Abstract
Our objective was to investigate the association of change of anthropometric measurements and the incidence of type 2 diabetes mellitus (T2DM) within a pooled sample of 2 population-based cohorts.
A final sample of 1324 women and 1278 men aged 31 to 83 years from 2 prospective cohorts in Germany, the CARLA (Cardiovascular Disease - Living and Ageing in Halle) and the SHIP study (Study of Health in Pomerania), were pooled. The association of change of body weight and waist circumference (WC) with incidence of T2DM was assessed by calculating sex-specific hazard ratios (HRs). We investigated the absolute change of markers of obesity as well as change relative to the baseline value and estimated crude and adjusted HRs. Furthermore, we conducted the analyses stratified by obesity status and age (<60 vs ≥60 years) at baseline.
Associations were found for both change of body weight and WC and incidence of T2DM in the crude and adjusted analyses. In the stratified study sample, those participants with a body mass index of <30 kg/m2 at baseline showed considerably lower HRs compared with obese women and men for both weight and WC. In the age-stratified analysis, we still found associations between change of weight and WC and incident T2DM with only marginal differences between the age groups.
Our study showed associations of change of weight and WC as markers of obesity with incidence of T2DM. Keeping a healthy and primarily stable weight should be the goal for preventing the development of T2DM.
INTRODUCTION
During the last decades, the prevalence and incidence of type 2 diabetes mellitus (T2DM) have risen dramatically,1 which cannot be explained by demographic change alone.2,3 There is strong need to further analyze the etiology of T2DM as well as investigate proper ways of prevention.
To date, the main risk factors for T2DM are well known-In addition to unchangeable risk factors like age, sex, and family history of diabetes, the most obvious risk factors lie within an unhealthy lifestyle, characterized by overweight/obesity,4,5 physical inactivity,6,7 smoking8 and an unbalanced diet.9,10 Health organizations like the World Health Organization, the International Diabetes Federation, or the American Diabetes Association recommend reaching for a stable and normal weight11–13 to reduce the risk of T2DM. Although the increase in risk due to excess body weight is well known, there are only a few studies that have scrutinized the role of weight change in the development of T2DM,4,14–21 and even less is known about the association between change of waist circumference (WC) and the incidence of T2DM.22
However, these studies have been done mostly in Asian or indigenous populations.18–20,23–25 As there are differences in body composition as well as in the risk of developing T2DM between different ethnicities,26,27 the results of these studies are not directly transferable to a European study population. Further studies of white populations, that are more comparable in terms of the composition of study participants, often used inappropriate methods like self-report of anthropometry.22,28 Moreover, these studies used different methods and the results were inconsistent. So the novelty of our study manifests itself in the structure of the study population as well as in the used methods and investigated markers of obesity.
Therefore, the objective of the present study was to investigate the association between changes in weight and WC with T2DM incidence in 2 independent population-based studies in Germany, where the prevalence and incidence of T2DM were found to be exceptionally high.29,30
METHODS
Study Population
We included data of 2 population-based longitudinal cohort studies from northeast Germany: The Cardiovascular Disease, Living and Ageing in Halle Study (CARLA), conducted in the urban area of Halle (Saale), and the Study of Health in Pomerania (SHIP) in rural West Pomerania.
Details on study design and methods have been described elsewhere.31,32 In brief, the baseline investigation was conducted between 2002 and 2006 in CARLA, obtaining a response of 64%, as well as between 1997 and 2001 in SHIP, achieving a response of 69%. Participants with an age ≤30 years were excluded from the analysis to minimize the risk of including cases of type 1 diabetes mellitus.
The first follow-up (FU1) investigations were conducted between 2007 and 2010 in CARLA and between 2002 and 2006 in SHIP. A second follow-up (FU2) examination was conducted in 2013 in CARLA and between 2008 and 2012 in SHIP. Thirty-five percent of the participants were lost to follow-up between baseline and FU2 (CARLA: 27%, SHIP: 38%).
Participants with diabetes at baseline or at FU1 (N = 827) were excluded from the study, as well as participants not attending the follow-up-examination (N = 1881) and those with missing data in the exposure or outcome variables (N = 99). Figure 1 shows the flow chart of our study population. The final study population consisted of 2602 individuals, 50.9% of whom were women.
FIGURE 1.
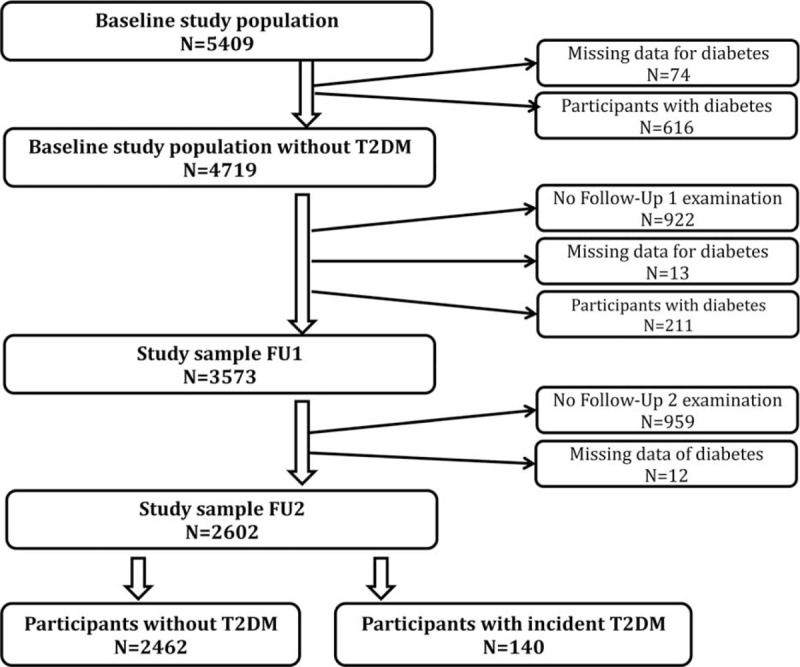
Study population and exclusions.
The studies were approved by the responsible ethic committees and the public data protection offices. All participants provided a written informed consent before study participation.
Definition of Incident T2DM
The incidence of T2DM was defined using self-reported physician's diagnoses of diabetes between FU1 and FU2, as well as newly prescribed antidiabetic medication, coded according to the Anatomical Therapeutic Chemical Classification (ATC) system, where code A10 was selected to define the current use of antidiabetic medication.
Ascertainment of Exposure and Covariates
In this study, exposure was defined as change of body weight and WC between baseline and FU1. Weight was measured with standardized measurement systems to an accuracy of 0.1 kg. WC was measured at the narrowest part between the lowest rib and the highest point of the iliac crest with a flexible but inelastic tape to the nearest 0.1 cm.
To determine social status, information on school education and professional education were coded using the “International Standard Classification of Education.”33 Information on current smoking status, duration of smoking, and number of cigarettes smoked was used to derive the variable of pack years (1 pack year ≙ 1 package of cigarettes smoked per day for 1 year). Alcohol consumption was dichotomized with values of either no or moderate consumption (male: 0–20 g/d; female: 0–10 g/d) or consumption outside of these limits. Sports activities during leisure time were also divided into 2 groups: participants with sports activities for at least 1 hour per week and a risk group with <1 hour of sports activities per week.
Based on the recommendations of the German Association for Nutrition,34 a simple score was generated out of the data of brown bread intake, red meat intake, and fruit and vegetable consumption because these nutritional components are known to be associated with T2DM. For each of these components, the participant received one score point if the intake corresponds to the recommendations. Consequently, the score had a range from “0” to “3” with each score-point corresponding to 1 item meeting the recommendations.
Statistical Analyses
The raw data of both studies were pooled to 1 dataset after harmonization in respect to content, categories, and units. Missing values for pack years (logarithmically transformed for the imputation process because of skewed distribution) (n = 3), alcohol consumption (n = 84), and physical activity (n = 1) were replaced by multiple imputation with 10 runs and age, sex, study region, education, and examination date as additional explanatory variables.35 Participants with missing values for anthropometric variables were excluded from the specific analyses that included these variables.
Because of a high dropout proportion, we tested the stability of the effects by conducting a weighted analysis. The use of these weights did not alter the effect estimates, so we only reported the unweighted results.
Descriptive baseline analyses of anthropometric markers, lifestyle factors, and socioeconomic aspects were performed for the final study population. For the calculation of incidence rates, we used the complete individual observation time for participants without incident T2DM and half of the individual observation time for participants with incident T2DM as we did not know the exact date of diagnosis.
We used change of weight and WC per year between baseline and FU1 as exposure variables and the incidence of T2DM between FU1 and FU2 as outcome. We estimated hazard ratios (HRs) as well as corresponding 95% confidence intervals (CIs), for the association of absolute change of markers of obesity and T2DM incidence. Due to the lack of information regarding the exact diagnosis date of T2DM, we used methods for interval censored time-to-event data. Age was used as time scale with age at baseline as the entry time. In a so-called delayed entry study, subjects are not observed until they are included in the study. Assuming that age at diabetes onset follows a Weibull distribution, we composed a proportional hazards model. It is similar to a generalized linear model, but includes a nonlinear term in the Weibull distribution for individual ages at baseline and at follow-up. Jain et al36 described these methods in another context. We tested for nonlinear relations by applying restricted cubic splines, indicating that the log-linearity transformation did fit the data best according to the Akaike information criterion.37
We selected confounders (weight or WC at baseline, study region, physical activity, alcohol consumption, education, dietary habits, and pack years) with the help of directed acyclic graphs38 and adjusted for these variables (Figure 2).
FIGURE 2.
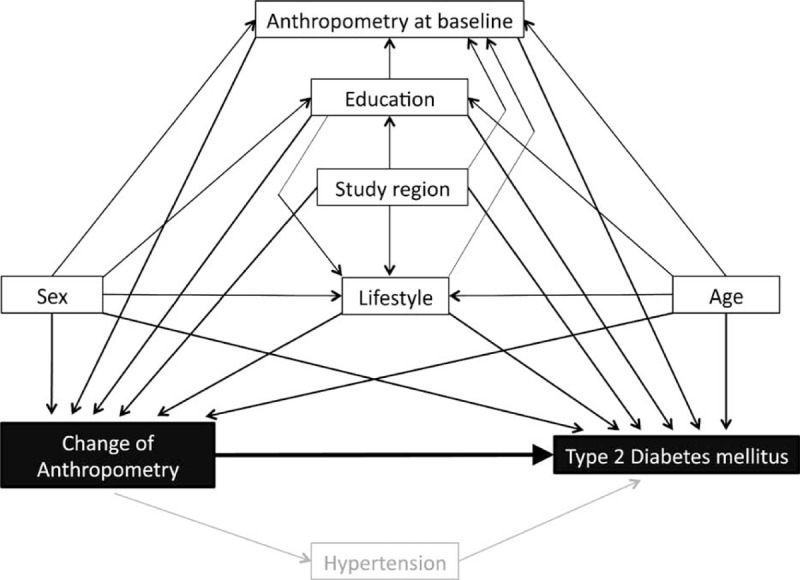
Model of directed acyclic graphs for the study question. Black boxes = exposition and outcome, white boxes with black frame = confounders, grey box = mediator.
To better account for baseline weight and WC, we repeated the analysis using relative units. The change of weight and WC was therefore analyzed in relation to the baseline value so that the difference was given as a percent of change per year instead of kilograms or centimeters per year. Again, we adjusted for the already mentioned confounders, with the exception of weight and WC at baseline, respectively.
Additionally, we conducted adjusted analyses stratified by baseline body mass index (BMI) (BMI ≤30 vs >30 kg/m2) and age at baseline (<60 vs ≥60 years) for absolute and relative change of obesity markers.
All analyses were conducted using SAS Version 9.3 (SAS Institute, Cary, NC) with the exception of the analysis for nonlinearity, which was conducted with R Version 3.0.2 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Population
Table 1 summarizes the characteristics of the study population at baseline separately for women and men and T2DM incidence status. Mean observation time was 4.8 years (95% CI: 4.7–4.8) and 5.5 years (95% CI: 5.5–5.6) for the timeframe between baseline and FU1 and between FU1 and FU2, respectively, leading to an observation time of 13,956 person years (py) for detecting T2DM. Of all participants included in the pooled study sample, 140 developed T2DM between FU1 and FU2, resulting in an overall incidence rate of 10.0/1000 py (95% CI: 8.4–11.7).
TABLE 1.
Characteristics of Study Population
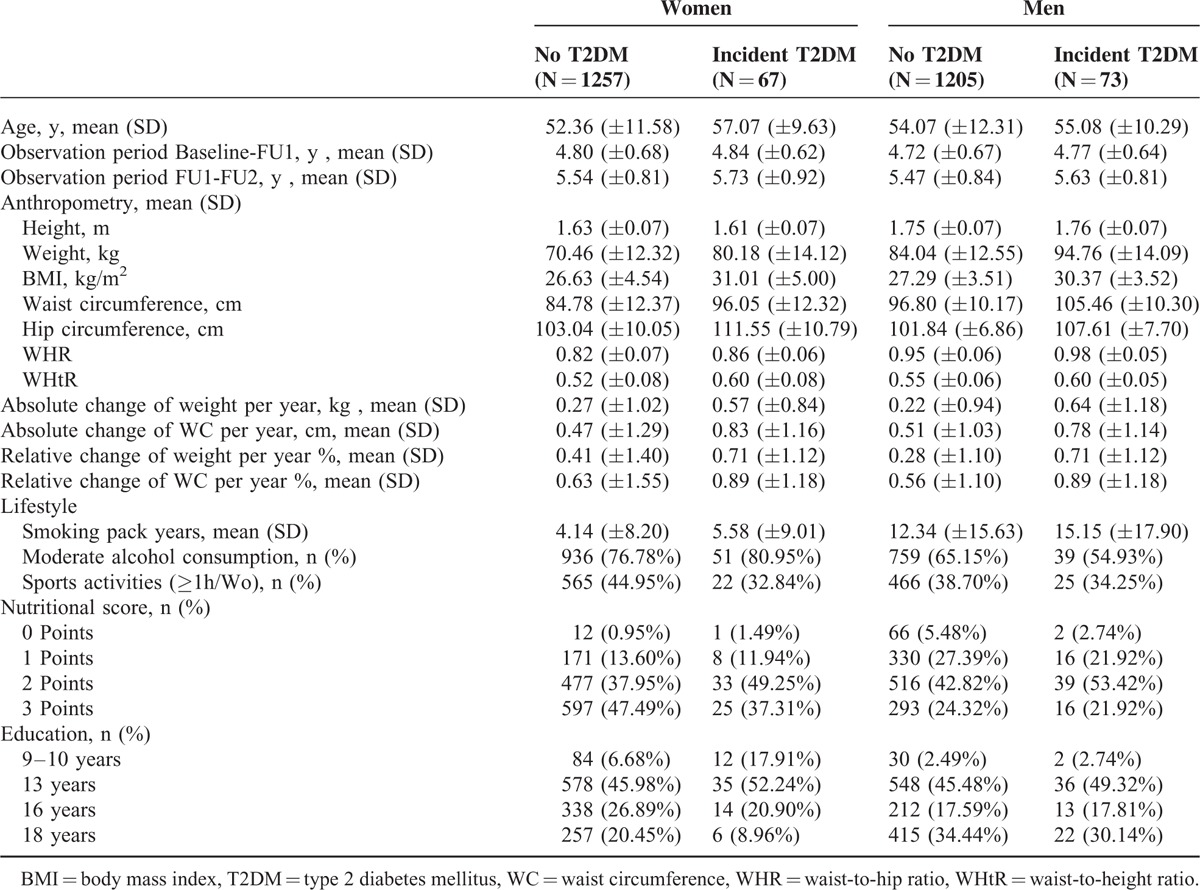
For both sexes, the mean BMI was above the recommendations (♀: 26.9 kg/m2, 95% CI: 26.6–27.1, ♂: 27.5 kg/m2, 95% CI: 27.3–27.7), and 22.5% (n = 586) of all participants were obese (BMI ≥30 kg/m2). The means BMI, WC, and waist-to-height-ratio (WHtR) were above the recommendations for women and men and were higher for those participants who later developed T2DM. Between baseline and FU1, weight and WC on average increased by 0.29 kg (95% CI: 0.23–0.34) and 0.49 cm (95% CI: 0.42–0.56) per year for women, and by 0.25 kg (95% CI: 0.19–0.30) and 0.53 cm (95% CI: 0.47–0.59) for men, respectively. The mean relative change of weight and WC per year was 0.43% (95% CI: 0.35–0.50) and 0.64% (95% CI: 0.56–0.72) for women and 0.30% (95% CI: 0.24–0.36) and 0.57% (95% CI: 0.51–0.63) for men, respectively. This indicates, for example, that on average a man with a weight of 70 kg gains 210 g per year and a man with a weight of 90 kg gains 270 g per year.
When we compared women with men, the latter showed a more unfavorable lifestyle because they smoked more, consumed alcohol outside the moderate limits more often, and were less active in leisure time sports.
Association Between Change of Markers of Obesity and Incidence of T2DM
In Figure 3, the individual annual change of weight and WC in dependence of the baseline value is displayed for women and men separately. Participants with incident T2DM gained more weight than did their nondiseased counterparts, especially when they had higher values at baseline. Regarding waist circumference, women with incident diabetes gained more (or lost less) WC compared with women without incident T2DM, with a tendency toward higher losses the higher the baseline WC was. For men, however, who developed incident T2DM showed an increase in WC across all levels of baseline WC, whereas in those without incident T2DM, WC tended to decrease, especially at higher baseline WC.
FIGURE 3.
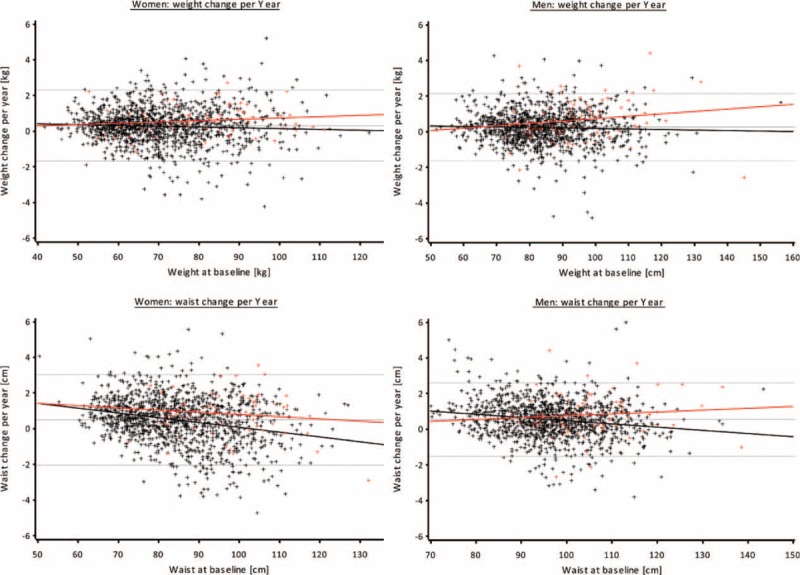
Change of weight and waist circumference (WC) by baseline weight or WC. Black crosses and line = participants without T2DM, red crosses and line = participants with incident T2DM, grey lines = 5% percentile, mean, 95% percentile.
Table 2 shows the results of the crude and adjusted regression analyses for the total study sample stratified by sex. For women, absolute weight change in kilograms per year and absolute change of WC in centimeter per year led to HRs of 1.28 (95% CI: 1.02–1.59) and 1.31 (95% CI: 1.07–1.60), respectively. The corresponding HRs for men were 1.34 (95% CI: 1.13–1.58) for weight change and 1.29 (95% CI: 1.06–1.57) for change of WC per year. This implies, for example, that a HR of 1.28 as the one for absolute weight change in women, the risk of incident T2DM for a woman who gains 1 kg of body weight in 1 year increases by 28%.
TABLE 2.
HRs and 95% CIs for the Association Between Change of Anthropometric Markers and Incident T2DM
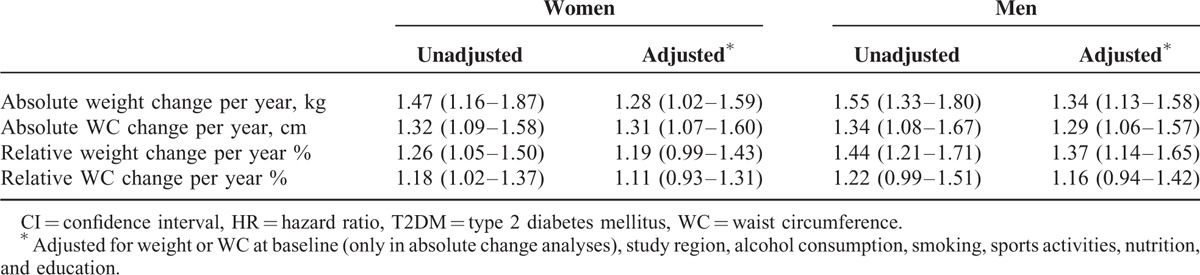
Adjusted HRs for women and men for relative weight change were 1.19 (95% CI: 0.99–1.43) and 1.37 (95% CI: 1.14–1.65), respectively. Relative change of WC resulted in HRs of 1.11 (95% CI: 0.93–1.31) and 1.16 (95% CI: 0.94–1.42) for women and men, respectively. This implies, for example, that for a man with a body weight of 70 kg, a weight gain of 700 g (≙1%) per year results in an increased risk for T2DM of 19%.
The results of the analyses after stratification by baseline BMI are presented in the upper part of Table 3. For women and men, a similar distribution was observed—about one-fourth of the participants were obese with a BMI ≥30 kg/m2, whereas the number of incident cases was comparable between the strata of BMI. Participants with a BMI <30 kg/m2 at baseline showed no or only small associations between change of anthropometric factors and incident T2DM. In contrast, participants with a BMI ≥30 kg/m2 showed stronger associations regarding the association of weight and WC and incident T2DM.
TABLE 3.
HRs for the Association Between Change of Anthropometric Markers and Incident T2DM, Stratified by Baseline BMI and Age, Respectively
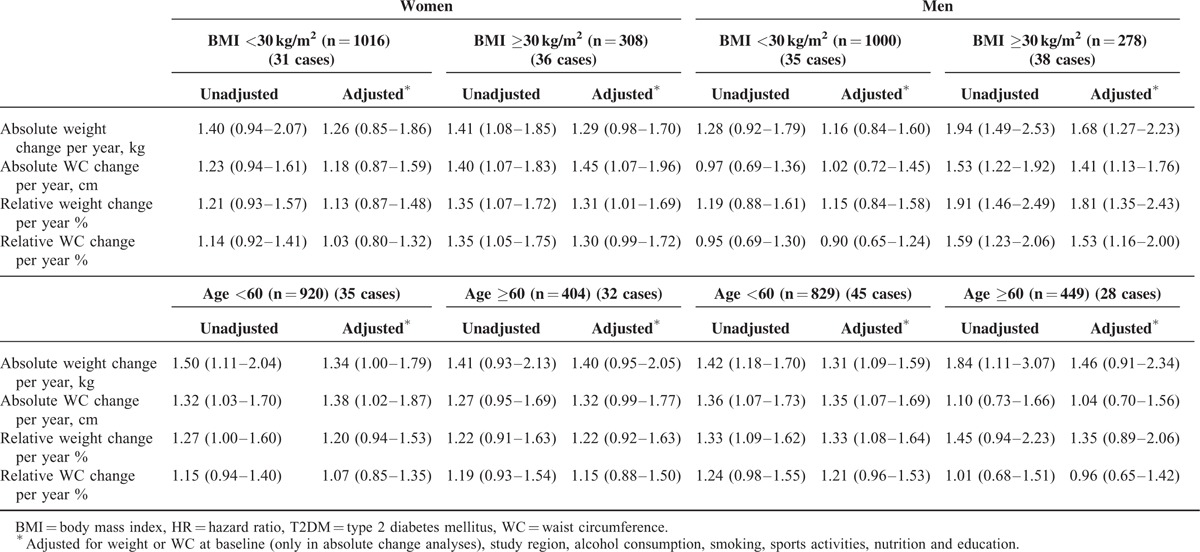
In the age-stratified analysis, in both women and men of all ages, we found positive associations of weight and WC gain with incident T2DM (lower section of Table 3).
DISCUSSION
In summary, we found effects for the association between change of anthropometric factors and incidence of T2DM. For the analyses, we decided to use weight and WC, but not BMI, waist-to-hip-ratio (WHR), and WHtR for several reasons. As height changes only marginal, changes of BMI and WHtR mainly reflect changes in weight and WC, respectively. Furthermore, WHR showed no changes because WC and hip circumference changed in similar dimensions.
As outlined in the descriptive analyses of the study population, participants with incident T2DM gained more weight and WC between baseline and FU1 as compared with the participants without incident T2DM. This finding was confirmed in the regression analyses. For the examined markers, we found considerably elevated HRs for both weight gain and WC gain in the unadjusted and adjusted models.
The calculation of HRs for relative change of weight and WC better takes into account the relationship with the baseline values than did HRs for the absolute changes. Again, in both, women and men, associations between relative change of weight and WC with incident T2DM were shown.
When we stratified the study sample by baseline BMI, we observed stronger associations in the participants who were obese at baseline (BMI ≥30 kg/m2) compared with those with a BMI <30 kg/m2, especially in men. For participants with a BMI < 30 kg/m2 at baseline, the estimated HRs were more imprecise and only relatively small associations were found. However, a trend toward increasing risk of T2DM with gaining weight or WC was still visible for the mentioned association for participants with a BMI <30 kg/m2. An exception to this was the change of WC in men with a BMI <30 kg/m2 wherein we observed no effects or reverse effects. One explanation for the weaker association in the nonobese participants could be the fact that the risk of T2DM increases with the duration and the degree of obesity,39 so participants who were already obese at baseline might have a higher risk of developing T2DM.
We also found a tendency toward increasing risk of T2DM with higher gain of weight and WC when we stratified the sample by baseline age. We did not find relevant differences between the younger and the older age groups, with the exception of change of waist in men, where older participants showed smaller associations between change of waist and incidence of T2DM compared with younger men. However, estimates are imprecise due to small sample sizes and therefore, further research is required.
When comparing our findings with those of other studies, differences between the study populations or the methods used need to be discussed. Some of the previous studies drew their samples from Asian or indigenous populations18–20,23–25 and their results are therefore not entirely comparable with our findings because risk of T2DM, as well as body fat distribution, differs by ethnicity.26,27 Other studies used self-report instead of direct measurements for assessing the weight of the participants.22,28 The mentioned studies showed conflicting results. Although some found an effect of weight change on incidence of T2DM,19,20,22,25,28 others did not.18,23,24
Comparing our results with those of the studies that are more comparable with our investigation in terms of study population and methods, we found accordance in most cases.4,14–17,21 Waring et al40 found no effect of change in weight on incident T2DM, which is contrary to our findings and those of the other mentioned studies. One reason for this could be the statistical methods that used only 3 weight change categories and no further distinction of weight gain in their analyses.
Koh-Banerjee et al22 were the only ones who investigated change of WC as an exposure variable and they also found an effect of increasing risk of T2DM with a gain of weight and WC.
Strengths, as well as some limitations, of our study need to be mentioned. A clear strength of our study is the direct and standardized measurement of anthropometric factors at both baseline and FU1 examination instead of using self-reported information. Furthermore, the clear temporal separation of changes in anthropometric markers and incidence of T2DM allow us to draw conclusions regarding weight change leading to T2DM. In particular, this means for the direction of association, it is more likely that changes in anthropometric parameters actually cause diabetes than it is to assume a reverse association. Finally, our study includes population-based samples from an urban and a rural area, making our results generalizable to a wide population.
One limitation of our study is the use of self-reported information to define T2DM instead of measurement from an oral glucose tolerance test. The use of self-report to define T2DM might underestimate the true incidence due to undetected cases. In general, the specificity of self-reported T2DM can be considered as high, whereas sensitivity is relatively low.41 This means that undetected cases could possibly have bias the results of this study. Another limitation is the missing information of the exact date of diabetes diagnosis, which led to the use of interval censored data. Despite the use of 2 pooled cohort studies, the sample size and number of incident cases of T2DM are still small, leading to imprecise estimates. Furthermore, the observation time for changes in anthropometry, as well as for incidence of T2DM, is limited, thus precluding conclusions regarding long-term risk due to weight gain or gain of WC. Both factors—small number of cases and short observation time—led to imprecise estimates, especially in the stratified analyses. Small variations in the measurement of WC are also worth mentioning when considering limitations of the study. Meanwhile, established measurement systems like photonic scanning would be more precise and also more expensive to implement.42 Additional research should be conducted to further verify the observed effects, especially regarding the change of WC.
Considering the practical use of the study, it becomes apparent that not only a high weight, BMI, or WC is a major risk factor for developing T2DM but also gaining weight or WC is hazardous. To keep the risk of developing T2DM low, a healthy weight should be achieved. If weight loss is not possible, especially in obese individuals, a stable weight should be the goal.
Acknowledgments
None.
Footnotes
Abbreviations: BMI = body mass index, CARLA = Cardiovascular Disease - Living and Ageing in Halle, CI = confidence interval, FU1 = first follow-up examination, FU2 = second follow-up examination, HR = hazard ratio, py = person years, SHIP = Study of Health in Pomerania, T2DM = type 2 diabetes mellitus, WC = waist circumference, WHR = waist-to-hip-ratio, WHtR = waist-to-height-ratio.
This work was supported by the Competence Network Diabetes mellitus of the German Federal Ministry of Education and Research (BMBF, grant 01GI1110C) and the Competence Network Obesity (BMBF, grant 01GI1121B)
The Cardiovascular Disease, Living and Ageing in Halle Study (CARLA) was supported by a grant from the Deutsche Forschungsgemeinschaft as part of the Collaborative Research Center 598 “Heart failure in the elderly—cellular mechanisms and therapy” at the Medical Faculty of the Martin-Luther-University Halle-Wittenberg, by a grant of the Wilhelm-Roux Programme of the Martin-Luther-University Halle-Wittenberg; by the Ministry of Education and Cultural Affairs of Saxony-Anhalt, and by the Federal Employment Office.
The Study of Health in Pomerania (SHIP) is part of the Community Medicine Research net (http://www.community-medicine.de) at the University of Greifswald, Germany. Funding was provided by grants from the German Federal Ministry of Education and Research (BMBF, grant 01ZZ0403); the Ministry for Education, Research, and Cultural Affairs; and the Ministry for Social Affairs of the Federal State of Mecklenburg–West Pomerania.
Parts of this study were presented in abstract form and orally at the 9th Annual Meeting of the German Society for Epidemiology (DGEpi) e.V., Ulm, Germany, 16–19 September 2013 and the Autumn Meeting of the German Diabetes Society and the Annual Meeting of the German Obesity Society, Leipzig, Germany, 21–22 November 2014.
The authors report no conflicts of interest.
REFERENCES
- 1.Danaei G, Finucane MM, Lu Y, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 2011; 378:31–40. [DOI] [PubMed] [Google Scholar]
- 2.Burke JP, Williams K, Gaskill SP, et al. Rapid rise in the incidence of type 2 diabetes from 1987 to 1996: results from the San Antonio Heart Study. Arch Intern Med 1999; 159:1450–1456. [DOI] [PubMed] [Google Scholar]
- 3.Geiss LS, Wang J, Cheng YJ, et al. Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA 2014; 312:1218–1226. [DOI] [PubMed] [Google Scholar]
- 4.Wannamethee SG, Shaper AG, Walker M. Overweight and obesity and weight change in middle aged men: impact on cardiovascular disease and diabetes. J Epidemiol Community Health 2005; 59:134–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinstein AR, Sesso HD, Lee IM, et al. Relationship of physical activity vs body mass index with type 2 diabetes in women. JAMA 2004; 292:1188–1194. [DOI] [PubMed] [Google Scholar]
- 6.Hu FB, Sigal RJ, Rich-Edwards JW, et al. Walking compared with vigorous physical activity and risk of type 2 diabetes in women: a prospective study. JAMA 1999; 282:1433–1439. [DOI] [PubMed] [Google Scholar]
- 7.Waller K, Kaprio J, Lehtovirta M, et al. Leisure-time physical activity and type 2 diabetes during a 28 year follow-up in twins. Diabetologia 2010; 53:2531–2537. [DOI] [PubMed] [Google Scholar]
- 8.Willi C, Bodenmann P, Ghali WA, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 2007; 298:2654–2664. [DOI] [PubMed] [Google Scholar]
- 9.Interact-Consortium. Association between dietary meat consumption and incident type 2 diabetes: the EPIC-InterAct study. Diabetologia 2013; 56:47–59. [DOI] [PubMed] [Google Scholar]
- 10.Brunner EJ, Mosdol A, Witte DR, et al. Dietary patterns and 15-y risks of major coronary events, diabetes, and mortality. Am J Clin Nutr 2008; 87:1414–1421. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Homepage World Health Organization. http://www.who.int/mediacentre/factsheets/fs312/en/ 28–5–2014. [Google Scholar]
- 12.International Diabetes Federation. Homepage International Diabetes Federation. http://www.idf.org/prevention 28–5–2014. [Google Scholar]
- 13.American Diabetes Association. Homepage American Diabetes Association. http://www.diabetes.org/are-you-at-risk/lower-your-risk/overweight.html?loc=atrisk-slabnav 28–5–2014. [Google Scholar]
- 14.Ford ES, Williamson DF, Liu S. Weight change and diabetes incidence: findings from a national cohort of US adults. Am J Epidemiol 1997; 146:214–222. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs-van der Bruggen MA, Spijkerman A, van Baal PH, et al. Weight change and incident diabetes: addressing an unresolved issue. Am J Epidemiol 2010; 172:263–270. [DOI] [PubMed] [Google Scholar]
- 16.Oguma Y, Sesso HD, Paffenbarger RS, Jr, et al. Weight change and risk of developing type 2 diabetes. Obes Res 2005; 13:945–951. [DOI] [PubMed] [Google Scholar]
- 17.Schienkiewitz A, Schulze MB, Hoffmann K, et al. Body mass index history and risk of type 2 diabetes: results from the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr 2006; 84:427–433. [DOI] [PubMed] [Google Scholar]
- 18.Ishikawa-Takata K, Ohta T, Moritaki K, et al. Obesity, weight change and risks for hypertension, diabetes and hypercholesterolemia in Japanese men. Eur J Clin Nutr 2002; 56:601–607. [DOI] [PubMed] [Google Scholar]
- 19.Hwang LC, Chen CJ, Lin BJ. Obesity and changes in body weight related to 10-year diabetes incidence in women in Taiwan. Asia Pac J Clin Nutr 2007; 16:677–682. [PubMed] [Google Scholar]
- 20.Kaneto C, Toyokawa S, Miyoshi Y, et al. Long-term weight change in adulthood and incident diabetes mellitus: MY Health Up Study. Diabetes Res Clin Pract 2013; 102:138–146. [DOI] [PubMed] [Google Scholar]
- 21.Biggs ML, Mukamal KJ, Luchsinger JA, et al. Association between adiposity in midlife and older age and risk of diabetes in older adults. JAMA 2010; 303:2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koh-Banerjee P, Wang Y, Hu FB, et al. Changes in body weight and body fat distribution as risk factors for clinical diabetes in US men. Am J Epidemiol 2004; 159:1150–1159. [DOI] [PubMed] [Google Scholar]
- 23.Li M, Campbell S, McDermott RA. Six year weight change and type 2 diabetes among Australian Indigenous adults. Diabetes Res Clin Pract 2010; 88:203–208. [DOI] [PubMed] [Google Scholar]
- 24.Mishra GD, Carrigan G, Brown WJ, et al. Short-term weight change and the incidence of diabetes in midlife: results from the Australian Longitudinal Study on Women's Health. Diabetes Care 2007; 30:1418–1424. [DOI] [PubMed] [Google Scholar]
- 25.Nanri A, Mizoue T, Takahashi Y, et al. Association of weight change in different periods of adulthood with risk of type 2 diabetes in Japanese men and women: the Japan Public Health Center-Based Prospective Study. J Epidemiol Community Health 2011; 65:1104–1110. [DOI] [PubMed] [Google Scholar]
- 26.Rosella LC, Mustard CA, Stukel TA, et al. The role of ethnicity in predicting diabetes risk at the population level. Ethn Health 2012; 17:419–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shai I, Jiang R, Manson JE, et al. Ethnicity, obesity, and risk of type 2 diabetes in women: a 20-year follow-up study. Diabetes Care 2006; 29:1585–1590. [DOI] [PubMed] [Google Scholar]
- 28.de Mutsert R, Sun Q, Willett WC, et al. Overweight in Early Adulthood, Adult Weight Change, and Risk of Type 2 Diabetes, Cardiovascular Diseases, and Certain Cancers in Men: a Cohort Study. Am J Epidemiol 2014; 179:1353–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schipf S, Werner A, Tamayo T, et al. Regional differences in the prevalence of known type 2 diabetes mellitus in 45-74 years old individuals: Results from six population-based studies in Germany (DIAB-CORE Consortium). Diabet Med 2012. [DOI] [PubMed] [Google Scholar]
- 30.Schipf S, Ittermann T, Tamayo T, et al. Regional differences in the incidence of self-reported type 2 diabetes in Germany: results from five population-based studies in Germany (DIAB-CORE Consortium). J Epidemiol Community Health 2014; 68:1088–1095. [DOI] [PubMed] [Google Scholar]
- 31.Greiser KH, Kluttig A, Schumann B, et al. Cardiovascular disease, risk factors and heart rate variability in the elderly general population: design and objectives of the CARdiovascular disease, Living and Ageing in Halle (CARLA) Study. BMC Cardiovasc Disord 2005; 5:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Volzke H, Alte D, Schmidt CO, et al. Cohort profile: the study of health in Pomerania. Int J Epidemiol 2011; 40:294–307. [DOI] [PubMed] [Google Scholar]
- 33.Schneider S. L. Applying the ISCED 97 to the German educational qualifications. In The International Standard Classification of Education. (Ed. Schneider S.L.) 77-102, Mannheim 2008. [Google Scholar]
- 34.Deutsche Gesellschaft für Ernährung e.V. Vollwertig Essen und Trinken nach den 10 Regeln der DGE. https://www.dge.de/ernaehrungspraxis/vollwertige-ernaehrung/10-regeln-der-dge/. 18-12-2013. [Google Scholar]
- 35.Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009; 338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jain V, Sucupira MC, Bacchetti P, et al. Differential persistence of transmitted HIV-1 drug resistance mutation classes. J Infect Dis 2011; 203:1174–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davison A.C., Statistical Models, Cambridge University Press, 2003. [Google Scholar]
- 38.Stang A. Causality and confounding in epidemiology. Gesundheitswesen 2011; 73:884–887. [DOI] [PubMed] [Google Scholar]
- 39.Bray GA. Medical consequences of obesity. J Clin Endocrinol Metab 2004; 89:2583–2589. [DOI] [PubMed] [Google Scholar]
- 40.Waring ME, Eaton CB, Lasater TM, et al. Incident diabetes in relation to weight patterns during middle age. Am J Epidemiol 2010; 171:550–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider AL, Pankow JS, Heiss G, et al. Validity and reliability of self-reported diabetes in the atherosclerosis risk in communities study. Am J Epidemiol 2012; 176:738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wells JC, Ruto A, Treleaven P. Whole-body three-dimensional photonic scanning: a new technique for obesity research and clinical practice. Int J Obes (Lond) 2008; 32:232–238. [DOI] [PubMed] [Google Scholar]


