Supplemental Digital Content is available in the text
Abstract
The angiosome concept provides practical information regarding the vascular anatomy of reconstructive and vascular surgery for the treatment of peripheral arterial occlusive disease and, particularly, critical lower limb ischemia.
The aim of the study was to confirm the efficacy of direct revascularization with the angiosome concept (DR) for lower limb ischemia.
Complementary manual searches were performed through the Pubmed, Cochrane Library, and EMBASE databases.
We searched all randomized and nonrandomized studies (NRSs) comparing DR with indirect revascularization (IR) (without the angiosome concept) for lower limb ischemia. Only 9 nonrandomized controlled retrospective cohort studies were found and included. Trials published in any language were included.
Primary endpoints were time to limb amputation and time to wound healing. Data extraction and trial quality assessment were performed by two authors independently. A third author was consulted for disagreements settlement and quality assurance.
Five NRSs involving 779 lower limbs revealed that DR significantly improved the overall survival of limbs (hazard ratio [HR] 0.61; 95% confidence interval [CI] = 0.46–0.80; P < 0.001; I2 = 0%). In addition, DR significantly improved time to wound healing (HR 1.38; 95% CI = 1.13–1.69; P = 0.002; I2 = 0%, in 5 studies including 605 limbs).
All included studies were retrospective comparative studies, and no consensus was obtained in describing wound conditions in the included studies.
Our results suggested that treatment of lower limb ischemia using DR is more effective in salvaging limbs and healing wounds than IR is. Additional randomized controlled studies are necessary to confirm these results.
INTRODUCTION
Peripheral arterial occlusive disease (PAOD) is a major disease that limits active aging in elderly people. Complications of PAOD are the leading cause of hospitalization and amputation for people with lower limb ischemia, and account for billion-dollar expenditures annually in the United States.1 Treatment goals for lower legs critical limb ischemia (CLI) patients are to increase wound healing, improve quality of life, prevent limb loss, and prolong survival. Guidelines from the Transatlantic Inter-Society Consensus II (TASC-II) and the American College of Cardiology/American Heart Association recommend multidisciplinary approaches to reduce the frequency of foot complications in CLI patients.2,3 Early revascularization intervention with bypass or endovascular surgery, particularly for high-risk patients, is considered to be the gold standard in reducing the possibility of hospitalization and amputation.2,4–6 Nevertheless, current revascularization intervention strategies, which restore circulation to a nontargeted artery, have a 15% failure rate in healing CLI wounds. Such a high rate suggests that increasing adequate blood supply to feeding arteries at the distal occluded lesion-site might be crucial in improving the results of intervention.
The concept of angiosomes, first described by Ian Taylor, provides practical information on the application of vascular anatomy for reconstruction and vascular surgery in the treatment of PAOD, and particularly on the treatment of CLI.7,8 According to the angiosome concept, the foot is divided into 6 distinct angiosomes fed by source arteries, 3 from posterior tibial, 2 from peroneal, and 1 from anterior tibial artery, with functional vascular interconnections between muscle, fascia, and skin.9 Numerous direct arterial-to-arterial connections exist between the main arteries of the foot, and these connections provide alternative routes of blood flow when the arteries that directly supply the angiosome is either disrupted or compromised.9 Therefore, the angiosome concept suggests that recanalization of the artery that is directly supplying the ischemic and/or ulcerated angiosome, instead of revascularizing one of the other 2 major arteries hoping that existing arterial-to-arterial connections will provide blood perfusion to the ischemic and/or ulcerated angiosome, might be more successful.2,9
It is unclear whether direct revascularization with the angiosome concept (DR) can provide superior results for CLI patients than that of conventional indirect revascularization (IR) without the angiosome concept. One recent review revealed that evidence is insufficient to recommend DR in CLI patients.10 However, we performed a systematic review and meta-analysis investigating the efficacy of DR, comparing it with conventional IR for the treatment of CLI patients.
METHODS
Search Strategy
This protocol-driven and systematic review was conducted in accordance with PROSPERO published protocol and analysis planning (PROSPERO 2013:CRD42013004401, http://www.crd.york.ac.uk/Prospero/).11 Searches were not restricted by publication status, date, or language. The search keywords included angiosome, angioplasty, endovascular, revascularization, endoluminal, transluminal, and bypass. In addition, MeSH terms were explored. The final results were combined with the following keywords: lower limbs, extremities, and foot. The databases we used to conduct our searches were Pubmed (from 1948 to March 2013), the Cochrane Library (latest issue published March 2013), and EMBASE (January 1980–March 2013). The search strategy is provided in Appendix 1. Databases of clinical trials (available at http://www.clinicaltrials.gov [accessed March 26, 2014]) reference lists of reviews had also been searched to identifying relevant trials.
Study Selection
In the literature search, titles, abstracts, and full texts of trials identified were independently screened by three authors (T-YH, T-SH, and C-HY). Articles with comparisons between DR and IR were included if adults (≥18 years old) with critical lower limb ischemia (as defined by TASC-II) had been treated using either endovascular surgery or conventional bypass surgery.12 Reviews, case series, case reports, and trials without comparisons between DR and IR were excluded. Primary outcomes included time to limb amputation, time to wound healing, and mortality rate.
Data Extraction
Characteristics of studies (year of publication, study design and setting, method of recruitment, inclusion and exclusion criteria), participants (sex, age, and underlying disease), interventions (operative techniques, endovascular, or traditional surgery), comparisons (types of control group), and outcomes (various outcome measurements and follow-up times) were recorded. In studies with multiple arm designs, head-to-head comparison data were extracted for data synthesis. Time to amputation and time to wound healing were used as the primary outcomes.
Assessment of Risk of Bias
Data extraction and quality assessment were performed by two authors (T-YH and C-HY) independently. A third author (T-SH) was consulted for disagreements settlement and quality assurance. The risk of bias tool of the Systematic Reviews of Interventions from the Cochrane Handbook was utilized for determination of methodological quality.12 Because the included studies were all nonrandomized studies (NRSs), we assessed methodological quality by using the Newcastle–Ottawa Quality Assessment Scale (NOS).13 Three determinants composed the NOS system, including selection scores, outcome scores, and comparability scores.13 Studies with NOS ≥8 points were defined as high-quality studies, whereas other studies as low-quality studies.
Data Synthesis and Statistical Analysis
For time to event outcome, hazard ratios (HRs) with 95% confidence intervals (CIs) were extracted in primary studies as the size to estimate the overall effect of treatment. If data were not provided, the effect size was calculated in accordance with methods suggested by Parmar et al14 by using a spreadsheet developed by Tierney et al,15 as described earlier. For 12-month amputation rate and 12-month wound healing rate, we defined amputation and wound healing as events. Clinical heterogeneity were assessed by comparing the protocols and methodologies of the included studies, and assessed statistical heterogeneity with the χ2 test results (using a cutoff value of P < 0.10), and the I2 statistic, where I2 < 25%, 25% ≤ I2 ≤ 50%, and I2 > 50% indicates mild, moderate, and substantial heterogeneity, respectively.16,17 Subgroup analysis based on study quality or intervention (surgical or endovascular) was conducted, and data synthesis and statistical analysis were conducted using Review Manager (RevMan Version 5.2; The Nordic Cochrane Center, Copenhagen, Denmark). A funnel plot was created to evaluate publication bias, and significance level was set at 0.05.
RESULTS
Search Results and Study Characteristics
Overall, 10 NRSs were included, as shown in Figure 1 and Table 1 .18–27 No randomized control trial was identified, and all studies were retrospective cohort study. Iida et al published two studies in 201020 and 2012.24 The study of 2010 analyzed patients from April 2003 to August 2008. The study of 2012 analyzed patients from April 2004 to October 2010 using matching method. Therefore, we used the study of 2012 to conduct meta-analysis. All the DR studies followed the Taylor's angiosome concept. Three studies (Azuma et al, Iida et al, and Söderström et al)23–25, which utilized propensity score matched comparison between DR and IR groups, were analyzed. Table 1 shows the major characteristics of the included NRSs, none of these were conducted before 2009. Eight NRSs provided outcome measurements indicating limb salvage rate (ie, free of above-ankle amputation) or free from major amputation rate. One NRS presented only the free from amputation rate and did not indicate major or minor amputation rate. Seven studies recorded the wound healing rate, and 1 study recorded the wound unhealing rate. The NRS by Rashid et al28 was excluded because their results provided subgroup data only, which could not be analyzed. All included trial patients were in either Rutherford Class 5 or 6 and Fontaine Stage 4.29,30 The key characteristics of patients included in all studies are shown in Table 2. Mean patient age was >67 years old and most patients were male. In addition, the majority of patients (at least 64%) had diabetes mellitus (DM), and 3 studies recruited only DM patients.21,25,26 We did not perform further subgroup analysis because only Varela et al19 had described the subgroup of IR with collateral vessels.
FIGURE 1.

Flow diagram of the article selection process in accordance with PRISMA guideline. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
TABLE 1.
Studies Included in the Systemic Review of the Effects of Angiosome Model Revascularization Treatment for Patients With Low Limb Ischemia

TABLE 1 (Continued).
Studies Included in the Systemic Review of the Effects of Angiosome Model Revascularization Treatment for Patients With Low Limb Ischemia

Quality Assessment
The risks of bias for all NRSs are shown in Table 3. The NOS of all trials ranged from 5 points to 9 points. Five NRSs had a score of >8 points.
TABLE 2.
The Patient Characteristics of the Included Studies

Meta-Analysis of Limb Salvage and Wound Healing
Reported outcomes in primary studies were shown in Table S1, http://links.lww.com/MD/A394, Table S2, http://links.lww.com/MD/A394, and Table S3, http://links.lww.com/MD/A394. Although 9 NRSs reported a limb salvage rate,18,19,21–27 and 8 NRSs reported the wound healing rate, the follow-up periods were different in individual study.18,19,21–23,25–27 To include more studies into our meta-analysis, we used time to amputation and time to wound healing as our primary endpoints. In addition, we could not find adequate information with regard to the data of loss-to-follow-up in primary studies. However, we analyzed 12-month amputation rate and 12-month wound healing rate using available information. A total of 719 limbs were treated with DR, and 493 limbs were treated with IR. Five studies included total endovascular treatment (EVT), another study included traditional bypass surgery, and 3 studies included both surgical and EVT interventions.
Fossaceca et al26 recorded the limbs of the DR and IR groups (247 limbs vs 52 limbs), but only presented outcomes by counting individual patients (167 patients vs 34 patients). Therefore, we calculated the data in our meta-analysis according to the number of patients.
Time to Amputation
Overall, we obtained 5 studies that reported 779 limbs. DR significantly improved time to amputation compared with IR (HR 0.61; 95% CI = 0.46–0.80; P < 0.001; Table 4; Figures 2 and 3). Little evidence of heterogeneity between studies was obtained (P = 0.69, I2 = 0%). According to study quality and intervention methods, subgroup analysis was performed, which showed little evidence of interaction (Table 4).
TABLE 3.
The Newcastle–Ottawa Scale of Included Studies

FIGURE 2.
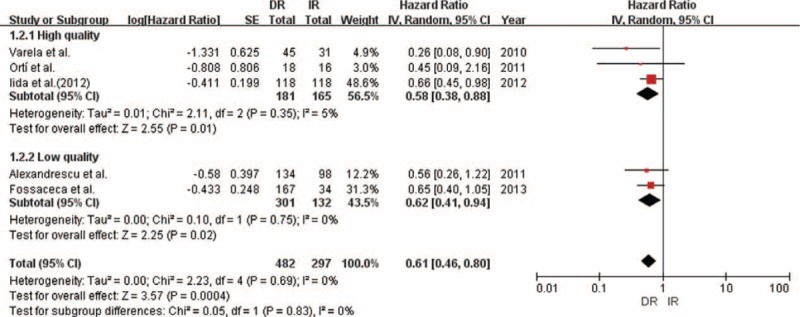
Forest plot comparing time to amputation, stratified by study quality.
FIGURE 3.

Forest plot comparing time to amputation, stratified by operation method.
TABLE 4.
Hazard Ratios for Time to Amputation and Time to Wound Healing for Patients Receiving Angiosome Model Target Revascularization Compared With Nonangiosome Group According to Meta-Analysis and Subgroup Analysis of All Trials

We analyzed 12-month amputation rate using 4 available studies. Our result showed the DR group significantly reduced 12-momth amputation rate compared with IR group (relative risk ratio [RR] 0.65; 95% CI = 0.54–0.79; P < 0.001; Figure S1, http://links.lww.com/MD/A394).
Time to Wound Healing
We obtained similar results for time to wound healing in the 5 studies with 605 limbs analyzed in which DR exerted a statistically significant effect (HR 1.38; 95% CI = 1.13–1.69; P = 0.002; Table 4; Figures 4 and 5). We found little heterogeneity between studies (P = 0.53; I2 = 0%). Subgroup analyses according to study quality and intervention methods showed little evidence of interaction (Table 4).
FIGURE 4.

Forest plot comparing time to wound healing, stratified by study quality.
FIGURE 5.

Forest plot comparing time to wound healing, stratified by operation method.
We analyzed 12-month wound healing rate using 4 available studies. Our result showed the DR group significantly improved 12-momth wound healing compared with IR group (RR 1.45; 95% CI = 1.26–1.66; P < 0.001; Figure S2, http://links.lww.com/MD/A394).
Mortality Rate
Mortality rate was only reported in 4 studies. Neville et al18 described a mortality rate of 13.6% over 100 days in the DR group and of 28.6% in the IR group. Kabra et al27 reported a mortality rate of 10.3% over 6 months in the DR group and of 20.0% in the IR group. Varela et al19 showed 13% mortality rate in the DR group and 27% in the IR over 12 months, as well as a P value of 0.17. Söderström et al25 showed 1-year survival rates of 74% in both the DR and IR groups (P = 0.65).
Publication Bias
Publication bias was analyzed using a funnel plot, which was symmetrical (Figures 6 and 7).
FIGURE 6.

Funnel plot of studies comparing time to amputation.
FIGURE 7.

Funnel plot of studies comparing time to wound healing.
DISCUSSION
Our meta-analysis provided evidence that DR significantly improves time to amputation and time to wound healing for CLI patients. However, insufficient information was available to conduct a meta-analysis on mortality. The angiosome concept was constructed based on the anatomy of blood circulation, which demonstrates that superior blood supply can improve tissue growth and wound healing.
Three studies performed matching in their data analysis (Azuma et al, Iida et al, and Söderström et al).23–25 The result of Iida et al showed that DR significantly decreased the amputation rate (HR 0.66; 95% CI = 0.45–0.98; P = 0.04). Azuma et al and Söderström et al were also comparing the outcome of time to wound healing. Söderström et al supported that DR group significantly increased wound healing rate (P < 0.001). However, Azuma et al showed no difference for wound healing between 2 groups (P = 0.185).
Varela et al confirmed that DR model treatment improved the wound healing rate 12 months following intervention (92% vs 73%; P < 0.01) and limb salvage rate 24 months following intervention (93% vs 72%; P = 0.02) for CLI patients. Varela et al further revealed that distal peroneal arterial connections (collateral vessels) and the patent pedal arch played a significant role in wound healing and limb salvagability in CLI patients who were treated without using the DR model.19 This suggested that the possible cause of IR treatment failure resulted from inadequate vascular connections between the revascularized arteries and the ischemic region. Therefore, a patent pedal arch or peroneal distal branches that restore blood flow to the ischemic area through collateral vessels might show a similar result in limb salvagability and wound healing as that obtained through the specific source arteries.19
Although current AHA/ACC guidelines suggest open bypass still the preferred operation for patients who would live for >2 years, traditional surgery and EVT have been compared in several studies.31–35 One meta-analysis performed by Romiti et al compared surgical and EVT interventions and demonstrated no difference in the limb salvage rate (endovascular, 82.4% ± 3.4%; surgery, 82.3% ± 3%).36 Advantages of EVT intervention include less surgical trauma, a smaller wound, fewer local complications, and shorter hospital stays.31,32 However, subgroup analysis for comparisons between EVT intervention and surgical intervention could not be assessed in this study.
DM has been recognized as a critical predicting factor for wound healing. Failure of ulcers to heal in the feet of diabetic patients might have resulted from poor vascular connections between angiosomes, which provided inadequate blood perfusion to the ischemic areas. In addition, TASC II guidelines indicate that the amputation rate was 5 to 10 times higher in diabetic patients than in nondiabetic patients because blood flow to the microvascular beds appears to be reduced in the feet of diabetic patients.2 Furthermore, diabetic patients have an impaired host defense system against infections.37,38 Azuma et al23 stated that diabetes is one of the risk factors in prolonged tissue healing time. Iida et al showed that higher hemoglobin A1c levels were a significant predictor of major amputation in a DR group. They postulated that the increased risk is most likely attributable to poor periprocedural blood glycemic control rather than to the presence of DM during the postoperative period.24 Three studies compared DR in diabetic patients.21,25,26 However, our study compared the outcomes of these 3 studies with the others, which had a distinct percentage of diabetic patients (>64%), and minimal heterogeneity was identified (P = 0.40; I2 = 0%). No other studies comparing diabetic and nondiabetic patients have been published. DR might have beneficial effects for wound healing in diabetic patients; however, the effects on nondiabetic patients require further investigation.
Azuma et al23 proved that DR treatment could significantly shorten the time needed for wound healing in the entire study cohort, and that it improved limb salvage rate in end-stage renal disease (ESRD) patients (P < 0.01). However, after propensity-score matching, the differences between limb salvage rate and wound healing rate in the DR and IR groups were lost. Azuma el al concluded that the angiosome concept might be unimportant in the field of bypass surgery, unlike EVT intervention. However, our analysis revealed that the DR model concept could be consistently applied to all patients, regardless of surgical or EVT intervention.
ESRD was a crucial risk factor for wound healing and limb salvagability,23 and several studies have reported that patients with ESRD have higher amputation rates.39–41 Johnson et al39 postulated that healing problems account for higher amputation rates rather than graft thrombosis. Thus, some studies have recommended that bypass surgery should be performed on carefully selected ESRD patients because of potential negative outcomes.39,42,43 However, no standard exists for selected ESRD patients.44 Some studies have reported that hypoalbuminemia, which might result from inflammation instead of malnutrition,45,46 detrimentally related to the life prognosis of ESRD patients.23,31,41 Azuma et al separated their patients into 3 groups: non-ERSD, ESRD without severely low albuminemia, and ESRD with severely low albuminemia (<3.0 g/dL). The wound healing rate of the ESRD in the low albuminemia group was significantly worse than in the other groups (P < 0.01). Their subgroup analysis demonstrated that DR significantly improved wound healing rate both in non-ESRD patients and in ESRD patients as compared with IR. However, comparing DR and IR in all patients showed no beneficial effect in their study (P = 0.185). They concluded that ESRD and the level of serum albumin were more critical than the angiosome concept.23
Cilostazol was typically used in PAOD patients as an antiplatelet drug.47 The improvement of microvascular circulation has been reported as one of the clinical benefits of cilostazol.48 Iida et al formed 2 groups and recorded the outcome of skin perfusion pressure (in mm Hg) on the time before and after surgery.24 Skin perfusion pressure was similar to that before intervention (1.6 ± 0.9 with cilostazol therapy vs 1.6 ± 0.8 without, P = 0.91), and it was statistically higher after EVT in the cilostazol-treated group than in the noncilostazol-treated group (51 ± 19 vs 45 ± 19, P = 0.04).24 Therefore, cilostazol might help to improve microcirculation; however, further evidence of amputation prevention or improvement in wound healing is necessary.
In this study, we performed throughout literature searches. For evaluation of time-to-event outcomes, we utilized HR as the metric of the summary effect size. The calculated HR was the relative hazard of an event occurring in the DR group compared with that of the IR group. From our result, it suggested that patients who received DR had decreasing risk of limb amputation and wound unhealing by 0.61 (95% CI = 0.46–0.80) and 1.38 (95% CI = 1.13–1.69) in the PAOD patients compared with patients received IR interventions. However, our study has several limitations. First, all included studies were retrospective comparative studies. Angiosome concept was delicate. It was trivial to approach the target feeding artery for the ischemia area. Therefore, there were too many operation methods for vascular access that all the retrospective studies could not have a very specific and appropriate design. Second, another source of bias might come from the confounding bias because of patients’ condition. Only Azuma et al, Iida et al, and Söderström et al performed matching in their analyses. The bias existed among these studies resulting from the differences of the selection and grouping criteria for these patients. The mortality rate of the patients treated with IR was higher than those treated with DR might imply that the patients in the IR had more comorbidities than those in the DR group. Third, no consensus was obtained in describing wound conditions in the included studies. The Rutherford and Fontaine stage was used in only 4 studies, but they only recorded stages. The locations, numbers, infection status, and surgical debridement procedures of these wounds were not recorded in numerous studies, which were major confounders in our analysis. The wound healing rate and the treatment strategy for a gangrenous wound would be much different than that of a superficial ulcer. None of the studies reported postoperative wound care programs. Infections, antibiotic treatment, and debridement surgery are all additional concerns in the included studies. Fourth, the detail description for the vascular lesions were absent. Future randomized study should report the detail of these vascular lesions, such as length, location, stenotic status, collateral vessel of the lesions as well as the status of the pedal arch. It is possible that the patients in the DR group exhibited superior vascular quality and that the target vessel was more easily approached, whereas patients in the IR group might have had either total occlusion or vessels that were small in diameter and had degenerated. Thus, the 2 groups were at distinct stages.
In conclusion, the angiosome model of revascularization was beneficial for patients with critical lower limb ischemia when considering limb salvagability and wound healing. Nonetheless, randomized controlled studies are necessary to confirm our results. Increasing the limb salvage rate is anticipated to improve daily activity and could prolong the survival of patients. Thus, a broad prospective study should be conducted to confirm the effect of the angiosome model concept. All the following characteristics should be recorded in detail, including the wound condition and location with standard recording system, the treatment of the wound (debridement, antibiotics, and the dressing of wound), the detail description of the stenotic status, the collateral vessels, and the condition of the pedal arch. Collateral vessels should be defined more carefully because some patients, especially diabetic patients, are collateral artery-dominant blood supply. Crucial confounding factors, such as DM, ESRD, serum albumin levels, and medications, should also be reported and analyzed.
Footnotes
Abbreviations: CI = confidence interval, CLI = critical limb ischemia, DM = diabetes mellitus, DR = direct revascularization with the angiosome concept, ESRD = end-stage renal disease, EVT = endovascular treatment, HR = hazard ratio, IR = indirect revascularization (nonangiosome model), NOS = Newcastle–Ottawa Quality Assessment Scale, NRS = nonrandomized study, PAOD = peripheral arterial occlusive disease, RR = relative risk.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Frykberg RG, Zgonis T, Armstrong DG, et al. Diabetic foot disorders: a clinical practice guideline (2006 revision). J Foot Ankle Surg 2006; 45 suppl:S1–S66. [DOI] [PubMed] [Google Scholar]
- 2.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg 2007; 45 suppl S:S5–S67. [DOI] [PubMed] [Google Scholar]
- 3.Olin JW, Allie DE, Belkin M, et al. ACCF/AHA/ACR/SCAI/SIR/SVM/SVN/SVS 2010 performance measures for adults with peripheral artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures, the American College of Radiology, the Society for Cardiac Angiography and Interventions, the Society for Interventional Radiology, the Society for Vascular Medicine, the Society for Vascular Nursing, and the Society for Vascular Surgery (Writing Committee to Develop Clinical Performance Measures for Peripheral Artery Disease). J Am Coll Cardiol 2010; 56:2147–2181. [DOI] [PubMed] [Google Scholar]
- 4.Cavanagh PR, Lipsky BA, Bradbury AW, et al. Treatment for diabetic foot ulcers. Lancet 2005; 366:1725–1735. [DOI] [PubMed] [Google Scholar]
- 5.Burns P, Gough S, Bradbury AW. Management of peripheral arterial disease in primary care. BMJ 2003; 326:584–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frykberg RG. Diabetic foot ulcerations: management and adjunctive therapy. Clin Podiatr Med Surg 2003; 20:709–728. [DOI] [PubMed] [Google Scholar]
- 7.Taylor GI, Palmer JH. The vascular territories (angiosomes) of the body: experimental study and clinical applications. Br J Plast Surg 1987; 40:113–141. [DOI] [PubMed] [Google Scholar]
- 8.Setacci C, De Donato G, Setacci F, et al. Ischemic foot: definition, etiology and angiosome concept. J Cardiovasc Surgery 2010; 51:223–231. [PubMed] [Google Scholar]
- 9.Clemens MW, Attinger CE. Angiosomes and wound care in the diabetic foot. Foot Ankle Clin 2010; 15:439–464. [DOI] [PubMed] [Google Scholar]
- 10.Alexandrescu V, Hubermont G. Primary infragenicular angioplasty for diabetic neuroischemic foot ulcers following the angiosome distribution: a new paradigm for the vascular interventionist? Diabetes Metab Syndr Obes 2011; 4:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang TY, Huang TS, Wang YC, et al. Angiosome versus non-angiosome target revascularization for treatment of lower limb ischemia. PROSPERO 2013; CRD42013004401. Available at: http://www.crd.york.ac.uk/Prospero/display_record.asp?ID=CRD42013004401 Accessed April 22, 2014. [Google Scholar]
- 12.Higgins JPT, Green S. editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. London: The Cochrane Collaboration; 2011. (http://handbook.cochrane.org/). [Google Scholar]
- 13.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [webpage on the Internet] Ottawa, ON: Ottawa Hospital Research Institute; 2011. [Accessed April 22, 2014]. [Google Scholar]
- 14.Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 1998; 17:2815–2834. [DOI] [PubMed] [Google Scholar]
- 15.Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007; 8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ioannidis JPA, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ 2007; 335:914–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neville RF, Attinger CE, Bulan EJ, et al. Revascularization of a specific angiosome for limb salvage: does the target artery matter? Ann Vasc Surg 2009; 23:367–373. [DOI] [PubMed] [Google Scholar]
- 19.Varela C, Acin F, de Haro J, et al. The role of foot collateral vessels on ulcer healing and limb salvage after successful endovascular and surgical distal procedures according to an angiosome model. Vasc Endovascular Surg 2010; 44:654–660. [DOI] [PubMed] [Google Scholar]
- 20.Iida O, Nanto S, Uematsu M, et al. Importance of the angiosome concept for endovascular therapy in patients with critical limb ischemia. Catheter Cardiovasc Interv 2010; 75:830–836. [DOI] [PubMed] [Google Scholar]
- 21.Alexandrescu V, Vincent G, Azdad K, et al. A reliable approach to diabetic neuroischemic foot wounds: below-the-knee angiosome-oriented angioplasty. J Endovasc Ther 2011; 18:376–387. [DOI] [PubMed] [Google Scholar]
- 22.Blanes Orti P, Riera Vazquez R, Puigmaci Minguell R, et al. Percutaneous revascularisation of specific angiosome in critical limb ischaemia. Angiologia 2011; 63:11–17. [Google Scholar]
- 23.Azuma N, Uchida H, Kokubo T, et al. Factors influencing wound healing of critical ischaemic foot after bypass surgery: is the angiosome important in selecting bypass target artery? Eur J Vasc Endovasc Surg 2012; 43:322–328. [DOI] [PubMed] [Google Scholar]
- 24.Iida O, Soga Y, Hirano K, et al. Long-term results of direct and indirect endovascular revascularization based on the angiosome concept in patients with critical limb ischemia presenting with isolated below-the-knee lesions. J Vasc Surg 2012; 55:363–370. [DOI] [PubMed] [Google Scholar]
- 25.Söderström M, Alback A, Biancari F, et al. Angiosome-targeted infrapopliteal endovascular revascularization for treatment of diabetic foot ulcers. J Vasc Surg 2013; 57:427–435. [DOI] [PubMed] [Google Scholar]
- 26.Fossaceca R, Guzzardi G, Cerini P, et al. Endovascular treatment of diabetic foot in a selected population of patients with below-the-knee disease: is the angiosome model effective? Cardiovasc Intervent Radiol 2013; 36:637–644. [DOI] [PubMed] [Google Scholar]
- 27.Kabra A, Suresh KR, Vivekanand V, et al. Outcomes of angiosome and non-angiosome targeted revascularization in critical lower limb ischemia. J Vasc Surg 2013; 57:44–49. [DOI] [PubMed] [Google Scholar]
- 28.Rashid H, Slim H, Zayed H, et al. The impact of arterial pedal arch quality and angiosome revascularization on foot tissue loss healing and infrapopliteal bypass outcome. J Vasc Surg 2013; 57:1219–1226. [DOI] [PubMed] [Google Scholar]
- 29.Fontaine R, Kim M, Kieny R. Surgical treatment of peripheral circulation disorders. Helv Chir Acta 1954; 21:499–533. [PubMed] [Google Scholar]
- 30.Rutherford RB, Baker JD, Ernst C, et al. Recommended standards for reports dealing with lower extremity ischemia: revised version. J Vasc Surg 1997; 26:517–538. [DOI] [PubMed] [Google Scholar]
- 31.Adam DJ, Beard JD, Cleveland T, et al. Bypass versus angioplasty in severe ischaemia of the leg (BASIL): multicentre, randomised controlled trial. Lancet 2005; 366:1925–1934. [DOI] [PubMed] [Google Scholar]
- 32.Bradbury AW, Adam DJ, Bell J, et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial: an intention-to-treat analysis of amputation-free and overall survival in patients randomized to a bypass surgery-first or a balloon angioplasty-first revascularization strategy. J Vasc Surg 2010; 51 (5 suppl):5S–17S. [DOI] [PubMed] [Google Scholar]
- 33.Albers M, Romiti M, Brochado-Neto FC, et al. Meta-analysis of alternate autologous vein bypass grafts to infrapopliteal arteries. J Vasc Surg 2005; 42:449–455. [DOI] [PubMed] [Google Scholar]
- 34.Wolf GL, Wilson SE, Cross AP, et al. Surgery or balloon angioplasty for peripheral vascular disease: a randomized clinical trial. Principal investigators and their Associates of Veterans Administration Cooperative Study Number 199. J Vasc Interv Radiol 1993; 4:639–648. [DOI] [PubMed] [Google Scholar]
- 35.van der Zaag ES, Legemate DA, Prins MH, et al. Angioplasty or bypass for superficial femoral artery disease? A randomised controlled trial. Eur J Vasc Endovasc Surg 2004; 28:132–137. [DOI] [PubMed] [Google Scholar]
- 36.Romiti M, Albers M, Brochado-Neto FC, et al. Meta-analysis of infrapopliteal angioplasty for chronic critical limb ischemia. J Vasc Surg 2008; 47:975–981. [DOI] [PubMed] [Google Scholar]
- 37.Turina M, Fry DE, Polk HC., Jr Acute hyperglycemia and the innate immune system: clinical, cellular, and molecular aspects. Crit Care Med 2005; 33:1624–1633. [DOI] [PubMed] [Google Scholar]
- 38.Peppa M, Stavroulakis P, Raptis SA. Advanced glycoxidation products and impaired diabetic wound healing. Wound Repair Regen 2009; 17:461–472. [DOI] [PubMed] [Google Scholar]
- 39.Johnson BL, Glickman MH, Bandyk DF, et al. Failure of foot salvage in patients with end-stage renal disease after surgical revascularization. J Vasc Surg 1995; 224:280–285. [DOI] [PubMed] [Google Scholar]
- 40.Ramdev P, Rayan SS, Sheahan M, et al. A decade experience with infrainguinal revascularization in a dialysis-dependent patient population. J Vasc Surg 2002; 36:969–974. [DOI] [PubMed] [Google Scholar]
- 41.Biancari F, Kantonen I, Matzke S, et al. Infrainguinal endovascular and bypass surgery for critical leg ischemia in patients on long-term dialysis. Ann Vasc Surg 2002; 16:210–214. [DOI] [PubMed] [Google Scholar]
- 42.Albers M, Romiti M, Braganca Pereira CA, et al. A meta-analysis of infrainguinal arterial reconstruction in patients with end-stage renal disease. Eur J Vasc Endovasc Surg 2001; 22:294–300. [DOI] [PubMed] [Google Scholar]
- 43.Albers M, Romiti M, De Luccia N, et al. An updated meta-analysis of infrainguinal arterial reconstruction in patients with end-stage renal disease. J Vasc Surg 2007; 45:536–542. [DOI] [PubMed] [Google Scholar]
- 44.Schanzer A, Goodney PP, Li Y, et al. Validation of the PIII CLI risk score for the prediction of amputation-free survival in patients undergoing infrainguinal autogenous vein bypass for critical limb ischemia. J Vasc Surg 2009; 50:769–775. [DOI] [PubMed] [Google Scholar]
- 45.de Mutsert R, Grootendorst DC, Indemans F, et al. Association between serum albumin and mortality in dialysis patients is partly explained by inflammation, and not by malnutrition. J Ren Nutr 2009; 19:127–135. [DOI] [PubMed] [Google Scholar]
- 46.Owen WF, Jr, Lew NL, Liu Y, et al. The urea reduction ratio and serum albumin concentration as predictors of mortality in patients undergoing hemodialysis. N Engl J Med 1993; 329:1001–1006. [DOI] [PubMed] [Google Scholar]
- 47.Takahashi S, Oida K, Fujiwara R, et al. Effect of cilostazol, a cyclic AMP phosphodiesterase inhibitor, on the proliferation of rat aortic smooth muscle cells in culture. J Cardiovasc Pharmacol 1992; 20:900–906. [DOI] [PubMed] [Google Scholar]
- 48.Miyashita Y, Saito S, Miyamoto A, et al. Cilostazol increases skin perfusion pressure in severely ischemic limbs. Angiology 2011; 62:15–17. [DOI] [PubMed] [Google Scholar]


