Abstract
This work is a retrospective study of magnetic resonance imaging (MRI) and T-stage subclassifications of nasopharyngeal carcinoma (NPC) involving the masticatory muscles (MMs). We examined how involvement of MMs influences the clinical T-stage classifications and the survival outcomes of NPC patients.
MRI data as well as the medical records from 816 NPC patients were analyzed retrospectively. All cases were restaged according to the seventh edition of American Joint Committee on Cancer staging system criteria. The overall survival (OS), local relapse-free survival (LRFS), and distant metastasis-free survival (DMFS) were analyzed by the Kaplan–Meier method, and their survival outcomes between different degrees of MM involvement and different T classifications were compared by using the log-rank test. All statistical analyses were conducted on SPSS 18.0 software. P > 0.05 was considered significant.
Of the 816 NPC patients analyzed, 283 (34.68%) had tumors that involved MMs. All of those 283 patients involved the medial pterygoid muscle, and 125 cases (15.32%) involved the lateral pterygoid muscle. Multivariate analysis identified MM involvement as an independent prognostic factor for patient's OS (P = 0.007) and LRFS (P = 0.024). MM involvement significantly correlated with a lower OS and LRFS (P < 0.01). In addition, compared with concurrent involvement of the medial and lateral pterygoid muscle, the medial pterygoid muscle involvement correlated with a higher OS and LRFS (P < 0.05). Among NPC patients, T-classifications 1 to 4 usually predicted the ultimate OS, LRFS, and DMFS (P > 0.1), unless the cancer involved the lateral pterygoid muscle.
NPC involving the lateral pterygoid muscle presents a worse survival outcome than that involving the medial pterygoid muscle. Any cancer involving the lateral pterygoid muscle should be classified in a higher T-stage subclassification.
INTRODUCTION
Nasopharyngeal carcinoma (NPC) is endemic to Southeast Asia and the southern part of China with an incidence of approximately 15 to 50 cases/105.1–4 NPC is generally considered a radiosensitive tumor, so radiotherapy is the primary treatment regimen for all stages of nonmetastatic disease. The 5-year overall survival (OS) rate of NPC has been reported to be between 58.6% and 83%, and tumor–node–metastasis (TNM) staging is regarded as one of the most important prognostic factors.5–7 Indeed, T staging should reflect the severity of the case and the ultimate prognosis for the patient. Currently, intensity-modulated radiation therapy (IMRT) is the standard radiation treatment for NPC,8 with an excellent local control rate (>90%) even for locally advanced cancers9,10 (eg, cases involving masticatory muscles [MMs]). For NPC, T staging depends in part on whether the MM is involved but this practice has never been properly evaluated.
A previous study reported that masticator space was involved in 19.7% of NPCs.11 When tumors extend laterally beyond the parapharyngeal space, they subsequently infiltrate the medial pterygoid muscle (MPM), lateral pterygoid muscle (LPM), and temporalis muscle.11,12 On the other hand, tumors extending into pterygopalatine fossa can involve masticator space indirectly,13 and this spread is linked to a greatly increased risk for distant metastasis and tumor recurrence. Therefore, involvement of the anatomical masticator space is generally considered a key, independent prognostic factor for OS and local relapse-free survival (LRFS), and currently renders an NPC a T4 classification.13 Indeed, according to the Chinese staging system devised in 2008,14 patients with MM involvement are often “upstaged” as T3 or T4 classifications. In practice, however, MM involvement does not always correlate with a poor prognosis, making T subclassification on the basis of MM involvement a vague prognosticator for NPC patients.
The anatomical masticator space is identified as a deep facial space that contains all of the MMs, while the infratemporal fossa is defined as the lateral part of anatomical masticator space including just the masseter muscle and temporalis muscle. According to the NPC staging guidelines in the seventh edition of American Joint Committee on Cancer (AJCC; Figure 1), both involvement of masticator space and infratemporal fossa are causes for a stage T4 classification.15 In contrast, in the Chinese NPC staging system (2008), T3 disease is any disease that infiltrates just the MPM, while T4 disease is any disease that involves the other MMs.14 Thus, T subclassification based on MM involvement remains controversial. To improve the prognostic power of T-stage classifications, we sought to resolve this controversy by retrospectively reevaluating the degree to which MM involvement influenced NPC's survival outcomes.
FIGURE 1.
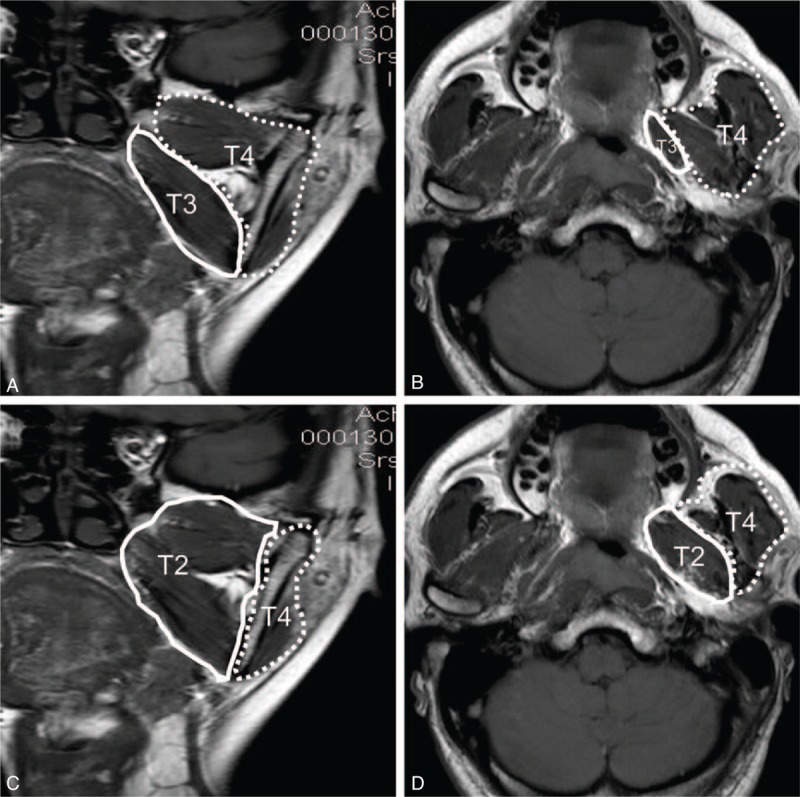
Coronal (A and C) and axial (B and D) images of T1-weighted fast spin echo imaging. According to guidelines for NPC from the seventh edition of the American Joint Committee on Cancer staging system (C and D), the anatomical masticator space includes the medial space (medial and lateral pterygoid muscle, solid line) and the lateral space (temporalis and masseter muscle, dotted line), and tumors involving the medial or lateral part are classified as T4. In contrast, the 2008 Chinese NPC staging system subdivides the masticator space into 2 independent spaces (A and B), including the medial part (only medial pterygoid muscle, solid line) and the lateral part (both lateral pterygoid muscle and other masticatory muscles, dotted line), and tumors involving the medial part are staged as T3 while those involving the lateral part are staged as T4. NPC = nasopharyngeal carcinoma.
Our retrospective study analyzed a cohort of consecutively evaluated, newly diagnosed NPC patients who had complete magnetic resonance imaging (MRI) and follow-up data. By comparing the survival outcomes between patients who had various degrees of MM involvement but were in different T-stage categories, we were able to judge whether basing T staging on involvement of MMs has prognostic value for patients with newly diagnosed NPCs.
MATERIALS AND METHODS
Ethical Statement
This retrospective study was approved by the institutional review board of Fujian Provincial Cancer Hospital (No. 200908). Permission was granted to conduct the study without first obtaining informed consent.
Patient Selection and Pretreatment Evaluation
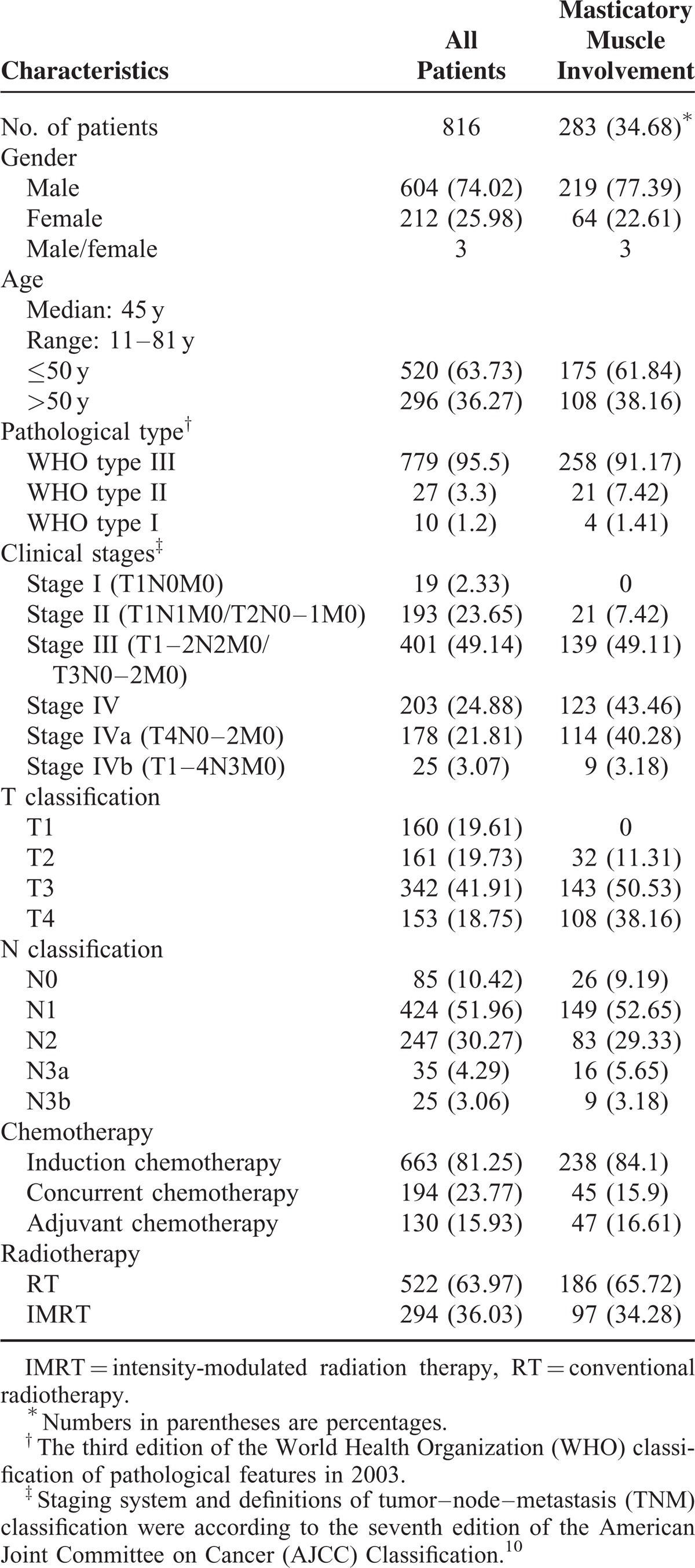
Data were compiled from 816 consecutive, newly diagnosed NPC patients who had no metastasis to distant organs and who were treated at Fujian Provincial Cancer Hospital from July 2005 to September 2007. All cases were histopathologically confirmed as NPC within 1 week before or after magnetic resonance (MR) scan. Among the 816 patients, 604 were males and 212 were females, and the median age was 45 years (range: 11–81 years). According to the histopathological patterns recognized by the World Health Organization (WHO), 779 (95.5%) patients were classified as type III, 27 (3.3%) as type II, and 10 (1.2%) as type I. We restaged these NPC cases according to MRI findings and on the basis of criteria set by the AJCC staging system (seventh edition).15 The patient characteristics, together with their corresponding staging categories and treatment regimens, are listed in Table 1.
TABLE 1.
Patient Characteristics, Staging Categories, and Treatment Regimens
The pretreatment evaluation for patients included a complete medical history, physical examination, nasopharyngoscopy examination, dental evaluation, standard laboratory tests, chest radiography or computed tomography (CT) scan, bone scan, abdominal ultrasound, and, most importantly, a head and neck MRI.14
MR Imaging
A head and neck MRI session was conducted according to accepted protocols, on a 1.5T MR system (Signa Excite 1.5T HD Twin Speed, GE Healthcare, Milwaukee, WI), which scanned from the lower temporal lobe to the supraclavicular. Before any antitumor treatments, all patients were subjected to the following 5 standard sequences: axial T1-weighted imaging-fast spin echo (Ax T1WI-FSE), axial proton density-weighted imaging (Ax PDWI) or axial T2-weighted imaging with short TI inversion recovery (Ax T2WI-STIR), coronal T2-weighted imaging with STIR (Cor T2WI-STIR), sagittal T1-weighted imaging-fast spin echo (Sag T1WI-FSE), and post–contrast-enhanced acquisition of axial and coronal T1WI-FSE with fat suppression (fs) (Ax and Cor T1WI-FSE fs +C). All acquired MR images were reinterpreted independently by 2 radiology specialists in head and neck MRI, who had 8 and 10 years of experience, respectively. Any disagreements were resolved by consensus.
Clinical Characteristics and Criterion of Masticatory Muscle Involvement
The diagnostic criterion of MM involvement on MRI was the primary tumor's extension beyond the parapharyngeal space or pterygopalatine fossa, and the spread into the anatomical masticator space. This causes MMs to present a higher signal intensity on T2WI-STIR images and significant enhancement on postcontrast T1WI-FSE images, both axial and coronal, in whole or in part; it also causes disappearance of high signal intensity of the parapharyngeal space on T1WI-FSE images. Clinical examination and reevaluation of patients with MM involvement showed that they present with a different degree of restriction of mouth opening as well as chewing dysfunction.
Treatment Regimens
All patients were treated with definitive radiotherapy: 522 patients (63.97%) received conventional radiotherapy, while the other 294 patients received IMRT. The mean dose of radiotherapy was 70 Gy, with the mean number of fractions at 33. For each of the above techniques, a detailed description and protocol has been published.16,17 Thirty-four patients (4.17%) were unable to withstand the entire radiotherapy protocol due to radiation intolerance, or complications due to infection or nasopharyngeal bleeding.
A total of 711 patients (87.13%) received platinum-based chemotherapy: induction in 663 (81.25%), concurrent in 194 (23.77%), adjuvant in 130 (15.93%), concurrent–adjuvant in 33 (4.049%), induction–concurrent in 149 (18.26%), induction–adjuvant in 123 (15.07%), and induction–concurrent–adjuvant in 29 (3.56%). Whenever possible, patients who failed to respond to the initial treatment received salvage treatments, including intracavitary brachytherapy, surgery, or adjuvant chemotherapy.
Follow-Up and Statistical Analysis
The median follow-up time was 66 months (range: 6–96 months), with 94.1% of patients finishing a complete 5-year follow-up. OS was calculated as the time between diagnosis and either death or the final follow-up visit. LRFS or regional relapse-free survival (RRFS) was calculated as the time between diagnosis and local or regional relapse. Distant metastasis-free survival (DMFS) was calculated as the time between diagnosis and the detection of metastasis to a distant site. All outcomes were evaluated in August 2013, and statistical analyses were conducted on SPSS software (version 18.0, SPSS Inc, Chicago, IL). Whether various T-stage prognoses correlated with involvement of MMs (as judged by MRI) was evaluated by the χ2 test. Survival curves were analyzed using the Kaplan–Meier method, and their differences were compared by the log-rank test. A 2-tailed P value of <0.05 was considered statistically significant.
RESULTS
Frequency of Masticatory Muscle Involvement
Of the 816 NPC patients we analyzed, MRI found tumors invading MMs in 283 (34.68%) cases. All of those cases had cancer involving the MPM, of which 125 (15.32% of the total) also had the cancer affecting the LPM. In addition, only 9 cases with temporalis muscle involvement and 1 case with masseter muscle involvement were diagnosed. Among those patients with MM involvement, 219 were males and 64 were females, and the median age was 46 years (range: 11–81 years) and, according to WHO guidelines, 258 of those patients (91.17%) displayed histopathological patterns that categorized their cancer as type III, 21 (7.42%) as type II, and 4 (1.41%) as type I. The characteristics, staging categories, and treatment regimens of patients with MM involvement are detailed in Table 1.
T Subclassifications and Treatment Regimens
Among cases involving the MPM versus LPM, 32 medial and 3 lateral cases were classified as T2 disease, 143 medial and 43 lateral classified as T3, and 108 medial and 79 lateral classified as T4. When other sites of the head or neck were involved with the cancer concurrently, the LPM was always more commonly affected than medial muscle: the mean frequencies of concurrent medial versus lateral involvement were, respectively, 29.68% versus 43.2% with cancers affecting the nasal cavity, 70.67% versus 78.4% with the vertebral anterior muscle, 35.34% versus 54.4% with the pterygopalatine fossa, 29.68% versus 38.4% with the paranasal sinus, 88.69% versus 97.6% with the skull base, 4.95% versus 10.4% with the orbit, 14.49% versus 25.6% with the cranial nerves, and 34.98% versus 58.4% with the cavernous sinus or intracranial erosion, respectively.
In addition, 255 patients (90.11%) were dosed with platinum-based chemotherapy, including induction in 238 (84.1%), concurrent in 67 (23.67%), adjuvant in 47 (16.61%), induction–concurrent in 51 (18.02%), induction–adjuvant in 45 (15.9%), concurrent–adjuvant in 10 (3.53%), and induction–concurrent–adjuvant in 9 (3.19%). As for radiotherapy, a total of 186 patients (65.72%) received conventional radiotherapy, while 97 patients (34.28%) were treated with IMRT. The mean dose of radiotherapy on nasopharynx tumors was 70 Gy, with 35 fractions of 2.0 Gy.
Prognosis of Patients With Masticatory Muscle Involvement
Of the 816 NPC patients, the 5-year OS, DMFS, and LRFS rates were 77.7%, 83.8%, and 91.0%, respectively. Corresponding local recurrences, regional recurrences, and distant metastases developed in 67 (8.21%), 24 (2.94%), and 128 (15.68%) patients, respectively. Only 6 patients (0.74%) presented with both local and regional recurrences. Eventually, about 179 of 816 patients (21.94%) died of the NPC-related diseases (eg, tumor recurrence, distant metastasis, and bleeding of tumor ulcer).
In the subgroups of NPC patients with masticator muscle involvement, the 5-year OS, DMFS, and LRFS rates were 69.61%, 82.69%, and 88.34%, respectively; and local recurrences, regional recurrences, and distant metastases developed in 33 (13.43%), 3 (1.06%), and 49 (17.31%) patients, respectively. By the time of the last scheduled follow-up, 86 patients (30.39%) had died, including 39 of distant metastases, 26 of local recurrences, 10 of treatment-related complications, 8 of other medical conditions, and 3 of unknown causes. Furthermore, cases with LPM involvement tended to have a worse outcome: the 5-year OS, DMFS, and LRFS were 63.2%, 81.6%, and 84.0%, respectively, with local recurrences, regional recurrences, and distant metastases developing in 20 (16.0%), 3 (1.06%), and 23 (18.4%) patients, respectively.
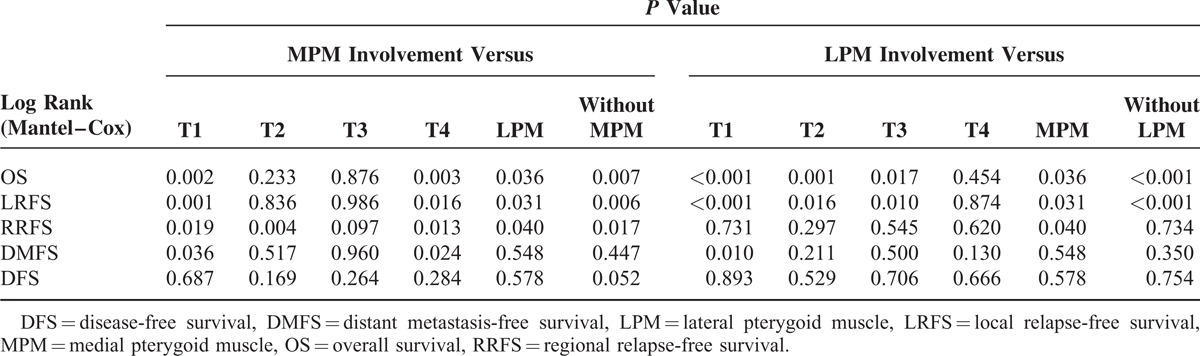
MM involvement was also found to worsen significantly the outcomes of OS (x2 = 7.246, P = 0.007), LRFS (x2 = 7.586, P = 0.006), and RRFS (x2 = 5.654, P = 0.017), but had no impact on DMFS (x2 = 0.506, P = 0.477; Figure 2). Furthermore, compared with patients with concurrent involvement of both MPM and LPMs, patients with MPM involvement had a better OS (x2 = 4.412, P = 0.036), LRFS (x2 = 4.626, P = 0.031), and RRFS (x2 = 4.238; P = 0.040), but had a similar DMFS (x2 = 0.361, P = 0.548; Figure 3). On the other hand, patients with tumors involving MPM had an OS, LRFS, and DMFS consistent with a T2 or T3 classification (P > 0.1), and their RRFS was more consistent with a T3 classification (P = 0.097; Figure 4). If, however, their tumor concurrently involved the LPM, then they presented OS and LRFS that were much worse and more consistent with a T4 tumor (P > 0.1); their RRFS was consistent with standard T staging (P > 0.1), and their DMFS was worse than that classified as T1 disease (P = 0.01; Figure 4). The survival outcomes of different T-stage subclassifications are compared in Table 2.
FIGURE 2.
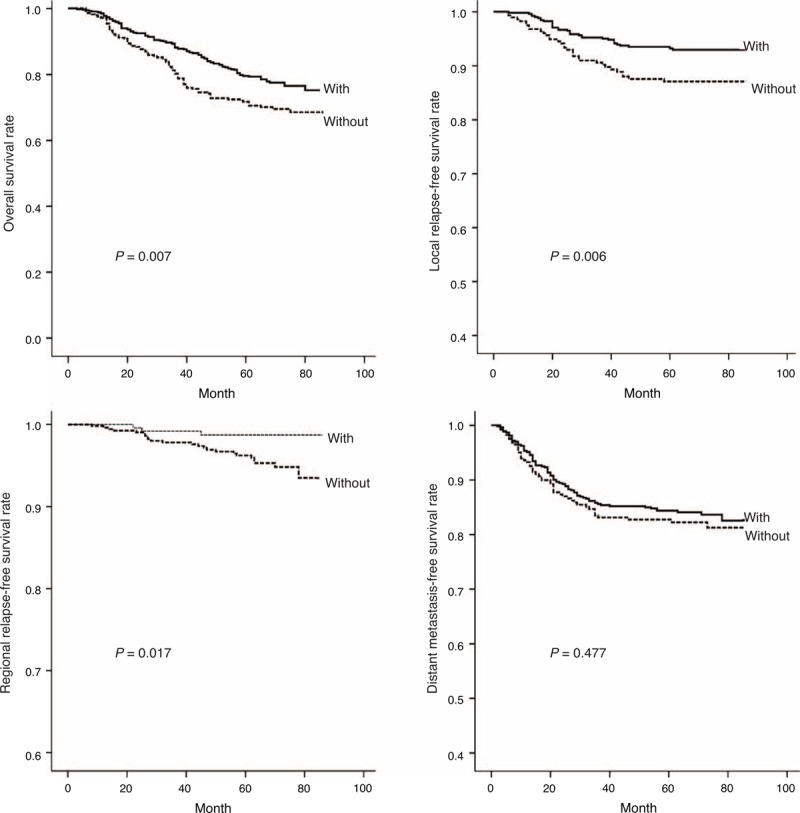
Curves show that NPC patients with masticatory muscle involvement presented significantly worse survival outcomes for OS (X2 = 7.246, P = 0.007), LRFS (X2 = 7.586, P = 0.006), and RRFS (X2 = 5.654, P = 0.017), but not for DMFS (X2 = 0.506, P = 0.477). DMFS = distant metastasis-free survival, LRFS = local relapse-free survival, NPC = nasopharyngeal carcinoma, OS = overall survival, RRFS = regional relapse-free survival.
FIGURE 3.
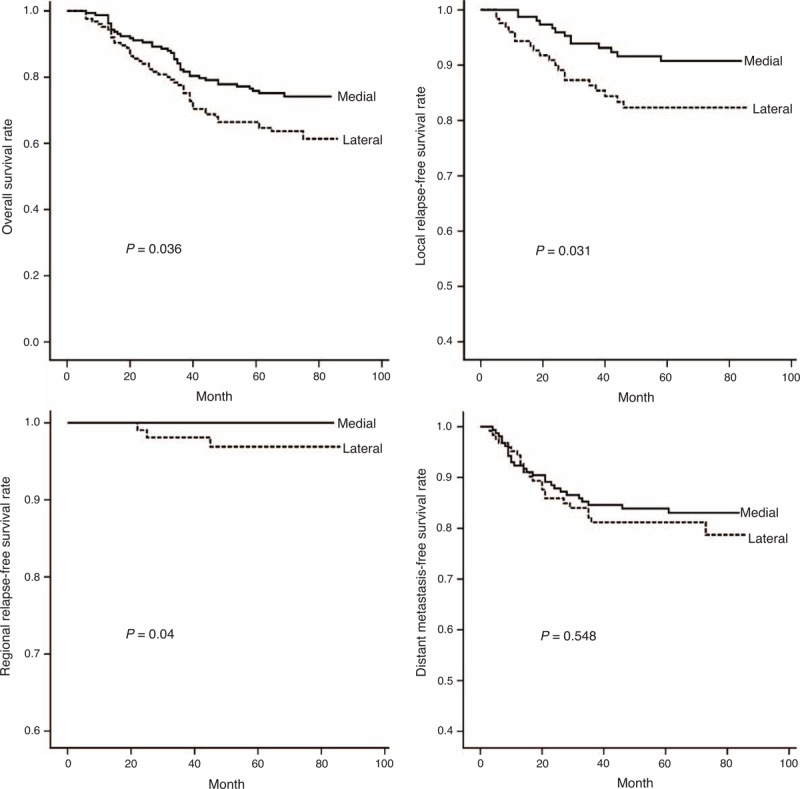
Curves show that NPC patients with medial pterygoid muscle involvement had a better OS (X2 = 4.412, P = 0.036), LRFS (X2 = 4.626, P = 0.031), and RRFS (X2 = 4.238, P = 0.040) than those with lateral pterygoid muscle involvement, but they had a similar DMFS (X2 = 0.361, P = 0.548). DMFS = distant metastasis-free survival, LRFS = local relapse-free survival, NPC = nasopharyngeal carcinoma, OS = overall survival, RRFS = regional relapse-free survival.
FIGURE 4.
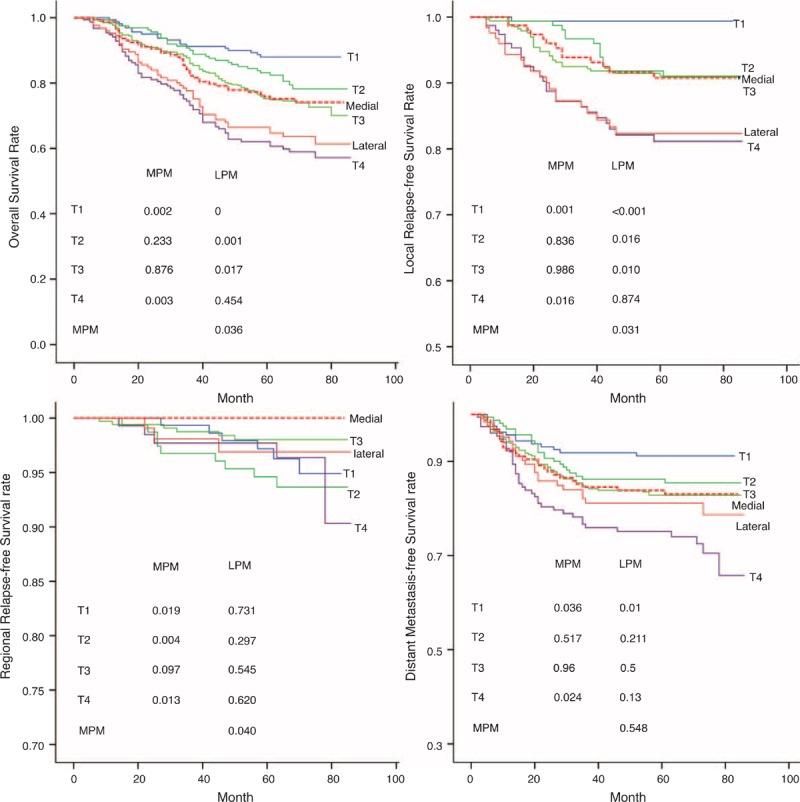
Five-year survival curves of OS, LRFS, RRFS, and DMFS show various outcomes for patients with tumors involving MPM and LPM involvement. With MPM involvement, the OS is similar to that of T2 and T3 classification, while cases involving the LPM are more consistent with cases classified as T4. The LRFS of patients with MPM involvement is consistent with those categorized as T3, whereas the LRFS of cases involving the LPM is consistent with T1, T2, T3, and T4 cases, but is worse than those involving MPM. The DMFS of cases involving MPM and LPM were more consistent with T2 and T3 classification. However, the RRFS of cases involving MPM and LPM are similar, regardless of the T-stage classification. DMFS = distant metastasis-free survival, LPM = lateral pterygoid muscle, LRFS = local relapse-free survival, MPM = medial pterygoid muscle, OS = overall survival, RRFS = regional relapse-free survival.
TABLE 2.
Comparison of Survival Outcomes and Different T Subclassifications for Tumors with Masticatory Muscle Involvement
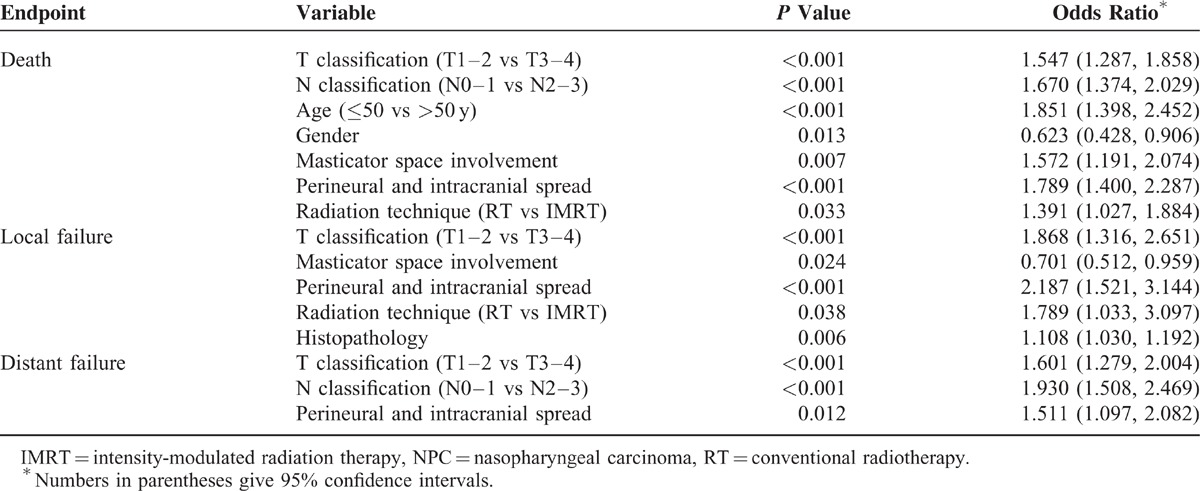
Multivariate analysis systematically calculated how various prognostic factors may affect survival outcomes of NPC patients. The following factors were taken into consideration in the Cox regression analysis: age (≥50 vs <50 years), gender, histopathology, T classification (T1–2 vs T3–4), N classification (N0–1 vs N2–3), parapharyngeal space extension, oropharyngeal or hypopharyngeal extension, MM involvement, skull base erosion, paranasal sinus erosion, perineural and intracranial spread, and chemotherapy. As a result, MM involvement was found to be one of the independent prognostic factors for both OS and LRFS (P = 0.007 and 0.024, respectively), but not for DMFS (P = 0.954). On the other hand, independent factors for OS were gender, age, T classification, N classification, perineural and intracranial spread, and radiation technique. For LRFS, independent factors were T classification, histopathology, perineural and intracranial spread, and radiation technique. For DMFS, the only independent factors were T classification, N classification, and perineural and intracranial spread. Not found as independent prognostic factors for OS, LRFS, and DMFS were the variables of vertebral anterior muscle involvement, parapharyngeal space extension, oropharyngeal or hypopharyngeal extension, skull base erosion, paranasal sinus erosion, and chemotherapy (Table 3).
TABLE 3.
Multivariate Analysis of Prognostic Factors in NPC Patients
DISCUSSION
Our results indicate that involvement of MMs was an independent prognostic factor for newly diagnosed NPC, and that patients with T1, T2, and T3 classifications or MPM involvement had better survival outcomes than those with T4 classification or LPM involvement. Thus, we highly recommend that LPM involvement should be regarded as an indicator for higher T subclassification.
Certain anatomical characteristics make it difficult to evaluate the masticatory space in a clinical examination, so to accurately evaluate this region, imaging modalities are essential.18 Currently, for newly diagnosed NPC, MRI and CT are valuable for TNM staging, and especially for T staging. Compared with CT, MRI scans provide better tissue contrast and multiplanar capability, allowing a more accurate evaluation of the anatomical information, in particular, the extent to which the primary nasopharynx tumor has invaded adjacent structures. For these reasons, MRI is widely considered the optimal imaging modality for T- and N-stage classifications for newly diagnosed NPC, in both the seventh edition of AJCC staging system and the Chinese NPC staging system.14,15 Accordingly, King et al demonstrated the excellent diagnostic power of MR imaging, which has a sensitivity of 100%, specificity of 93%, and accuracy of 95%.19,20 The routine MRIs with T2WI-STIR and contrast-enhanced T1WI-FSE in axial and coronal planes can assess whether the tumor has spread into the parapharyngeal or masticator space. Taking these advances in MRI techniques into consideration, staging of patients in our study was reevaluated based on MRI, to assess prognostic value of T staging.
Anatomically, the masticator space is the deep cervical fascia (Figure 1) that contains the muscles involved in mastication, posterior body and ramus of the mandible, and the mandibular division of the trigeminal nerve (V3).21,22 Even if masticator space involvement was regarded as an independent factor for T-stage classification and prognosis,12 under most circumstances, different degrees of MM involvement predict different outcomes. A previous study by Tang et al reported involvement of the masticator space at a frequency of 19.7%,12 with frequencies of MPM and LPM involvement at only 18.7% and 10.0%, respectively. In our study, however, all cancers that involved the LPM also involved the MPM. Moreover, we diagnosed 283 (34.68%) patients as having MPM involvement and 125 (15.32%) as having LPM involvement (Table 1).
NPC patients with MM involvement did not more often develop distant metastasis (eg, liver, bone, or lung) compared with those without involvement (17.31% vs 15.95%, P > 0.1); however, they did present a higher 5-year local recurrence rate (11.66% vs 6.38%, P < 0.05) and a higher death rate (30.39% vs 22.14%, P < 0.05). In accord with the results of multivariate analysis of NPC patients, the MM involvement was an independent prognostic factor for OS and LRFS (P = 0.007 and 0.024, respectively), but not for DMFS. This is also consistent with a previous study.12 In addition, NPC patients with tumors involving the MMs showed a higher frequency of involvement of surrounding structures, which may dramatically worsen the OS rate and boost local relapse rate. Table 1 shows that patients with MM involvement were mostly categorized as T3 and T4 (50.53% and 38.16%, respectively) and only 32 of 283 (11.31%) patients with MM involvement were categorized as T2. Moreover, patients with MM involvement appeared to have a higher frequency of concurrent involvement of pterygopalatine fossa, orbit, paranasal sinuses, the maxillary division of the trigeminal nerve (V2) and V3, etc.
Generally, NPC tumor extends step-by-step, originating from the nasopharyngeal mucosa and spreading laterally into the parapharyngeal space or/and masticator space. The parapharyngeal space and pterygopalatine fossa are both routes through which tumors might invade masticator space directly. In this study, even though the invasion of the MPM always originated with erosion of the parapharyngeal space, NPCs infiltrating the pterygopalatine fossa could invade the medial and lateral anterior pterygoid muscles concurrently. For our NPC cases involving the MMs, all presented concurrent involvement of parapharyngeal space. Among those with MPM and LPM involvement, concurrent involvement of pterygopalatine fossa was seen in 35.34% (100/283) and 54.4% (68/125), respectively. Despite the fact that a higher risk of distant metastasis and tumor recurrence was seen with tumors affecting the parapharyngeal space, pterygopalatine fossa, and masticator space, tumors extending through the parapharyngeal space tended to be less severe. In contrast, the pterygopalatine fossa contains the maxillary division of the trigeminal nerve (V2), which increases the risk for V2 infiltration. Therefore, concurrent involvement of pterygopalatine fossa should be considered an important prognostic factor. Thus, we recommend that any T-stage subclassification should consider the routes through which NPC tumors extend to the MMs.
Another cranial nerve, the mandibular division of the trigeminal nerve (V3), runs through an intervening space that lies between the MPM and LPM. In NPC tumors infiltrating the LPM, the risk for V3 infiltration was dramatically increased. In our study, one of the most significant prognostic factors for NPC and a factor in T4 classification was involvement of the LPM. This finding agrees with the seventh edition of AJCC staging system. Of our subjects, among those with LPM involvement, the frequency of V3 infiltration was 12.8% (16/125), which was higher than that for patients with MPM involvement (7.42%, 21/283; P = 0.008). Furthermore, for those with tumors involving the LPM, the likelihood that cranial nerves were involved (103/125, 82.4%) was much higher than those with the MPM involvement (165/283, 58.3%; P < 0.001). This shows that prognostic outcomes partly depend on the degree of involvement of MMs: compared with tumors involving the MPM, tumors that involve the LPM are linked to a poorer prognosis.
For NPC diagnoses, it remains controversial whether T classifications should be based on whether the MMs are involved.11–14 In this retrospective study, patients with NPC tumors involving the MMs eventually had poorer survival outcomes, primarily because other sites with the same or worse prognosis were concurrently involved (eg, the pterygopalatine fossa, V2 or V3, and orbit). Therefore, concurrent involvement of other structures should be considered during T subclassification. Furthermore, although our results show a relatively low frequency of temporalis and masseter muscle involvement, when these structures were involved, so were other MMs, and the survival outcome was far worse. We found that of 9 patients with temporalis and masseter muscle involvement, the OS, LRFS, and DMFS rates were 4/9 (44.44%), 6/9 (66.67%), and 6/9 (66.67%), respectively. These results are consistent with part of previous studies.12,23,24
Our results suggest that, when diagnosing NPC, an important prognostic factor for the OS and LRFS is whether the tumor involves the MMs. We recommend that T-stage subclassification based on MM involvement should be considered as a factor in the AJCC staging system. Since involvement of MPM presented a better survival outcome (Figure 2), it should trigger a lower T-stage subclassification (ie, T2 or T3). The retrospective study by Chen et al23 even demonstrated that those with a T4a subclassification (involvement of the masticator space only) had a significantly higher OS (P = 0.033) and DMFS (P = 0.036) than those with a T4b subclassification (involvement of intracranial region, cranial nerves, hypopharynx, and/or orbit). Currently, the Chinese system considers LPM involvement grounds for T4 classification. (Indeed, LPM involvement comes with a high frequency of concurrent involvement of pterygopalatine fossa, cranial nerves, and orbit.) Since tumors involving the temporalis and masseter muscles carry a high risk for extensive tumor invasion, we believe that they should also be categorized as T4.
MPM and LPM involvement, in our study, did not significantly affect survival outcomes (regardless of whether the Chinese system or the AJCC system would subclassify them as stage T2, T3, or T4). Few patients with lateral muscle involvement are categorized as T2 or T3, which is also a limitation of this study.
Another limitation of this study is that it drew from a relatively small sample size for a retrospective evaluation of NPC patients’ prognosis. Moreover, only a fraction of the recruited patients had received the IMRT treatment regimen. A further study with a larger sample size and based on IMRT is now being conducted in our center. We expect that the results from this study will further refine our prognostic model.
CONCLUSION
Our retrospective study concluded that MRI can determine whether a tumor involves the MMs and that MM involvement is an independent prognostic factor for NPC. NPCs involving the LPM were linked to a worse survival outcome than those involving the MPM, but have generally been classified similarly. We highly recommend that LPM involvement should be considered cause for a T subclassification higher than MPM involvement, and that concurrent involvement at other sites should also guide T-stage subclassification.
Acknowledgment
We thank Dr Diana Colgan for proofreading and editing this article.
Footnotes
Abbreviations: AJCC = American Joint Committee on Cancer, CT = computed tomography, DMFS = distant metastasis-free survival, IMRT = intensity-modulated radiation therapy, LPM = lateral pterygoid muscle, LRFS = local relapse-free survival, MM = masticatory muscle, MPM = medial pterygoid muscle, MRI = magnetic resonance imaging, NPC = nasopharyngeal carcinoma, OS = overall survival, RRFS = regional relapse-free survival.
All listed authors contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Razak AR, Siu LL, Liu FF, et al. Nasopharyngeal carcinoma: the next challenges. Eur J Cancer 2010; 46:1967–1978. [DOI] [PubMed] [Google Scholar]
- 2.Chan AT, Teo PM, Johnson PJ. Nasopharyngeal carcinoma. Ann Oncol 2002; 13:1007–1015. [DOI] [PubMed] [Google Scholar]
- 3.Chan AT. Nasopharyngeal carcinoma. Ann Oncol 2010; 21 suppl 7:vii308–vii312. [DOI] [PubMed] [Google Scholar]
- 4.Wei KR, Yu YL, Yang YY, et al. Epidemiological trends of nasopharyngeal carcinoma in China. Asian Pac J Cancer Prev 2010; 11:29–32. [PubMed] [Google Scholar]
- 5.El-Sherbieny E, Rashwan H, Lubis SH, et al. Prognostic factors in patients with nasopharyngeal carcinoma treated in Hospital Kuala Lumpur. Asian Pac J Cancer Prev 2011; 12:1739–1743. [PubMed] [Google Scholar]
- 6.Zhao LN, Zhou B, Shi M, et al. Clinical outcome for nasopharyngeal carcinoma with predominantly WHO II histology treated with intensity-modulated radiation therapy in non-endemic region of China. Oral Oncol 2012; 48:864–869. [DOI] [PubMed] [Google Scholar]
- 7.Mao YP, Xie FY, Liu LZ, et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys 2009; 73:1326–1334. [DOI] [PubMed] [Google Scholar]
- 8.Perri F, Bosso D, Buonerba C, et al. Locally advanced nasopharyngeal carcinoma: current and emerging treatment strategies. World J Clin Oncol 2011; 2:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kwong DL, Sham JS, Leung LH, et al. Preliminary results of radiation dose escalation for locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 2006; 64:374–381. [DOI] [PubMed] [Google Scholar]
- 10.Su SF, Han F, Zhao C, et al. Long-term outcomes of early-stage nasopharyngeal carcinoma patients treated with intensity-modulated radiotherapy alone. Int J Radiat Oncol Biol Phys 2012; 82:327–333. [DOI] [PubMed] [Google Scholar]
- 11.Dubrulle F, Souillard R, Hermans R. Extension patterns of nasopharyngeal carcinoma. Eur Radiol 2007; 17:2622–2630. [DOI] [PubMed] [Google Scholar]
- 12.Tang LL, Li WF, Chen L, et al. Prognostic value and staging categories of anatomic masticator space involvement in nasopharyngeal carcinoma: a study of 924 cases with MR imaging. Radiology 2010; 257:151–157. [DOI] [PubMed] [Google Scholar]
- 13.Lai V, Khong PL. Updates on MR imaging and 18F-FDG PET/CT imaging in nasopharyngeal carcinoma. Oral Oncol 2014; 50:539–548. [DOI] [PubMed] [Google Scholar]
- 14.Pan J, Xu Y, Qiu S, et al. A comparison between the Chinese 2008 and the 7th edition AJCC staging systems for nasopharyngeal carcinoma. Am J Clin Oncol 2013; 00:1–8. [DOI] [PubMed] [Google Scholar]
- 15.Edge SB, Byrd DR, Compton CC, et al. American Joint Committee on Cancer Staging Manual. 7th ed.2010; New York: Springer-Verlag, 41–49. [Google Scholar]
- 16.Xu L, Pan J, Wu J, et al. Factors associated with overall survival in 1706 patients with nasopharyngeal carcinoma: significance of intensive neoadjuvant chemotherapy and radiation break. Radiother Oncol 2010; 96:94–99. [DOI] [PubMed] [Google Scholar]
- 17.Lin SJ, Pan JJ, Han L, et al. Nasopharyngeal carcinoma treated with reduced-volume intensity-modulated radiation therapy: report on the outcome of a prospective series. Int J Radiat Oncol Bio Phys 2009; 75:1071–1078. [DOI] [PubMed] [Google Scholar]
- 18.Meltzer DE, Shatzkes DR. Masticator space: imaging anatomy for diagnosis. Otolaryngol Clin North Am 2012; 45:1233–1251. [DOI] [PubMed] [Google Scholar]
- 19.Abdel Khalek Abdel Razek A, King A. MRI and CT of nasopharyngeal carcinoma. AJR Am J Roentgenol 2012; 198:11–18. [DOI] [PubMed] [Google Scholar]
- 20.King AD, Vlantis AC, Bhatia KS, et al. Primary nasopharyngeal carcinoma: diagnostic accuracy of MR imaging versus that of endoscopy and endoscopic biopsy. Radiology 2011; 258:531–537. [DOI] [PubMed] [Google Scholar]
- 21.Galli F, Flor N, Villa C, et al. The masticator space. Value of computed tomography and magnetic resonance imaging in localisation and characterisation of lesions. Acta Otorhinolaryngol Ital 2010; 30:94–99. [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandes T, Lobo JC, Castro R, et al. Anatomy and pathology of the masticator space. Insights Imaging 2013; 4:605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen L, Liu LZ, Chen M, et al. Prognostic value of subclassification using MRI in the T4 classification nasopharyngeal carcinoma intensity-modulated radiotherapy treatment. Int J Radiat Oncol Biol Phys 2012; 84:196–202. [DOI] [PubMed] [Google Scholar]
- 24.Zhang GY, Huang Y, Cai XY, et al. Prognostic value of grading masticator space involvement in nasopharyngeal carcinoma according to MR imaging findings. Radiology 2014; 273:136–143. [DOI] [PubMed] [Google Scholar]


