Supplemental Digital Content is available in the text
Abstract
Survivin is a biomarker of cancer known for its anti-apoptotic and cell-cycle regulating properties. In the context of non-cancer pathology, high levels of survivin may be measured in blood and synovial fluid of patients with rheumatoid arthritis (RA) and associate with early joint damage and poor therapy response.
The aim of the study was to investigate the value of survivin measurements in blood for diagnosis of RA in the frame of the Malaysian epidemiological investigation of rheumatoid arthritis (MyEIRA) study. The study enrolled RA patients from eight rheumatology centres in Peninsular Malaysia. The healthy controls matched by age, gender and ethnicity were recruited on the community basis from the residential area of the patients. Levels of survivin were measured in blood of RA patients (n = 1233) and controls (n = 1566) by an enzyme-linked immuno-sorbent assay (ELISA). The risk for RA was calculated as odds ratio (OR) and 95% confidence intervals in the individuals with high levels of survivin. The risk was calculated in relation to antibodies against cyclic citrullinated peptides (ACPA), detected by ELISA and HLA-DRB1 shared epitope (SE) alleles, identified by the polymerase chain reaction using sequence specific oligonucleotide method.
High levels of survivin were detected in 625 of 1233 (50.7%) RA cases and in 85 of 1566 (5.4%) controls, indicating its high specificity for RA. Survivin was association with an increase in RA risk in the patients having neither SE-alleles nor ACPA (OR = 5.40, 95% CI 3.81–7.66). For the patients combining survivin, SE, and ACPA, the estimated risk for RA was 16-folds higher compared to the survivin negative patients with SE and ACPA(OR = 16.21, 95% CI 5.70–46.18).
To conclude, detection of survivin in blood provides a simple test to improve diagnostic and to increase predictability for RA.
INTRODUCTION
Rheumatoid arthritis (RA) is a progressive debilitating autoimmune disease, which affects 0.5% to 1% people among all ethnic groups.1–3 Heritability of RA according to twin studies is about 60%,4 whereas familial aggregation of RA with siblings has a recurrent risk ratio of between 2 and 17.5
Risk of RA is strongly associated with major histocompatibility complex and carriage of the HLA Class II locus, in particular the HLA DR beta chain 1 (HLA-DRB1) gene.6 Several whole-genome studies have confirmed the linkage between the HLA-DRB1 gene and RA with an estimated contribution of 30% to 35% of the total genetic effect in RA.7–9 A group of RA-related HLA-DRB1 alleles encoding a conserved amino acid sequence (70QRRAA74 or 70KRRAA74 or 70RRRAA74) at position 70 to 74 in the third hypervariable region of the first domain of the DR beta chain was defined as shared epitope (SE).10 These SE alleles appear to confer high risk of severe joint damaging disease. In contrast, the HLA-DRB1 alleles with negatively charged aspartic acid at residue 70 (70DERAA74) were considered nonpredisposing DRB1 alleles, potentially protecting from RA or favoring less bone destruction.11–15
The exact biological mechanism connecting the SE alleles and RA remains unknown. The activation of CD4+ T cells by autologous antigens is considered an early event in RA pathogenesis. The current view on mechanisms underlying the effect of SE include the activation of CD4+ T cells with arthritogenic self-peptide sequences wherein the amino acid arginine is deiminated to citrulline (citrullinated peptides)16,17 and expansion of these self-specific T cells in the joints.10,18 Antibodies specific for citrullinated peptides (ACPAs) have been recognized as important prognostic and diagnostic tool for RA.19 Though the association of SE alleles with ACPA is documented,19 the induction of T-cell proliferation and cytokine response was not always associated with ACPA production.20 Furthermore, the presence of HLA-DRB1 alleles may also be found in the ACPA-negative RA.21 This suggests the existence of other factors enhancing the molecular link between SE and aberrant immunological responses in RA.
In the present study, we evaluated survivin, an oncoprotein known as a tissue marker of cancer. Following the initial description of survivin,22 the studies on survivin are concentrated on its anti-apoptotic and cell cycle-regulatory properties in malignancies.23 During recent years, the role of survivin in nonmalignant cells attracts increasing attention. Survivin has been shown essential for differentiation, growth, and regeneration of healthy tissues including hematopoetic stem cells.24 In the immune-competent cells, survivin is required for functional antigen presentation, the hallmark of aberrant immunity in RA disease. Survivin controls the maturation of antigen-presenting dendritic cells and expression of MHC Class II molecules.25,26 Survivin is important for the formation of a functional T-cell receptor in the developing thymocytes27,28 and for the differentiation into effector and memory T cells.29,30 At preclinical phase of RA, high levels of survivin correlate with cytokines assuring formation of aggressive Th1 and Th17 cells.31 In the patients early after the RA diagnosis, survivin predicts joint destructive course of disease,32 and resistance to anti-rheumatic treatment.33 Survivin may be measured in blood and synovial fluid of a substantial part of RA patients.31,32 Thus, we hypothesized that survivin may be specific diagnostic test for RA and for estimation of risk of RA development.
MATERIAL AND METHODS
Study Population
The Malaysian Epidemiological Investigation of Rheumatoid Arthritis (MyEIRA) study was approved by the Medical Research and Ethics Committee, Ministry of Health, Malaysia (KKM/JEPP/02 Jld.1 (86);(14)dlm.KKM/NIHSEC/08/0804/ MRG-2005-12). Informed written consent was obtained from all the participants. MyEIRA is a population-based case–control study, which addresses the influence of genes and environment on the development of RA, and comprises 1233 RA patients and 1566 controls enrolled in the study during the period between August 2005 and December 2009. RA cases who fulfilled the American College of Rheumatology classification criteria of RA34 and had the disease duration <2 years were identified within 8 rheumatology centers in Peninsular Malaysia. The recruitment of controls was done on the community basis with the support of the State and District Health Department and covered the regions corresponding to residential area of the patients. Nurses and healthcare professionals identified the controls matched to the RA cases by the residential area, age, sex, and ethnicity in house-by-house visits. The RA cases and the controls had a structured interview based on the study questionnaire, which collected information on a broad range of issues including lifestyle factors, occupational exposures, health aspects, socioeconomic factors, and demographic data.35 Blood samples were collected from all the participants.
Assessment of Survivin Protein in Blood
Survivin levels were measured in serum samples diluted 1:10 using a sandwich enzyme-linked immunoassay (DY647, R&D Systems, Minneapolis, MN, USA). The absolute level of survivin in each sample was calculated after the serial dilution of human recombinant survivin (R&D Systems), the detection limit was 100 pg/mL. Samples containing >450 pg/mL of survivin were considered positive based on the cut-off level determined in 104 healthy blood donors.26
HLA-DRB1 Genotyping
High-resolution genotyping of HLA-DRB1∗01–DRB1∗016 alleles was performed in the DNA extracted from the leukocytes of EDTA preserved peripheral blood using Ficoll Hypaque (Lymphoprep™, Axis-Shield PoC AS, Oslo, Norway), by the QIAamp DNA Blood Mini kit (Qiagen, Hilden, Germany). The PCR sequence-specific oligonucleotide probe hybridization method was applied using the LABType∗HD Class II DRB1 (One Lambda Inc, CA, USA) as described previously.36 HLA-DRB1 allele frequencies were obtained by direct counting. The carriers of SE were recognized by the presence of HLA alleles, DRB1∗01 (∗01:01, ∗01:02, ∗01:07), DRB1∗04 (∗04:01, ∗04:04, ∗04:05, ∗04:08, ∗04:10), and DRB1∗10 (∗10:01, ∗10:03). Individuals carrying 1 or 2 SE alleles were classified as SE-positive.
Autoantibody Measurements
ACPA was measured in serum samples using Immunoscan-RA Mark2 ELISA test (anti-CCP test, Malmö, Sweden). Samples with ACPA ≥25 AU/mL were defined as positive.37
Statistical Analysis
The post-hoc calculation of the study power (alpha = 0.05) found that the collected material was sufficient to attain study power of 97%. Student t test was calculated for the comparison of the mean age in the patient and control groups. Prevalence of survivin >450 pg/mL among the RA patients and the controls allowed calculation of specificity and positive predictive value of survivin for RA. The probability of RA among the subjects with high survivin was calculated as odds ratio (OR) and corresponded to the relative risk of RA. The ethnic heterogeneity of the material was taken as a potential confounding factor; therefore, association between survivin and SE alleles and SE sequences was calculated separately for the 3 major ethnical groups comprising MyEIRA Malay, Chinese, and Indian. Samples obtained from individuals with different ethnical background (controls, n = 94, 6.0% and RA, n = 82, 6.7%) were therefore excluded from this analysis. To investigate possible diagnostic basis associated with survivin testing, we repeated the primary analysis among the subjects stratified after ACPA tests.
Ninety-five percent confidence intervals were calculated and P values were 2-sided, with statistical significance set at P < 0.05. All analyses were performed using the IBM SPSS Statistics 20.0 software (SPSS Inc, Chicago, IL, USA).
RESULTS
The Prevalence of Survivin in MyEIRA Study Population
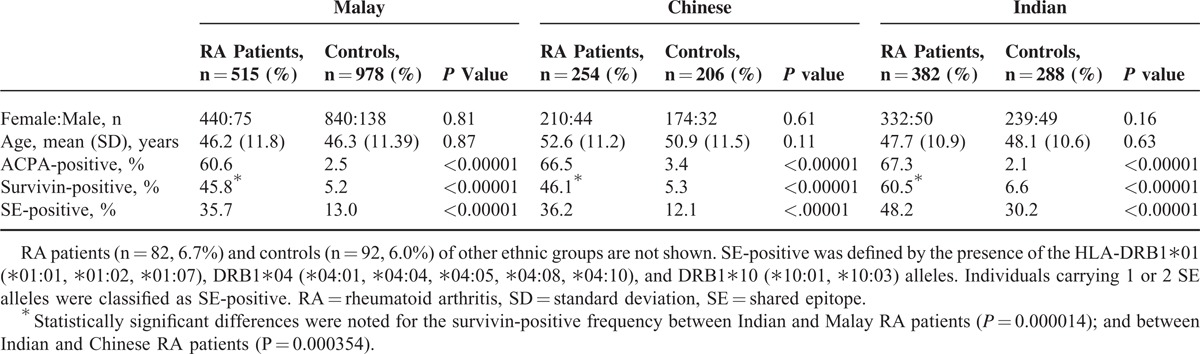
Six-hundred twenty-five of 1233 (50.7%) RA patients were survivin-positive and so were 85 of 1566 (5.4%) controls (P = 2.66 × 10E-164). Survivin positivity appeared to have a specificity of 0.95 and positive predictive value of 0.88 for RA. The prevalence of survivin-positive RA patients was comparable between the sexes (50% women and 55% men). Demographic and biomarker characteristics of the RA patients and the controls of 3 major ethnic groups within MyEIRA are presented in Table 1. The prevalence of survivin-positive RA patients and controls was significantly higher in the Indian ethnic subgroup (60.5% and 6.6%, respectively) compared with Malays and Chinese (all P values <0.0001).
TABLE 1.
Demographic and Biomarker Characteristics of Patients With RA and Controls in the MyEIRA Study
The Association Between Survivin and SE Alleles in RA Patients
The SE carriers comprised 40% of the RA patients and 16% of the controls. Among the SE-positive RA patients, 59% (291/494) were also survivin-positive. The complete genotype of HLA-DRB1∗01-HLA-DRB1∗16 alleles for all RA patients stratified by the presence of survivin and the controls is shown in Supplementary Table S1 http://links.lww.com/MD/A179. The presence of survivin significantly increased the risk of RA within the Malays, Chinese, and Indian ethnic groups (Figure 1). The combination of survivin positivity and SE positivity recognized the group wherein the risk of RA was highest. The presence of either survivin or SE separately was associated with lower, but still increased, risk of RA.
FIGURE 1.
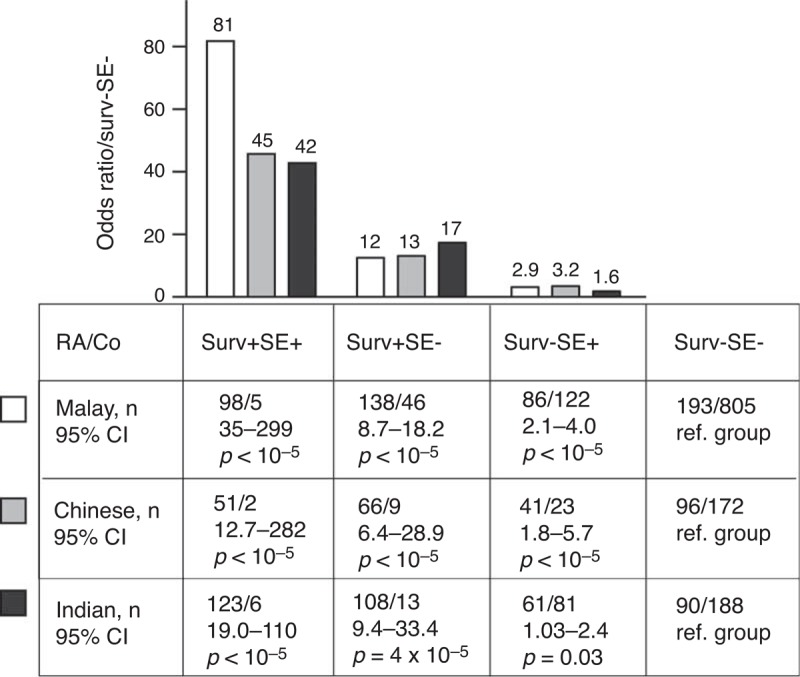
The relative risk of RA in subjects with high survivin in 3 major ethnic populations of the MyEIRA study. The study material was stratified after survivin levels (surv+, >450 pg/mL) and the carriage of HLA-DRB1 shared epitope (SE+) alleles within Malay (RA patients, 515, Co 1493), Chinese (RA patients, 254, Co 460), and Indian subjects (RA patients, 382, Co 670). The probability of RA for the subjects with high survivin was calculated as odds ratio in relation to surv-SE- reference group. This corresponded to the relative risk of RA. 95% CI = 95% confidence interval, Co = controls, RA = rheumatoid arthritis.
The estimated risk of RA related to the presence of specific SE alleles and survivin was also calculated within the Malays, Chinese, and Indian ethnic groups. Overall, the risk of RA was significantly increased in the survivin-positive carriers of DRB1∗01 (OR 2.09, 95%CI 1.13–3.88, P = 0.024) and DRB1∗04 (OR 4.85, 95% CI 2.79–6.20, P < 0.00001). In addition, the carriers of DRB1∗10 SE alleles had increased RA risk in the survivin-positive (OR 4.77, 95% CI 3.49–6.53, P < 0.00001) and in the survivin-negative RA (OR 3.64, 95% CI 2.63–5.04, P < 0.00001).
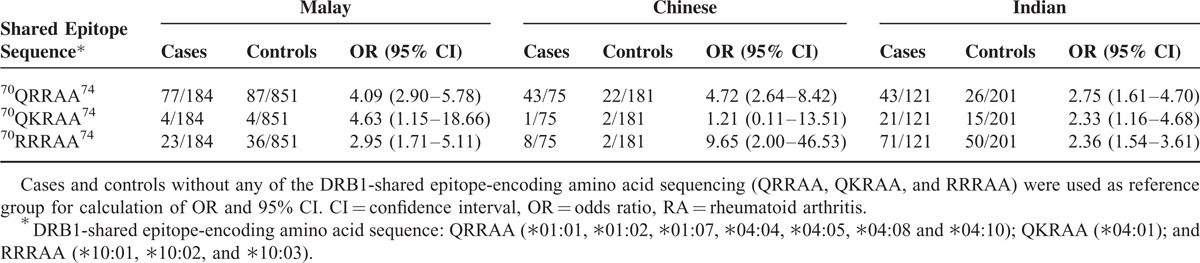
The analysis of RA risk was performed for the survivin-positive SE carriers in relation to the amino acid sequence of different ethnic subgroups (Table 2). In the Malays survivin-positive patients, the RA risk was associated with the 70QRRAA74 and 70QKRAA74 SE-sequences; in the Chinese survivin-positive patients, the presence of 70RRRAA74 SE-sequence indicated the highest RA risk; and in the Indian survivin-positive patients, the RA risk was comparable between the sequences. The evaluation of other HLA-DRB1 alleles frequent among Asians showed a slight accumulation of survivin-positive patients within the DRB1∗09 carriers (OR 2.20, 95% CI 1.50–3.22, P = 5.9 × 10E-5). The DRB1∗14 alleles (eg, ∗14:02 and ∗14:06) common among American Indians who originated in northeast Asia were prevalent among the RA patients and were equally distributed in the survivin-positive and survivin-negative groups. The DRB1 alleles containing sequences nonpredisposing to RA DRB1∗03, ∗08, ∗12, and ∗13 were equally represented among the survivin-positive and survivin-negative RA patients (Supplementary Table S1 http://links.lww.com/MD/A179).
TABLE 2.
The Frequency of Shared Epitope Sequences (QRRAA, QKRAA, and RRRAA) in the Survivin-positive RA Patients
The Combination of Survivin and ACPA Increases the Risk of RA
The high levels of ACPA were detected in 83% of the survivin-positive and in 44.7% of the survivin-negative RA patients (Figure 2). The survivin positivity was associated with higher risk of RA when present in combination with ACPA (OR = 16.21, 95% CI 5.70–46.18, P < 0.0001); however, the presence of survivin in the ACPA-negative RA patients was associated with a significantly increased risk of RA when compared with the controls (OR = 5.64, 95% CI 4.12–7.70), P < 0.0001). The specificity of survivin positivity was comparable between the ACPA-positive RA and ACPA-negative RA (0.89 and 0.95, respectively).
FIGURE 2.
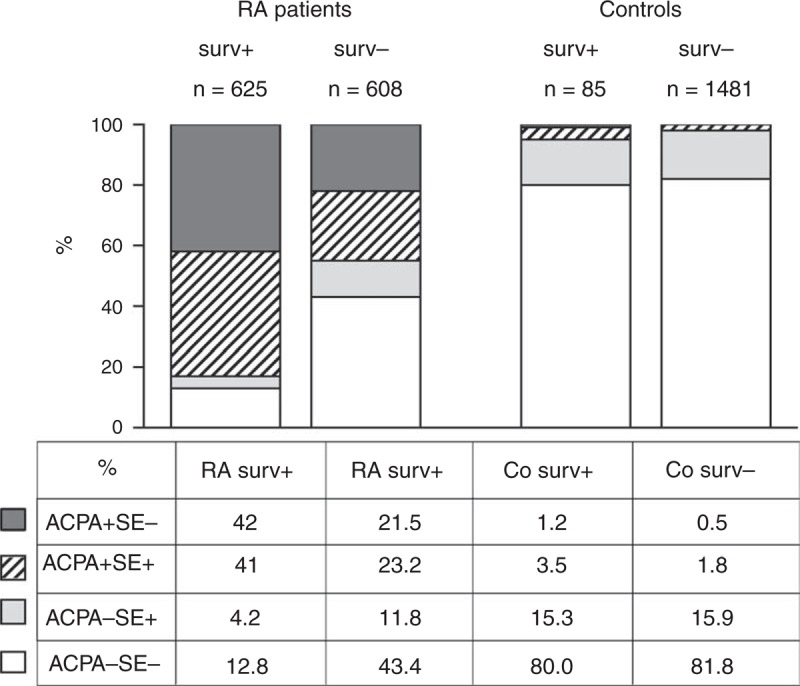
The composition of survivin-positive and survivin-negative groups among the RA patients and Co of the MyEIRA study. The prevalence of HLA-DRB1 shared epitope alleles (SE) and ACPA is presented in per cent within survivin positive and negative RA patients and Co. ACPA = antibodies to citrullinated peptides, Co = controls, RA = rheumatoid arthritis.
The Added Value of Survivin For Risk of RA in the Presence of HLA-DRB1 SE Alleles and ACPA
Survivin positivity was associated with the overall risk for RA (OR 17.91, 95% CI 14.01–22.89, P < 10E-10). The survivin positivity was prevalent in the SE-positive (SE+) and in ACPA-positive (ACPA+) RA patients (Figure 2). The association of survivin with any of these factors indicated the increased risk of RA. To analyze the added value of survivin positivity to the risk of RA, the OR was calculated for the survivin-positive compared with survivin-negative subjects within the groups of SE+ACPA-, SE-ACPA+, SE+ACPA+, and those having neither SE nor ACPA (Figure 3). We observed that survivin positivity was consistently associated with the increased RA risk in each of the studied subgroups (Figure 3). The presence of survivin in the SE- and ACPA-negative subjects was associated with the 5.4-fold higher estimated risk of RA compared with the survivin-negative subjects (OR 5.40, 95% CI 3.81–7.66), whereas the highest estimated risk of RA was in the group of combining all 3 biomarkers (Survivin+SE+ACPA+) (OR 16.21, 95% CI 5.70–46.18).
FIGURE 3.
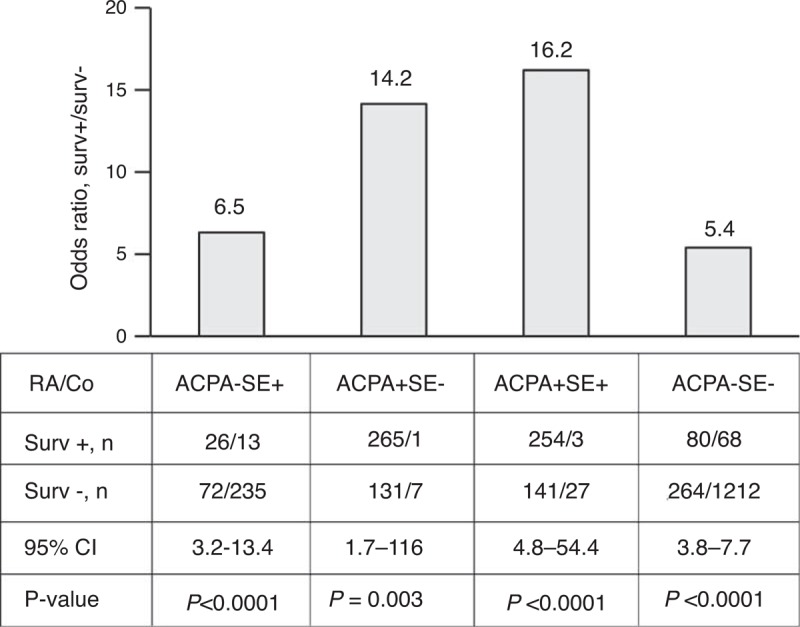
The added value of high survivin to the relative risk of RA. The added risk of RA was estimated as the OR calculated for the survivin-positive subjects (Surv+) within the ACPA-negative noncarriers of SE (SE−ACPA−), ACPA-positive carriers of SE (SE+ACPA+); ACPA-negative carriers of SE (SE+ACPA−); and ACPA-positive noncarriers of SE (ACPA+SE−). ACPA = antibodies to citrullinated peptides, Co = controls, OR = odds ratio, RA = rheumatoid arthritis, SE = shared epitope.
DISCUSSION
The MyEIRA study is the first large Asian case–control population-based epidemiologic study estimating the predictive value of survivin for RA. The study demonstrates the prevalence of high serum levels of survivin in 50.7% of RA patients and in 5.4% of the controls, indicating its high specificity for RA, which allowed to consistently distinguish RA patients from controls. The MyEIRA study comprises patients with short duration of RA, which explains the high prevalence of survivin similar to the one previously shown in the Swedish cohort of early RA patients.32
The study demonstrates that survivin is highly associated with 2 established biomarkers predicting development of RA namely the HLA-DRB1 SE alleles and ACPA. The substantial part of survivin-positive RA cases is carriers of the SE alleles (45%) and additional 42% has production of ACPA. We have previously shown that the presence of DRB1∗04 and ∗10 alleles is associated with the increased risk of ACPA-positive RA in the population of Malaysia.36 The survivin-positive RA patients are over-represented within the carriers of DRB1∗01 and DRB1∗04 alleles, whereas the DRB1∗10 is associated with survivin-positive and with survivin-negative RA. Additionally, survivin-positive RA patients are shown to be frequent carriers of the SE alleles containing 70QRRAA74, 70QKRAA74, and 70RRRAA74 sequences, whereas DRB1∗14 (∗14:02 and ∗14:06) alleles containing the identical SE sequence are not enriched in the survivin-positive patients. In contrast to ACPA-positive RA,14,15 we observe no negative association between the presence of survivin and the frequency of nonpredisposing DRB1 alleles containing 70DERAA74 sequence.
The prevalence of SE and ACPA alone or in combination was significantly higher in the survivin-positive RA patients and may be considered a limitation in this study. To estimate the value of survivin for the development of RA, we validate its role in ACPA-negative patients. The specificity of survivin for RA appears to be high and shows no decline in the absence of ACPA, which indicates that survivin-positivity accounts for a significantly increased risk for development of RA irrespective of the SE and ACPA status. The measurement of survivin may be of unique value for ACPA-negative RA patients where specific biomarkers of the disease are missing.
Selection of RA patients among the hospital-referred subjects with similar baseline characteristics, in which the presence of autoantibodies has high diagnostic value, could be a major concern of this observational study. The internal validity is assured by the broad enrolment programme, which included 8 rheumatology centers of Malaysia. Carefully matched controls are recruited to the study in the residential areas of the patients. Importantly, the ethnic differences of the Malaysian population are represented in the RA cases and in the control group. The obtained results are consistent within all ethnic subgroups. This strengthens the external validity of the study and permits extrapolation of the results to more universal population of RA patients.
In the clinical context, simultaneous presence of SE alleles with survivin and ACPA may reflect common biological pathways in RA. Indeed, survivin-positive RA patients demonstrate common features to ACPA-positive subset of patients. Similar with ACPA, the high levels of survivin may be measured in the preclinical stage of the disease and predicted development of RA.31 At early stages of RA, survivin predicts joint destructive course of disease,32 and resistance to antirheumatic treatment.33 Interestingly, high levels of survivin are frequently found in association with smoking and ACPA, and a combination of these parameters predicts high levels of survivin.38
Taken together, the present study demonstrates that survivin is a valuable biomarker specific for RA. Survivin positivity accounts for the increased susceptibility for RA in the subjects negative for SE and ACPA. Survivin is frequently detected in association with SE alleles and ACPA and combination of these parameters increases further the risk of RA and may assist its preclinical diagnosis.
ACKNOWLEDGMENTS
The authors would like to thank the Director General of Health, Ministry of Health Malaysia for supporting the work described in this article and permission to publish the article. The authors are also indebted to patients and controls for their kind participation. We also thank the MyEIRA study group members for data collection, practical help and assistance at the hospitals, in the laboratory and at site.
Footnotes
Abbreviations: ACPA = antibodies against cyclic citrullinated peptides, ELISA = enzyme linked immunosorbent assay, HLA = human leucocyte antigen, IL = Interleukin, MyEIRA = Malaysian epidemiological investigation of rheumatoid arthritis, OR = odds ratio, RA = rheumatoid arthritis, SE = shared epitope.
The MyEIRA study was financially supported by Ministry of Health, Malaysia: MRG 05-007, JPP-IMR 07–017 and JPP-IMR 08–012. The Swedish Research Council (521-2011-2414), Arne Lundberg's Foundation, Torsten Söderbergs Stiftelse, the Regional agreement on medical training and clinical research between the Western Götaland county council and the University of Göteborg (LUA/ALF FoU/ALFGBG-138661).
The authors declare no conflict of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Eriksson JK, Neovius M, Ernestam S, et al. Incidence of rheumatoid arthritis in Sweden: a nationwide population-based assessment of incidence, its determinants, and treatment penetration. Arthritis Care Res (Hoboken) 2013; 65:870–878. [DOI] [PubMed] [Google Scholar]
- 2.Richman NC, Yazdany J, Graf J, et al. Extraarticular manifestations of rheumatoid arthritis in a multiethnic cohort of predominantly Hispanic and Asian patients. Medicine (Baltimore) 2013; 92:92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.See LC, Kuo CF, Chou IJ, et al. Sex- and age-specific incidence of autoimmune rheumatic diseases in the Chinese population: a Taiwan population-based study. Semin Arthritis Rheum 2013; 43:381–386. [DOI] [PubMed] [Google Scholar]
- 4.MacGregor AJ, Snieder H, Rigby AS, et al. Characterizing the quantitative genetic contribution to rheumatoid arthritis using data from twins. Arthritis Rheum 2000; 43:30–37. [DOI] [PubMed] [Google Scholar]
- 5.Lin JP, Cash JM, Doyle SZ, et al. Familial clustering of rheumatoid arthritis with other autoimmune diseases. Hum Genet 1998; 103:475–482. [DOI] [PubMed] [Google Scholar]
- 6.Deighton CM, Walker DJ, Griffiths ID, et al. The contribution of HLA to rheumatoid arthritis. Clin Genet 1989; 36:178–182. [DOI] [PubMed] [Google Scholar]
- 7.Lundstrom E, Kallberg H, Smolnikova M, et al. Opposing effects of HLA-DRB1∗13 alleles on the risk of developing anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis. Arthritis and rheumatism 2009; 60:924–930. [DOI] [PubMed] [Google Scholar]
- 8.Mackie SL, Taylor JC, Martin SG, et al. A spectrum of susceptibility to rheumatoid arthritis within HLA-DRB1: stratification by autoantibody status in a large UK population. Genes Immun 2012; 13:120–128. [DOI] [PubMed] [Google Scholar]
- 9.van der Helm-van Mil AH, Huizinga TW, de Vries RR, et al. Emerging patterns of risk factor make-up enable subclassification of rheumatoid arthritis. Arthritis Rheum 2007; 56:1728–1735. [DOI] [PubMed] [Google Scholar]
- 10.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum 1987; 30:1205–1213. [DOI] [PubMed] [Google Scholar]
- 11.Mattey DL, Dawes PT, Gonzalez-Gay MA, et al. HLA-DRB1 alleles encoding an aspartic acid at position 70 protect against development of rheumatoid arthritis. J Rheumatol 2001; 28:232–239. [PubMed] [Google Scholar]
- 12.Raychaudhuri S, Sandor C, Stahl EA, et al. Five amino acids in three HLA proteins explain most of the association between MHC and seropositive rheumatoid arthritis. Nat Genet 2012; 44:291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Helm-van Mil AH, Huizinga TW, Schreuder GM, et al. An independent role of protective HLA class II alleles in rheumatoid arthritis severity and susceptibility. Arthritis Rheum 2005; 52:2637–2644. [DOI] [PubMed] [Google Scholar]
- 14.van der Woude D, Lie BA, Lundstrom E, et al. Protection against anti-citrullinated protein antibody-positive rheumatoid arthritis is predominantly associated with HLA-DRB1∗1301: a meta-analysis of HLA-DRB1 associations with anti-citrullinated protein antibody-positive and anti-citrullinated protein antibody-negative rheumatoid arthritis in four European populations. Arthritis Rheum 2010; 62:1236–1245. [DOI] [PubMed] [Google Scholar]
- 15.Zanelli E, Huizinga TW, Guerne PA, et al. An extended HLA-DQ-DR haplotype rather than DRB1 alone contributes to RA predisposition. Immunogenetics 1998; 48:394–401. [DOI] [PubMed] [Google Scholar]
- 16.Hill JA, Bell DA, Brintnell W, et al. Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. J Exp Med 2008; 205:967–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scally SW, Petersen J, Law SC, et al. A molecular basis for the association of the HLA-DRB1 locus, citrullination, and rheumatoid arthritis. J Exp Med 2013; 210:2569–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhayani HR, Hedrick SM. The role of polymorphic amino acids of the MHC molecule in the selection of the T cell repertoire. J Immunol 1991; 146:1093–1098. [PubMed] [Google Scholar]
- 19.Klareskog L, Ronnelid J, Lundberg K, et al. Immunity to citrullinated proteins in rheumatoid arthritis. Annu Rev Immunol 2008; 26:651–675. [DOI] [PubMed] [Google Scholar]
- 20.Law SC, Street S, Yu CH, et al. T-cell autoreactivity to citrullinated autoantigenic peptides in rheumatoid arthritis patients carrying HLA-DRB1 shared epitope alleles. Arthritis Res Ther 2012; 14:R118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bang SY, Lee KH, Cho SK, et al. Smoking increases rheumatoid arthritis susceptibility in individuals carrying the HLA-DRB1 shared epitope, regardless of rheumatoid factor or anti-cyclic citrullinated peptide antibody status. Arthritis Rheum 2010; 62:369–377. [DOI] [PubMed] [Google Scholar]
- 22.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med 1997; 3:917–921. [DOI] [PubMed] [Google Scholar]
- 23.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer 2008; 8:61–70. [DOI] [PubMed] [Google Scholar]
- 24.Fukuda S, Pelus LM. Survivin, a cancer target with an emerging role in normal adult tissues. Mol Cancer Ther 2006; 5:1087–1098. [DOI] [PubMed] [Google Scholar]
- 25.Singh P, Hoggatt J, Hu P, et al. Blockade of prostaglandin E2 signaling through EP1 and EP3 receptors attenuates Flt3L-dependent dendritic cell development from hematopoietic progenitor cells. Blood 2012; 119:1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andersson SE, Svensson MN, Erlandsson MC, et al. Activation of Fms-like tyrosine kinase 3 signaling enhances survivin expression in a mouse model of rheumatoid arthritis. PLoS One 2012; 7:e47668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okada H, Bakal C, Shahinian A, et al. Survivin loss in thymocytes triggers p53-mediated growth arrest and p53-independent cell death. J Exp Med 2004; 199:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xing Z, Conway EM, Kang C, et al. Essential role of survivin, an inhibitor of apoptosis protein, in T cell development, maturation, and homeostasis. J Exp Med 2004; 199:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song J, So T, Croft M. Activation of NF-kappaB1 by OX40 contributes to antigen-driven T cell expansion and survival. J Immunol 2008; 180:7240–7248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei F, Song J, Haque R, et al. Transgenic expression of survivin compensates for OX40-deficiency in driving Th2 development and allergic inflammation. Eur J Immunol 2013; 43:1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bokarewa M, Brink M, Erlandsson M, et al. Survivin but not Fms-like tyrosine kinase 3 ligand is up-regulated before the onset of rheumatoid arthritis: a pilot study. Arthritis Res Ther 2014; 16:R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Svensson B, Hafstrom I, Forslind K, et al. Increased expression of proto-oncogene survivin predicts Joint destruction and persistent disease activity in early rheumatoid arthritis. Ann Med 2010; 42:45–54. [DOI] [PubMed] [Google Scholar]
- 33.Isgren A, Forslind K, Erlandsson M, et al. High survivin levels predict poor clinical response to infliximab treatment in patients with rheumatoid arthritis. Semin Arthritis Rheum 2012; 41:652–657. [DOI] [PubMed] [Google Scholar]
- 34.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31:315–324. [DOI] [PubMed] [Google Scholar]
- 35.Too CL, Yahya A, Murad S, et al. Smoking interacts with HLA-DRB1 shared epitope in the development of anti-citrullinated protein antibody-positive rheumatoid arthritis: results from the Malaysian Epidemiological Investigation of Rheumatoid Arthritis (MyEIRA). Arthritis Res Ther 2012; 14:R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chun-Lai T, Padyukov L, Dhaliwal JS, et al. Shared epitope alleles remain a risk factor for anti-citrullinated proteins antibody (ACPA)-positive rheumatoid arthritis in three Asian ethnic groups. PLoS One 2011; 6:e21069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klareskog L, Stolt P, Lundberg K, et al. A new model for an etiology of rheumatoid arthritis: smoking may trigger HLA-DR (shared epitope)-restricted immune reactions to autoantigens modified by citrullination. Arthritis Rheum 2006; 54:38–46. [DOI] [PubMed] [Google Scholar]
- 38.Svensson B, Hafstrom I, Erlandsson MC, et al. Smoking in combination with antibodies to cyclic citrullinated peptides is associated with persistently high levels of survivin in early rheumatoid arthritis: a prospective cohort study. Arthritis Res Ther 2014; 16:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]


