Abstract
Cisplatin, a major antineoplastic drug used in the treatment of solid tumors, is a known nephrotoxin. This retrospective cohort study evaluated the prevalence and severity of cisplatin nephrotoxicity in 54 children and its impact on height and weight.
We recorded the weight, height, serum creatinine, and electrolytes in each cisplatin cycle and after 12 months of treatment. Nephrotoxicity was graded as follows: normal renal function (Grade 0); asymptomatic electrolyte disorders, including an increase in serum creatinine, up to 1.5 times baseline value (Grade 1); need for electrolyte supplementation <3 months and/or increase in serum creatinine 1.5 to 1.9 times from baseline (Grade 2); increase in serum creatinine 2 to 2.9 times from baseline or need for electrolyte supplementation for more than 3 months after treatment completion (Grade 3); and increase in serum creatinine ≥3 times from baseline or renal replacement therapy (Grade 4).
Nephrotoxicity was observed in 41 subjects (75.9%). Grade 1 nephrotoxicity was observed in 18 patients (33.3%), Grade 2 in 5 patients (9.2%), and Grade 3 in 18 patients (33.3%). None had Grade 4 nephrotoxicity. Nephrotoxicity patients were younger and received higher cisplatin dose, they also had impairment in longitudinal growth manifested as statistically significant worsening on the height Z Score at 12 months after treatment. We used a multiple logistic regression model using the delta of height Z Score (baseline-12 months) as dependent variable in order to adjust for the main confounder variables such as: germ cell tumor, cisplatin total dose, serum magnesium levels at 12 months, gender, and nephrotoxicity grade. Patients with nephrotoxicity Grade 1 where at higher risk of not growing (OR 5.1, 95% CI 1.07–24.3, P = 0.04). The cisplatin total dose had a significant negative relationship with magnesium levels at 12 months (Spearman r = −0.527, P = <0.001).
INTRODUCTION
The success in cancer therapy has increased the remission rate and survival time of patients, it is now common that cancer survivors face the chronic effects of treatment such as ototoxicity, neurotoxicity, nephrotoxicity, and skeletal dysfunction.1–6
Cisplatin is a commonly used antineoplastic drug in the treatment of solid tumors. It binds to deoxyribonucleic acid (DNA), leading to intra- and interstrand cross-links that result in defective DNA templates and interfere with DNA synthesis and replication.7,8 It also affects mitochondria by inhibiting ATPase activity and causing apoptosis, oxidative stress, inflammation, and necrosis. It can be ototoxic, myelotoxic, gastrotoxic, neurotoxic, and nephrotoxic.9,10
In the kidney, the main damage occurs in the renal tubules.11 The most common adverse effects of tubular damage include hypomagnesemia, hypophosphatemia, hyponatremia, hypocalcemia, normoglycaemic-glucosuria, proteinuria, metabolic acidosis including Fanconi syndrome, and nephrogenic diabetes insipidus. Cisplatin can also produce glomerular damage (thrombotic microangiopathy) and acute kidney injury (AKI) as a result of acute tubular injury/necrosis, which can further progress to chronic kidney disease.3,12
Cytotoxic chemotherapy treatment has been linked to delayed longitudinal growth in children with cancer.13 Bone is the main repository of minerals in the body including calcium, phosphorus, magnesium, and trace elements. Bone formation is directly linked to longitudinal growth and in animal models cisplatin has shown to inhibit new bone formation.14 Data have also been published indicating that after standard cisplatin chemotherapy is completed, platinum exposure continues for at least 20 years. Whether chronically elevated platinum concentrations influence the development of these or other late toxicities including secondary malignancies is unknown.15
The aim of our study was to evaluate the prevalence and severity of cisplatin nephrotoxicity in children and to determine the impact of nephrotoxicity on growth.
PATIENTS AND METHODS
Study Participants and Design
Retrospective study of children treated with cisplatin for solid tumors that were part of a cohort studied for adverse reactions to chemotherapy, approved by the Hospital IRB and Ethics Committee at the Hospital Infantil de México Federico Gómez. This large public hospital receives patients referred from across the country. All patients or parents provided written informed consent.
Patients treated for childhood cancer (≤18 years of age at the start of therapy) received cisplatin therapy between June 2002 and May 2013.
The information about weight, height, serum creatinine, and electrolytes (magnesium, phosphorus, and potassium) before each cisplatin cycle and after 12 months of treatment was obtained from the medical record. We excluded patients with incomplete information.
The standard deviation score (Z Score) for height and weight was calculated with the app STAT GrowthCharts version 3.2 that uses the National Center for Health Statistics 2000 Center for Disease Control Growth Data.16 The Z Score was obtained by subtracting the actual patient weight or height from the mean weight or height of the population of that chronological age and gender divided by the appropriate standard deviation. A Z Score of 0 means that the patient has the average height or weight for age and gender. Values below −2.0 or +2.0 are outside normal growth charts.
Glomerular filtration rate (GFR) was estimated using Schwartz formula as serum creatinine was measured by Jaffe method.17 Hypomagnesemia was defined as Mg values <1.5 mg/dL. It was considered severe when Mg is ≤1.0 mg/dL.18 Hypophosphatemia was defined when phosphate values below age recommended values by the National Kidney Foundation clinical practice guidelines (KDIGO):19 6 to 12 months 5.0 to 7.8 mg/dL; 1 to 5 years 4.5 to 6.5 mg/dL; 6 to 12 years 3.6 to 5.8 mg/dL; and 13 to 20 years 2.3 to 4.5 mg/dL. Hypokalemia was defined when potassium values below age recommended values:20 1 month to 2 years 3.7 to 5.9 mEq/L; >2 years to 18 years 3.5 to 5.0 mEq/L.
Nephrotoxicity Definition
There are no standards to identify drug-induced kidney disease. We decided to grade nephrotoxicity considering a score that combines AKI definition21 and tubulopathy, manifested as electrolyte disturbances as follows: normal renal function (Grade 0); asymptomatic electrolyte disorders (hypomagnesemia, hypokalemia or hypophosphatemia), including an increase in serum creatinine, up to 1.5 times baseline value (Grade 1); need for electrolyte supplementation (magnesium, potassium, or phosphate) <3 months and/or increase in serum creatinine 1.5 to 1.9 times from baseline (Grade 2); increase in serum creatinine 2 to 2.9 times from baseline or need for electrolyte supplementation (magnesium, potassium, or phosphate) for more than 3 months after treatment completion (Grade 3); and increase in serum creatinine ≥3 times from baseline or renal replacement therapy (Grade 4).
The decision to initiate electrolyte supplementation was made by the treating oncologist.
A clinical pharmacologist, a pediatric nephrologist, a pediatric oncologist, and an adverse drug reaction surveillance clinician performed the clinical characterization.
Statistical Analysis
Descriptive statistics is reported as means and standard deviations or median (25th, 75th percentile) for continuous measures and percentages for binary/categorical measures. Differences between nephrotoxicity and nonnephrotoxicity patients were performed using Student's t-test, Mann–Whitney, or Fisher exact test depending upon the variable. Cisplatin total dose and nephrotoxicity grading was compared by Kruskal–Wallis test. Height and weight Z Score at baseline versus Z Score at 12 months of treatment was compared using paired t-test.
Repeated measures analysis of variance with Bonferroni post-test or Friedman test, depending upon the distribution of the variable, was used to analyze electrolyte, creatinine, and estimated GFR (eGFR) changes overtime.
Correlations between cisplatin dose and electrolyte levels at 12 months were determined by Pearson correlation coefficient. For all tests a P value <0.05 (2-sided) was considered statistically significant. All statistical analyses were performed using statistical analysis software (GraphPad Prism for MacOs X version 5.0).
For multivariate analysis we used a multiple logistic regression model using the delta of height Z score (baseline–12 months) as dependent variable in order to adjust for the main confounder variables such as germ cell tumor, cisplatin total dose, serum magnesium at 12 months, gender, and nephrotoxicity grade. We used the SPSS version 20 for Mac (IBM Corporation, Armonk, NY).
RESULTS
Patient Characteristics
We collected data on 61 cisplatin-treated patients that participated in the adverse drug event studies. Seven patients were excluded, 3 of them because of incomplete medical information in the clinical chart, and 4 because they did not complete cisplatin treatment. We have complete information for 54 patients (Figure 1). No patient received growth hormone.
FIGURE 1.
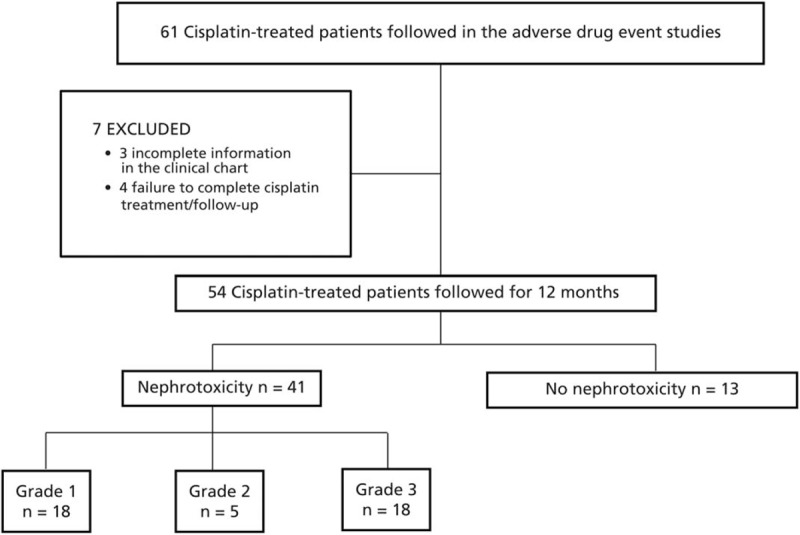
Flow diagram of the patients enrolled in the study.
Nephrotoxicity
Patient demographics are outlined in Table 1. Nephrotoxicity was observed in 41 patients (76%). No nephrotoxicity (Grade 0) was observed in 13 patients (24%). Grade 1 nephrotoxicity was observed in 18 patients (33.3%), Grade 2 in 5 patients (9.2%), and Grade 3 in 18 patients (33.3%). None had Grade 4 nephrotoxicity. Only 5 patients showed an increase in serum creatinine, 4 of them 1.5 to 1.9 from baseline and only one 2.5 times from baseline, all of them required electrolyte supplements, one patient recovered to baseline serum creatinine at 12 months of follow-up. The severity of nephrotoxicity was related to the cisplatin total dose (Figure 2), Kruskal–Wallis P = 0.008.
TABLE 1.
Patient Characteristics
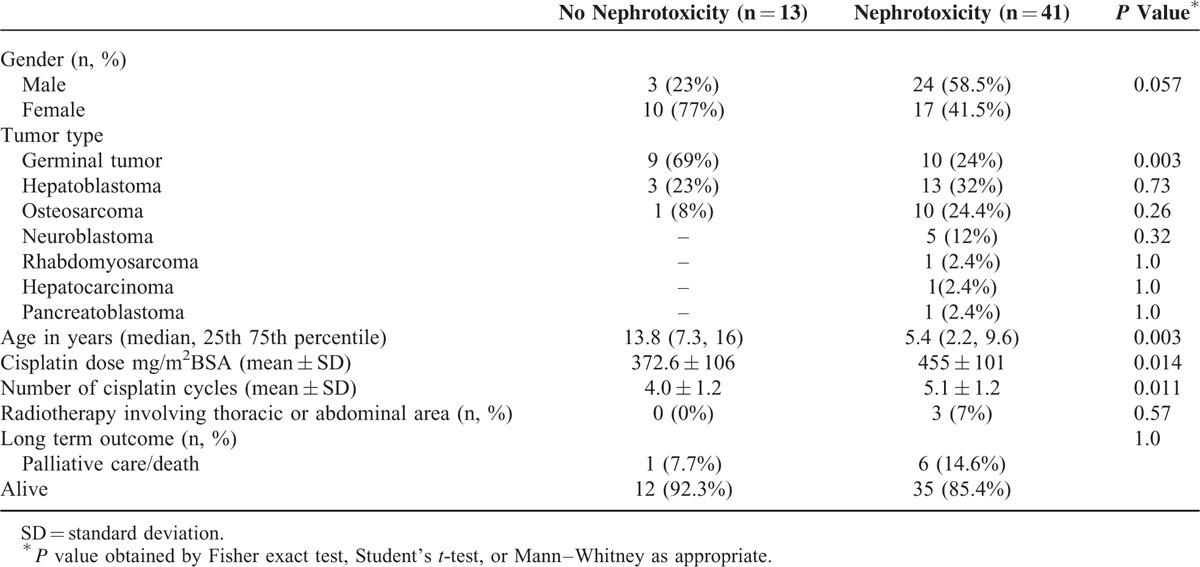
FIGURE 2.
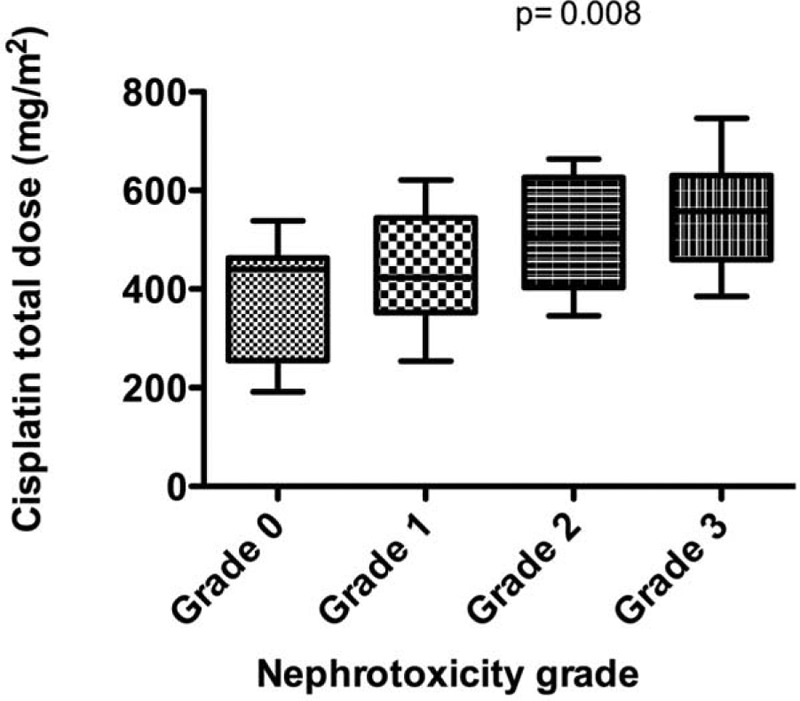
Cisplatin total dose and nephrotoxicity grade. Box and whisker plots show the 10th, 25th, 50th (median), 75th, and 90th percentile. The severity of nephrotoxicity increased with the cumulative dose (Kruskal–Wallis P = 0.008).
Hypophosphatemia was found in 35 patients (65%), hypomagnesemia in 22 (40.7%), and hypokalemia in 20 patients (37%). Combined hypomagnesemia and hypophosphatemia was observed in 19 patients (35%) and 8 had hypomagnesemia, hypophosphatemia, and hypokalemia (14.8%).
Patients with nephrotoxicity were younger than patients with nonnephrotoxicity, median age 5.4 versus 13.8 years, respectively (P = 0.003), they received higher number of cisplatin cycles and therefore higher cisplatin total dose (mean accumulated dose in nephrotoxicity 455 versus 372 mg/m2 in nonnephrotoxicity, P = 0.01). Patients with nephrotoxicity had more diverse tumors than nonnephrotoxicity patients in whom germinal tumors were more frequent (Table 1).
Concomitant Medications
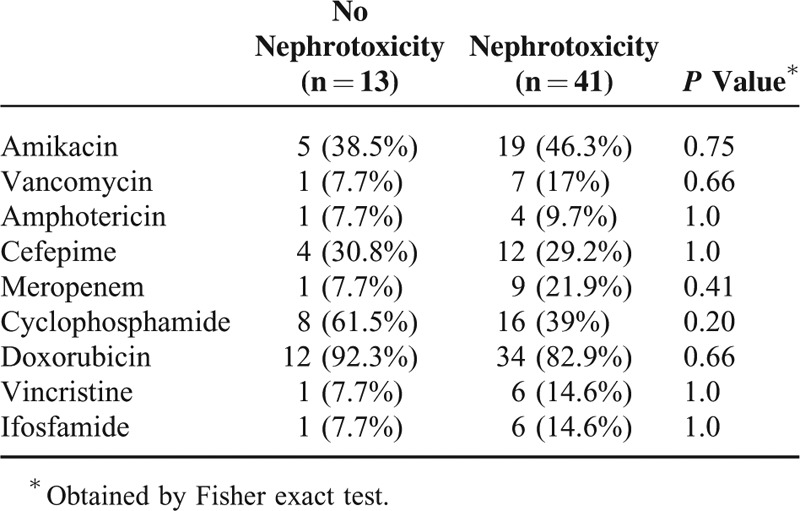
There was no difference in the concomitant medications administered to nephrotoxicity and nonnephrotoxicity patients (Table 2). Three patients from nonnephrotoxicity and 7 patients from nephrotoxicity group received amikacin/cefipime twice during the 1st year of chemotherapy.
TABLE 2.
Cisplatin Nephrotoxicity and Concomitant Medications (n, %)
Serum Electrolytes and Glomerular Filtration Rate Follow-up
Values of serum electrolytes, creatinine, and eGFR by Schwartz formula at baseline, 2nd, 3rd, 4th cisplatin cycle, and 12 months of follow-up are shown in Table 3. Phosphorus values showed a “U” shape overtime, starting with 4.43 ± 0.77 mEq/L, reaching the lowest value at the 3rd cisplatin cycle with 4.0 ± 0.90 mEq/L and the highest value at 12 months (4.74 ± 1.13 mEq/L). Phosphorus values were statistically significant different at the 2nd, 3rd, and 4th cisplatin cycles than at 12 months.
TABLE 3.
Cisplatin Cycle, Renal Function, and Electrolyte Levels
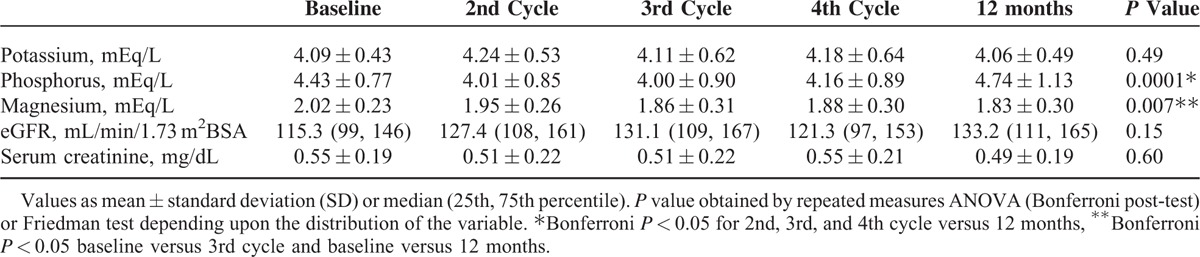
Magnesium level decreased each visit in a progressive way, being significantly lower at the 3rd cycle and at 12 months compared to baseline. Patients started cisplatin treatment with a magnesium level of 2.02 ± 0.23 and 1.83 ± 0.30 mg/dL at 12 months.
There was no statistically significant difference in serum potassium, creatinine, and eGFR. It is worth noting eGFR was not normally distributed and 12 patients had hyperfiltration (eGFR >150 mL/min/1.73 m2BSA).
Weight and Height Z Score
Patients without nephrotoxicity maintained their height Z Score after 12 months of treatment (baseline −1.37 ± 1.0, 12 months −1.56 ± 0.8, paired t-test P = 0.95, Figure 3A), whereas those with nephrotoxicity had worse height Z Scores after 12 months. Z Scores were more pronounced in those with nephrotoxicity Grade 1 (baseline −0.33 ± 1.2, 12 months −1.15 ± 1.1, P = 0.01, Figure 3B) than Grades 2 and 3 (baseline −0.34 ± 1.0 vs 12 months −0.8 ± 1.1, P = 0.04, Figure 3C). There was no difference in weight Z Score (basal vs 12 months) in all groups (Figure 3D–F).
FIGURE 3.
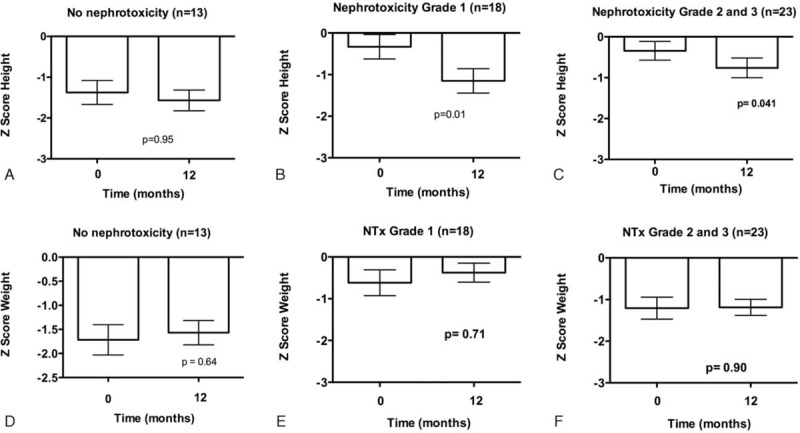
Nephrotoxicity grading and mean ± SE of height Z Score (A, B, C) and weight (D, E, F) at baseline and after 12 months of cisplatin treatment. P value obtained by paired t-test.
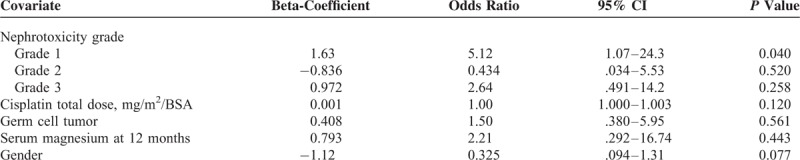
The results of the multivariate logistic analysis of risk factors for the lack of longitudinal growth are shown in Table 4. Patients with nephrotoxicity Grade 1 where at higher risk of not growing (OR 5.1, 95% CI 1.07–24.3, P = .04).
TABLE 4.
Multivariate Logistic Analysis of Risk Factors for the Lack of Longitudinal Growth at 12 months After Cisplatin Therapy
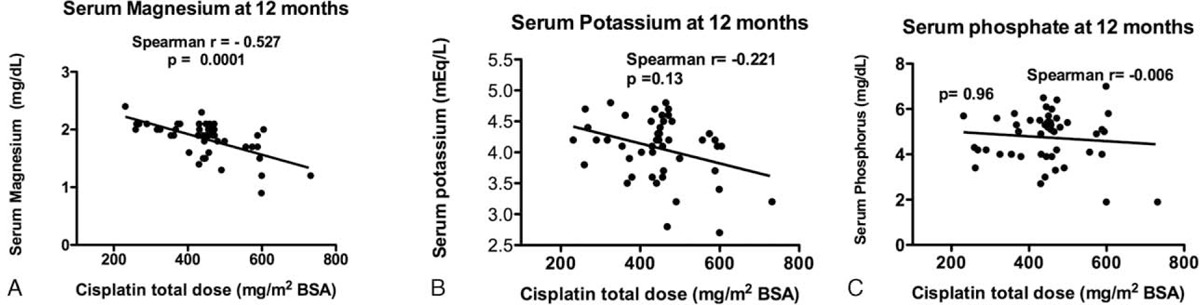
Cisplatin Total Dose and Correlation With Electrolytes at 12 months
The cisplatin total dose had a significant negative relationship with magnesium, potassium, and phosphate levels at 12 months, being statistically significant only for magnesium (Figure 4A, Spearman correlation −0.527, P = <0.001).
FIGURE 4.
Correlation of cisplatin total dose and serum electrolyte levels at 12 months. (A) Magnesium, (B) potassium, and (C) phosphate.
Patient Follow-Up
After the 12 months, 1 patient in the nonnephrotoxicity (Grade 0) group was in palliative care, 4 patients in the group with nephrotoxicity died, and 2 went to palliative care.
DISCUSSION
Our study confirms a high frequency (76%) of nephrotoxicity in cisplatin-treated children. In order to evaluate the cisplatin nephrotoxicity we propose a score that combines the AKI criteria considering the increase in baseline creatinine21 with an evaluation of the tubulopathy manifested as electrolyte disturbances, as this latter is the main and most frequent manifestation of cisplatin nephrotoxicity, but we included not only magnesium as Skinner et al have suggested11,22 but also phosphate and potassium.
Grade 1 (asymptomatic, nontreated electrolyte disturbances) and Grade 3 nephrotoxicity (increase in serum creatinine at 12 months 2–2.9 times from baseline or need for electrolyte supplementation for more than 3 months after treatment completion) had the same frequency, 33.3% in our study.
Nephrotoxicity patients received higher cisplatin dose as has been reported in other studies.11,23
An acute reduction in GFR with the respective increase in serum creatinine has been reported in 20% to 80% of cisplatin-treated children, with a GFR at 10 years after treatment <60 mL/min/1.73 m2 in 11%.22 In our study we found that 9.2% (5 patients) had an acute reduction in eGFR. Probably the proportion would be higher if we were able to use the new Schwartz bedside formula, a more precise equation to estimate GFR, unfortunately in our center serum creatinine is still measured by Jaffe method.
Hypomagnesemia has been pointed out as the most common manifestation of tubular toxicity present in 12% to 100% of cisplatin-treated patients.23 Nevertheless in the present study, using the KDIGO age-dependent phosphate values,19 hypophosphatemia was more frequent (65%) and persistent than hypomagnesemia (40.7%) in cisplatin-treated children. Ariceta et al23 demonstrated that all children treated with cisplatin present increased magnesiuria immediately after therapy, with a minimal dose to induce hypomagnesemia of 300 mg/m2BSA. We found that cisplatin total dose had a significant negative correlation with magnesium serum levels after 12 months of treatment.
We found that patients with nephrotoxicity have impairment in longitudinal growth manifested as statistically significant worsening on the height Z Score at 12 months after treatment, interestingly, patients with nephrotoxicity Grade 1 (asymptomatic, not treated) were the most affected.
Our study has limitations such as the retrospective design, the small number of patients, single center, the lack of a control group of children with solid tumors not treated with platinum compounds, and absence of information about Tanner stage and nutritional status. The strength is that all patients had serum electrolyte and creatinine results before each cisplatin cycle and in the 12-month follow-up period.
Cisplatin was introduced in medical practice in 1970. The importance of hydration and electrolyte supplementation during infusion to prevent adverse events was described and integrated to management protocols in the 1980s and 1990s. During the study period (2002–2013) cisplatin infusion itself was provided in similar way, nevertheless there have been other improvements in the treatment of cancer patients at our Institution such as the related to surgical techniques, the use of filgastrim for neutropenia and the availability of a linear accelerator for targeted radiation therapy since 2010. Any of these changes could improve cancer outcomes and reduce complete reliance on cisplatin to treat solid tumors.
Childhood and adolescence are crucial times to acquire the peak bone mass. Phosphate is a key component of the hydroxiapatite crystals (Ca10(PO4)6(OH)2) in the bone mineral phase.24 Magnesium is also important mineral in bone health, and its depletion is related to low parathyroid hormone and bone demineralization.25–27
We suggest that the observed impairment in longitudinal growth in patients with nephrotoxicity may be due to phosphate and magnesium urinary losses; patients with asymptomatic, nontreated electrolyte abnormalities (nephrotoxicity Grade 1) are at higher risk of lack of longitudinal growth, cisplatin total dose has a negative relationship with magnesium serum levels at 12 months. There are studies in animal models that suggest that hypomagnesemia enhances cisplatin accumulation in renal tissue by upregulating the organic cation transporter 2 (OCT2) transporter.28 Circulating levels of cisplatin have been shown up to 20 years after therapy, thus long-term renal effects are expected.15,29
The molecular mechanisms of cisplatin-induced damage in the nonproliferating renal tubular cells are not fully understood. Cisplatin clearance by the kidney depends upon glomerular filtration and tubular secretion. Cisplatin accumulates in the kidney at higher concentrations than in the blood and other organs, contributing to kidney injury. This high drug concentration is attributed to several membrane transporters (Copper transporter 1 -CTR1/SLC31A1-, copper-transporting ATPase 1 -ATP7A-, copper-transporting ATPase 2 –ATP7B-, multidrug and toxin extrusion protein 1 – MATE1/SLC47A1-, OCT2).30–32
Further studies are needed to identify if genetic variants can predict the nephrotoxicity risk, as occur with the ototoxicity,33,34 and importantly if electrolyte supplementation can prevent the deleterious effect of nephrotoxicity on longitudinal growth and bone health.
Acknowledgments
The authors thank Fondos Federales Hospital Infantil de México Federico Gómez, HIM/2011/016 and HIM/2013/026. Mara Medeiros received a CONACYT grant 205627 for the support. The authors also thank the contributions of the Canadian Pharmacogenomics Network for Drug Safety (CPNDS) consortium for the discussion of the definition of cisplatin nephrotoxicity. The CPNDS consortium includes 13 pediatric academic health centres across Canada. Participants are arranged geographically by institution across Canada. Vancouver: BC Children's Hospital, Child & Family Research Institute, CMMT, POPi: Michael Hayden, Bruce Carleton, Colin Ross, Stuart MacLeod, Wyeth Wasserman, Craig Mitton, Anne Smith, Claudette Hildebrand, Lucila Castro Pastrana, Reza Ghannadan, Shahrad Rod Rassekh, Jonathan Lim, Fudan Miao, Henk Visscher, Kusala Pussegoda, Folefac Aminkeng, Michelle Higginson, Nasim Massah, Mojgan Yazdanpanah, Johanna Sistonen, Ricardo Jimenez, Adrienne Borrie, Ursula Amstutz, Shevaun Hughes, Kaitlyn Shaw, Marie deHaan, Satvir Dhoot, Amit P Bhavsar, Yuling Li, Jong W Lee. Calgary: Alberta Children's Hospital: Cheri Nijssen-Jordan, David Johnson, Linda Verbeek, Rick Kaczowka, Patti Stevenson, Andrea Hurton, Carnation Zhuwaki. Edmonton: Stollery Children's Hospital: Paul Grundy, Kent Stobart, Bev Wilson, Sunil Desai, Maria Spavor, Linda Churcher, Terence Chow. Winnipeg: Winnipeg Health Sciences Centre: Kevin Hall, Nick Honcharik, Sara Israels, Shanna Chan, Byron Garnham, Michelle Staub. London: London Health Sciences Centre: Michael Rieder, Becky Malkin. Hamilton: McMaster Children's Hospital: Carol Portwine, Amy Cranston. Toronto: Hospital for Sick Children: Gideon Koren, Shinya Ito, Paul Nathan, Mark Greenberg, Facundo Garcia Bournissen, Miho Inoue, Sachi Sakaguchi, Toshihiro Tanaka, Hisaki Fujii, Mina Ogawa, Ryoko Ingram, Taro Kamiya, Smita Karande, Sholeh Ghayoori. Kingston: Kingston General Hospital: Mariana Silva, Stephanie Willing. Ottawa: Children's Hospital of Eastern Ontario: Régis Vaillancourt, Pat Elliott-Miller, Donna Johnston, Herpreet Mankoo, Elaine Wong, Brenda Wilson, Lauren O’Connor, Caleb Hui, Cindy Yeun. Health Canada: Maurica Maher. Montréal: Hôpital Sainte-Justine: Jean-Francois Bussières, Denis Lebel, Pierre Barret, Aurélie Closon, Eve Coulson, Lena Cerruti. Montreal Heart Institute: Marie-Pierre Dubé, Michael Phillips. McGill University Health Centre-Montréal Children's Hospital: Nada Jabado, Anelise Espirito Santo, Martine Nagy. McGill University: Denise Avard. Halifax: IWK Health Centre: Margaret Murray, Darlene Boliver, Marilyn Tiller, and Carol-Anne Osborne. St. John's: Janeway Children's Hospital: Lisa Goodyear, Jack Hand and Lynette Bowes, and Norma Kean.
Footnotes
Abbreviations: AKI = acute kidney injury, GFR = glomerular filtration rate, Z Score = standard deviation score.
A complete list of members listed at the end of the paper.
The study was supported by the Fondos Federales Hospital Infantil de México Federico Gómez, HIM/2011/016 and HIM/2013/026. Mara Medeiros received a CONACYT grant 205627.
The authors have no conflict of interest to disclose.
REFERENCES
- 1.Jones DP, Spunt SL, Green D, et al. Renal late effects in patients treated for cancer in childhood: a report from the Children's Oncology Group. Pediatr Blood Cancer 2008; 51:724–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lam AQ, Humphreys BD. Onco-nephrology: AKI in the cancer patient. Clin J Am Soc Nephrol 2012; 7:1692–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Hudson MM. Long-term complications following childhood and adolescent cancer: foundations for providing risk-based health care for survivors. CA Cancer J Clin 2004; 54:208–236. [DOI] [PubMed] [Google Scholar]
- 4.Perazella MA. Onco-nephrology: renal toxicities of chemotherapeutic agents. Clin J Am Soc Nephrol 2012; 7:1713–1721. [DOI] [PubMed] [Google Scholar]
- 5.Stava CJ, Jimenez C, Hu MI, et al. Skeletal sequelae of cancer and cancer treatment. J Cancer Surviv 2009; 3:75–88. [DOI] [PubMed] [Google Scholar]
- 6.Travis LB, Fossa SD, Sesso HD, et al. Chemotherapy-induced peripheral neurotoxicity and ototoxicity: new paradigms for translational genomics. J Natl Cancer Inst 2014; 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeConti RC, Toftness BR, Lange RC, et al. Clinical and pharmacological studies with cis-diamminedichloroplatinum (II). Cancer Res 1973; 33:1310–1315. [PubMed] [Google Scholar]
- 8.Marullo R, Werner E, Degtyareva N, et al. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One 2013; 8:e81162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coradini PP, Cigana L, Selistre SG, et al. Ototoxicity from cisplatin therapy in childhood cancer. J Pediatr Hematol Oncol 2007; 29:355–360. [DOI] [PubMed] [Google Scholar]
- 10.Miller RP, Tadagavadi RK, Ramesh G, et al. Mechanisms of cisplatin nephrotoxicity. Toxins 2010; 2:2490–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Skinner R, Pearson AD, English MW, et al. Cisplatin dose rate as a risk factor for nephrotoxicity in children. Br J Cancer 1998; 77:1677–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujieda M, Matsunaga A, Hayashi A, et al. Children's toxicology from bench to bed-drug-induced renal injury (2): nephrotoxicity induced by cisplatin and ifosfamide in children. J Toxicol Sci 2009; 34 Suppl 2:S251–S257. [DOI] [PubMed] [Google Scholar]
- 13.Cool WP, Grimer RJ, Carter SR, et al. Longitudinal growth following treatment for osteosarcoma. Sarcoma 1998; 2:115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stine KC, Wahl EC, Liu L, et al. Cisplatin inhibits bone healing during distraction osteogenesis. J Orthop Res 2014; 32:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gietema JA, Meinardi MT, Messerschmidt J, et al. Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet 2000; 355:1075–1076. [DOI] [PubMed] [Google Scholar]
- 16.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics 2002; 109:45–60. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am 1987; 34:571–590. [DOI] [PubMed] [Google Scholar]
- 18.Kaplinsky C, Alon US. Magnesium homeostasis and hypomagnesemia in children with malignancy. Pediatr Blood Cancer 2013; 60:734–740. [DOI] [PubMed] [Google Scholar]
- 19.National Kidney Foundation. KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. Am J Kidney Dis 2009; 53:S11–104. [DOI] [PubMed] [Google Scholar]
- 20.Arcara K, Tschudy M. The Harriet Lane Handbook: A Manual for Pediatric House Officers/the Harriet Lane Service, Children's Medical and Surgical Center of the Johns Hopkins Hospital. 2012; Philadelphia, PA: Elsevier Mosby, 1132. [Google Scholar]
- 21.Acute Kidney Injury Work Group. KDIGO clinical practice for acute kidney injury. Kidney Int Suppl 2012; 2:8–12. [Google Scholar]
- 22.Skinner R, Parry A, Price L, et al. Persistent nephrotoxicity during 10-year follow-up after cisplatin or carboplatin treatment in childhood: relevance of age and dose as risk factors. Eur J Cancer 2009; 45:3213–3219. [DOI] [PubMed] [Google Scholar]
- 23.Ariceta G, Rodriguez-Soriano J, Vallo A, et al. Acute and chronic effects of cisplatin therapy on renal magnesium homeostasis. Med Pediatr Oncol 1997; 28:35–40. [DOI] [PubMed] [Google Scholar]
- 24.Boskey AL. Bone composition: relationship to bone fragility and antiosteoporotic drug effects. Bonekey Rep 2013; 2:447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abrams SA, Chen Z, Hawthorne KM. Magnesium metabolism in 4-year-old to 8-year-old children. J Bone Miner Res 2014; 29:118–122. [DOI] [PubMed] [Google Scholar]
- 26.Orchard TS, Larson JC, Alghothani N, et al. Magnesium intake, bone mineral density, and fractures: results from the Women's Health Initiative Observational Study. Am J Clin Nutr 2014; 99:926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rude RK, Singer FR, Gruber HE. Skeletal and hormonal effects of magnesium deficiency. J Am Coll Nutr 2009; 28:131–141. [DOI] [PubMed] [Google Scholar]
- 28.Yokoo K, Murakami R, Matsuzaki T, et al. Enhanced renal accumulation of cisplatin via renal organic cation transporter deteriorates acute kidney injury in hypomagnesemic rats. Clin Exp Nephrol 2009; 13:578–584. [DOI] [PubMed] [Google Scholar]
- 29.Hjelle LV, Gundersen PO, Oldenburg J, et al. Long-term platinum retention after platinum-based chemotherapy in testicular cancer survivors: a 20-year follow-up study. Anticancer Res 2015; 35:1619–1625. [PubMed] [Google Scholar]
- 30.Filipski KK, Mathijssen RH, Mikkelsen TS, et al. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther 2009; 86:396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howell SB, Safaei R, Larson CA, et al. Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol 2010; 77:887–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Motohashi H, Inui K. Multidrug and toxin extrusion family SLC47: physiological, pharmacokinetic and toxicokinetic importance of MATE1 and MATE2-K. Mol Aspects Med 2013; 34:661–668. [DOI] [PubMed] [Google Scholar]
- 33.Pussegoda K, Ross CJ, Visscher H, et al. Replication of TPMT and ABCC3 genetic variants highly associated with cisplatin-induced hearing loss in children. Clin Pharmacol Ther 2013; 94:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wensing KU, Ciarimboli G. Saving ears and kidneys from cisplatin. Anticancer Res 2013; 33:4183–4188. [PubMed] [Google Scholar]


