognostic clinicopathologic factors associated with survival in these patients.
This is a single-arm retrospective study conducted in 2 tertiary medical centers; 140 mRCC patients were enrolled between January 2007 and June 2014. Sorafenib was administered at a dose of 400 mg twice daily, and continued until disease progression, at which point the dose was increased to 600 or 800 mg twice daily, or the onset of an intolerable adverse drug event (ADE) that required dose reduction or temporary suspension of treatment.
The primary endpoint was overall survival (OS), and the secondary endpoints included progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), and safety.
The median follow-up time was 32 months. The median OS and PFS were 24 months (range, 3–88 months) and 16 months (range, 0–88 months), respectively. Patients with clear cell carcinoma had a greater OS (P = 0.001) whereas sarcomatoid differentiation (P = 0.045) and disease progression (P = 0.010) negatively impacted OS; time from kidney surgery or biopsy to initiation of sorafenib treatment was associated with PFS (P = 0.027). Efficacy analysis revealed that 3 (2.1%) patients achieved complete responses, 28 (20.0%) patients experienced partial responses, 88 (62.9%) patients had stable disease, and 21 (15.0%) patients developed progressive disease. Moreover, the ORR was 22.1%, and the DCR was 85.0%. Most ADEs were classified as grades 1 or 2 with only 14 (10.0%) patients experiencing a severe ADE (grade 3).
Sorafenib monotherapy can achieve promising OS and PFS for Chinese patients with mRCC, especially in those with clear cell carcinoma, with manageable adverse events.
INTRODUCTION
Renal cell carcinoma (RCC) accounts for nearly 3% of all adult malignancies, and its incidence is increasing in China.1,2 The poor prognosis of these patients is due in part to its late detection; approximately one-third of patients are diagnosed with metastatic RCC (mRCC) at first presentation.3 In addition, although surgery is the most effective treatment, approximately 20% to 40% of patients experience distant metastasis or local recurrence after primary nephrectomy4 with a 5-year survival rate of <10%.5 Furthermore, mRCC is highly resistant to chemotherapy and radiotherapy; therefore, immunotherapy had been the main treatment option for these patients. However, low tumor response and high toxicities limit this treatment option to only a select few patients.6,7 Surgical resection of mRCC at multiple sites also improves long-term survival, but it is not always technically feasible.8
The advent of targeted therapy that inhibit specific signaling pathways important for tumor growth and metastasis, including the vascular endothelial growth factor (VEGF) signaling pathway,9 has changed the treatment paradigms of mRCC. Since the use of the first VEGF tyrosine kinase inhibitor, sunitinib, the treatment algorithm of mRCC has dramatically changed. Sorafenib (Nexavar, Bayer Pharmaceuticals, West Haven, Conn and Onyx Pharmaceuticals, Emeryville, Calif) is another tyrosine kinase inhibitor, targeting the VEGF receptor, platelet-derived growth factor receptor, Raf kinase 1, KIT, and Fms-like tyrosine kinase 3.10–12 Previous studies have shown that sorafenib improves progression-free survival (PFS) in mRCC by 2.7 months11 as well as overall survival (OS)13; therefore, it has been recommended by several guidelines, including those of the National Comprehensive Cancer Network, as a second-line treatment option for those who failed prior immunotherapy.14 However, its efficacy in Chinese patients is not fully known. Therefore, this retrospective study analyzed the characteristics and outcomes of 140 patients with mRCC who were treated with sorafenib at 2 large-volume centers in China to evaluate the efficacy and safety of sorafenib and identify any prognostic factors related to the efficacy of sorafenib.
METHODS
Patients
Between January 2007 and June 2014, 191 mRCC patients treated with sorafenib were included in this retrospective study. All the patients were enrolled from 2 large-volume Chinese centers: the Peking University First Hospital and the Chinese People's Liberation Army General Hospital. Among the 191 patients, 28 were considered being at high risk for recurrence (ie, ≥T3, ≥G3, N1-2, larger tumor diameter, with tumor necrosis, higher grade of the cell nucleus) and were treated with sorafenib as adjuvant therapy. The remaining 163 patients had mRCC, which was confirmed by histopathological methods. Of these 163 mRCC cases, 16 were lost at follow-up and 7 were excluded because of incomplete data. Thus, 140 patients were analyzed. This study was approved by the Ethics Committees of Peking University First Hospital and Chinese People's Liberation Army General Hospital, and informed consent was obtained from each participant.
Treatment and Follow-Up
Sorafenib monotherapy was administered as first-line therapy for 106 (75.7%) patients, second-line therapy after immunotherapy for 23(16.4%) patients, and second-line therapy following sunitinib for 11 (79%) patients. Sorafenib was administered at a dose of 400 mg twice daily over 1 cycle, which consisted of 4 weeks, and continued until disease progression, defined as new metastases outbreak or a 20% increase in size over baseline, or the onset of an intolerable adverse drug event (ADE). The dosage was increased to 600 mg twice daily or 800 mg twice daily in patients with disease progression and manageable toxicity as previously described.15 Dose reduction or temporary suspension was allowed according to the ADE grade and individual tolerability. The median of duration of sorafenib treatment was 20 months (range, 3–88 months), and the median follow-up time was 32 months (range, 3–88 months).
The primary endpoint was OS, and secondary endpoints included PFS, objective response rate (ORR), disease control rate (DCR), and safety. ORR was calculated as complete response (CR) + partial response (PR), and the DCR was determined by CR + PR + stable disease (SD). CR, PR, SD, and progressive disease (PD) were defined according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria.16 We assessed the tumor response by computed tomography or magnetic resonance intensity once every other cycle. Efficacy was defined according to the RECIST criteria16; a 30% reduction in tumor size was deemed effective. The PFS was calculated from the time of sorafenib initiation to the occurrence of PD. ADEs were examined and graded every cycle according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0.17
Statistical Analysis
Patients’ clinical and pathological characteristics were represented as mean with range (minimum to maximum) for continuous variables and n (%) for categorical variables. To evaluate the association of OS and PFS with patients’ clinical and pathological characteristics, patients’ clinical and pathological characteristic data were summarized as n (%) for given OS and PFS values. Univariate and multivariate Cox-regression model analyses were applied, and the results were shown as hazard ratios (HRs) with corresponding 95% confidence intervals (95% CIs). Variables with significance level of P < 0.1 in the univariate Cox-regression model were selected for multivariate analysis. Kaplan–Meier survival curves with a log-rank test were performed to identify the OS and PFS among the clinical and pathological characteristics.18 All statistical assessments were 2-tailed, and P values <0.05 were considered significant. All statistical analyses were carried out with IBM SPSS statistical software version 22 for Windows (IBM Corp., New York, NY).
RESULTS
Patients’ Clinical and Pathological Characteristics
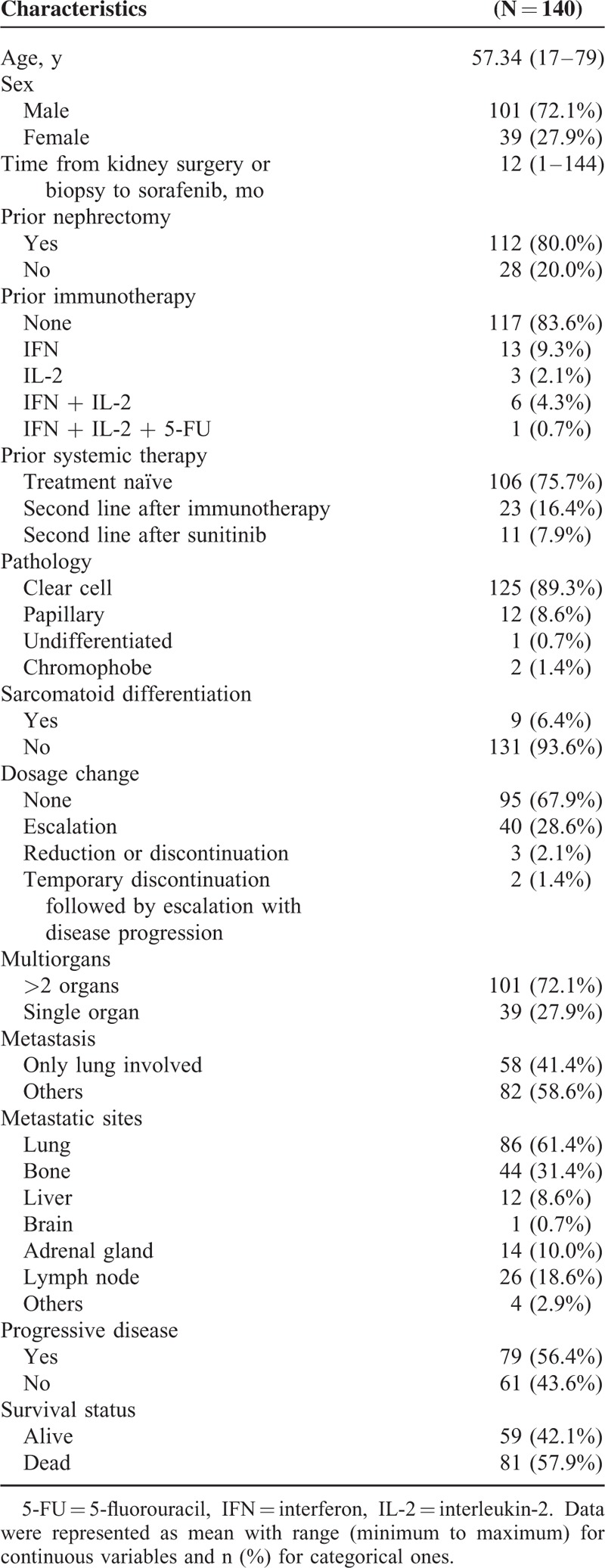
A total of 140 mRCC patients (101 men and 39 women) with a mean age of 57.3 years (range, 17–79 years) receiving sorafenib monotherapy (median dosage of 400 mg twice daily) were enrolled in this study. All the clinical and pathological characteristics of patients are shown in Table 1. The median time from kidney surgery or biopsy to initiation of sorafenib therapy was 12 months (range, 1–144 months); 112 (80%) patients underwent nephrectomy and 23 (16.4%) patients received prior immunotherapy, including combinations of interferon, interleukin-2 (IL-2), and 5-fluorouracil (Table 1). Histologic analysis revealed that 125 (89.3%) patients had clear cell carcinoma and 9 (6.4%) patients had sarcomatoid differentiation. In addition, 101 (72.1%) patients had metastasis to >2 organs, including the lung, bone, lymph node, adrenal gland, liver, and brain, whereas 58 (41.4%) patients had metastatic involvement in the lung alone (Table 1). During the treatment period, 40 (28.6) patients had their sorafenib dosage increased; 3 (2.1%) patients had their dosage reduced or their treatment discontinued altogether, and 2 (1.4%) patients had a mixture of dosage escalation, reduction, and treatment discontinuation (Table 1). Moreover, 79 (56.4%) patients experienced PD at least once during the follow-up period using the RECIST criteria, and 59 (42.1%) patients were alive at the last follow-up (Table 1).
TABLE 1.
Patients’ Clinical and Pathological Characteristics
Clinicopathological Factors Associated With OS and Disease Progression in mRCC Patients Treated With Sorafenib
The median OS was 24 months (range, 3–88 months; Figure 1). The association between OS and the patients’ clinical and pathological characteristics were presented in Table 2. Univariate and multivariate analyses suggest that patients with clear cell carcinoma have a better OS than those with other types of mRCC (ie, papillary, undifferentiated, chromophobe) (HR = 0.33, 95% CI = 0.182–0.596, P < 0.001). In addition, sarcomatoid differentiation (HR = 2.23, 95% CI = 1.02–4.89, P = 0.045) and disease progression (HR = 1.88, 95% CI = 1.16–3.03, P = 0.010) were also associated with higher risk for death during the follow-up period (Table 2). These characteristics continued to be associated with OS by multivariate analysis that included all relevant variables (Supplementary Table S1, http://links.lww.com/MD/A382). Kaplan–Meier survival curves with a log-rank analysis of OS by pathological results, sarcomatoid differentiation, and disease progression are shown in Figure 2.
FIGURE 1.
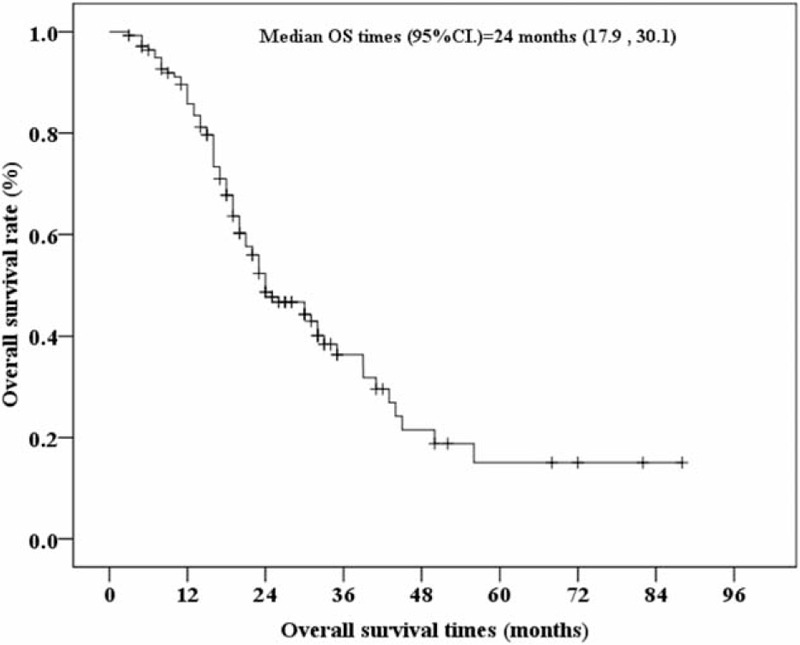
Kaplan–Meier curve of overall survival (OS) for 140 Chinese metastatic renal cell carcinoma patients receiving sorafenib. 95% CI, 95% confidence intervals of median OS times. “+” indicates censored cases. The median OS times were derived at 24 months with 95% CI = 17.9–30.1 months.
TABLE 2.
Association of Overall Survival With Patients’ Clinical and Pathological Characteristics (N = 140)
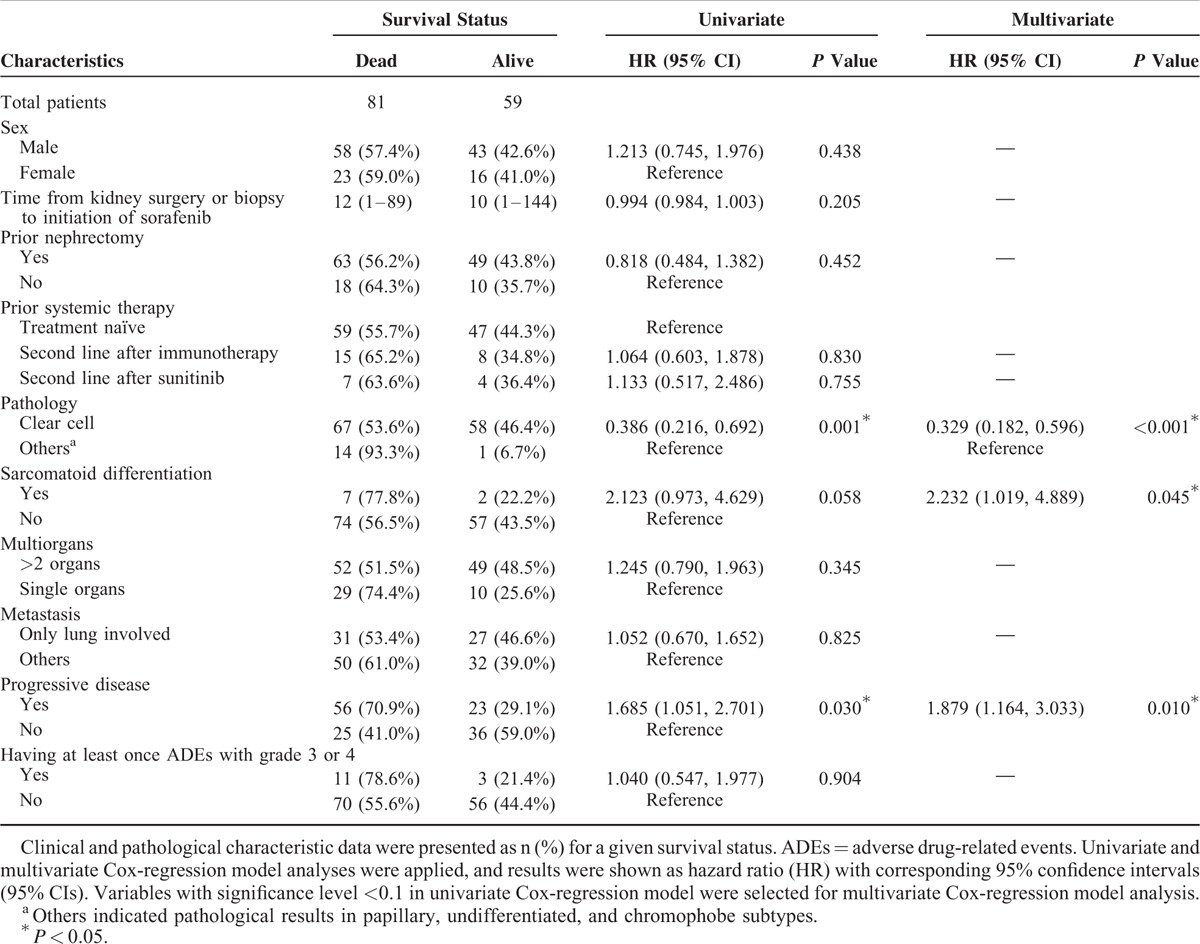
FIGURE 2.
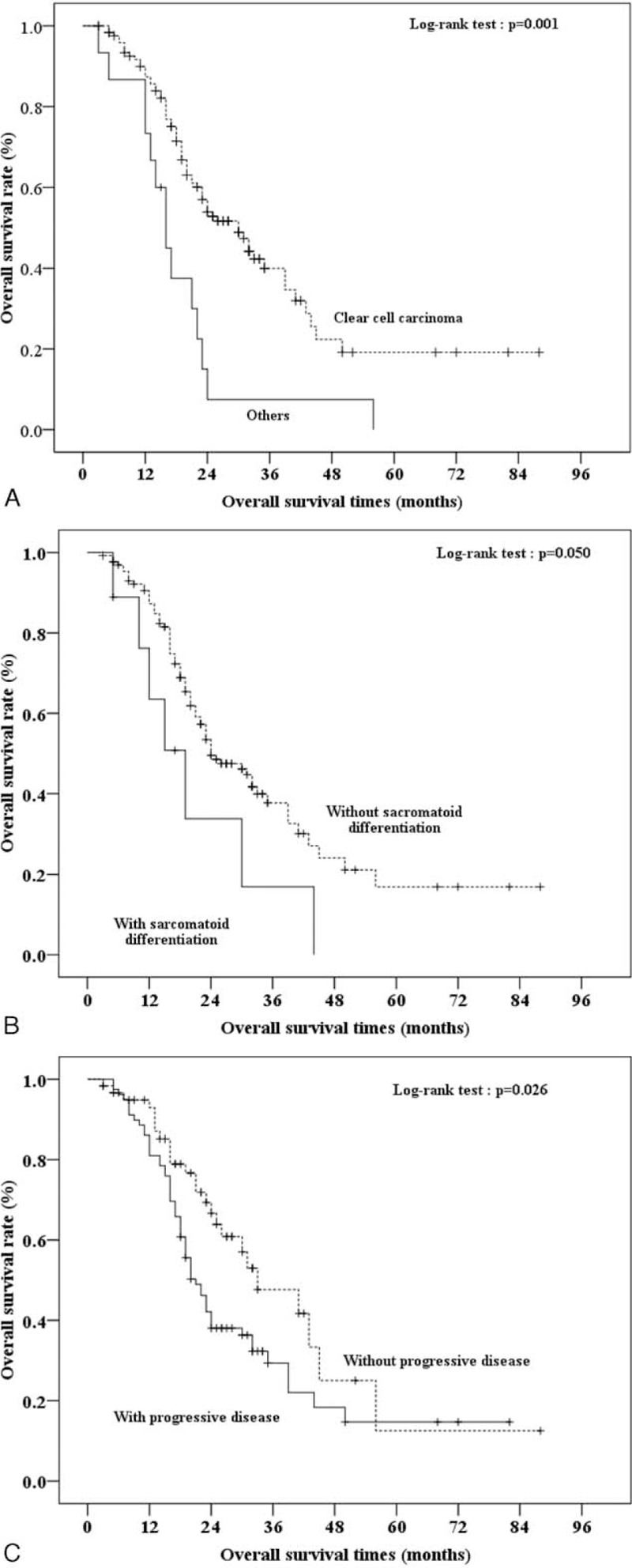
Kaplan–Meier curves of overall survival (OS) times for 140 Chinese metastatic renal cell carcinoma patients receiving sorafenib by pathological result. The log-rank test was performed to identify the significance of OS by specific characteristics, including (A) clear cell carcinoma, (B) sarcomatoid differentiation, and (C) progressive disease. 95% CI, 95% confidence intervals of median OS times. “+” indicates censored cases.
As shown in Table 3, the clinicopathologic variables associated with PFS were next analyzed. Progressive status (PS) included patients with PD as well as those that had died at last follow-up. A total of 104 (74.2%) patients had PS, including patients who experienced PD (the aforementioned 79 patients) along with patients without PD that had died of mRCC, and Kaplan–Meier curves of the PFS among the 140 patients were illustrated in Figure 3. Univariate Cox-regression analysis revealed that time from kidney surgery or biopsy to initiation of sorafenib treatment was associated with PFS (HR = 0.990, 95% CI = 0.981–0.999, P = 0.028; Table 3). This variable continued to be associated with PFS in a multivariate analysis that included all relevant variables (P < 0.027; Table S2, http://links.lww.com/MD/A382).
TABLE 3.
Association of Progression-Free Survival With Patients’ Clinical and Pathological Characteristics (N = 140)
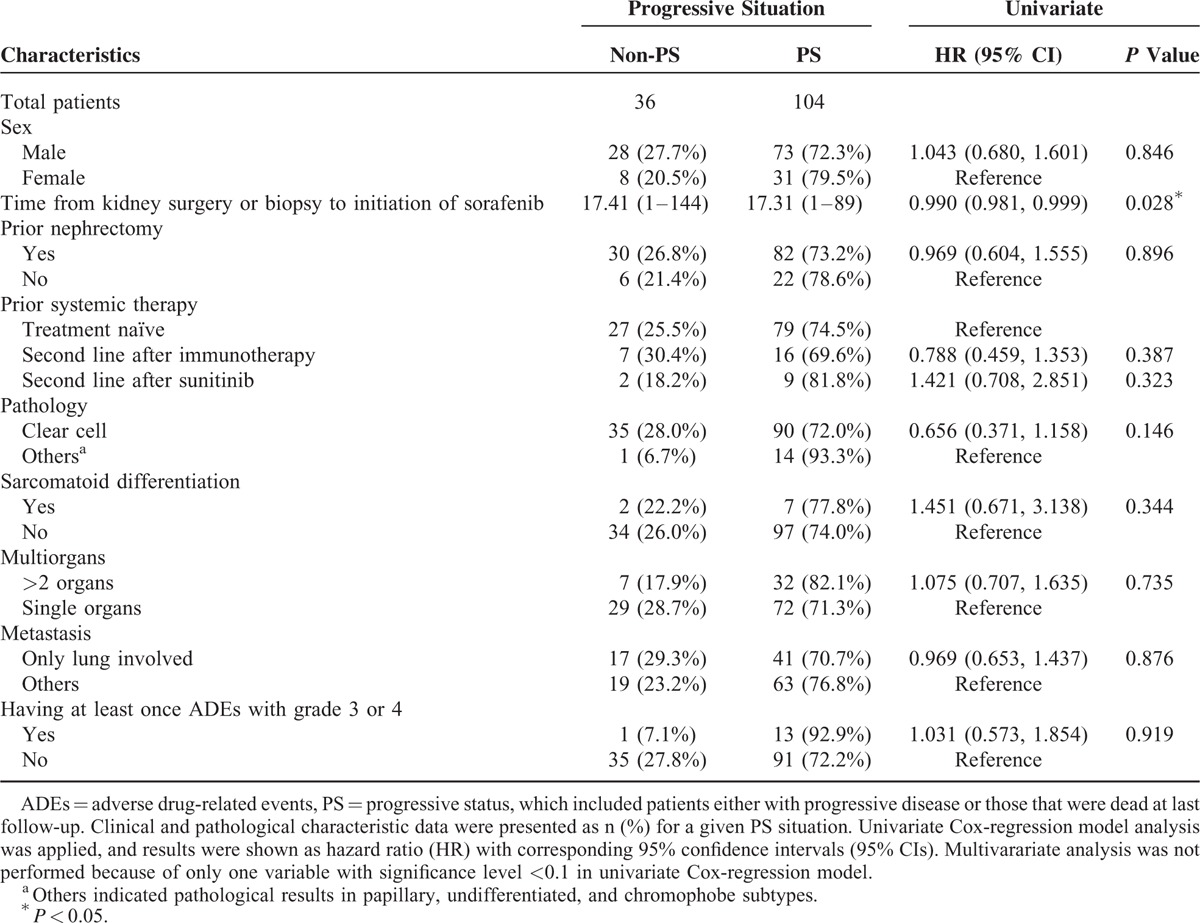
FIGURE 3.
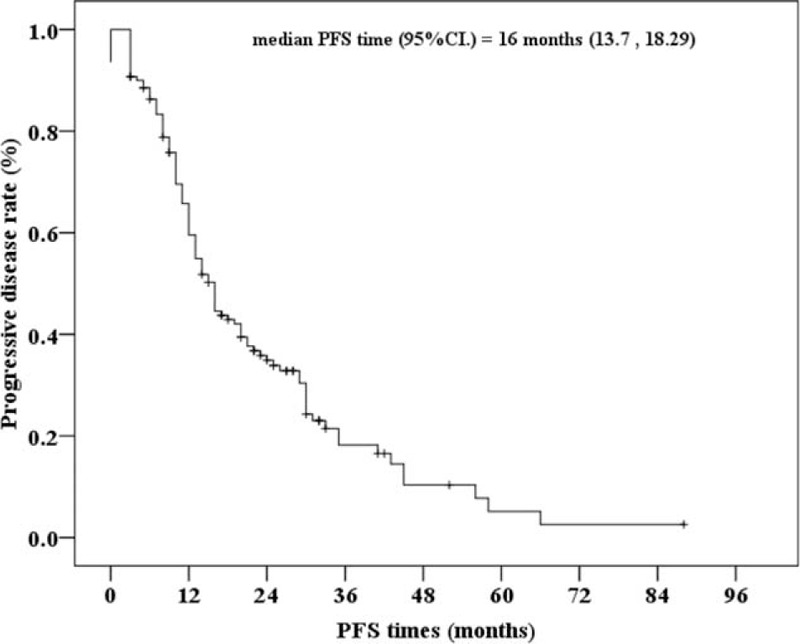
Kaplan–Meier curve of progressive-free survival (PFS) for 140 Chinese metastatic renal cell carcinoma patients treated with sorafenib. 95% CI, 95% confidence intervals of median PFS times. “+” indicates censored cases. The median PFS times were derived at 16 months with 95% CI = 13.7–18.3 months.
Efficacy Evaluation
All of the 140 patients enrolled received sorafenib for at least 2 cycles and were included in the evaluation of treatment efficacy. The ORR was 22.1%. In addition, 3 (2.1%) patients achieved CRs, 28 (20.0%) patients reached PRs, 88 (62.9%) patients experienced SD for >2 cycles, and 21 (15.0%) patients developed PD. The ORR included patients with CR and PR. Moreover, the DCR, including patients with CR, PR, or SD, was 85.0%.
Analysis of Sorafenib Safety in mRCC Patients
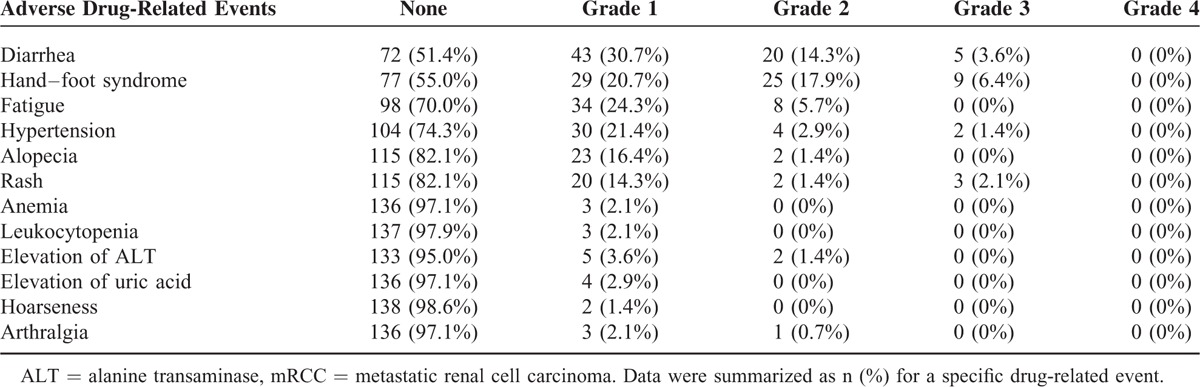
As shown in Table 4, the 6 most common ADEs after sorafenib initiation were diarrhea (48.6% of patients), hand–foot syndrome (45.0% of patients), fatigue (30.0% of patients), hypertension (25.7% of patients), alopecia (17.9% of patients), and rash (17.9% of patients). Other ADEs included anemia, leukocytopenia, elevated alanine transaminase, elevated uric acid, hoarseness, and arthralgia. Most ADEs were mild to moderate (grades 1 or 2; range, 1.4%–45%). However, some ADEs were severe (grades 3; range, 0%–6.4%; Table 4). In this study, a total of 40 patients received dose escalation (higher dose group); however, no correlation between higher sorafenib doses and severe ADEs were observed in this study cohort (data not shown).
TABLE 4.
Summary of the Adverse Drug-Related Events in Chinese mRCC Patients (N = 140)
DISCUSSION
Sorafenib improves PFS11 and OS13 in mRCC patients and is currently recommended as a second-line treatment option for those who failed prior immunotherapy.14 However, few studies have analyzed its efficacy in Chinese patients.19,20 In this retrospective study, the outcomes were analyzed in 140 Chinese mRCC patients treated with sorafenib with a median follow-up time of 32 months. The median OS was 24 months, and PFS was 16 months. Moreover, the ORR was 22.1%, and the DCR was 85.0% with manageable ADEs. The presence of clear cell carcinoma and the absence of sarcomatoid differentiation or disease progression might be associated with increased OS whereas the time from kidney surgery or biopsy to initiation of sorafenib treatment may be associated with PFS.
In the Treatment Approaches in Renal Cancer Global Evaluation Trial study, a phase III clinical trial involving 903 RCC patients treated in centers in Europe and in the United States, sorafenib significantly improved the PFS (5.5 vs 2.7 months)11 and OS (17.8 vs 14.3 months)13 of patients refractory to prior immunotherapy as compared to the placebo group. Moreover, the DCR in the sorafenib and placebo groups was 62% and 37%, respectively. However, studies in Chinese patients suggest that the efficacy of sorafenib may be even greater.2 In a study that included 98 mRCC patients, Zhang et al19 reported a PFS of 15 months, but the median follow-up time was relatively short, and the impact on OS was not evaluated. In another small study of 30 Chinese patients with advanced RCC, Yang et al20 reported that the OS and PFS was 16 and 14 months, respectively. Consistent with the previous studies evaluating the efficacy of sorafenib in Chinese mRCC patients,2 the median PFS in the present study was 16 months with ORR and DCR of 22.1% and 85.0%, respectively, which is relatively higher than previous studies in Western populations.11,13 It is also higher than a study of 82 Chinese patients with advanced kidney cancer treated with gemcitabine and IL-2, oxaliplatin and capecitabine, or sorafenib alone with PFS rates of 9.1 (95% CI = 7.9–10.3), 7.5 (95% CI = 5.5–9.5), and 10.9 (95% CI = 10.5–11.3) months, respectively.21 The improved response to sorafenib in Chinese patients may be attributed to the inherent genetic differences between the ethnic groups, which may result in differences in drug pharmacokinetics and pharmacodynamics. This theory is supported by similar findings in Japanese22,23 and Korean24 patients with advanced RCC. However, as shown in a recently published systemic review, the median OS and PFS for sorafenib-treated mRCC patients in Western countries varies.25 For example, the TIVO trial26 reported a median OS of 29.3 months, which is similar to the OS in the present study (24 months). Alternatively, the greater proportion of patients receiving dose escalation in this study may have contributed to better OS as dose escalation therapy may prove superior over therapy discontinuation in the case of disease progression.15,27,28 Therefore, further investigations are needed to determine if there are truly significantly different prognoses for various ethnic groups as well as to investigate the effects of documented tumor marker differences and varying molecular characteristics in different ethnic groups in order to select the best candidates for different targeted drugs.
In a small study that included 37 Chinese mRCC patients, Zhao et al29 found that the absence of symptoms, the absence of bone or pancreatic metastasis, and a relative dose intensity of targeting agents in the first month were all independently associated with OS. In the present study, 3 independent factors were identified that impacted the survival of mRCC patients treated with sorafenib. First, clear cell carcinoma appeared to offer a benefit to OS of 14 months, which was similar to previous studies in which nonclear cell histology was an adverse prognostic factor for predicting OS.30,31 In addition, we found that sarcomatoid differentiation and PD negatively impacted OS, and the time from kidney surgery or biopsy to initiation of sorafenib treatment may be associated with PFS. Although the association of tumor sarcomatoid differentiation with poor prognosis was consistent with the previous reports,32–34 this result should not be overinterpreted considering the small sample of patients with sarcomatoid differentiation (9 out of 140 patients). Thus, further studies are required to confirm this association.
In terms of ADEs related to sorafenib, the most commonly reported include hand–foot syndrome, rashes, allopecia, diarrhea, hypertension, and fatigue,35,36 which is consistent with those observed in the present study. However, the ADEs were manageable in that most were mild or moderate. Only 14 (10.0%) patients experienced severe, 19 grade 3 ADEs. Several previous studies have explored the correlation between ADEs and efficacy of tyrosine kinase inhibitors and suggest that the incidence of ADEs (even high-grade ADEs) may indicate better outcome.37–39 DiFiore et al40 suggested that severe clinical toxicities or grades 3 and 4 ADEs may result in better prognosis, with a median OS benefit of 24 months as compared to those without grades 3 and 4 ADEs. In contrast, ADEs were not associated with a longer OS in the present study, which may be because of differences in the pharmacokinetic and pharmacodynamic parameters between ethnic groups.
The present study is limited by its retrospective nature. In addition, although the patients lost to last follow-up was <10%, some ADEs may have been missed and some data were incomplete (eg, Eastern Cooperative Oncology Group performance status, Karnofsky performance scale, hemoglobin, and calcium concentration). Moreover, we did not investigate the specific biomarkers related to clear cell carcinoma and OS. Despite these limitations, our study is the largest multicenter retrospective study of Chinese patients treated with sorafenib with the longest follow-up to date. Further studies are needed to elucidate the underlying mechanism and biomarkers associated with efficacy or survival.
In summary, sorafenib monotherapy can achieve promising OS and PFS for Chinese patients with mRCC, especially for those with clear cell carcinoma, resulting in a satisfactory DCR and manageable ADEs.
Acknowledgments
The authors would like to thank the entire staff of the Department of Urology, Peking University First Hospital and Chinese People's Liberation Army General Hospital, Beijing, China, for their assistance.
Footnotes
Abbreviations: ADE = adverse drug event, CI = confidence interval, CR = complete response, DCR = disease control rate, HR = hazard ratio, IL-2 = interleukin-2, mRCC = metastatic renal cell carcinoma, ORR = objective response rate, OS = overall survival, PD = progressive disease, PFS = progression-free survival, PR = partial response, RCC = renal cell carcinoma, RECIST = Response Evaluation Criteria in Solid Tumors, SD = stable disease.
YX, GG, and LX equally contributed to this study.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30. [DOI] [PubMed] [Google Scholar]
- 2.Ye DW, Zhang HL. Critical appraisal of sorafenib in the treatment of Chinese patients with renal cell carcinoma. Onco Targets Ther 2014; 7:925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Staehler M, Haseke N, Schoeppler G, et al. Modern therapeutic approaches in metastatic renal cell carcinoma. EAU-EBU Update Series 2007; 5:26. [Google Scholar]
- 4.Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med 2005; 353:2477–2490. [DOI] [PubMed] [Google Scholar]
- 5.Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet 2009; 373:1119–1132. [DOI] [PubMed] [Google Scholar]
- 6.Coppin C, Porzsolt F, Awa A, et al. Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev 2005; CD001425. [DOI] [PubMed] [Google Scholar]
- 7.Bukowski RM. Cytokine therapy for metastatic renal cell carcinoma. Semin Urol Oncol 2001; 19:148. [PubMed] [Google Scholar]
- 8.Alt AL, Boorjian SA, Lohse CM, et al. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer 2011; 117:2873–2882. [DOI] [PubMed] [Google Scholar]
- 9.Meadows KL, Hurwitz HI. Anti-VEGF therapies in the clinic. Cold Spring Harb Perspect Med 2012; 2:a006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilhelm SM, Carter C, Tang L, et al. BAY43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004; 64:7009–7109. [DOI] [PubMed] [Google Scholar]
- 11.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007; 356:125–134. [DOI] [PubMed] [Google Scholar]
- 12.Rini BI. Vascular endothelial growth factor-targeted therapy in metastatic renal cell carcinoma. Cancer 2009; 115:2306–2312. [DOI] [PubMed] [Google Scholar]
- 13.Escudier B, Eisen T, Stadler WM, et al. Sorafenib for treatment of renal cell carcinoma: final efficacy and safety results of the phase III treatment approaches in renal cancer global evaluation trial. J Clin Oncol 2009; 27:3312–3318. [DOI] [PubMed] [Google Scholar]
- 14.Ljungberg B, Cowan NC, Hanbury DC, et al. EAU guidelines on renal cell carcinoma: the 2010 update. Eur Urol 2010; 58:398–406. [DOI] [PubMed] [Google Scholar]
- 15.Wang HK, Zhang HL, Zhu Y, et al. A Phase II trial of dosage escalation of sorafenib in Asian patients with metastatic renal cell carcinoma. Future Oncol 2014; 10:1941–1951. [DOI] [PubMed] [Google Scholar]
- 16.Duffaud F, Therasse P. New guidelines to evaluate the response to treatment in solid tumors. Bull Cancer 2000; 87:881–886. [PubMed] [Google Scholar]
- 17.Cancer Therapy Evaluation Program. Common Terminology Criteria for Adverse Events, Version 3.0, DCTD, NCI, NIH, DHHS. http://ctep.cancer.gov. August 9, 2006. [Google Scholar]
- 18.Jing W, Chen Y, Lu L, et al. Human umbilical cord blood-derived mesenchymal stem cells producing IL15 eradicate established pancreatic tumor in syngeneic mice. Mol Cancer Ther 2014; 13:2127–2137. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Dong B, Lu JJ, et al. Efficacy of sorafenib on metastatic renal cell carcinoma in Asian patients: results from a multicenter study. BMC Cancer 2009; 9:249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang L, Shi L, Fu Q, et al. Efficacy and safety of sorafenib in advanced renal cell carcinoma patients: results from a long-term study. Oncol Lett 2012; 3:935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng ZD, Qu SX, Liu YY, et al. Clinical controlled trial of first-line treatment for advanced kidney cancer. Natl Med J China 2012; 92:2984–2987. [DOI] [PubMed] [Google Scholar]
- 22.Akaza H, Tsukamoto T, Murai M, et al. Phase II study to investigate the efficacy, safety, and pharmacokinetics of sorafenib in Japanese patients with advanced renal cell carcinoma. Jpn J Clin Oncol 2007; 37:755–762. [DOI] [PubMed] [Google Scholar]
- 23.Naito S, Tsukamoto T, Murai M, et al. Overall survival and good tolerability of long-term use of sorafenib after cytokine treatment: final results of a phase II trial of sorafenib in Japanese patients with metastatic renal cell carcinoma. BJU Int 2011; 108:1813–1819. [DOI] [PubMed] [Google Scholar]
- 24.Park SJ, Lee JL, Park I, et al. Comparative efficacy of sunitinib versus sorafenib as first-line treatment for patients with metastatic renal cell carcinoma. Chemotherapy 2012; 58:468–474. [DOI] [PubMed] [Google Scholar]
- 25.Mayer N, Fishman MN, Tomshine J, et al. A systematic review of the efficacy and safety experience reported for sorafenib in advanced renal cell carcinoma (RCC) in the post-approval setting. PLoS One 2015; 10:e0120877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Motzer RJ, Bhargava P, Esteves B, et al. A phase III, randomized, controlled study to compare tivozanib with sorafenib in patients (pts) with advanced renal cell carcinoma (RCC). J Clin Oncol 2011; 29:310.21149663 [Google Scholar]
- 27.Amato R, Zhai J, Willis J, et al. A phase II trial of intra-patient dose-escalated sorafenib in patients with metastatic renal cell carcinoma. Clin Genitourin Cancer 2012; 10:153–158. [DOI] [PubMed] [Google Scholar]
- 28.Gore ME, Jones RJ, Ravaud A, et al. Efficacy and safety of intrapatient dose escalation of sorafenib as first-line treatment for metastatic renal cell carcinoma (mRCC). J Clin Oncol 2011; 29: Abstr 4609. [Google Scholar]
- 29.Zhao J, Huang X, Sun F, et al. Prognostic factors for overall survival with targeted therapy in Chinese patients with metastatic renal cell carcinoma. Can Urol Assoc J 2014; 8:E821–E827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol 2005; 23:832–841. [DOI] [PubMed] [Google Scholar]
- 31.Porta C, Procopio G, Carteni G, et al. Sequential use of sorafenib and sunitinib in advanced renal-cell carcinoma (RCC): an Italian multicenter retrospective analysis of 189 patient cases. BJU Int 2011; 108:E250–E257. [DOI] [PubMed] [Google Scholar]
- 32.Molina AM, Tickoo SK, Ishill N, et al. Sarcomatoid-variant renal cell carcinoma: treatment outcome and survival in advanced disease. Am J Clin Oncol 2011; 34:454–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Golshayan AR, George S, Heng DY, et al. Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. J Clin Oncol 2009; 27:235–241. [DOI] [PubMed] [Google Scholar]
- 34.Beuselinck B, Lerut E, Wolter P, et al. Sarcomatoid dedifferentiation in metastatic clear cell renal cell carcinoma and outcome on treatment with anti-vascular endothelial growth factor receptor tyrosine kinase inhibitors: a retrospective analysis. Clin Genitourin Cancer 2014; 12:e205–e214. [DOI] [PubMed] [Google Scholar]
- 35.Gollob JA, Rathmell K, Richmond TM, et al. Phase II trial of sorafenib plus interferon alfa-2b as first- or second-line therapy in patients with metastatic renal cell cancer. J Clin Oncol 2007; 25:3288–3295. [DOI] [PubMed] [Google Scholar]
- 36.Bhojani N, Jeldres C, Patard JJ, et al. Toxicities associated with the administration of sorafenib, sunitinib, and temsirolimus and their management in patients with metastatic renal cell carcinoma. Eur Urol 2008; 53:917–930. [DOI] [PubMed] [Google Scholar]
- 37.Li X-S, Wu X, Zhao P-J, et al. Efficacy and safety of sunitinib in the treatment of metastatic renal cell carcinoma. Chin Med J 2011; 124:2920–2924. [PubMed] [Google Scholar]
- 38.Rini BI, Cohen DP, Lu DR, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst 2011; 103:763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Poprach A, Pavlik T, Melichar B, et al. Skintoxicity and efficacy of sunitinib and sorafenibin metastatic renal cell carcinoma: a national registry-based study. Ann Oncol 2012; 23:3137. [DOI] [PubMed] [Google Scholar]
- 40.DiFiore F, Rigal O, Menager C, et al. Severe clinical toxicities are correlated with survival in patients with advanced renal cell carcinoma treated with sunitinib and sorafenib. Br J Cancer 2011; 105:1811–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]


