Supplemental Digital Content is available in the text
Abstract
The purpose of this study was to describe the patient characteristics, computed tomography (CT) and 18F-fluorodeoxyglucose positron emission tomography (FDG PET) findings, and clinical outcomes of primary pulmonary synovial sarcoma (PPSS), together with their pathologic correlations. The medical records of 14 patients with pathologically proven PPSS in a tertiary hospital from January 1997 to December 2014 were retrospectively reviewed. The CT findings were evaluated. The maximum standardized uptake value (maxSUV) of the tumors was obtained, and clinical outcomes with respect to tumor recurrence and mortality were assessed by Kaplan–Meier analysis. The median tumor size was 10.2 cm and the most common anatomic location was the lung followed by the pleura/chest wall and mediastinum. Most of the tumors appeared as single lesions and had circumscribed margins. All the cases showed heterogeneous enhancement with necrotic or cystic portions, and intratumoral vessels were frequently seen. Half of the tumors had intratumoral calcifications, and tumor rupture, pleural/chest wall extension, and pleural effusion occurred frequently. However, lymph node enlargement was rare. The median maxSUV of the tumors was 4.35. Patient outcomes with respect to tumor recurrence (n = 8, 57.1%) and death (n = 3, 21.4%) were poor despite their young age, and the mean follow-up period was 28.5 months.
In conclusion, PPSS usually occurs in young adults, generally in the lung, presents as a large, circumscribed mass, and tumor rupture or extension of the pleura/chest wall may occur. The tumors often contain calcifications and vessels; they may exhibit triple attenuation on enhanced CT images, and clinical outcomes are poor.
INTRODUCTION
Primary pulmonary synovial sarcoma (PPSS) is known as a distinct histologic subtype of pulmonary sarcoma after the identification of a pathognomonic chromosomal translocation in synovial sarcoma.1 The first case was reported in 1995.2 Despite its name, PPSS does not originate from the synovium but from immature mesenchymal elements.3 Increasingly advanced diagnostic techniques of immunohistochemistry, cytogenetic analysis, and electron microscopy have enabled improved recognition of PPSS.1,4
Among all primary lung malignancies, pulmonary sarcomas account for only 0.1% to 0.5%. The most commonly reported subtypes are leiomyosarcoma, undifferentiated pleomorphic sarcoma, fibrosarcoma, and, more recently, synovial sarcoma.3
Soft-tissue synovial sarcoma constitutes about 5% to 10% of all soft tissue sarcomas.5 The most frequent sites of origin are the lower and upper extremities.4 Extrathoracic soft-tissue synovial sarcoma is much more frequent than PPSS and usually presents as a paraarticular soft-tissue mass, affects young and middle-aged adults, and has well-recognized imaging features. PPSS may arise in the lung, pleura, chest wall, mediastinum, or heart, and has the same patient demographics and some radiologic characteristics as its soft-tissue counterpart.4 Over the past decade, the radiologic features of PPSS have been described in histopathologic and surgical articles, and in a few case reports.1,4,6–14 To the best of our knowledge, there have been no detailed findings of PPSS from CT and 18F-fluorodeoxy glucose positron emission tomography (FDG PET). The purpose of this study was to describe the patient characteristics, CT and FDG PET findings, and clinical outcomes of PPSS with their pathologic correlations, using consecutive data from a tertiary referral center.
MATERIALS AND METHODS
Patient Characteristics
This study was approved by the Institutional Review Board, and informed consent was waived. All confirmed cases of PPSS from January 1997 to December 2014 were retrospectively identified from the patient records based on the pathological diagnosis and using our inhouse search software (ABLE and PETAgate; Asan Medical Center, Seoul, Korea). The terms used were “chest or pulmonary sarcoma,” “chest CT,” and “synovial sarcoma”. Fourteen cases of PPSS were identified after excluding 148 cases of sarcomatoid carcinoma, 29 of metastatic sarcoma, and 17 of undetermined type (Figure 1). At the same time, 217 cases of pathologically proven synovial sarcoma involving all anatomic locations were identified according to their origin, and the 14 cases of PPSS were also included among these.
FIGURE 1.
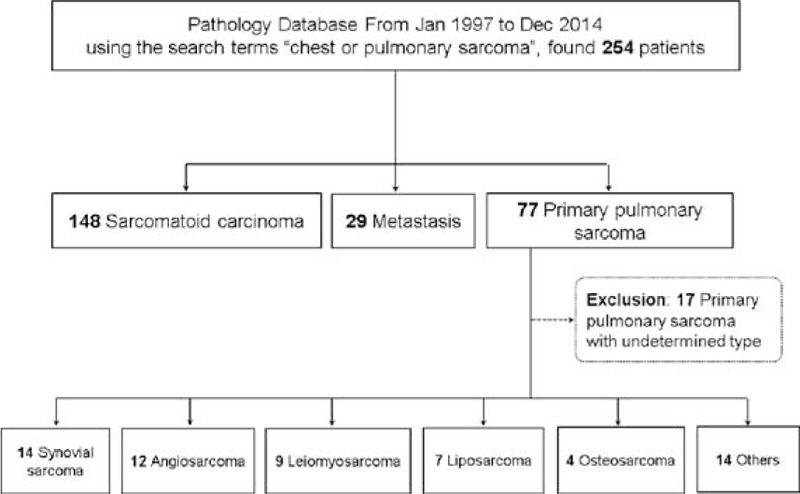
Flow diagram for selecting cases of primary pulmonary synovial sarcoma.
Patients with pulmonary metastasis were excluded by a systemic review of their medical records or of other studies such as whole-body FDG PET, bone scans, and radiological examinations of the extremities, involving a combined approach by oncologists, radiologists, pathologists, and pulmonologists. Finally, 14 cases in 8 women and 6 men, with a median age of 37 years (range: 18–65 years) at diagnosis, were included in this study. Clinical information such as chief complaints, smoking habits, treatment methods, and outcomes were obtained by reviewing electronic medical records.
CT Evaluation
CT Scanning Protocol
CT imaging was performed using a SOMATOM (Siemens Medical Solutions, Forchheim, Germany) (n = 10) or Lightspeed Volume CT (General Electric Medical Systems, Milwaukee, WI) (n = 4). With the SOMATOM, scanning parameters were as follows: 120 kVp; 100 mA with automatic tube current modulation; and reconstruction intervals, 3/5 mm, with no gap in the standard algorithm, and 1 mm, with a 5-mm gap in the high-frequency algorithm. With the Lightspeed Volume CT, scanning parameters were as follows: 120 kVp; 100 to 300 mA with automatic tube current modulation; and reconstruction intervals, 2.5/5 mm, with no gap for the lung algorithm, and 1.25-mm, with a 5-mm gap for the bone algorithm. All images were routinely reformatted on a coronal plane. CT contrast and 50 mL normal saline were injected at a rate of 2.5 mL/s for contrast-enhanced CT. All images were examined on the mediastinal window (width, 450 Hounsfield units; level, 50 Hounsfield units) and lung window (width, 1,500 Hounsfield units; level, 700 Hounsfield units) settings in the picture archiving and communication system.
CT Evaluation
Two radiologists (M.Y.K., with 18 years of thoracic radiology experience and G.H.K., with 2 years of radiology experience) who were blind to the data, except for the diagnosis of PPSS, evaluated the CT images in consensus. One radiologist (23 years of clinical experience in thoracic radiology, non author) verified the data set.
The CT findings were well described, including tumor size, location (in lung, pleura/chest wall, or mediastinum), number of lesions, margins, internal features such as internal necrosis or cystic portions, enhancement, intratumoral vessels, and calcification, and external features such as tumor rupture, pleural or chest wall extension, and pleural effusion. The presence of lymphadenopathy with a >1-cm short-axis diameter on the axial CT scan was also evaluated. If the lesion showed an internal low-attenuation component, other than a calcification, it was considered as having a heterogeneous enhancement. For multiple masses, the largest lesion was selected to assess the CT findings.
FDG PET Scanning Protocol
FDG PET was performed in 6 patients using a PET/CT scanner (Discovery PET/CT 690; GE Health Care, Milwaukee, WI). After the patient had fasted for at least 6 hours, he or she was given an 18F-FDG (5.2 MBq/kg) intravenous injection and a PET/CT scan was performed 50 minutes later. Three-dimensional PET acquisition was carried out and attenuation correction was achieved with CT attenuation maps. Using the patient's lean body mass, the standardized uptake value (SUV) was calculated. A maximum SUV of 2.5 was regarded as the cutoff value for a hypermetabolic lesion. The median interval between the CT and FDG PET studies was 9 days (range: 1–14).
Pathologic Diagnosis
The pathologic specimens were obtained by surgical resection (n = 9) or CT-guided (n = 3), fluoroscopy-guided (n = 1), or bronchoscopy-guided (n = 1) core-needle biopsy (Table 1). The CT-guided procedures were performed using a 16-channel SOMATOM Sensation (Siemens Medical Solutions, Forchheim, Germany). To perform the fluoroscopy-guided procedures, the Siemens Artis zee multipurpose system (Siemens AG, Muenchen, Germany) was used. Biopsies were carried out with a 20-gauge semiautomatic core biopsy needle (Stericut with a coaxial guide, TSK Stericut; TSK, Laboratory, Soja, Japan). After the pleura had been punctured, biopsies were performed 2 or 3 times with the patient holding their breath to get sufficient tissue for diagnosis.
TABLE 1.
Detailed Clinical Characteristics of 14 Patients With Primary Pulmonary Synovial Sarcoma
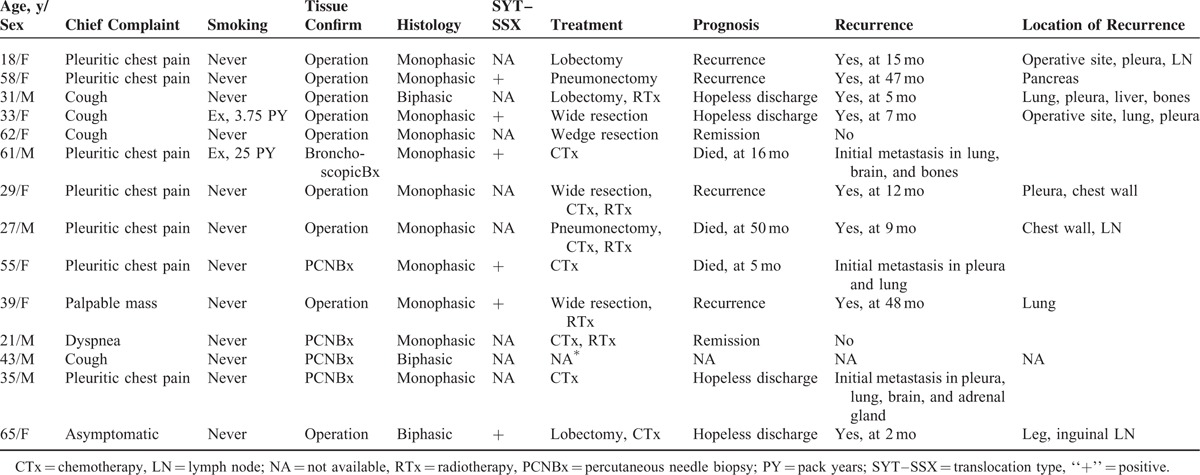
The pathologic specimens were fixed in 10% formalin, paraffin-embedded, and sectioned for hematoxylin and eosin staining and immunohistochemistry. Two pathologists primarily reviewed all the specimens, and 1 expert thoracic pathologist (J.S.S.) examined them to obtain detailed pathologic findings. PPSS was diagnosed based on morphology and immunohistochemical findings. Reverse transcriptase polymerase chain reaction was used to detect SYT-SSX1/2 fusion transcripts in 6 patients (Supplementary Table 1, http://links.lww.com/MD/A385).
Statistical Analysis
Patient characteristics and radiological findings are descriptively presented. Clinical outcomes with respect to disease-free survival were analyzed by the Kaplan–Meier method, using commercial statistical software (SPSS for Windows, version 21.0; SPSS Inc., Chicago, IL).
RESULTS
Patient Characteristics
The incidence of PPSS among all the subtypes of primary pulmonary sarcoma was 18.2% (14/77). Also, only 5.9% (14/217) of the synovial sarcomas originating from all anatomic locations were of primary pulmonary origin.
No definite gender predominance was noted, and most of the patients had never smoked (85.7%). The patients’ complaints included pleuritic chest pain (n = 7, 50.0%), cough (n = 4, 28.6%), a palpable mass (n = 1, 7.1%), and dyspnea (n = 1, 7.1%). Tumor recurrence or mortality (n = 11, 78.6%) occurred in many of the patients despite their young age, and the 2-year disease-free survival rate was 35.7% (Figure 2). The clinical findings, including the outcomes, are summarized in Table 1.
FIGURE 2.
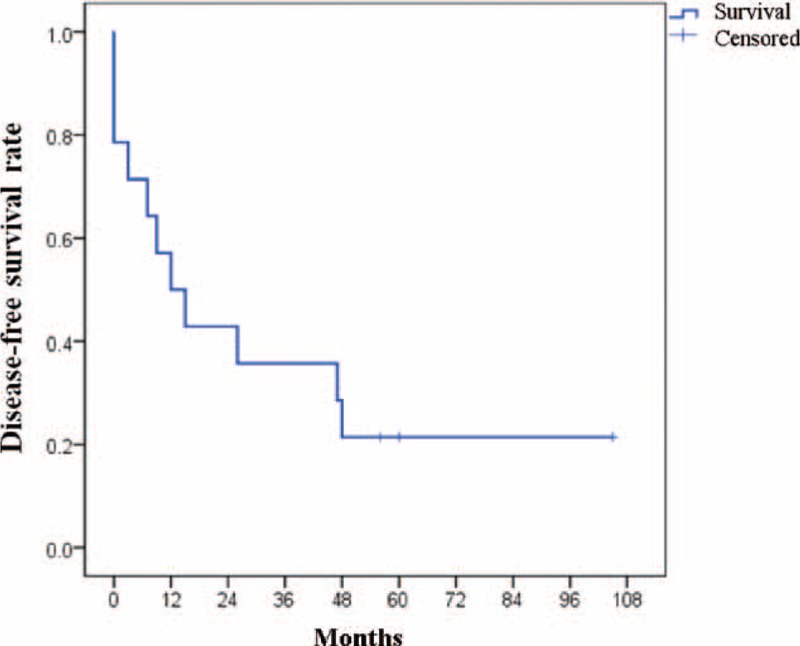
Disease-free survival for tumor recurrence or mortality analyzed by the Kaplan–Meier method; 1 and 2-year disease-free survival rates were 50% and 35.7%, respectively.
CT and FDG PET Findings
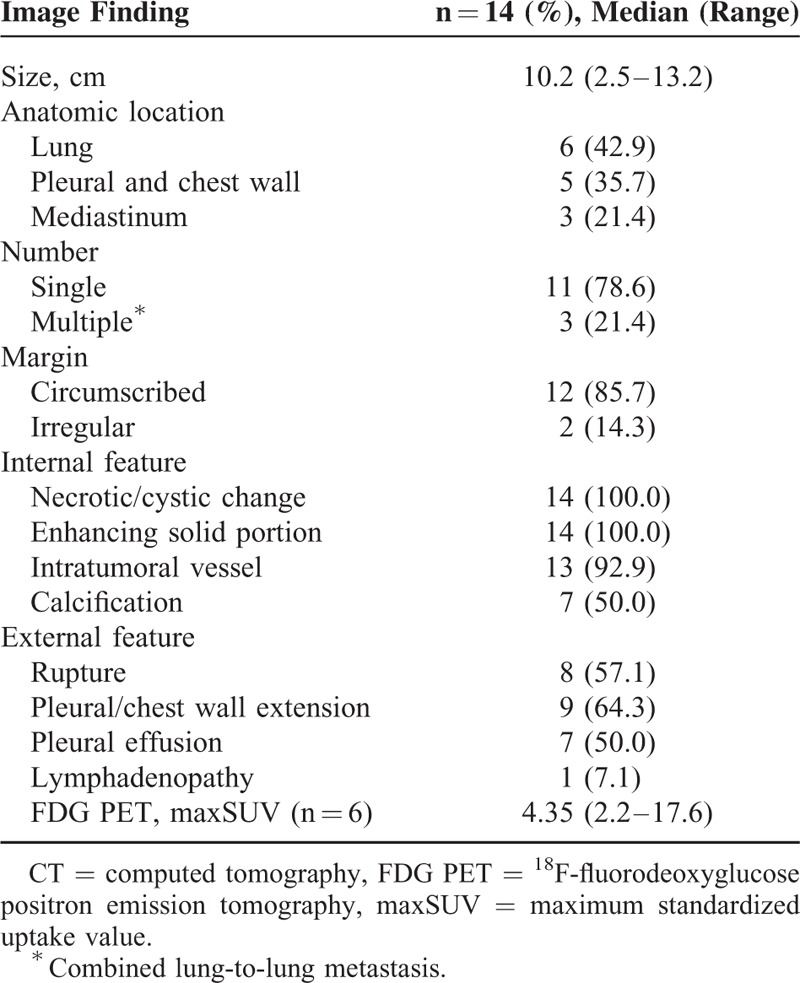
The median longest diameter of tumor on the axial CT images was 10.2 cm (range: 2.5–13.2 cm). The most common anatomic location was the lung (n = 6, 42.9%) (Figures 3 and 4), followed by the pleura/chest wall (n = 5, 35.7%) and the mediastinum (n = 3, 21.4%) (Figure 5). Most of the tumors presented as single lesions (n = 11, 78.6%). The tumors generally had circumscribed margins (n = 12, 85.7%)), and all showed heterogeneous enhancement with internal necrotic or cystic changes. Intratumoral vessels (n = 13, 92.9%), or punctate or amorphous calcification (Figure 4) (n = 7, 50.0%), were also noted. Tumor rupture, pleural or chest wall extension, or pleural effusion occurred in half of the patients, but lymph node enlargement (n = 1) was rare.
FIGURE 3.
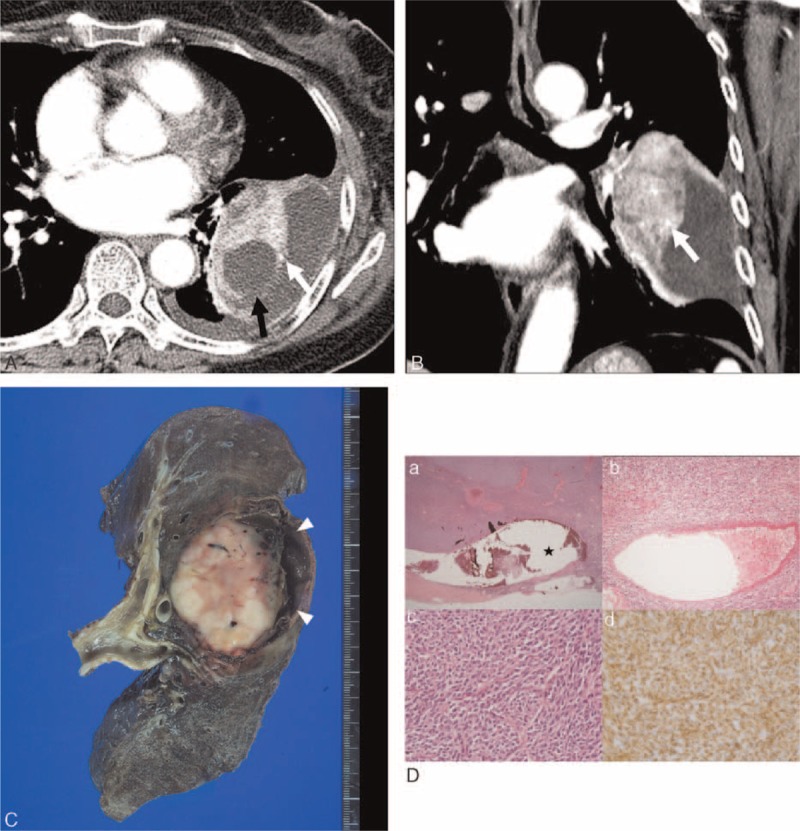
Computed tomography (CT) images obtained in a 58-year-old woman with monophasic primary pulmonary synovial sarcoma. (A and B) Conventional transverse mediastinal CT image (5-mm thick) obtained at the level of the left pulmonary vein, and coronal mediastinal CT image (5-mm thick) obtained at the level of the left upper pulmonary vein. CT images show a 7.7-cm-sized heterogenously enhancing mass in the left lower lobe with circumscribed margin and internal cystic or necrotic portion, and an intratumoral vessel (white arrows in A and B). Note the obliterated tumor margin (black arrow in A) and pleural effusion, indicating rupture of the tumor. FDG PET reveals an FDG-avid lung mass with a maxSUV of 3.8 (not shown). (C) Pneumonectomy was performed and the photograph shows the creamy white and soft solid portion with focal hemorrhage. The cystic portion is filled with a blood clot (arrowheads). (D) On histologic analysis, (a) the periphery of the tumor shows cystic change (★); (b) a thick-walled blood vessel is present in the center of the tumor; (c) the tumor has elevated cellularity and is composed of short spindle-shaped cells with hyperchromatic nuclei, and high mitotic activity (hematoxylin–eosin stain, original magnifications 12.5, ×100, ×400, respectively); and (d) the tumor cells are positive for vimentin by immunohistochemistry. FDG PET = 18F-fluorodeoxyglucose positron emission tomography, maxSUV = maximum standardized uptake value.
FIGURE 4.
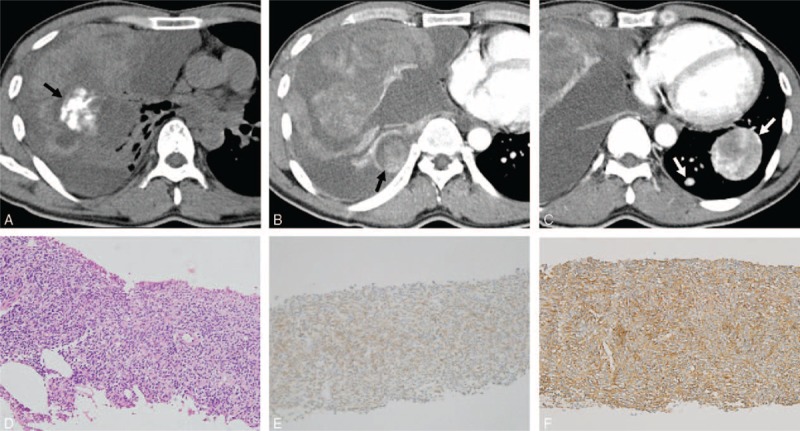
Computed tomography (CT) images obtained in a 35-year-old man with monophasic primary pulmonary synovial sarcoma. (A) Nonenhanced conventional transverse mediastinal CT image (3-mm thick) obtained at the level of the bronchus intermedius. The CT image shows amorphous calcification (black arrow in A) in the right middle lobe. (B and C) Contrast-enhanced conventional transverse mediastinal CT images (3-mm thick) obtained at the level of left atrial roof and at the level of inferior vena cava. The CT images reveal a 13.2-cm heterogenously enhancing mass with an internally cystic or necrotic portion, and an intratumoral vessel. Note the enhancing pleural-based nodule in the right lower hemithorax (black arrow in B), suggestive of ipsilateral pleural metastasis, and the tumor rupture with fluid effusion with high attenuation in the pleural space, indicating hemothorax. The CT image shows a heterogenously enhancing mass with an intratumoral vessel in the left lower lobe, and another small enhancing nodule (white arrows in C), suggestive of contralateral lung metastases. FDG PET shows an FDG-avid lung mass with a maxSUV of 3.7 (not shown). (D) Pathology after core-needle biopsy shows a cellular mass with spindle-shaped cells with hyperchromatic nuclei (hematoxylin–eosin stain, original magnification ×200). (E) The tumor cells are weakly diffusely positive for Bcl-2 by immunohistochemistry, supporting the diagnosis of synovial sarcoma (original magnification ×200). (F) The tumor cells are positive for CD99 by immunohistochemistry, supporting the diagnosis of synovial sarcoma (original magnification ×200). Bcl-2 = B-cell lymphoma-2, FDG PET = 18F-fluorodeoxyglucose positron emission tomography, maxSUV = maximum standardized uptake value.
FIGURE 5.
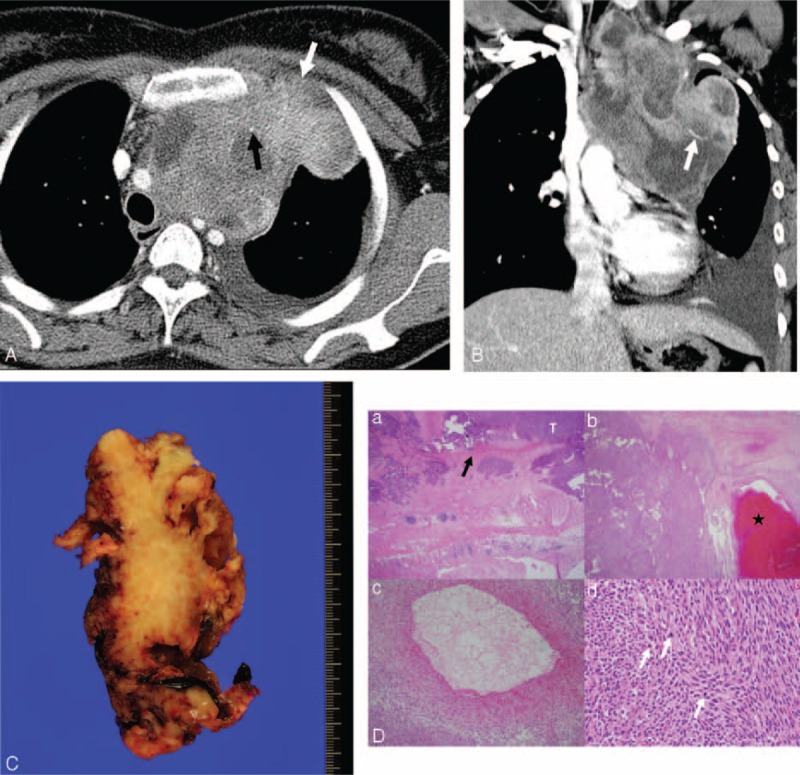
Computed tomography (CT) images obtained in a 33-year-old woman with monophasic primary pulmonary synovial sarcoma in the mediastinum. (A and B) Conventional transverse mediastinal CT image (5-mm thick) obtained at the level of the upper trachea, and coronal mediastinal CT image (5-mm thick) obtained at the level of the superior vena cava. CT images show a 12.8-cm heterogenously enhancing mass with lobulated margin and an internal cystic or necrotic portion that extends to the thoracic inlet. Chest wall invasion (white arrow in A) and pleural effusion are evident. Note the punctate calcification (black arrow in A) and intratumoral vessel (white arrow in B). FDG PET shows an FDG-avid mediastinal mass with a maxSUV of 4.9 (not shown). (C) Incomplete wide resection was performed because of severe adhesion with the left innominate vein and chest wall. The photograph shows the cut surface of the mass with a multifocal hemorrhage. (D) On histopathologic analysis, (a) the tumor (T) invades the pleural wall (black arrow); (b) it shows cystic change with hemorrhage (★); (c) a 1-mm-diameter blood vessel can be seen in the tumor; and (d) the tumor contains hypercellular short spindle-shaped cells with high mitotic activity (white arrows) (hematoxylin–eosin stain, original magnification ×12.5, ×12.5, ×100, ×400, respectively). FDG PET = 18F-fluorodeoxyglucose positron emission tomography, maxSUV = maximum standardized uptake value.
The median value of the maxSUV in the 6 patients with available FDG PET scans was 4.35 (range: 2.2–17.6), and 5 of the lesions showed hypermetabolic activity (>2.5 maxSUV). The CT and FDG PET findings in the 14 PPSS cases are summarized in Table 2 (Supplementary Table 2, http://links.lww.com/MD/A385).
TABLE 2.
CT and FDG PET Findings in 14 Patients With Primary Pulmonary Synovial Sarcoma
Histopathological Analysis
Of the 14 PPSS cases, 11 were of the monophasic type and 3 of the biphasic type. The monophasic PPSS cases were highly cellularized and composed of short spindle-shaped cells with hyperchromatic nuclei and high mitotic activity. In most of the cases, increased vascularity and frequent cystic change with hemorrhage were observed. The biphasic synovial sarcomas were composed of spindle-shaped cells and epithelial components.
Immunohistochemical staining revealed that the tumor cells were positive for CD 99 (6/7), B-cell lymphoma-2 (Bcl-2) (6/7), vimentin (4/4), cytokeratin (3/11), CD56 (3/3), epithelial membrane antigen (EMA) (1/7), and S-100 protein (1/8). They were negative for CD34 (0/10), desmin (0/2), smooth muscle actin (SMA) (0/5), and calretinin (0/3).
In addition STY-SSX fusion transcripts were identified in 6 patients, which confirmed their diagnosis of PPSS. The histopathologic findings for the PPSS are summarized in Supplementary Table 1, http://links.lww.com/MD/A385.
DISCUSSION
PPSS is an extremely rare primary lung tumor. In this study, the relative incidence of PPSS among the cases of primary pulmonary sarcoma was 18.2%, and among the cases of synovial sarcoma that originated from all anatomic locations, it was approximately 6%. The median age at initial presentation was 37 years with no sex predilection, similar to that in other studies and in extrathoracic soft tissue sarcoma.15 The most common symptoms were pleuritic chest pain and cough, and most patients were nonsmokers.
The CT manifestations of PPSS are similar to those of many other common pulmonary and pleural neoplasms of the lung and pleura, including primary and metastatic lung cancers, solitary fibrous tumors of the pleura, malignant mesothelioma, and other rare primary pulmonary sarcomas such as undifferentiated pleomorphic sarcoma, leiomyosarcoma, fibrosarcoma, malignant nerve sheath tumor, hemangiopericytoma, and sarcomatoid carcinoma.4 In our formal radiological reports before tissue confirmation, tumors such as nonspecific intrathoracic sarcoma, solitary fibrous tumor of the pleura, thymic neoplasm, and lung cancer are considered as differential diagnoses.
In addition, as synovial sarcoma of extrathoracic soft tissue origin is relatively common, and distant metastases occur in 40% to 50% of such patients, with lung metastasis being the most frequent, metastatic synovial sarcoma can be considered one of the candidates for differential diagnosis.4 During the study period, 3 pathologically proven cases of metastatic synovial sarcoma of the lung as well as a case of extrathoracic soft tissue synovial sarcoma occurred. As the radiologic characteristics of primary and metastatic synovial sarcomas are indistinguishable, meticulous clinical and radiologic assessment is necessary to exclude the presence of an extrathoracic primary tumor.
In this study, the most common anatomic location was the lung (42.9%). Even though a lesion might appear to be located mainly within the lung or the pleura, its origin is sometimes uncertain because of its large size.4 Most of the tumors in this study presented as solitary (78.6%), large circumscribed masses (85.7%) with mass effect, often with calcifications, and most were heterogeneous with triple attenuation on the enhanced CT images (ie, soft tissue attenuation, a well-enhanced solid portion, and a cystic or necrotic portion). All the tumors had hypoattenuated regions with no enhancement on the CT images, and these corresponded to the hemorrhagic, necrotic, and cystic, myxoid, or friable components of the pathologic findings. Regions of cellular solid tissue components appeared as well-enhanced lesions with high attenuation on contrast-enhanced CT, and intratumoral enhanced vessels were very common internal features (92.9%). As for the external features of the tumors, they had frequent extrapleural fat or chest wall extensions (64.3%), but no bony involvement. The tumors showed a propensity to rupture in the pleural space, with a rupture site visible sometimes, which resulted in frequent hemothorax or pleural effusion (50.0%). However, hilar or mediastinal lymphadenopathy was very rare.
Primary lung cancer can be indistinguishable from PPSS in CT in routine practice. However, the absence of significant lymphadenopathy with a relatively large circumscribed tumor in a young adult may indicate PPSS rather than lung cancer. Because percutaneous or bronchoscopic core biopsy of a tumor may result in tumor rupture in the pleural space, careful preprocedural evaluation is needed.
Three main histologic variants of PPSS have been described: the classic biphasic type that consists of spindle-shaped cells and epithelial cells (usually forming glandular structures); the monophasic type made up of only spindle-shaped cells; and the poorly differentiated type that consists of cells that resemble those of small round cell tumors.16 Although biphasic synovial sarcoma was the first to be recognized, monophasic synovial sarcomas are much more common in the respiratory system.
In addition, secondary changes including tumoral calcification or ossification, cystic changes, and necrosis may accompany the tumors.16
In immunohistochemical studies, PPSS is often positive for cytokeratin, EMA, Bcl-2, and vimentin, and negative for S-100, desmin, SMA, and vascular tumor markers.9 In our cases, the tumors were positive for CD99 and Bcl-2 in 85.7% of the cases, for vimentin and CD56 in 100.0% of the cases, and for CK in 27.3% and EMA in 14.3%. Only 1 case was positive for S-100, and all were negative for CD34, desmin, SMA, and calretinin.
Synovial sarcoma is associated with a specific t(X;18) (p11;q11) translocation that involves SS18 (also known as SYT) and SSX1, SSX2, or SSX4. A balanced reciprocal translocation is found in >90% of synovial sarcomas. As a result, the SYT gene on chromosome 18 is fused with the SSX1 or SSX2 gene on the X chromosome.17 In our study, this translocation was identified in all 6 patients tested (100.0%).
In the present series, most patients received surgery with or without chemotherapy or radiation therapy. Because the current treatment for PPSS is surgical resection, followed by chemotherapy, radiation therapy, or a combination of these modalities,9,16 meticulous preoperative chest CT evaluation is essential for planning treatment. Although differential diagnosis of PPSS is difficult using only radiologic findings, CT can help delineate the extent of tumor involvement and determine the potential for surgical resection and can monitor the effects of chemotherapy or radiation therapy.
The overall 5-year survival rate of soft tissue synovial sarcoma is about 50% to 80%.5 Tumor size of <5 cm, a low mitotic rate, absence of necrosis, presence of the SYT-SSX2 fusion gene, and local eradication are recognized favorable prognostic factors for survival.14 Long-term follow-up results of the patients in this study indicated that the outcomes in terms of recurrence and death was poor with a 2-year disease-free survival rate of 35.7%. In addition, the results also showed that the most common site of recurrence was thorax. These results are possibly due to late detection, bulky tumor, and the difficulty in obtaining wide surgical margins.4,7,9,14 Hence, early diagnosis and surgery with adequate resection margins are of key importance.
This study had several limitations. First, because PPSS is a rare tumor, the cases were retrospectively collected in a tertiary referral center. Hence, selection bias is possible. Second, in 5 patients, pathologic specimens were obtained through percutaneous core-needle biopsy, and may not have contained enough tissue to represent the whole tumors. Finally, the number of FDG PET examinations was small and led to variable metabolic uptake. Large-series studies are required to establish the potential of metabolic uptake in the diagnosis of PPSS.
In conclusion, PPSS usually occurs in young adults, commonly in the lung, presents as a large, circumscribed mass, and may involve tumor rupture or extension into the pleura or chest wall. Intratumoral calcification and vessels that may exhibit triple attenuation on enhanced CT images are frequent, and the clinical outcomes of the PPSS were poor.
Footnotes
Abbreviations: CT = Computed tomography, FDG PET = 18F-fluorodeoxyglucose positron emission tomography, LN = lymph node, maxSUV = maximum standardized uptake value, PPSS = primary pulmonary synovial sarcoma.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Hartel PH, Fanburg-Smith JC, Frazier AA, et al. Primary pulmonary and mediastinal synovial sarcoma: a clinicopathologic study of 60 cases and comparison with five prior series. Mod Pathol 2007; 20:760–769. [DOI] [PubMed] [Google Scholar]
- 2.Zeren H, Moran CA, Suster S, et al. Primary pulmonary sarcomas with features of monophasic synovial sarcoma: a clinicopathological, immunohistochemical, and ultrastructural study of 25 cases. Hum Pathol 1995; 26:474–480. [DOI] [PubMed] [Google Scholar]
- 3.Keel SB, Bacha E, Mark EJ, et al. Primary pulmonary sarcoma: a clinicopathologic study of 26 cases. Mod Pathol 1999; 12:1124–1131. [PubMed] [Google Scholar]
- 4.Frazier AA, Franks TJ, Pugatch RD, et al. From the archives of the AFIP: pleuropulmonary synovial sarcoma. Radiographics 2006; 26:923–940. [DOI] [PubMed] [Google Scholar]
- 5.Raney RB. Synovial sarcoma in young people: background, prognostic factors, and therapeutic questions. J Pediatr Hematol/Oncol 2005; 27:207–211. [DOI] [PubMed] [Google Scholar]
- 6.Duran-Mendicuti A, Costello P, Vargas SO. Primary synovial sarcoma of the chest: radiographic and clinicopathologic correlation. J Thorac Imaging 2003; 18:87–93. [DOI] [PubMed] [Google Scholar]
- 7.Zhang WD, Guan YB, Chen YF, et al. CT imaging of primary pleuropulmonary synovial sarcoma. Clin Radiol 2012; 67:884–888. [DOI] [PubMed] [Google Scholar]
- 8.Cabuk D, Ustuner B, Akgul AG, et al. Primary synovial sarcoma of lung. Korean J Thorac cardiovascular surgery 2014; 47:306–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dennison S, Weppler E, Giacoppe G. Primary pulmonary synovial sarcoma: a case report and review of current diagnostic and therapeutic standards. Oncologist 2004; 9:339–342. [DOI] [PubMed] [Google Scholar]
- 10.Park JS, Min BR, Park SH, et al. Primary pulmonary biphasic synovial sarcoma confirmed by molecular detection of a SYT-SSX2 fusion gene: report of 1 case. Korean J Intern Med 2010; 25:331–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mermigkis CM, Kopanakis A, Patentalakis G, et al. Primary monophasic synovial sarcoma presenting as a pulmonary mass: a case report. J Med Case Rep 2008; 2:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosono T, Hironaka M, Kobayashi A, et al. Primary pulmonary synovial sarcoma confirmed by molecular detection of SYT-SSX1 fusion gene transcripts: a case report and review of the literature. Jpn J Clin Oncol 2005; 35:274–279. [DOI] [PubMed] [Google Scholar]
- 13.Gaertner E, Zeren EH, Fleming MV, et al. Biphasic synovial sarcomas arising in the pleural cavity. A clinicopathologic study of five cases. Am J Surg Pathol 1996; 20:36–45. [DOI] [PubMed] [Google Scholar]
- 14.Essary LR, Vargas SO, Fletcher CD. Primary pleuropulmonary synovial sarcoma: reappraisal of a recently described anatomic subset. Cancer 2002; 94:459–469. [DOI] [PubMed] [Google Scholar]
- 15.Kransdorf MJ. Malignant soft-tissue tumors in a large referral population: distribution of diagnoses by age, sex, and location. AJR Am J Roentgenol 1995; 164:129–134. [DOI] [PubMed] [Google Scholar]
- 16.Eilber FC, Dry SM. Diagnosis and management of synovial sarcoma. J Surg Oncol 2008; 97:314–320. [DOI] [PubMed] [Google Scholar]
- 17.Dal Cin P, Rao U, Jani-Sait S, et al. Chromosomes in the diagnosis of soft tissue tumors. I. Synovial sarcoma. Mod Pathol 1992; 5:357–362. [PubMed] [Google Scholar]


