Supplemental Digital Content is available in the text
Abstract
Although epidermal growth factor receptor (EGFR) monoclonal antibodies (mAbs) have been proved synergistic effect when combined with cytotoxic agents for advanced nonsmall cell lung cancer (NSCLC), the results of relevant clinical trials remain controversial. The purpose of this meta-analysis was to assess the advantage and toxicity profile of chemotherapy plus EGFR-mAbs versus chemotherapy alone for patients with NSCLC.
We rigorously searched electronic databases for eligible studies reporting EGFR-mAbs combined with chemotherapy versus chemotherapy alone for patients with advanced NSCLC. The primary outcome was overall survival (OS). Pooled results were calculated using proper statistical methods.
Nine phase II/III randomized controlled trials involved a total of 4949 participants were included. In general, compared with chemotherapy alone, the addition of EGFR-mAbs significantly improved OS (hazard ratio [HR] = 0.91, 95% confidence interval [CI]: 0.86–0.97, P = 0.006), progression-free survival (HR = 0.83, 95% CI: 0.87–0.98, P = 0.01), response rate (odd ratio [OR] = 1.28, 95% CI: 1.12–1.47, P = 0.0003), and disease control rate (OR = 1.17, 95% CI: 1.01–1.36, P = 0.04). Subgroup analysis showed that apparent OS benefit present in patients with squamous NSCLC (HR = 0.83, 95% CI: 0.74–0.93, P = 0.001), and those treatment-naive population (HR = 0.88, 95% CI: 0.82–0.95, P = 0.0006). Several manageable adverse events were markedly increased by EGFR-mAbs, such as acne-like rash, infusion reactions, and diarrhea. The risk for some ≥Grade 3 toxicities, such as leukopenia, febrile neutropenia, and thromboembolic events were slightly increased by the addition of EGFR-mAbs. In general, the toxicities of the combination strategy were tolerable and manageable.
The addition of EGFR-mAbs to chemotherapy provided superior clinical benefit along with acceptable toxicities to patients with advanced NSCLC, especially those harboring squamous cancer and treatment-naive. Further validation in front-line investigation, proper selection of the potential benefit population by tumor histology, and development of prognostic biomarkers are warranted for future research and clinical application of EGFR-mAbs.
INTRODUCTION
For patients with advanced nonsmall cell lung cancer (NSCLC), the efficacy of chemotherapeutic has reached “therapeutic plateau” with a median overall survival (OS) of around 8 to 10 months.1–2 Despite the fact that the prognosis of patients with epidermal growth factor receptor (EGFR) or anaplastic lymphoma linase (ALK) positive mutation is significantly improved by targeted therapies, more than half of the patients without known driver mutations have no choice for target therapies mentioned above.3–6 Therefore, novel treatment strategies for patients with advanced NSCLC are still urgently required.
Since aberrant function of the EGFR pathway is vital in the development of NSCLC,7–9 and the expression rate of EGFR is relatively high (40% to 80%) in NSCLC,10–11 another kind of EGFR-targeting agents, including cetuximab, panitumumab, matuzumab and more recently, necitumumab, classified as monoclonal antibodies (mAbs), have been currently under extensive investigation.12–15 They have shown impressive activity when combined with radiation therapy and the potential to increase the effectiveness of some cytotoxic agents have been confirmed by preclinical data.8,16
Previous clinical trials have shown that the addition of EGFR-mAbs to platinum-based chemotherapy is both tolerable and feasible.17,18 However, other clinical trials, including recent study INSPIRE, failed to validate this conclusion.19–21 These conflicting results impede the interpretation and translation of EGFR-mAbs to clinical practice. Therefore, we conducted this systemic review and meta-analysis to evaluate the efficacy and safety of the addition of EGFR-mAbs to chemotherapy, compared with chemotherapy alone in patients with advanced NSCLC. Predefined subgroup analysis was conducted to identify the potential proper patient population.
METHODS
Search Strategy and Study Selection
This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement. No ethical approval and patient consent are required as all analysis were based on previous published studies.
We systematically searched the electronic databases including PubMed, Embase, and the Central Registry of Controlled Trials of the Cochrane Library (between inception to January 1, 2015), as well as the meeting records related to lung cancer from ASCO and ESMO databases (2010 to January 1, 2015). The keywords used in the literature search include “chemotherapy,” “NSCLC,” “cetuximab,” “nectitumumab,” “panitumumab,” “matuzumab,” and “combination.”
The purpose of this meta-analysis was to evaluate the efficacy and toxicity profile of standard chemotherapy plus EGFR-mAbs, compared with chemotherapy alone. Therefore, only randomized controlled trials (RCTs) that met the following criteria were included: Prospective phase II or III RCTs designed for patients with advanced NSCLC. Randomized assignment of participants to EGFR-mAbs (cetuximab, nectitumumab, panitumumab, or matuzumab) plus standard chemotherapy as experimental group or the corresponding chemotherapy as parallel control. No concurrent or sequential radiotherapy is allowed during the trial. One of the following outcomes must be reported: OS, progression-free survival (PFS), objective response rate (ORR), disease control rate (DCR), or toxicity profile.
Besides, the search was limited initially to English publications in humans. All potentially relevant publications were further retrieved and evaluated for inclusion. We also hand-searched references of relevant publications for additional studies. After rigorous screening, only eligibility studies were included in this meta-analysis.
Data Extraction and Quality Assessment
Our primary outcome was OS. Other measure outcomes included PFS, ORR, DCR, and toxicity profile. Two authors (WF and YM) performed the search independently to avoid bias in the data extraction process. Disagreement over eligibility of a study was resolved by consensus or by the third investigators. For each study, we extracted the key information as following: first author's name, year of publication, trial phase, line of treatment, number of participants, regimens for intervention and control arms, as well as the outcomes mentioned above.
Assessment of Risk of Bias in Included Studies
For each included study, we assessed the risk of bias following the Cochrane Collaboration guidelines (http://www.cochrane.de). Six domains were employed for this part including sequence generation, allocation concealment, blinding of participants or outcome assessment, incomplete outcome data, selective outcome reporting, and other sources of bias.
Statistical Analysis
Heterogeneity across studies was assessed with a forest plot and the inconsistency statistic (I2). A random-effects model was employed in case of the existence of potential heterogeneity (I2 ≥ 50%); otherwise, the fixed-effect model would be applied. We calculated the pooled hazard ratio (HR) for survival outcomes (PFS, OS) and pooled odd ratio (OR) for dichotomous data (ORR, DCR) with proper algorithm. Graphical funnel plots were generated to visually inspect for publication bias. All calculations were performed using Review Manager (version 5.2 for Windows; the Cochrane Collaboration, Oxford, UK). P < 0.05 was considered statistically significant for all analysis.
RESULTS
Study Characteristics
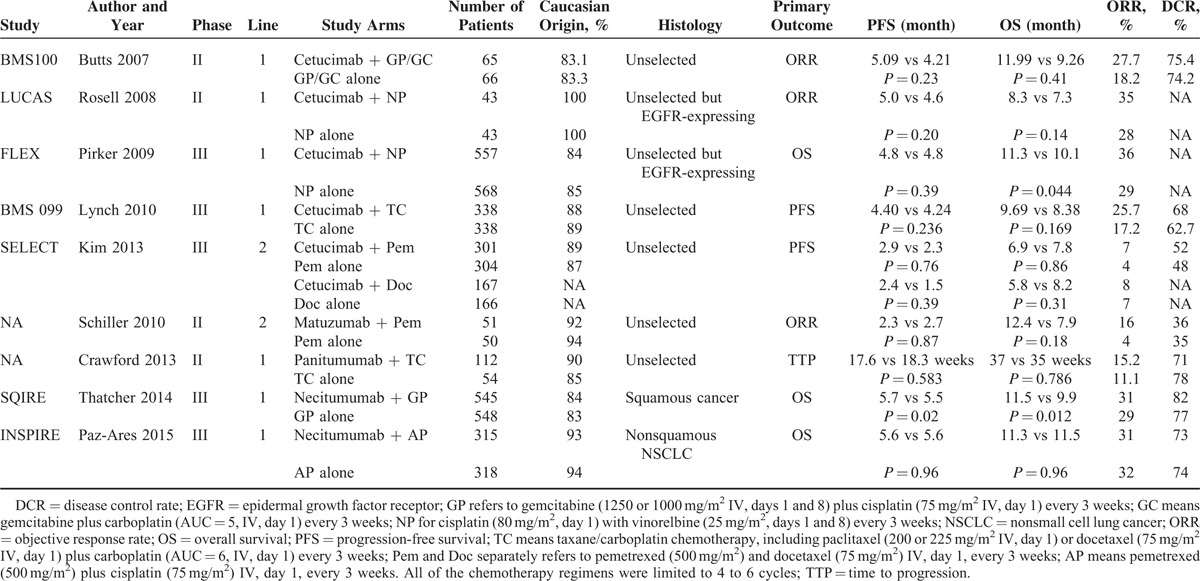
Figure 1 shows the flow chart reflecting the selection process for eligible RCTs. Among the potentially eligible trials, 9 studies with 4949 patients met the inclusion criteria after rigorously identification. Other potential eligible studies were excluded for reasons of single-armed, without chemotherapy combination or involved of radiotherapy. Among the included studies, there were 5 phase III RCTs.17–21 Seven trials9,10,17–19,21,23 were investigation in front-line, while the rest were second-line trial.20,22 Two RCTs9,17 conducted in selected population according to the expression of EGFR (immunohistochemical method, IHC). Two studies18,21 selected patients according to histological type. Furthermore, 4 agents (cetuximab,9,10,17,19,20 nectitumumab,18,21 panitumumab,23 or matuzumab22) with comparable data were identified. Only 3 studies17,18,21 were designed with OS as the primary outcome. All studies were designed with 2 arms except one20 phase III trial, which evaluated the efficacy and toxicity of the combination of cetuximab with docetaxel or pemetrexed, compared with docetaxel or pemetrexed alone. As regard histological type, 4 studies17–19,21 provided relevant subgroup information. The specific number of included study may vary according to the corresponding outcomes. All of the included studies provided outcomes about OS, PFS, and ORR. Data for DCR were available in 7 trials. Complete characteristics of selected trials were summarized in Table 1.
FIGURE 1.
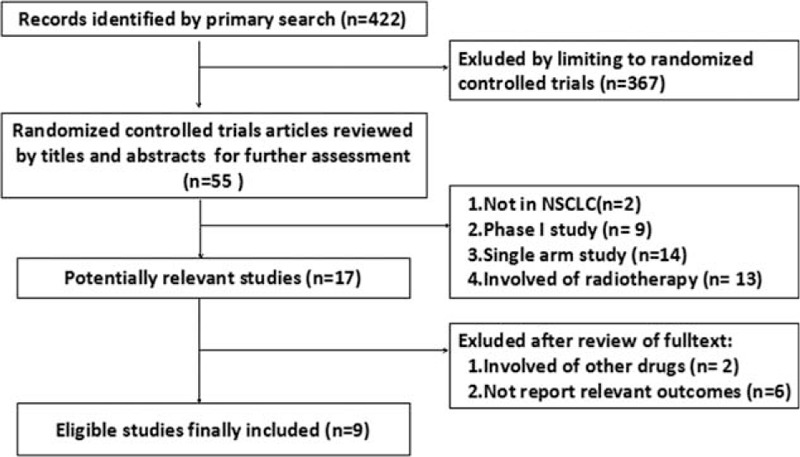
The flowchart of the process for selecting relevant articles.
TABLE 1.
Characteristics of Included Studies and Agents
Risk of Bias
All the eligible trials reported “randomization” and 3 studies provided the conduction details of the randomization. All of the included studies were marked with “open-label,” however, given the fact that the outcomes were assess by independent reviewers, the risk for blinding of participants or outcome assessment were defined as “unclear risk of bias.” Moreover, for most studies included in these meta-analyses, low risk of bias existed for other key domains, including incomplete outcome data, selective outcome reporting and other sources of bias. In general, no high risk of bias was detected as shown in Figure S1, http://links.lww.com/MD/A388.
Primary Outcome: OS
In general, the median OS of patients treated with EGFR-mAbs plus chemotherapy was superior to those treated with chemotherapy alone (HR was 0.91, 95% confidence interval [CI]: 0.86–0.97, P = 0.006). The result was shown in Figure 2. No significant heterogeneity was detected among the studies included for OS analysis (I2 = 15%).
FIGURE 2.
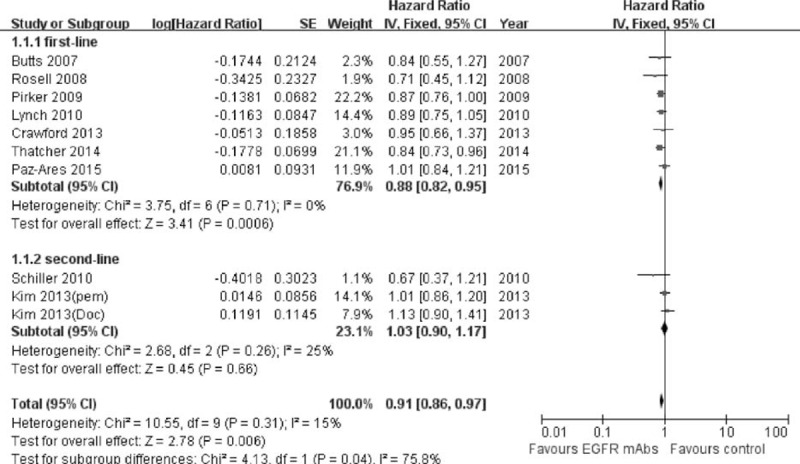
Forest plot and pooled HR and 95% CI for OS: Chemotherapy plus EGFR-mAbs versus chemotherapy alone for advanced NSCLC. EGFR = epidermal growth factor receptor; HR = hazard ratio; CI = confidence interval; NSCLC = nonsmall cell lung cancer; OS = overall survival.
Seven studies provided the detailed analysis in chemotherapy-naive patients. The median OS were 8.3 to 12.0 months for the combination group, compared with 7.3 to 11.5 months among the chemotherapy alone group in first-line setting. The pooled HR for OS was 0.88 (95% CI: 0.82–0.95, P = 0.0006) in favor of the addition of EGFR-mAbs to the first-line standard chemotherapy. However, it failed to provided additional survival benefit in second-line setting. The pooled HR was 1.03 (95% CI: 0.88–1.17, P = 0.66) according to the subgroup data of 2 studies.20,22
As shown in Figure 3, the addition of EGFR-mAbs to chemotherapy produced a significant OS improvement for patients with squamous cancer (HR = 0.83, 95% CI: 0.74–0.93, P = 0.001). The risk of death was decreased 17% by combination with EGFR-mAbs. Similarly, there were 3 studies provided the result of the adenocarcinoma subgroup. However, this group population only got slightly survival improvement from the addition of EGFR-mAbs and the pooled HR was 0.95 (95% CI: 0.85–1.07, P = 0.43).
FIGURE 3.
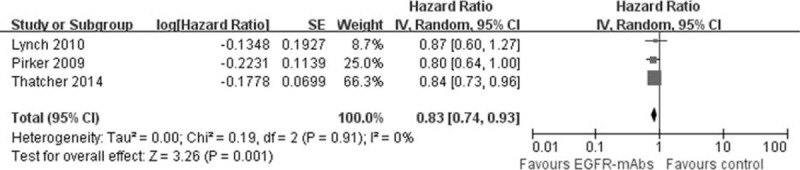
Forest plot and pooled HR and 95% CI for OS according to histology: (A) squamous cell carcinoma; (B) adenocarcinoma. HR were calculated for chemotherapy plus EGFR-mAbs versus chemotherapy alone. CI = confidence interval; EGFR = epidermal growth factor receptor; HR = hazard ratio; OS = overall survival.
Secondary Outcomes: PFS, ORR, DCR, and Serious Adverse Effects
There was a favorable trend for the addition of EGFR-mAbs to the present standard chemotherapy in PFS, ORR, and DCR. As shown in Figure 4, the risk of disease progression was slightly but significantly decreased by 7% compared with the control group (pooled HR was 0.93, 95% CI: 0.87–0.98, P = 0.01). Meanwhile, the addition of EGFR-mAbs to chemotherapy also significantly improved the ORR (pooled OR was 1.28, 95% CI: 1.12–1.47, P = 0.0003) and DCR (pooled OR was 1.17, 95% CI: 1.01–1.36, P = 0.04). Detailed description can be found in Figures 5 and 6.
FIGURE 4.
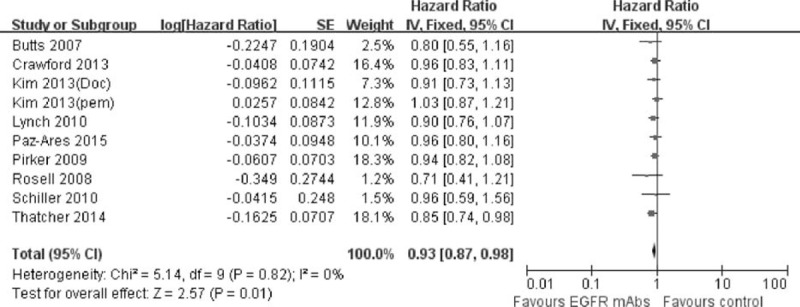
Forest plot and pooled HR and 95% CI for PFS: chemotherapy plus EGFR-mAbs versus chemotherapy alone for advanced NSCLC. CI = confidence interval; EGFR = epidermal growth factor receptor; HR = hazard ratio; NSCLC = nonsmall cell lung cancer; PFS = progression-free survival.
FIGURE 5.
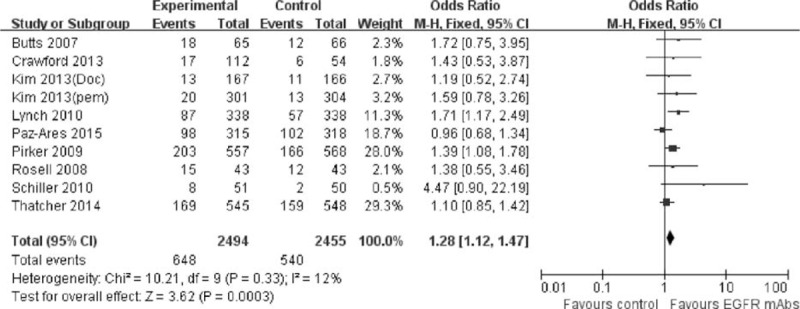
Forest plot and pooled OR and 95% CI for ORR: chemotherapy plus EGFR-mAbs versus chemotherapy alone for advanced NSCLC. CI = confidence interval; EGFR = epidermal growth factor receptor; NSCLC = nonsmall cell lung cancer; OR = odd ratio; ORR = objective response rate.
FIGURE 6.
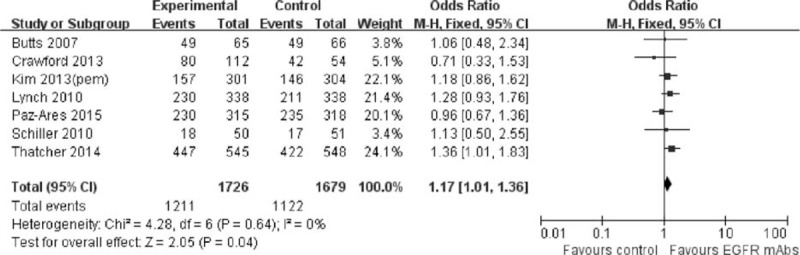
Forest plot and pooled OR and 95% CI for DCR: chemotherapy plus EGFR-mAbs versus chemotherapy alone for advanced NSCLC. CI = confidence interval; DCR = disease control rate; EGFR = epidermal growth factor receptor; NSCLC = nonsmall cell lung cancer; OR = odd ratio.
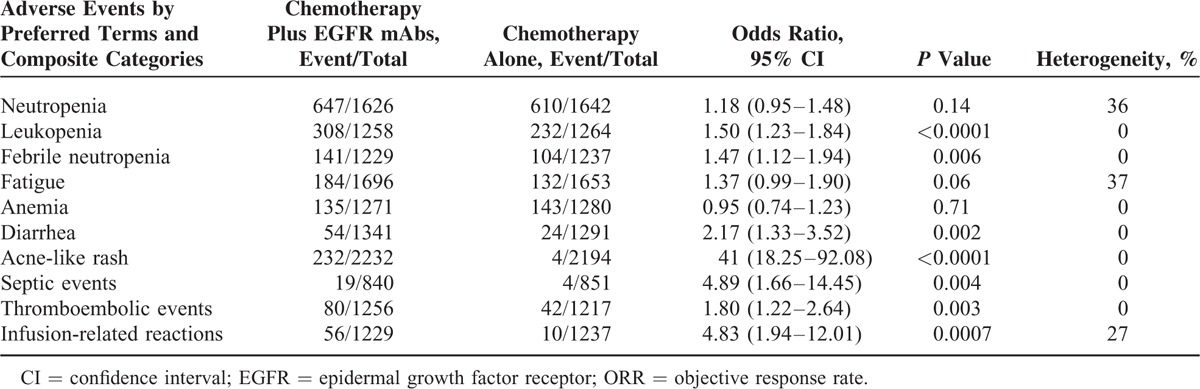
All of the included studies reported the serious adverse effects. We analyzed the adverse events by preferred terms and composite categories as shown in Table 2. In general, the addition of EGFR-mAbs was tolerable and manageable. Serious adverse effects for patients receiving chemotherapy plus EGFR-mAbs were mainly acne-like rash (weighted rate: 10.39% vs 0.18%; OR 41.00, 95% CI: 18.25–92.08, P < 0.0001), infusion-related reactions (weighted rate: 4.56% vs 0.81%; OR 4.83, 95% CI: 1.94–12.01, P = 0.0007) and diarrhea (weighted rate: 4.03% vs 1.86%; OR 2.17, 95% CI: 1.33–3.52, P = 0.002). Besides, the risk for some ≥Grade 3 toxicities, such as leukopenia, febrile neutropenia, and thromboembolic events also slightly increased by the addition of EGFR-mAbs, compared with chemotherapy alone. The combination regimens did not significantly increased the incidence of neutropenia, anemia, or fatigue.
TABLE 2.
Pooled ORR and 95% CI for Adverse Events by Preferred Terms and Composite Categories
Publication Bias
Highly sensitive search strategy and rigorous inclusion criteria have been applied to minimize the potential publication bias. Furthermore, according to the funnel plot conducted for assessment of publication bias, no significant asymmetry was detected for our primary outcome (Figure 7).
FIGURE 7.
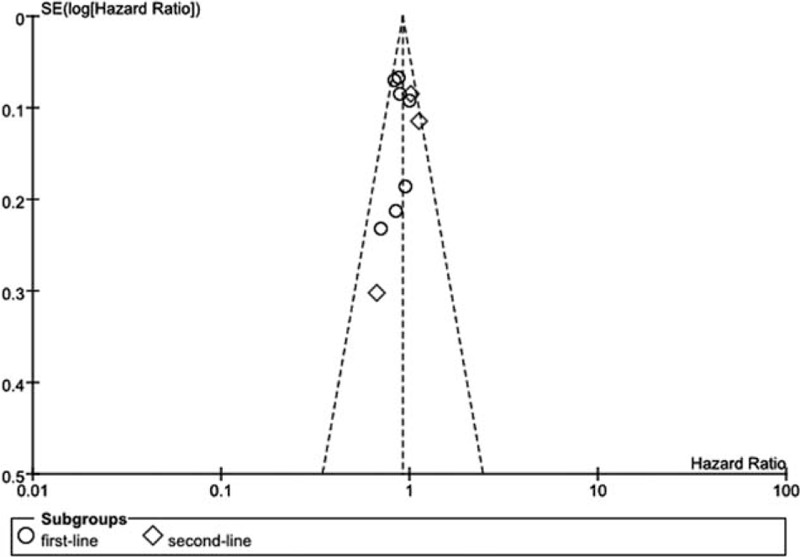
Funnel plot of included studies for primary outcome overall survival.
DISCUSSION
Nowadays, the role of EGFR as a therapeutic target has been well established. There are rational basis for EGFR mAbs to be combined with chemotherapy for advanced NSCLC in clinical practice. First, effective anti-EGFR-mAbs compete with endogenous ligands, primarily EGF, for receptor ligand-binding sites. This competitive binding blocks critical signaling pathways and suppress the growth of tumors expressing EGFR, which does not usually happen when TKIs are used.24 Second, preclinical research reveals that some EGFR mAbs can induce immunological reaction through antibody-dependent cell-mediated and complement-dependent pathway and enhance the cytotoxic effect of chemotherapy.24,25 However, results of clinical trials evaluating the effectiveness of addition of EGFR-mAbs to chemotherapy were controversial. Our meta-analysis confirmed that the addition of EGFR-mAbs to chemotherapy resulted in prolonged OS, progression-delaying effect, better response rate, and DCR than standard chemotherapy.
To our knowledge, our study is the first meta-analysis to collect data of all available RCTs on EGFR-mAbs combined with chemotherapy. Pujol et al26 had performed a meta-analysis of individual patient data from randomized trials of chemotherapy plus cetuximab as first-line treatment. However, our study included all the available EGFR-mAbs agents (cetuximab, nectitumumab, panitumumab, and matuzumab) and relevant high-quality RCTs to further explore the efficacy of EGFR-mAbs combined with standard chemotherapy. Yang et al27 also conducted a meta-analysis on similar subject, which found that the OS, 1-year survival rate, and ORR with chemotherapy plus cetuximab were apparently better than those with chemotherapy alone, but the differences in PFS were not significant. Our study, nevertheless, confirmed the apparent greater progression-delaying effect of addition of EGFR-mAbs. Possible explanation for this inconsistency was that another 5 RCTs were incorporated and the number of participant was doubled in our meta-analysis, the potential improvement trend in PFS was therefore demonstrated.
At present, there is no robust evidence for selecting the potential benefit population from EGFR-mAbs treatment by tumor histology. Results of recent studies implied that patients with squamous NSCLC might gain benefit from EGFR-mAbs. INSPIRE is a phase III RCT about the pemetrexed and cisplatin plus necitumumab (a second-generation recombinant human immunoglobulin G1 EGFR-mAbs that competitively inhibits ligand binding) as first-line therapy in patients with advanced nonsquamous NSCLC.21 This study fails to prove the efficacy benefit of necitumumab plus pemetrexed and cisplatin chemotherapy for above population setting. However, in study SQIRE, a similar trial designed for patients with squamous NSCLC, the addition of necitumumab to gemcitabine/cisplatin regimen produced significant OS and PFS improvement.18 Our study also found that patient harboring squamous NSCLC were the potential population to benefit from the addition of EGFR-mAbs (HR = 0.83, 95% CI: 0.74–0.93, P = 0.001) while those with adenocarcinoma were not (HR = 0.95, 95% CI: 0.85–1.07, P = 0.43). There are 2 explanations for this finding. First, it has been reported that the expression rate of EGFR is higher in patients with squamous-cell compared with nonsquamous-cell carcinomas.28 Meanwhile, further analysis of study FLEX based on prospectively collected data indicated only high EGFR expression (IHC score ≥200; score 0–300) could predict survival benefit associated with the addition of cetuximab to chemotherapy.17 Second, as the genomic complexity of squamous NSCLC is much more complicated than lung adenocarcinoma,29 the immunogenicity might be stronger in former subset. A recent study found that the stronger immunogenicity of squamous NSCLC led to better response to ipilimumab treatment than nonsquamous subset.30 Therefore, it is reasonable to assume that patients with squamous NSCLC may obtain more benefit from EGFR mAbs therapy due to the function of antibody-dependent cell-mediated cytotoxicity and complement activation.
Although robust evidence favor the addition of EGFR-mAbs to chemotherapy for treatment-naive patients, whether the addition of EGFR-mAbs is of value in second-line setting remains unknown. Therefore, we provided preliminary analysis based on 2 included studies to answer this question. In contrast to first-line setting, our result indicted that combination of EGFR-mAbs with standard second-line chemotherapy failed to provided additional survival benefit (pooled HR was 1.03, 95% CI: 0.88–1.17, P = 0.66). The underlying mechanism is still unclear. It is noteworthy that patients’ tolerability to treatment usually deteriorated after they failed from first-line chemotherapy. According to the result of SELECT study, the toxic effects were significantly worse for the cetuximab plus chemotherapy group than for the chemotherapy group alone in the second-line setting.22 This might compromise the potential benefit from the additional EGFR-mAbs treatment. Furthermore, the chemotherapy regimen given to the majority of patients in these 2 trails was single-agent pemetrexed. However, a preclinical study found anticancer synergy between cetuximab and docetaxel, gemcitabine, cisplatin, rather than pemetrexed.31 Therefore, ineffectiveness of the clinical combination of cetuximab and pemetrexed might also lead to the negative result in OS.
Given the safety concerns, our study revealed that serious adverse effects (≥Grade3) for patients receiving chemotherapy plus EGFR-mAbs were mainly acne-like rash, infusion-related reaction, diarrhea, leukopenia, febrile neutropenia, and thromboembolic. The combination regimens did not significantly increased the incidence of neutropenia, anemia, or fatigue. This toxicity profile of combination of EGFR-mAbs with chemotherapy was consistent with those described in previous reports. In general, the safety profile of this combination was acceptable and manageable according to original studies.
The present meta-analyses are limited by the heterogeneity of various agents employed in the individual trials. Besides, our work was not based on individual patient data. Other limitations include publication status as ongoing studies were ineligible for inclusion. However, here we presented the first meta-analysis illustrating the clinical efficacy of combining EGFR-mAbs with chemotherapy over chemotherapy alone based on available data from recent 9 RCTs.
CONCLUSION
The addition of EGFR-mAbs to chemotherapy could provide superior clinical benefit to patients with advanced NSCLC, especially those harboring squamous cancer and in first-line setting. Further validation in front-line investigation, proper selection of the potential benefit population by tumor histology, and development of prognostic biomarkers are warranted for future research and clinical application of EGFR-mAbs.
Footnotes
Abbreviations: ADCC = antibody-dependent cell-mediated cytotoxicity, ALK = anaplastic lymphoma linase, AP = pemetrexed plus cisplatin, CI = confidence interval, CTLA-4 = cytotoxic T-cell lymphocyte antigen-4, DCR = disease control rate, Doc = docetaxel, EGFR = epidermal growth factor receptor, GC = gemcitabine plus carboplatin, GP = gemcitabine plus cisplatin, HR = hazard ratio, mAbs = monoclonal antibodies, NP = cisplatin with vinorelbine, NSCLC = nonsmall cell lung cancer, OR = odd ratio, ORR = objective response rate, OS = overall survival, Pem = pemetrexed, PFS = progression-free survival, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analysis, RCTs = randomized controlled trials, TC = taxane plus carboplatin.
Author contributions: Conceived and designed the experiments: JS. Contributed analysis tools: Y-XM and Y-XZ. Performed the experiments: Z-HH, TZ, and S-DH. Conducted the statistical analysis: Y-YZ and TQ. The manuscript was written by JS and Y-PY. Proofreading was provided by H-YZ and YH. The covering letter was from LZ.
This work was supported by the following funds: National High Technology Research and Development Program of China (Grant No. 2012AA02A502). Innovative drug R&D center based on real-time high-throughput cell-based screening platform and large capacity compound library (Grant No. 2013ZX09401003-002). National Natural Science Funds of China (Grant No. 81372502). Wu Jieping Medical Foundation Project (Grant No. 320.6750.131). All the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Azzoli CG, Temin S, Giaccone G. 2011 Focused update of 2009 American Society of Clinical Oncology clinical practice guideline update on chemotherapy for stage IV non-small-cell lung cancer. J Oncol Pract 2012; 8:63–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med 2002; 346:92–98. [DOI] [PubMed] [Google Scholar]
- 3.Garassino MC, Martelli O, Broggini M, et al. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol 2013; 14:981–988. [DOI] [PubMed] [Google Scholar]
- 4.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13:239–246. [DOI] [PubMed] [Google Scholar]
- 5.Dillon B, Naidoo B, Knight H, et al. NICE guidance on erlotinib for first-line treatment of EGFR-TK mutation-positive advanced or metastatic non-small-cell lung cancer. Lancet Oncol 2012; 13:764–765. [DOI] [PubMed] [Google Scholar]
- 6.Shaw AT, Yeap BY, Solomon BJ, et al. Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol 2011; 12:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kari C, Chan TO, de Rocha QM, et al. Targeting the epidermal growth factor receptor in cancer: apoptosis takes center stage. Cancer Res 2003; 63:1–5. [PubMed] [Google Scholar]
- 8.Baselga J, Arteaga CL. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol 2005; 23:2445–2459. [DOI] [PubMed] [Google Scholar]
- 9.Rosell R, Robinet G, Szczesna A, et al. Randomized phase II study of cetuximab plus cisplatin/vinorelbine compared with cisplatin/vinorelbine alone as first-line therapy in EGFR-expressing advanced non-small-cell lung cancer. Ann Oncol 2008; 19:362–369. [DOI] [PubMed] [Google Scholar]
- 10.Butts CA, Bodkin D, Middleman EL, et al. Randomized phase II study of gemcitabine plus cisplatin or carboplatin, with or without cetuximab, as first-line therapy for patients with advanced or metastatic non small-cell lung cancer. J Clin Oncol 2007; 25:5777–5784. [DOI] [PubMed] [Google Scholar]
- 11.Herbst RS, Shin DM. Monoclonal antibodies to target epidermal growth factor receptor-positive tumors: a new paradigm for cancer therapy. Cancer 2002; 94:1593–1611. [DOI] [PubMed] [Google Scholar]
- 12.Ciardiello F, Tortora G. EGFR antagonists in cancer treatment. N Engl J Med 2008; 358:1160–1174. [DOI] [PubMed] [Google Scholar]
- 13.Schiller JH. Developments in epidermal growth factor receptor-targeting therapy for solid tumors: focus on matuzumab (EMD 72000). Cancer Invest 2008; 26:81–95. [DOI] [PubMed] [Google Scholar]
- 14.Weiner LM, Belldegrun AS, Crawford J, et al. Dose and schedule study of panitumumab monotherapy in patients with advanced solid malignancies. Clin Cancer Res 2008; 14:502–508. [DOI] [PubMed] [Google Scholar]
- 15.Kuenen B, Witteveen PO, Ruijter R, et al. A phase I pharmacologic study of necitumumab (IMC-11F8), a fully human IgG1 monoclonal antibody directed against EGFR in patients with advanced solid malignancies. Clin Cancer Res 2010; 16:1915–1923. [DOI] [PubMed] [Google Scholar]
- 16.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene 2000; 19:6550–6565. [DOI] [PubMed] [Google Scholar]
- 17.Pirker R, Pereira JR, Szczesna A, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009; 373:1525–1531. [DOI] [PubMed] [Google Scholar]
- 18.Thatcher N, Hirsch FR, Szczesna A, et al. A randomized, multicenter, open-label, phase III study of gemcitabine-cisplatin (GC) chemotherapy plus necitumumab (IMC-11F8/LY3012211) versus GC alone in the first-line treatment of patients (pts) with stage IV squamous non-small cell lung cancer (sq-NSCLC). J Clin Oncol 2014; 32:5s.(Abstr 8008). [Google Scholar]
- 19.Lynch TJ, Patel T, Dreisbach L, et al. Cetuximab and first-line taxane/carboplatin chemotherapy in advanced non-small-cell lung cancer: results of the randomized multicenter phase III trial BMS099. J Clin Oncol 2010; 28:911–917. [DOI] [PubMed] [Google Scholar]
- 20.Kim ES, Neubauer M, Cohn A, et al. Docetaxel or pemetrexed with or without cetuximab in recurrent or progressive non-small-cell lung cancer after platinum-based therapy: a phase 3, open-label, randomised trial. Lancet Oncol 2013; 14:1326–1336. [DOI] [PubMed] [Google Scholar]
- 21.Paz-Ares L, Mezger J, Ciuleanu TE, et al. Necitumumab plus pemetrexed and cisplatin as first-line therapy in patients with stage IV non-squamous non-small-cell lung cancer (INSPIRE): an open-label, randomised, controlled phase 3 study. Lancet Oncol 2015; 16:328–337. [DOI] [PubMed] [Google Scholar]
- 22.Schiller JH, von Pawel J, Schutt P, et al. Pemetrexed with or without matuzumab as second-line treatment for patients with stage IIIB/IV non-small cell lung cancer. J Thorac Oncol 2010; 5:1977–1985. [DOI] [PubMed] [Google Scholar]
- 23.Crawford J, Swanson P, Schwarzenberger P, et al. A phase 2 randomized trial of paclitaxel and carboplatin with or without panitumumab for first-line treatment of advanced non-small-cell lung cancer. J Thorac Oncol 2013; 8:1510–1518. [DOI] [PubMed] [Google Scholar]
- 24.Houghton AN, Scheinberg DA. Monoclonal antibody therapies-a “constant” threat to cancer. Nat Med 2000; 6:373–374. [DOI] [PubMed] [Google Scholar]
- 25.Dienstmann R, Tabernero J. Necitumumab, a fully human IgG1 mAb directed against the EGFR for the potential treatment of cancer. Curr Opin Investig Drugs 2010; 11:1434–1441. [PubMed] [Google Scholar]
- 26.Pujol JL, Pirker R, Lynch TJ, et al. Meta-analysis of individual patient data from randomized trials of chemotherapy plus cetuximab as first-line treatment for advanced non-small cell lung cancer. Lung Cancer J Iaslc 2014; 83:211–218. [DOI] [PubMed] [Google Scholar]
- 27.Yang ZY, Liu L, Mao C, et al. Chemotherapy with cetuximab versus chemotherapy alone for chemotherapy-naive advanced non-small cell lung cancer. Cochrane Database Syst Rev 2014; 11:D9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jeon YK, Sung SW, Chung JH, et al. Clinicopathologic features and prognostic implications of epidermal growth factor receptor (EGFR) gene copy number and protein expression in non-small cell lung cancer. Lung Cancer J Iaslc 2006; 54:387–398. [DOI] [PubMed] [Google Scholar]
- 29.Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012; 489:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lynch TJ, Bondarenko I, Luft A, et al. Ipilimumab in combination with paclitaxel and carboplatin as first-line treatment in stage IIIB/IV non-small-cell lung cancer: results from a randomized, double-blind, multicenter phase II study. J Clin Oncol 2012; 30:2046–2054. [DOI] [PubMed] [Google Scholar]
- 31.Steiner P, Joynes C, Bassi R, et al. Tumor growth inhibition with cetuximab and chemotherapy in non-small cell lung cancer xenografts expressing wild-type and mutated epidermal growth factor receptor. Clin Cancer Res 2007; 13:1540–1551. [DOI] [PubMed] [Google Scholar]


