Abstract
Strength training has, in recent years, been shown to be beneficial for people with Parkinson disease and multiple sclerosis. Consensus regarding its utility for these disorders nevertheless remains contentious among healthcare professionals. Greater clarity is required, especially in regards to the type and magnitude of effects as well as the response differences to strength training between individuals with Parkinson disease or multiple sclerosis.
This study examines the effects, magnitude of those effects, and response differences to strength training between patients with Parkinson disease or multiple sclerosis.
A comprehensive search of electronic databases including Physiotherapy Evidence Database scale, PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and CINAHL was conducted from inception to July 2014.
English articles investigating the effect of strength training for individuals with neurodegenerative disorders were selected. Strength training trials that met the inclusion criteria were found for individuals with Parkinson disease or multiple sclerosis.
Individuals with Parkinson disease or multiple sclerosis were included in the study. Strength training interventions included traditional (free weights/machine exercises) and nontraditional programs (eccentric cycling).
Included articles were critically appraised using the Physiotherapy Evidence Database scale.
Of the 507 articles retrieved, only 20 articles met the inclusion criteria. Of these, 14 were randomized and 6 were nonrandomized controlled articles in Parkinson disease or multiple sclerosis. Six randomized and 2 nonrandomized controlled articles originated from 3 trials and were subsequently pooled for systematic analysis. Strength training was found to significantly improve muscle strength in people with Parkinson disease (15%–83.2%) and multiple sclerosis (4.5%–36%). Significant improvements in mobility (11.4%) and disease progression were also reported in people with Parkinson disease after strength training. Furthermore, significant improvements in fatigue (8.2%), functional capacity (21.5%), quality of life (8.3%), power (17.6%), and electromyography activity (24.4%) were found in individuals with multiple sclerosis after strength training.
The limitations of the study were the heterogeneity of interventions and study outcomes in Parkinson disease and multiple sclerosis trials. Strength training is useful for increasing muscle strength in Parkinson disease and to a lesser extent multiple sclerosis.
INTRODUCTION
Neurodegenerative disorders such as Parkinson disease and multiple sclerosis represent a major medical concern for health professionals and national healthcare bodies.1 Both disorders result from progressive neuronal dysfunction and neuronal cell death leading to progressive disability and eventual death.2 Classical signs and symptoms customary to both disorders include motor problems, cognitive impairment, behavioral disturbances, and systemic abnormalities.3–5
There is no cure and few cost-effective drug agents for treating people with Parkinson disease or multiple sclerosis.6,7 Recent advances in understanding the pathogenic mechanisms responsible for each disorder may aid in the identification and development of cost-effective disease-modifying agents in the future.8 However, cost-effective treatments, with disease-modifying properties and symptomatic benefits are required in the short term.
Accumulating evidence suggests that strength training is a useful therapy for addressing many of the clinical features that present in individuals with neurodegenerative disorders.9–11 By definition, strength training refers to an intervention in which participants train a muscle or group of muscles against an external resistance.12 Whereas evidence suggests that lower limb strength training (ie, leg press, knee extension, and knee flexion) is beneficial for individuals with Parkinson disease and multiple sclerosis,13–19 consensus regarding the effects, magnitude of those effects, and disease-dependent responses remain contentious. By contrast, the therapeutic utility of strength training is well recognized in the elderly,20 individuals with mild cognitive impairment and in those that have suffered a stroke. Health benefits associated with strength training in elderly individuals include improvements in strength,21,22 cardiorespiratory capacity,23 functional capacity,23,24 muscle activity,25 body composition,26 mood,27 cognition,28,29 health-related quality of life,30 and enhanced hemodynamic activity on functional magnetic resonance imaging tasks.31 In individuals who have suffered a stroke, strength training has been found to improve muscular strength, upper and lower limb function and performance on functional tasks.32–34 Improvements in selective attention, conflict resolution, associative memory, and regional patterns of functional brain activity have also been observed after strength training in seniors with mild cognitive impairment.31
In the last 2 years, 3 systematic reviews have evaluated the effects of strength training in either Parkinson disease or multiple sclerosis.9,35,36 Findings from these reviews suggest that strength training is useful for improving muscle strength and some measures of functional capacity in these disorders. Since the publication of these reviews, a number of randomized controlled trials have been published,9,35,36 somewhat limiting the informative capacity of previous reviews. Previous systematic reviews have also included trials with confounding supplementary interventions (ie, creatine monohydrate and balance training)35,36 as well as trials without a disease control or comparison group.9,36 These methodological limitations may have led to an inaccurate appraisal of the effects of strength training as a therapy in individuals with Parkinson disease or multiple sclerosis. It is of vital importance that systematic reviews accurately evaluate experimental therapies like strength training because such documents inform health professionals.
In this systematic review, we provide the most recent evidence to support a robust evaluation of the effect of strength training in people with Parkinson disease or multiple sclerosis. Unlike previous reviews, our study evaluates the effect of strength training alone, in people with Parkinson disease or multiple sclerosis. In addition, our study only selects trials that included individuals with multiple sclerosis or Parkinson disease in the control or comparison group. Moreover, our study evaluates through a meta-analysis, the magnitude of strength improvements in individuals with multiple sclerosis or Parkinson disease in response to strength training. Finally, unlike previous reviews, our study explores whether differences in response to strength training exist between individuals with multiple sclerosis or Parkinson disease.
MATERIAL AND METHODS
Search Strategy
A comprehensive search of electronic databases was conducted from inception to July 2014. Electronic searches were performed using Physiotherapy Evidence Database (PEDro) scale, PubMed, EMBASE, Cochrane Central Register of Controlled Trials, and CINAHL databases. The search strategy utilized a population, intervention, comparison, and outcome approach.37 The population key words were “Parkinson disease,” “multiple sclerosis,” Alzheimer disease, amyotrophic lateral sclerosis, Huntington disease, and spinocerebellar ataxia; the intervention key words were “strength training,” “progressive strength training,” “resistance training,” “weight training,” and “strengthening programs”; and the outcome key words included “strength,” “disease severity,” “gait,” “balance,” “fatigue,” “functional capacity,” “mood,” and “quality of life”. This initial search only found trials on strength training in individuals with Parkinson disease or multiple sclerosis.
As this was a literature review and did not involve the recruitment and assessment of patients, ethical approval was not necessary.
Eligibility Criteria
Randomized controlled trials and nonrandomized controlled trials that examined the effect of strength training in individuals suffering with multiple sclerosis or Parkinson disease were included in the review. Strength training was defined as an intervention in which participants exercised a muscle or group of muscles against an external resistance.12 Eligible studies included those examining the effect of strength training in individuals with multiple sclerosis and Parkinson disease. Exclusion criteria were as follows: case studies; observational studies; studies with healthy controls or healthy comparison groups; and studies employing supplementary intervention therapies in addition to or different from strength training.
Data Extraction
Two independent authors (T.M.C. and A.R.R) extracted data from the included studies. A specialized extraction form was designed and recorded the following methodological details for each study as described below.
Publication details: authors and year of publication; details of the study: study design and number of participants, experimental and control interventions, and reported outcomes (controls and experimental); participant characteristics: disease population, disease status, and age; specific intervention details: intervention groups, mode of strength training, targeted anatomical regions, setting in which the study was conducted, level of supervision, duration of the intervention (weeks), frequency of strength training, specific exercises employed, exercise intensity, number of sets and repetitions performed for each exercise, rest taken between sets and exercises, and the progression method used for strength training interventions; moderator variables: participant retention and dropouts, participant adherence, and adverse effects associated with strength training.
Corresponding authors of studies were contacted as necessary for supplementary information not detailed in the publication. In cases wherein authors did not respond or did not provide supplementary methodological information pertaining to their publication, a not reported statement was assigned.
Quality Assessment
All articles that satisfied the predefined inclusion criteria were independently rated for quality by 2 reviewers (T.C. and A.R.) using the PEDro scale.38 The PEDro scale is an 11 points scale designed to examine the methodological quality of intervention studies. The scale evaluates the following methodological aspects: specific eligibility criteria, randomization allocation, concealed allocation, baseline demographic similarities, participant blinding, therapist blinding, outcome assessor blinding, whether more than 85% of participants completed follow-up for at least 1 primary outcome, intention to treat analysis, between group statistical comparisons, and point estimates and variability for at least one of the primary outcome measures. When rating each study, only criteria 2 and 11 are considered for the PEDro scale. Initial discrepancies between the independent authors were resolved by consensus. In instances wherein discrepancies could not be resolved, a final decision was made by another independent author (M.Z.).
Data Analysis and Synthesis
For analysis, studies were categorized according to disease. The heterogeneity of populations and extensive variety of reported outcomes prevented a meta-analysis for all outcomes, with the exception of strength. Whereas 15 articles reported on strength as an outcome,13–16,18,19,39–45 3 articles by Dalgas et al16,18,19 and 2 articles by Dibble et al42,43 appeared to originate from the same trial. Strength data from 3 articles by Dalgas et al16,18,19 were pooled together into a single effect size for a better interpretation of the effects of strength training on strength as an outcome. Standardized effect sizes were calculated for the meta-analysis using pre- and poststrength mean values for each group (intervention and comparison) (Hedges and Olkin, 1985). Effect sizes were corrected for the magnitude of sample size of each study as suggested by Hedges and Olkin (1985). The risk of publication bias in trials was examined statistically using the egger regression test, with a significant publication bias considered to be P ≤ 0.10. All statistical analyses were performed using STATA 9.1 (StataCorp LC, Texas, USA).
RESULTS
Articles Included
The database search strategy and results are presented in Figure 1. Five hundred seven articles were identified by the initial search strategy. Four hundred seventy one of the identified articles were excluded based on their title. The abstracts of the remaining 36 articles were evaluated and 6 articles were excluded (Figure 1). Full texts of the remaining 30 articles were retrieved and reviewed, resulting in the exclusion of 10 articles (Figure 1). Of the 20 articles included in the systematic review, 8 appeared to originate from 3 separate trials. Subsequently, the extracted and reviewed data is representative of 15 independent trials.
FIGURE 1.
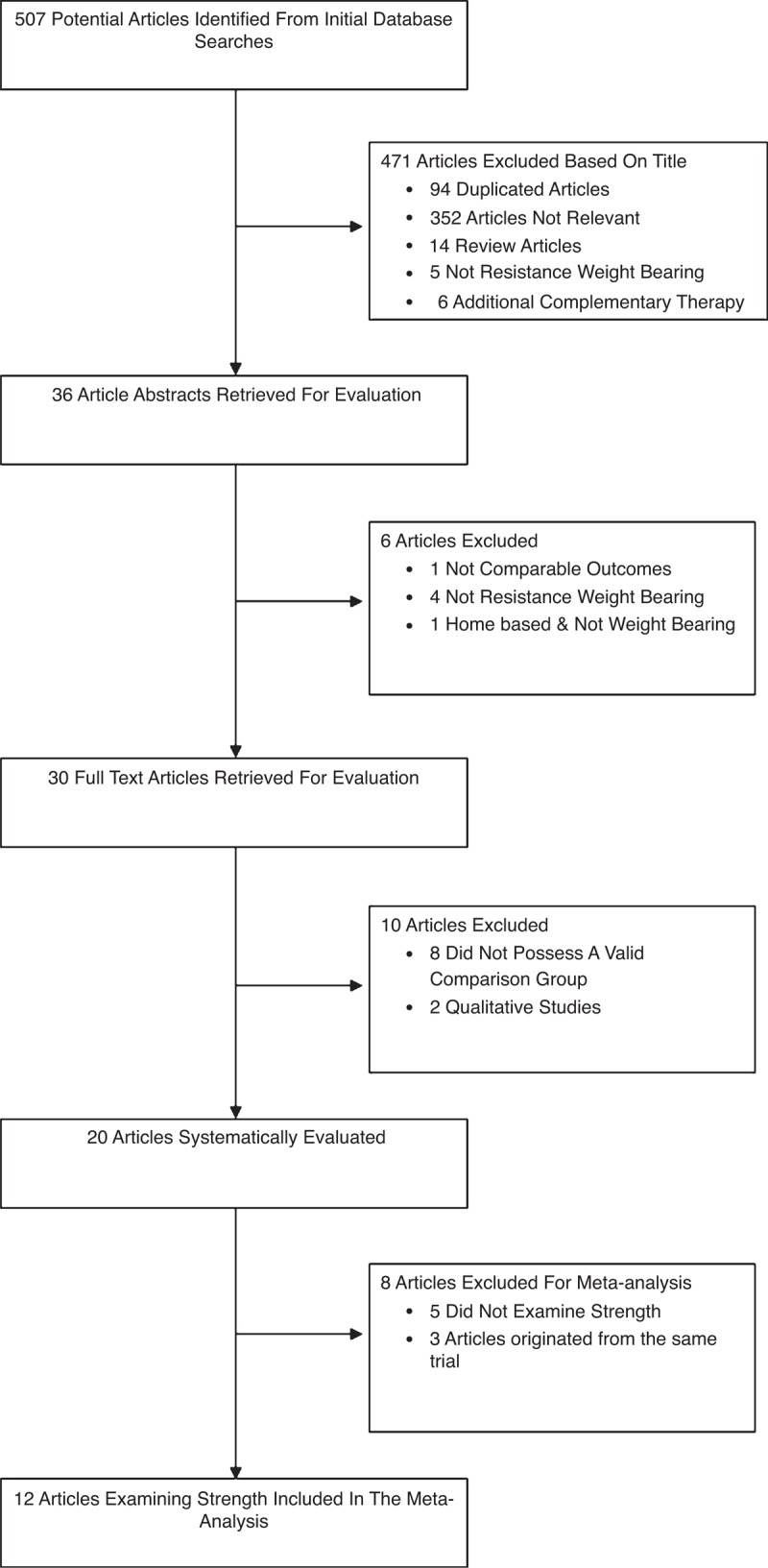
Flowchart for selection of trials included in the systematic review and meta-analysis.
Methodological Quality
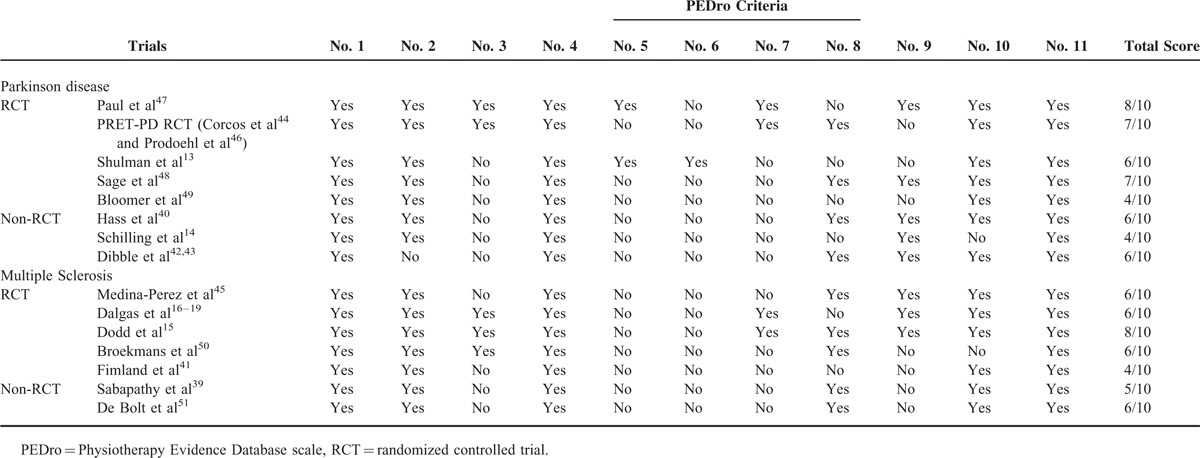
The methodological quality of included trials varied considerably in both Parkinson disease and multiple sclerosis populations. PEDro scores ranged from 4 to 8 points in both Parkinson disease13,14,40,42–44,46–49 and multiple sclerosis trials15–19,39,41,45,50,51 (Table 1).
TABLE 1.
Trial Inclusions Rated According to the Physiotherapy Evidence Database Scale
Participants Characteristics
The number of trials included was 8 in Parkinson disease13,14,40,42–44,46–49 and 7 in multiple sclerosis.15–19,39,41,45,50,51 Disease population, study design, number of participants, stage of disease, mean age and standard deviation, trial intervention, and trial outcomes are shown in Tables 2 and 3 .
TABLE 2.
Overview of Trials of Strength Training Interventions in Individuals With Parkinson Disease or Multiple Sclerosis
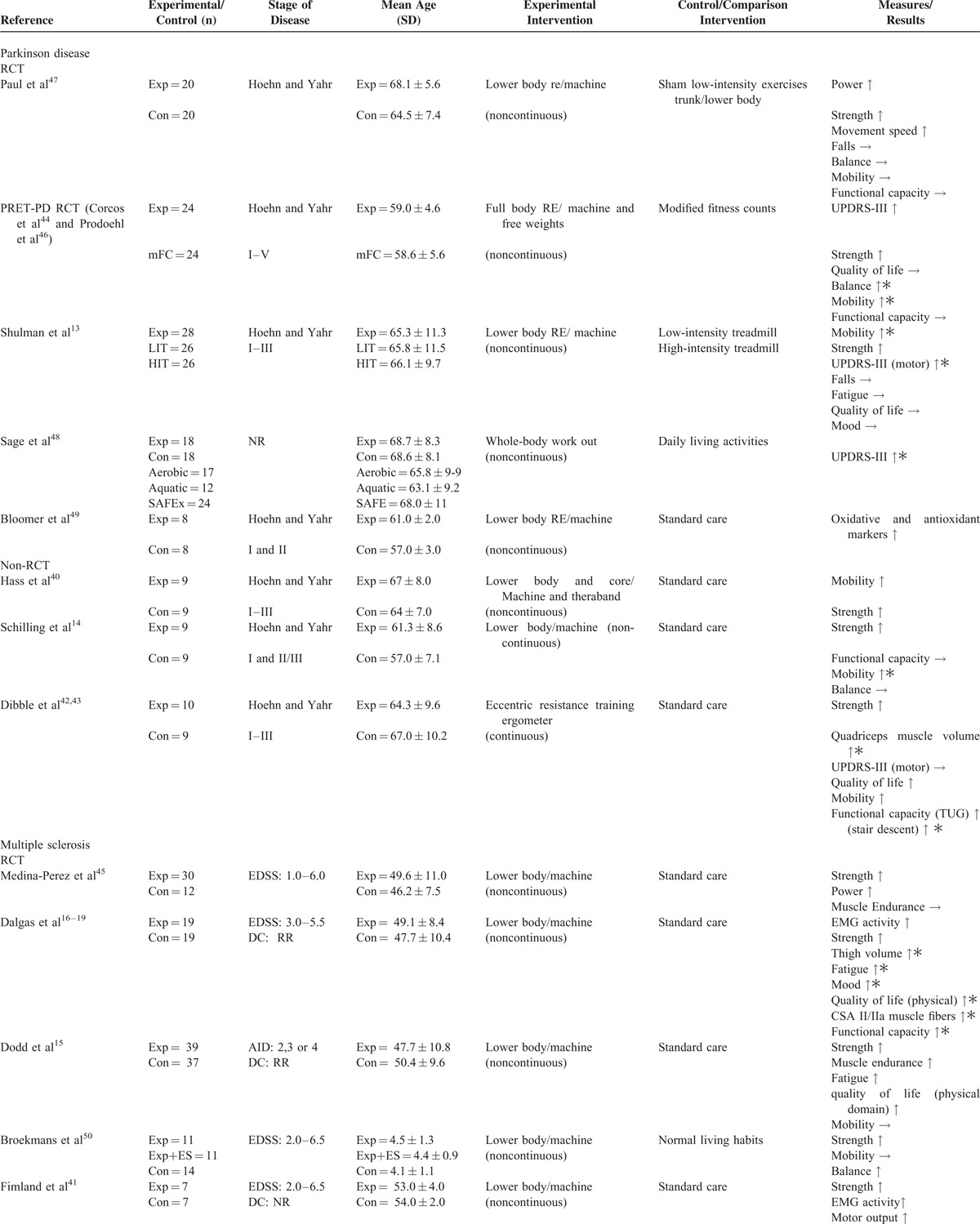
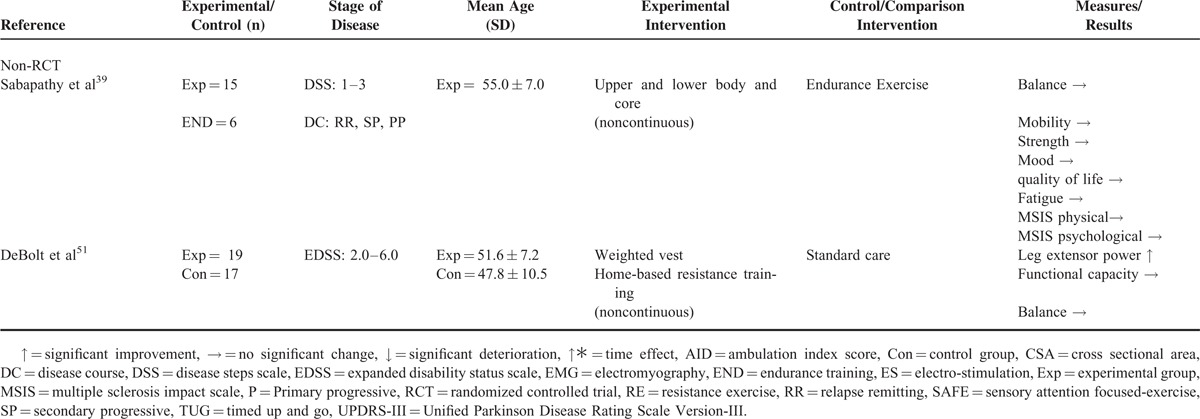
TABLE 2 (Continued).
Overview of Trials of Strength Training Interventions in Individuals With Parkinson Disease or Multiple Sclerosis
Intervention Characteristics
Of the 8 trials conducted in individuals with Parkinson disease13,14,40,42–44,46–49 (5 randomized controlled trials13,44,46–49 and 3 nonrandomized controlled trials14,40,42,43), 5 used lower body strength training interventions,13,14,42,43,47,49 2 used a full-body strength training intervention,44,46,48 and 1 used a lower body and core strength training intervention40 (Tables 2 and 3 ). Training protocols ranged from 2 to 24 months of twice to thrice weekly training.13,14,40,42–44,46–49 Only 2 trials conducted in individuals with Parkinson disease reported on the level of supervision for strength training interventions.44,46,48
Of the 7 trials conducted in multiple sclerosis15–19,39,41,45,50,51 (5 randomized controlled trials15–19,41,45,50 and 2 nonrandomized controlled trials39,51), 5 trials trained the lower body15–19,41,45,50 and 2 trials trained the full body39,51 (Tables 2 and 3 ). Intervention protocols utilized in multiple sclerosis trials ranged from 3 weeks to 6 months of 2 to 5 times weekly training.15–19,39,41,45,50,51 Of the 7 trials conducted in individuals with multiple sclerosis, only 3 trials reported on the level of supervision for strength training interventions.15–19,50
Risk of Bias
Statistical examination using the egger regression test revealed no publication bias (P = 0.131).
Intensity and Progression of Strength Training
Two randomized44,46,47 and 2 nonrandomized controlled trials40,42,43 conducted in Parkinson disease reported on the intensity of strength training performed throughout the intervention, whereas 3 randomized controlled trials41,45,50 reported on the intensity of strength training in multiple sclerosis. The progression of strength training was reported by 3 randomized44,46,47,49 and 3 nonrandomized controlled trials14,40,42,43 in Parkinson disease. In contrast, there were no trials that reported on the progression of strength training in multiple sclerosis.
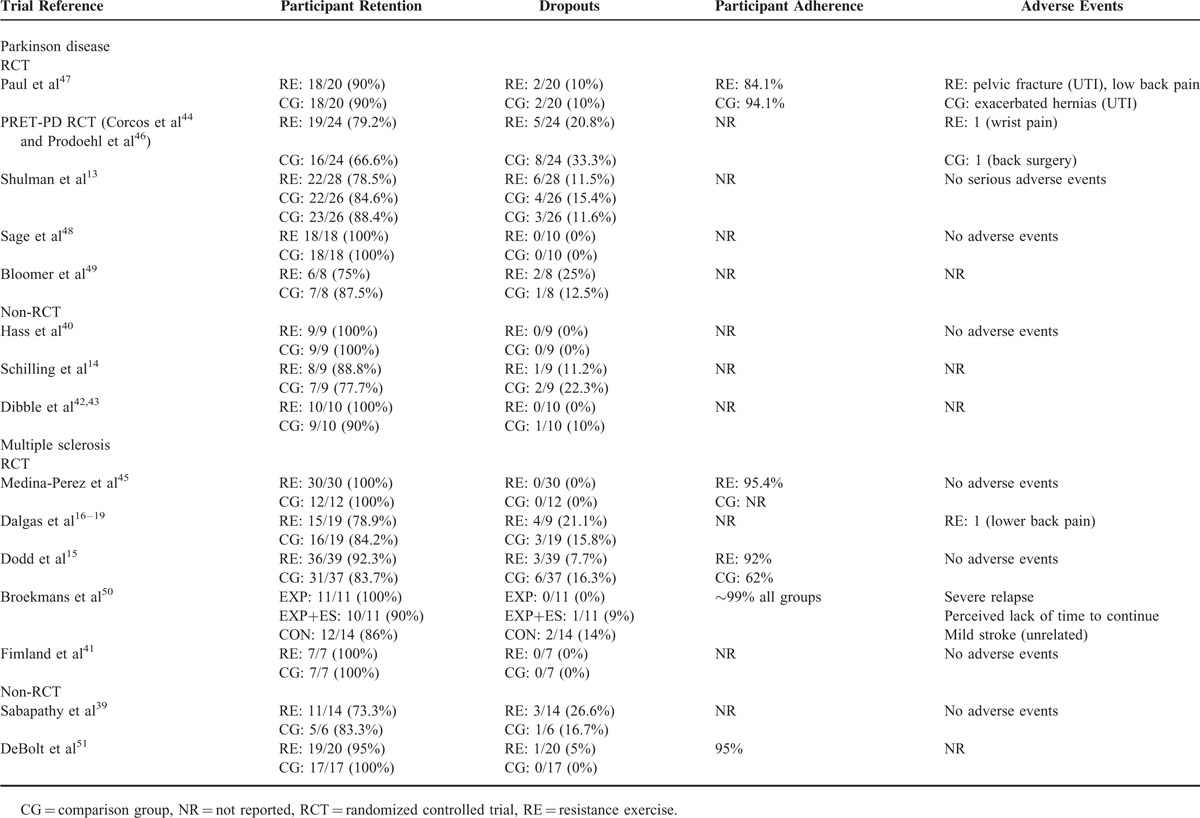
Participant Retention, Adherence, and Adverse Events
Participant retention ranged from 75% to 100% in Parkinson disease trials13,14,40,42–44,46–49 and from 73.3% to 100% in multiple sclerosis trials15–19,39,41,45,50,51 (Table 4). Four trials in multiple sclerosis ([Medina-Perez et al45 strength training group 95.4%; control group not reported], [Dodd et al15 strength training group 92%; control group 62%], [Broekmans et al50 ∼99% all groups] and [DeBolt et al51 strength training group 95%]), and 1 trial in Parkinson disease reported on participant adherence (Paul et al47 strength training group 84.1%; control group 94.1%) (Table 4). Five trials in Parkinson disease13,40,44,46–48 and 6 trials in multiple sclerosis15–19,39,41,45,50 reported on adverse events,13,40,44,46–48 with only minor or clinically unrelated medical issues reported (Table 4).
TABLE 3.
Summary Details of the Specific Strength Training Interventions Used in Parkinson Disease or Multiple Sclerosis Trials
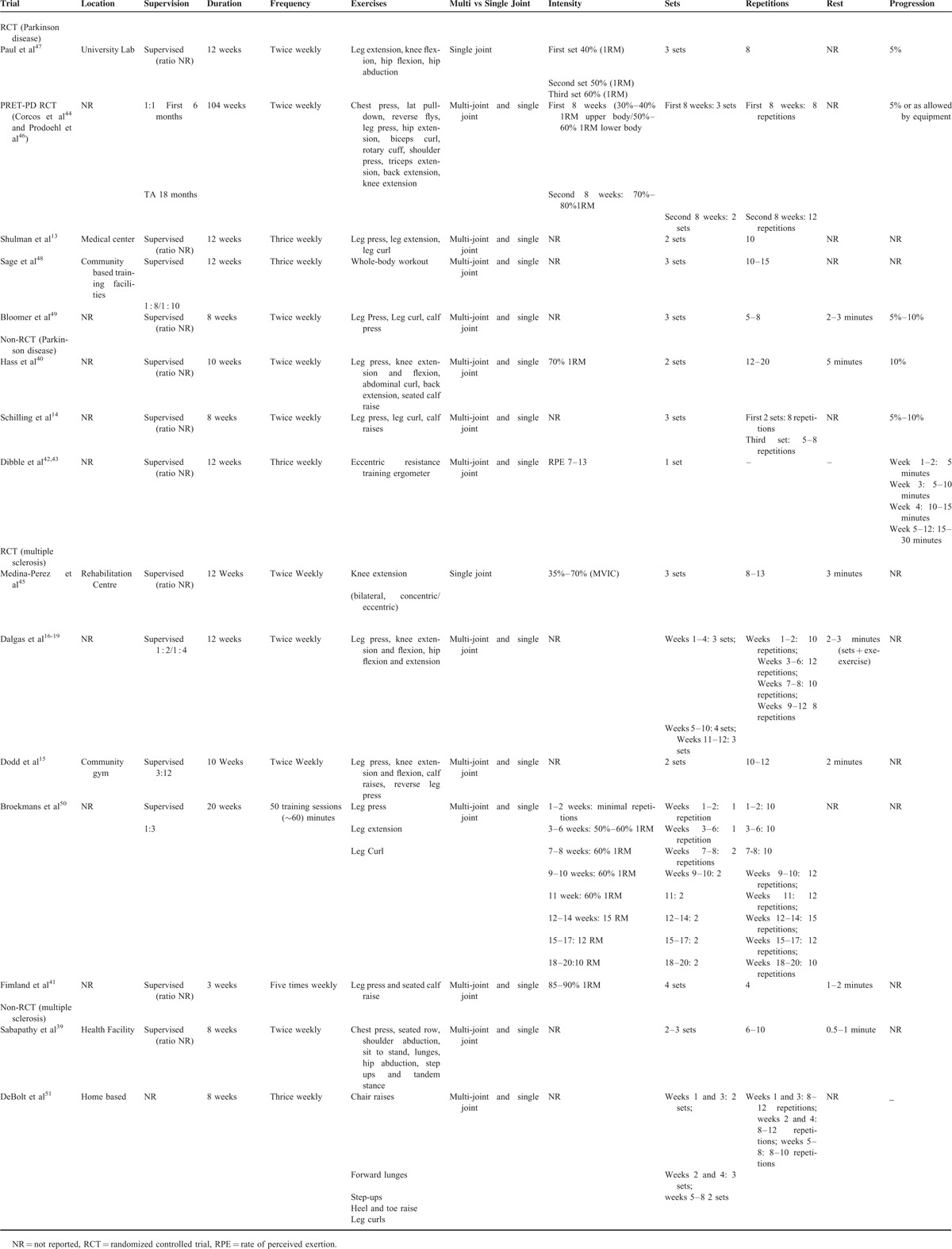
TABLE 4.
Summary of Retention, Adherence and Adverse Events in Parkinson Disease or Multiple Sclerosis Strength Training Trials
Outcomes Measures
Strength As an Outcome Measure in Parkinson Disease
Three randomized controlled trials evaluated the effect of strength training on strength in people with Parkinson disease.13,44,47 Strength was evaluated across trials using 1 repetition maximum (1RM) and maximum voluntary isometric contraction (MVIC) protocols with torque transducers, pneumatic resistance machines, and dynamometers. Corcos et al44 found a significant improvement in elbow flexor muscle strength (1RM, 15%) in the strength training group, while off medication, after 24 months of upper and lower body resistance training. No significant differences in strength were found for the control group in this trial. Shulman et al13 in another trial found a significant improvement in leg press and leg extension strength (1RM, 16%) in individuals within the strength training group, but not in the high or low intensity treadmill training groups, after 3 months of thrice weekly resistance training. Paul et al47 also reported a significant improvement in lower limb strength (1RM, leg extension, 14.6%; knee flexion, 18.6%; hip flexion, 39.8%; hip abduction, 33.9%) and power (leg extension, 17.3%; knee flexion, 20.6%; hip flexion, 46.3%; hip abduction, 43.1%) in the strength training group in comparison to the sham comparison group after 12 weeks of lower body resistance training.
Three nonrandomized controlled trials also evaluated the effect of strength training on strength and found significant improvements.14,40,42,43 Hass et al,40 after 10 weeks of twice weekly lower body strength training, found a significant improvement in knee extension (1RM, 76%) and knee flexion (1RM, 57%) strength in the intervention group, but not in the control group. Schilling et al14 in another trial reported a significant improvement in leg press strength (1RM, 22%) in the intervention group, whereas the control group showed no significant differences. Dibble et al42,43 similarly reported a significant improvement in quadriceps muscle strength (MVIC) in the more (average torque 23%; peak torque 18%) and less-affected leg (average torque 16%; peak torque 83.2%) in the strength training intervention group only.
Strength As an Outcome in Multiple Sclerosis
Five randomized controlled trials reported on strength as an outcome after strength training,15,16,18,19,41,45,50 with all 5 trials reporting significant improvements in strength. Strength was evaluated across trials using MVIC, maximum voluntary dynamic contraction, and 1RM strength protocols with pneumatic resistance machines, dynamometers and the Leg Extensor Power Rig. Medina-Perez et al45 reported a significant improvement in knee extension strength (MVIC, 7.7%) and power (40% MVIC, 15.6%) in the intervention group, but not in the control group after 12 weeks of strength training. Significant improvements in leg press strength (1RM, 15%) in the intervention group, but not the control group were also reported by Dodd et al15 after strength training. Broekmans et al50 in line with Medina-Perez et al,45 reported significant improvements in isometric strength in the knee flexors and extensors (MVIC, average knee extension 45° change: 10.8, average knee extension 90° change: 10, average knee flexor 45° change: 4, average knee flexion 90° change: 2.3) in the intervention group as a result of strength training. In another trial, Dalgas et al16,18,19 reported significant improvements in isokinetic, isometric, and angular impulse knee extensor and flexor strength in the intervention group ([Dalgas et al,19 MVIC at 70° knee flexion; knee extension: 13.2%, knee flexion: 13.8%], [Dalgas et al18; maximum voluntary dynamic contraction, knee extension 90°: 4.5%; knee extension 180°:10.2%; knee flexion 90°: 21.3%; knee flexion 180°: 18.6%], [Dalgas et al,16 MVIC, knee extension: 15.7%, knee flexion: 21.3%]), but not in the control group as a result of resistance training. Dalgas et al16 additionally reported a significant improvement in leg press strength. Fimland et al41 in another trial reported a significant improvement in plantar flexion strength (MVIC, 36%) in the strength training intervention group, but not in the control group. In a nonrandomized controlled trial, DeBolt et al51 reported a significant improvement in leg extensor power (24%) in the intervention group, whereas the disease control group showed no changes after strength training.
In addition to muscle strength, significant study-specific improvements in gait, clinical disease progression, functional capacity, quality of life, oxidative biomarkers, mood, fatigue, falls, skeletal muscle volume, and electromyography activity were observed after strength training in individuals with multiple sclerosis or Parkinson disease.13–19,39–51
Parkinson Disease Measures
Unified Parkinson Disease Rating Scale Version 3
Three randomized13,44,48 and 1 nonrandomized controlled trial42 conducted in Parkinson disease evaluated the effect of strength training on clinical disease progression using the Unified Parkinson Disease Rating Scale Version 3. Corcos et al44 reported a significant improvement on the Unified Parkinson Disease Rating Scale Version 3 in the intervention group (7.4 point decrease), but not in the control group after 24 months of strength training. Shulman et al13 in another study similarly reported a significant improvement on the motor subscale of the Unified Parkinson Disease Rating Scale Version 3 in the strength training group. Furthermore, Sage et al48 found a significant improvement on the Unified Parkinson Disease Rating Scale Version 3 in the strength training group. Dibble et al42 by contrast found no improvement on the Unified Parkinson Disease Rating Scale Version 3 in the intervention group after strength training.
Functional Mobility
Three randomized13,46,47 and 3 nonrandomized controlled trials14,40,42,43 evaluated the effect of strength training on mobility in individuals with Parkinson disease. Mobility was assessed across trials using the 10 meter timed walk test, 6 minute walk test, 50 feet walk test and timed up and go. Paul et al47 did not report significant changes in mobility after strength training. In contrast, Prodoehl et al46 and Shulman et al13 found significant improvements in mobility as a result of strength training. The 3 nonrandomized controlled trials12,39,41,42 that reported on mobility as an outcome also documented improvements.
Balance
Two randomized46,47 and 2 nonrandomized controlled trials14,39 examined the effect of strength training on balance outcomes in Parkinson disease. Balance was evaluated across trials using a variety of outcomes including the single leg stance, choice stepping task, berg balance scale, functional reach test, 5 time sit to stand test, and the activities-specific balance confidence scale. Paul et al47 did not find a significant improvement in balance as a result of strength training. Prodoehl et al46 by contrast reported a significant improvement in balance after strength training. Both nonrandomized controlled trials14,39 were unable to find a significant improvement in balance after strength training.
Functional Capacity
One randomized trial44 examined the effect of strength training on functional capacity. Corcos et al44 assessed functional capacity using the modified Physical Performance Test and reported no significant changes after strength training in the intervention or control group.
Quality of Life
Two randomized13,44 and 1 nonrandomized controlled trial42 evaluated the effect of strength training on quality of life. All 3 trials assessed quality of life using the 39-Item Parkinson Disease Questionnaire. Both randomized controlled trials10,11 did not report a significant improvement in quality of life after strength training. Dibble et al42 by contrast reported a significant improvement in quality of life in the intervention group after strength training.
Oxidative and Antioxidant Markers
One randomized controlled trial49 in Parkinson disease measured changes in blood oxidant and antioxidant marker levels and reported significant increases in antioxidant marker levels (superoxide dismutase [9.9%] and glutathione peroxidase [1.8%]) and a significant reduction in oxidative stress marker levels (malondialdehyde [15%] and hydrogen peroxide [16%]).
Mood
One randomized controlled trial13 evaluated the effect of strength training on mood in Parkinson disease. Shulman et al13 found no significant changes in mood after strength training using the Beck Depression Inventory.
Fatigue
One randomized controlled trial13 evaluated the effect of strength training on fatigue in Parkinson disease. Shulman et al13 used the 16-item Parkinson Fatigue Scale and found no significant change in fatigue after strength training in the strength training intervention group or high- and low-intensity treadmill intervention groups.
Falls
Two randomized controlled trials11,45 evaluated the effect of strength training on falls in people with Parkinson disease.13,47 Falls were assessed using the New Freezing of Gait Questionnaire47 and Falls Efficacy Scale.13 No trial reported a significant effect on falls outcomes after strength training.
Skeletal Muscle Volume
One nonrandomized controlled trial43 evaluated the effect of strength training on quadriceps muscle volume in Parkinson disease. Dibble et al43 found a significant increase in quadriceps muscle volume using magnetic resonance imaging after strength training in the intervention group only.
Multiple Sclerosis
Functional Mobility
Two randomized15,50 and 2 nonrandomized controlled trials39,51 evaluated the effect of strength training on functional mobility in multiple sclerosis. Functional mobility was assessed across trials using the 2 minute walk test, 10 meter walk test, timed 25 foot walk and timed up and go. No trial reported a significant improvement in mobility as a result of strength training.
Balance
One randomized50 and 2 nonrandomized39,51 controlled trials evaluated the effect of strength training on balance in multiple sclerosis. Balance was evaluated across trials using the Functional Reach Test,39,50 Four Square Step Test,39 and AccuswayPLUS force platform.51 Broekmans et al50 reported a significant improvement in balance in the intervention group only as a result of strength training. However, Sabapathy et al39 and DeBolt et al51 did not find significant improvements in balance after strength training.
Functional Capacity
One randomized controlled trial16 evaluated the effect of strength training on functional capacity outcomes in multiple sclerosis. Dalgas et al16 reported a significant improvement in functional capacity (computed as 1/4 [chair stand test post/chair stand test pre] + [stair climb test post/stair climb test pre] + [10 meter walk test post /10 meter walk test pre] + [6 minute walk test post / 6 minute walk test pre] × 100) as a result of strength training.
Quality of Life
Two randomized15,17 and 1 nonrandomized controlled trial39 reported on quality of life outcomes after strength training in multiple sclerosis. Quality of life was assessed across trials using the Short Form-3617,39 and the World Health Organisation Quality of Life-BREF.15 Dodd et al15 and Dalgas et al17 reported a significant improvement in quality of life in the intervention group as a result of strength training. In contrast, Sabapathy et al39 found no significant improvement in quality of life after strength training.
Electromyography Activity
Two randomized controlled trials17,40 assessed the effect of strength training on electromyography activity during maximum voluntary isometric contractions. Dalgas et al recorded surface electromyography signals from the Vastus Lateralis, Rectus Femoris, and Semitendinosus during maximal voluntary isometric contractions of the knee flexors and extensors (assessed at 70° knee flexion), using bipolar electrodes. The upper electrode of each pair was placed at the midpoint between the Spina Iliaca anterior superior and patellar basis. After 12 weeks of strength training, Dalgas et al found significant improvements in maximal isometric (μV) knee extension and knee flexion activity (semitendinosus: 27.6%; vastus lateralis: 27%; rectus femoris: 28%) in the intervention group, but not the control group. Fimland et al41 recorded surface electromyography activity during maximum voluntary isometric contractions of the plantar flexors (ankle positioned at 90°), using bipolar surface electrodes placed according to Surface Electromyography for the Noninvasive Assessment of Muscles recommendations. Fimland et al41 reported significant improvements (15%) in surface electromyography activity of the plantar flexors after 3 weeks of strength training in the intervention group in comparison to the control group.
Skeletal Muscle Volume and Architecture
Only 1 randomized controlled trial18 measured changes to thigh volume, muscle fiber numbers, type, and size. Muscle biopsies of the vastus lateralis (middle portion) were taken to assess changes in muscle fiber number, type, and size. Dalgas et al18 reported a significant increase in the cross sectional area of type II and IIa vastus lateralis muscle fibers after strength training in the intervention group only.
Fatigue
Two randomized15,17 and 1 nonrandomized controlled trial39 evaluated the effect of strength training on fatigue in multiple sclerosis. Fatigue was assessed across trials using a variety of outcomes including the Modified Fatigue Scale and Fatigue Severity Scale, Multidimensional Fatigue Inventory. Dodd et al15 reported a significant improvement in the level of fatigue experienced (24%) after 10 weeks of twice weekly strength training. Similar findings were reported by Dalgas et al,17 who reported a 10% improvement in the level of fatigue experienced after strength training. Sabapathy et al39 also reported a significant improvement in the level of fatigue experienced as a result of strength training.
Mood
One randomized17 and 1 nonrandomized controlled trial39 examined the effect of strength training on mood outcomes in multiple sclerosis. Dalgas et al17 reported significant improvements (−2.4 points) in mood using the Major Depression Inventory as a result of strength training. In contrast, Sabapathy et al39 found no significant changes in mood using the Beck Depression Inventory after strength training.
Muscle Endurance
Two randomized controlled trials15,45 evaluated the effect of strength training on muscle endurance in multiple sclerosis. Medina-Perez et al45 measured muscle endurance as the maximum number of repetitions that a participant could perform during a single set of knee extension using a load of 40% of the maximum voluntary isometric contraction, whereas Dodd et al15 measured endurance by counting the number of repetitions that a participant could complete on the seated leg press and reverse leg press using a load of 50% of 1RM. Medina-Perez et al45 did not find a significant change in muscle endurance in the intervention or control group after strength training. In contrast, Dodd et al15 reported a significant improvement in muscle endurance in the intervention group relative to the control group after strength training.
DISCUSSION
This review found that strength training is useful for improving muscle strength in Parkinson disease and to a lesser extent multiple sclerosis. Evidence also showed that strength training is helpful for improving clinical measures of disease progression and mobility in Parkinson disease. However, the evidence is unclear regarding the efficacy of strength training on falls, quality of life, fatigue, functional capacity, and balance in Parkinson disease. In multiple sclerosis, strength training was also found to improve fatigue, quality of life, muscle power, electromyography activity, and functional capacity. However, its effect on balance and mood remains equivocal.
An increase in strength was the most consistently reported benefit of strength training in people with Parkinson disease and multiple sclerosis. A meta-analysis of the extracted strength data revealed that strength training had a larger effect on strength in people with Parkinson disease (d = 0.87) than multiple sclerosis (d = 0.33) (Figure 2). Different pathological mechanisms underpinning impairments in strength in each disease are likely to account for this discrepancy. For instance, impairments in strength in multiple sclerosis are thought to be mediated by central52,53 (spinal and supraspinal mechanisms) and muscular deficits,54–56 whereas in Parkinson disease impairments in strength are thought to result from central deficits only.57–59 This finding suggests that strength training may only produce meaningful benefits in strength in people with Parkinson disease.
FIGURE 2.
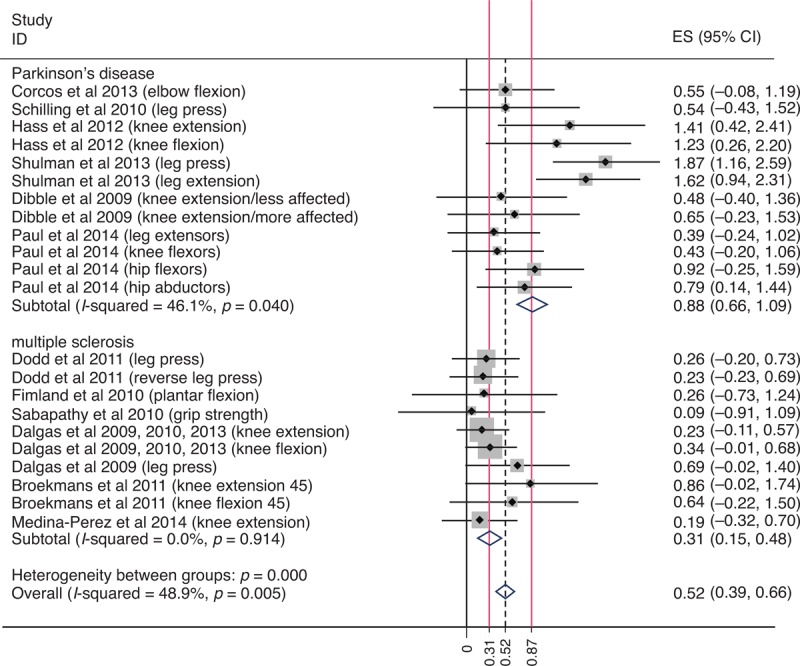
Meta-analysis of trials that measured muscle strength.
Strength training trials in Parkinson disease also reported improvements in mobility. The improvements were reported on short and longer duration mobility assessments, suggesting that strength training has a favorable effect on multiple aspects of mobility. This finding is consistent with the supposition that muscle strength strongly predicts mobility in people with Parkinson disease.60,61 Surprisingly, no improvements in mobility were reported in individuals with multiple sclerosis after strength training. This finding was unexpected, as the strength training interventions in Parkinson disease and multiple sclerosis trials, for the most part, used similar training frequencies (2–3 times per week), resistance exercises (leg press, knee extension, knee flexion, and calf raises), and sets per exercise (2–3). This may indicate that strength training is not capable of improving mobility in individuals with multiple sclerosis. The inability to improve mobility may be explained by the smaller improvements in strength observed in individuals with multiple sclerosis. Indeed, recent findings show that muscle strength significantly predicts performance on mobility tasks in individuals with multiple sclerosis.62 Alternatively, it is possible that the strength training interventions used in the multiple sclerosis trials were unable to provide a stimulus sufficient to improve mobility in multiple sclerosis, and perhaps more intense or specific training interventions may be required.
In addition, strength training was found to have a positive effect on disease progression in people with Parkinson disease (Unified Parkinson Disease Rating Scale-Version 3). Interestingly, improvements in disease progression were observed in a cohort with mild-to-advanced disability that were not on medication, suggesting that strength training alone may be capable of positively impacting on disease progression in individuals at all stages of Parkinson disease. The positive effect of strength training on disease progression may have been mediated by favorable central changes. For instance, recent evidence shows that repetitive force generation increases neuronal activation in the basal ganglia, thalamus, parietal cortex, cerebellum, and motor cortex.63–66 Furthermore, emerging evidence has shown that exercise interventions can increase regional brain volume and structural connectivity in patients with Parkinson disease and other neurodegenerative disorders.67–70 Further studies are required to confirm the latter remarks.
In multiple sclerosis trials, improvements in strength were accompanied by significant improvements in fatigue, quality of life, muscle power, maximal electromyography activity, and functional capacity. The reported improvements in fatigue are of clinical interest given that 33%–75% of individuals with multiple sclerosis suffer from fatigue.71–73 Nevertheless, this finding was not surprising, given that exercise has previously been reported to improve fatigue in multiple sclerosis.74 The improvements in fatigue may in part explain the benefits observed in quality of life, especially considering that fatigue is an important predictor of quality of life in people with multiple sclerosis.75,76 The increases in muscle power and maximal electromyography activity are consistent with the observed improvements in strength. The reported improvements in lower limb strength, fatigue, and muscle power likely contributed to the improvement in functional capacity documented by Dalgas et al.16 Indeed, recent findings have shown that strength,77 fatigue,78 and muscle power61 significantly influences functional capacity in individuals with multiple sclerosis and other neurodegenerative disorders.
It is important to note that most trials included in this systematic review recruited individuals with mild-to-moderate disability. The higher level of disability in individuals at advanced stages of Parkinson disease or multiple sclerosis may have led researchers to only include individuals at early-to-middle stages of both diseases. The same level of benefits after strength training may not be possible in individuals at more advanced stages of Parkinson disease or multiple sclerosis. Future trials assessing the effect of strength training in individuals with Parkinson disease and multiple sclerosis with a severe level of disability are therefore warranted.
In general, the trials displayed adequate methodological quality, with PEDro scores ranging from 4 to 8 in both diseases. The major methodological shortcomings found using the PEDro scale included a failure to report concealed allocation (criteria 3), participant blinding (criteria 5), therapist blinding (criteria 6), and outcome assessor blinding (criteria 7). It is important to acknowledge that it is often not possible to blind participants or therapists to exercise or group allocation.79 Trial scores generated using the PEDro scale may therefore underestimate the quality of evidence.
In addition to evaluating trials using the PEDro scale, we also performed a critical appraisal of specific intervention characteristics important to strength training trials. This appraisal found that specific intervention characteristics were typically well detailed, with the exception of the level of supervision and strength training intensity. The lack of data reported on the level of supervision and the intensity of strength training performed is of concern in particular, as a high level of supervision as well as an appropriate intensity of strength training is required to maximize therapeutic benefits and avoid potential injury.80 The poor level of reporting on strength training progression in multiple sclerosis trials is also concerning, given that modulating the progression of strength training is important to avoid injury and training plateaus.81 The inadequate reporting of participant adherence in both disease populations was also worrisome, as it does not enable internal and external examination of what dose of strength training is required to maximize therapeutic benefits and avoid injury in such populations.
Based on our findings and American College of Sports Medicine guidelines, we recommend that individuals with multiple sclerosis or Parkinson disease perform progressive submaximal strength training (whole-body single and multi-joint resistance exercises) on at least 2 nonconsecutive days per week for an hour under direct supervision (eg, physiotherapist, exercise physiologist, strength and conditioning specialist) to improve muscle strength and other disease specific clinical features (Parkinson disease: mobility and disease progression; multiple sclerosis: fatigue, quality of life, muscle power, maximal electromyography activity, and functional capacity).
Limitations
Lack of consistent reporting and heterogeneity of study outcomes between trials made it difficult to draw firm conclusions beyond improvements in muscle strength with respect to the benefits of strength training for individuals with multiple sclerosis or Parkinson disease.
CONCLUSION
Trials investigating the effect of strength training in individuals with Parkinson disease or multiple sclerosis are in their infancy. Nevertheless, benefits in strength were found after strength training in individuals with Parkinson disease and, to a lesser extent, in multiple sclerosis. Some evidence was also found to suggest that strength training has a positive effect on clinical disease progression and mobility in individuals with Parkinson disease. Similarly, some evidence showed that strength training is beneficial for muscle power, maximum electromyography activity, fatigue, functional capacity, and quality of life in individuals with multiple sclerosis. Additional trials employing high-quality methodological designs are required to confirm and expand on these findings. Such trials may provide evidence-based rationale for using strength training as a therapy for other neurodegenerative disorders such as Alzheimer disease and Huntington disease.
Acknowledgments
The authors of this article would like to thank Prof Roger Barker for his comments on the article.
Footnotes
Abbreviations: 1RM = one repetition maximum, NR = not reported, MVIC = maximum voluntary isometric contraction, PEDro scale = Physiotherapy Evidence Database scale.
Drs TMC and ARR contributed equally to the writing of this article.
TMC and ARR contributed equally to the concept of the study, development of the search strategy analysis, analysis of the results, and writing of the article. Both authors had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analysis. MRZ contributed to the interpretation of the results, critical revision of the article for important intellectual content, editing of the article and final approval of the version to be published.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Nance MA. Therapy in Huntington's disease: where are we? Curr Neurol Neurosci Rep 2012; 12:359–366. [DOI] [PubMed] [Google Scholar]
- 2.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006; 443:787–795. [DOI] [PubMed] [Google Scholar]
- 3.Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology 2009; 72 suppl 4:S1–S136. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet 2007; 369:2031–2041. [DOI] [PubMed] [Google Scholar]
- 5.Benedict RH, Zivadinov R. Risk factors for and management of cognitive dysfunction in multiple sclerosis. Nat Rev Neurol 2011; 7:332–342. [DOI] [PubMed] [Google Scholar]
- 6.Lu E, Wang BW, Guimond C, et al. Disease-modifying drugs for multiple sclerosis in pregnancy: a systematic review. Neurology 2012; 79:1130–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Evans JR, Mason SL, Williams-Gray CH, et al. The natural history of treated Parkinson's disease in an incident, community based cohort. J Neurol Neurosurg Psychiatry 2011; 82:1112–1118. [DOI] [PubMed] [Google Scholar]
- 8.Noyes K, Bajorska A, Chappel A, et al. Cost-effectiveness of disease-modifying therapy for multiple sclerosis: a population-based study. Neurology 2011; 77:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjølhede T, Vissing K, Dalgas U. Multiple sclerosis and progressive resistance training: a systematic review. Mult Scler 2012; 18:1215–1243. [DOI] [PubMed] [Google Scholar]
- 10.Falvo MJ, Schilling BK, Earhart GM. Parkinson's disease and resistive exercise: rationale, review, and recommendations. Mov Disord 2008; 23:1–11. [DOI] [PubMed] [Google Scholar]
- 11.Hindle JV, Petrelli A, Clare L, et al. Nonpharmacological enhancement of cognitive function in Parkinson's disease: a systematic review. Mov Disord 2013; 28:1034–1049. [DOI] [PubMed] [Google Scholar]
- 12.Esco MR. Resistance Training for Health and Fitness. In: Medicine ACoS, ed. American College of Sports Medicine. Indianapolis: American College of Sport Medicine; 2013:1–2. [Google Scholar]
- 13.Shulman LM, Katzel LI, Ivey FM, et al. Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease physical exercise for patients with PD. JAMA Neurol 2013; 70:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schilling BK, Pfeiffer RF, LeDoux MS, et al. Effects of moderate-volume, high-load lower-body resistance training on strength and function in persons with Parkinson's disease: a pilot study. Parkinsons Dis 2010; 2010:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodd K, Taylor N, Shields N, et al. Progressive resistance training did not improve walking but can improve muscle performance, quality of life and fatigue in adults with multiple sclerosis: a randomized controlled trial. Mult Scler 2011; 17:1362–1374. [DOI] [PubMed] [Google Scholar]
- 16.Dalgas U, Stenager E, Jakobsen J, et al. Resistance training improves muscle strength and functional capacity in multiple sclerosis. Neurology 2009; 73:1478–1484. [DOI] [PubMed] [Google Scholar]
- 17.Dalgas U, Stenager E, Jakobsen J, et al. Fatigue, mood and quality of life improve in MS patients after progressive resistance training. Mult Scler 2010; 16:480–490. [DOI] [PubMed] [Google Scholar]
- 18.Dalgas U, Stenager E, Jakobsen J, et al. Muscle fiber size increases following resistance training in multiple sclerosis. Mult Scler 2010; 16:1367–1376. [DOI] [PubMed] [Google Scholar]
- 19.Dalgas U, Stenager E, Lund C, et al. Neural drive increases following resistance training in patients with multiple sclerosis. J Neurol Jul 2013; 260:1822–1832. [DOI] [PubMed] [Google Scholar]
- 20.Nelson ME, Rejeski WJ, Blair SN, et al. Physical activity and public health in older adults: recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 2007; 116:1094. [DOI] [PubMed] [Google Scholar]
- 21.Nelson ME, Fiatarone MA, Morganti CM, et al. Effects of high-intensity strength training on multiple risk factors for osteoporotic fractures: a randomized controlled trial. JAMA 1994; 272:1909–1914. [DOI] [PubMed] [Google Scholar]
- 22.Fiatarone MA, Marks EC, Ryan ND, et al. High-intensity strength training in nonagenarians: effects on skeletal muscle. JAMA 1990; 263:3029–3034. [PubMed] [Google Scholar]
- 23.Pereira A, Izquierdo M, Silva AJ, et al. Effects of high-speed power training on functional capacity and muscle performance in older women. Exp Gerontol 2012; 47:250–255. [DOI] [PubMed] [Google Scholar]
- 24.Mangione KK, Miller AH, Naughton IV. Cochrane review: improving physical function and performance with progressive resistance strength training in older adults. Phys Ther 2010; 90:1711–1715. [DOI] [PubMed] [Google Scholar]
- 25.Cadore EL, Izquierdo M, Pinto SS, et al. Neuromuscular adaptations to concurrent training in the elderly: effects of intrasession exercise sequence. Age 2013; 35:891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Avila JJ, Gutierres JA, Sheehy ME, et al. Effect of moderate intensity resistance training during weight loss on body composition and physical performance in overweight older adults. Eur J Appl Physiol 2010; 109:517–525. [DOI] [PubMed] [Google Scholar]
- 27.Pereira DS, de Queiroz BZ, Miranda AS, et al. Effects of physical exercise on plasma levels of brain-derived neurotrophic factor and depressive symptoms in elderly women: a randomized clinical trial. Arch Phys Med Rehabil 2013; 94:1443–1450. [DOI] [PubMed] [Google Scholar]
- 28.Liu-Ambrose T, Nagamatsu LS, Graf P, et al. Resistance training and executive functions: a 12-month randomized controlled trial. Arch Int Med 2010; 170:170–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cassilhas RC, Viana VA, Grassmann V, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc 2007; 39:1401. [DOI] [PubMed] [Google Scholar]
- 30.Levinger I, Goodman C, Hare DL, et al. The effect of resistance training on functional capacity and quality of life in individuals with high and low numbers of metabolic risk factors. Diabetes Care 2007; 30:2205–2210. [DOI] [PubMed] [Google Scholar]
- 31.Nagamatsu LS, Handy TC, Hsu CL, et al. Resistance training promotes cognitive and functional brain plasticity in seniors with probable mild cognitive impairment. Arch Int Med 2012; 172:666–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouellette MM, LeBrasseur NK, Bean JF, et al. High-intensity resistance training improves muscle strength, self-reported function, and disability in long-term stroke survivors. Stroke 2004; 35:1404–1409. [DOI] [PubMed] [Google Scholar]
- 33.Harris JE, Eng JJ. Strength training improves upper-limb function in individuals with stroke a meta-analysis. Stroke 2010; 41:136–140. [DOI] [PubMed] [Google Scholar]
- 34.Ada L, Dorsch S, Canning CG. Strengthening interventions increase strength and improve activity after stroke: a systematic review. Aust J Physiother 2006; 52:241–248. [DOI] [PubMed] [Google Scholar]
- 35.Lima LO, Scianni A, Rodrigues-de-Paula F. Progressive resistance exercise improves strength and physical performance in people with mild to moderate Parkinson's disease: a systematic review. J Physiother 2013; 59:7–13. [DOI] [PubMed] [Google Scholar]
- 36.Brienesse LA, Emerson MN. Effects of resistance training for people with Parkinson's disease: a systematic review. J Am Med Dir Assoc 2013; 14:236–241. [DOI] [PubMed] [Google Scholar]
- 37.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8:336–341. [DOI] [PubMed] [Google Scholar]
- 38.Maher CG, Sherrington C, Herbert RD, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 2003; 83:713–721. [PubMed] [Google Scholar]
- 39.Sabapathy NM, Minahan CL, Turner GT, et al. Comparing endurance-and resistance-exercise training in people with multiple sclerosis: a randomized pilot study. Clin Rehabil 2011; 25:14–24. [DOI] [PubMed] [Google Scholar]
- 40.Hass CJ, Buckley TA, Pitsikoulis C, et al. Progressive resistance training improves gait initiation in individuals with Parkinson's disease. Gait Posture 2012; 35:669–673. [DOI] [PubMed] [Google Scholar]
- 41.Fimland MS, Helgerud J, Gruber M, et al. Enhanced neural drive after maximal strength training in multiple sclerosis patients. Eur J Appl Physiol 2010; 110:435–443. [DOI] [PubMed] [Google Scholar]
- 42.Dibble LE, Hale TF, Marcus RL, et al. High intensity eccentric resistance training decreases bradykinesia and improves quality of life in persons with Parkinson's disease: a preliminary study. Parkinsonism Relat Disord 2009; 15:752–757. [DOI] [PubMed] [Google Scholar]
- 43.Dibble LE, Hale TF, Marcus RL, et al. High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson's disease. Mov Disord 2006; 21:1444–1452. [DOI] [PubMed] [Google Scholar]
- 44.Corcos DM, Robichaud JA, David FJ, et al. A two-year randomized controlled trial of progressive resistance exercise for Parkinson's disease. Mov Disord 2013; 28:1230–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Medina-Perez C, de Souza-Teixeira F, Fernandez-Gonzalo R, et al. Effects of a resistance training program and subsequent detraining on muscle strength and muscle power in multiple sclerosis patients. NeuroRehabilitation 2014; 34:523–553. [DOI] [PubMed] [Google Scholar]
- 46.Prodoehl J, Rafferty MR, David FJ, et al. Two-year exercise program improves physical function in Parkinson's disease the PRET-PD randomized clinical trial. Neurorehabil Neural Repair 2015; 29:112–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paul SS, Canning CG, Song J, et al. Leg muscle power is enhanced by training in people with Parkinson's disease: a randomized controlled trial. Clin Rehabil 2014; 28:275–288. [DOI] [PubMed] [Google Scholar]
- 48.Sage MD, Johnston RE, Almeida QJ. Comparison of exercise strategies for motor symptom improvement in Parkinson's disease. Neurodegener Dis Manag 2011; 1:387–395. [Google Scholar]
- 49.Bloomer RJ, Schilling BK, Karlage RE, et al. Effect of resistance training on blood oxidative stress in Parkinson disease. Med Sci Sports Exerc 2008; 40:1385–1389. [DOI] [PubMed] [Google Scholar]
- 50.Broekmans T, Roelants M, Feys P, et al. Effects of long-term resistance training and simultaneous electro-stimulation on muscle strength and functional mobility in multiple sclerosis. Mult Scler 2011; 17:468–477. [DOI] [PubMed] [Google Scholar]
- 51.DeBolt LS, McCubbin JA. The effects of home-based resistance exercise on balance, power, and mobility in adults with multiple sclerosis. Arch Phys Med Rehabil 2004; 85:290–297. [DOI] [PubMed] [Google Scholar]
- 52.de Haan A, de Ruiter CJ, van der Woude LH, et al. Contractile properties and fatigue of quadriceps muscles in multiple sclerosis. Muscle Nerve 2000; 23:1534–1541. [DOI] [PubMed] [Google Scholar]
- 53.Ng A, Miller R, Gelinas D, et al. Functional relationships of central and peripheral muscle alterations in multiple sclerosis. Muscle Nerve 2004; 29:843–852. [DOI] [PubMed] [Google Scholar]
- 54.Kent-Braun J, Ng A, Castro M, et al. Strength, skeletal muscle composition, and enzyme activity in multiple sclerosis. J Appl Physiol 1997; 83:1998–2004. [DOI] [PubMed] [Google Scholar]
- 55.Garner DJ, Widrick JJ. Cross-bridge mechanisms of muscle weakness in multiple sclerosis. Muscle Nerve 2003; 27:456–464. [DOI] [PubMed] [Google Scholar]
- 56.Carroll CC, Gallagher PM, Seidle ME, et al. Skeletal muscle characteristics of people with multiple sclerosis. Arch Phys Med Rehabil 2005; 86:224–229. [DOI] [PubMed] [Google Scholar]
- 57.Bridgewater KJ, Sharpe MH. Trunk muscle performance in early Parkinson's disease. Phys Ther 1998; 78:566–576. [DOI] [PubMed] [Google Scholar]
- 58.Corcos DM, Chen CM, Quinn NP, et al. Strength in Parkinson's disease: relationship to rate of force generation and clinical status. Ann Neurol 1996; 39:79–88. [DOI] [PubMed] [Google Scholar]
- 59.Yanagawa S, Shindo M, Yanagisawa N. Muscular weakness in Parkinson's disease. Adv Neurol 1989; 53:259–269. [PubMed] [Google Scholar]
- 60.Allen N, Sherrington C, Canning C, et al. Reduced muscle power is associated with slower walking velocity and falls in people with Parkinson's disease. Parkinsonism Relat Disord 2010; 16:261–264. [DOI] [PubMed] [Google Scholar]
- 61.Paul SS, Sherrington C, Fung VS, et al. Motor and cognitive impairments in Parkinson disease: relationships with specific balance and mobility tasks. Neurorehabil Neural Repair 2013; 27:63–71. [DOI] [PubMed] [Google Scholar]
- 62.Broekmans T, Gijbels D, Eijnde BO, et al. The relationship between upper leg muscle strength and walking capacity in persons with multiple sclerosis. Mult Scler 2013; 19:112–121. [DOI] [PubMed] [Google Scholar]
- 63.Dai TH, Liu JZ, Sahgal V, et al. Relationship between muscle output and functional MRI-measured brain activation. Exp Brain Res 2001; 140:290–300. [DOI] [PubMed] [Google Scholar]
- 64.Dettmers C, Fink GR, Lemon RN, et al. Relation between cerebral activity and force in the motor areas of the human brain. J Neurophysiol 1995; 74:802–815. [DOI] [PubMed] [Google Scholar]
- 65.Florin E, Dafsari H, Reck C, et al. Modulation of local field potential power of the subthalamic nucleus during isometric force generation in patients with Parkinson's disease. Neuroscience 2013; 240:106–116. [DOI] [PubMed] [Google Scholar]
- 66.Ehrsson HH, Fagergren A, Jonsson T, et al. Cortical activity in precision-versus power-grip tasks: an fMRI study. J Neurophysiol 2000; 83:528–536. [DOI] [PubMed] [Google Scholar]
- 67.Sehm B, Taubert M, Conde V, et al. Structural brain plasticity in Parkinson's disease induced by balance training. Neurobiol Aging 2014; 35:232–239. [DOI] [PubMed] [Google Scholar]
- 68.Prosperini L, Fanelli F, Petsas N, et al. Multiple sclerosis: changes in microarchitecture of white matter tracts after training with a video game balance board. Radiology 2014; 273:529–538. [DOI] [PubMed] [Google Scholar]
- 69.Bonzano L, Tacchino A, Brichetto G, et al. Upper limb motor rehabilitation impacts white matter microstructure in multiple sclerosis. NeuroImage 2014; 90:107–116. [DOI] [PubMed] [Google Scholar]
- 70.Burciu RG, Fritsche N, Granert O, et al. Brain changes associated with postural training in patients with cerebellar degeneration: a voxel-based morphometry study. J Neurosci 2013; 33:4594–4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Berger JR, Pocoski J, Preblick R, et al. Fatigue heralding multiple sclerosis. Mult Scler 2013; 19:1526–1532. [DOI] [PubMed] [Google Scholar]
- 72.Comi G, Leocani L, Rossi P, et al. Physiopathology and treatment of fatigue in multiple sclerosis. J Neurol 2001; 248:174–179. [DOI] [PubMed] [Google Scholar]
- 73.Freal J, Kraft G, Coryell J. Symptomatic fatigue in multiple sclerosis. Arch Phys Med Rehabil 1984; 65:135–138. [PubMed] [Google Scholar]
- 74.Andreasen A, Stenager E, Dalgas U. The effect of exercise therapy on fatigue in multiple sclerosis. Mult Scler 2011; 17:1041–1054. [DOI] [PubMed] [Google Scholar]
- 75.Amato M, Ponziani G, Rossi F, et al. Quality of life in multiple sclerosis: the impact of depression, fatigue and disability. Mult Scler 2001; 7:340–344. [DOI] [PubMed] [Google Scholar]
- 76.Kargarfard M, Eetemadifar M, Mehrabi M, et al. Fatigue depression and health-related quality of life in patients with multiple sclerosis in Isfahan, Iran. Eur J Neurol 2012; 19:431–437. [DOI] [PubMed] [Google Scholar]
- 77.Broekmans T, Gijbels D, Eijnde BO, et al. The relationship between upper leg muscle strength and walking capacity in persons with multiple sclerosis. Mult Scler 2013; 19:112–119. [DOI] [PubMed] [Google Scholar]
- 78.Motl R, Balantrapu S, Pilutti L, et al. Symptomatic correlates of six-minute walk performance in persons with multiple sclerosis. Eur J Phys Rehabil Med 2013; 49:59–66. [PubMed] [Google Scholar]
- 79.Foley NC, Bhogal SK, Teasell RW, et al. Estimates of quality and reliability with the physiotherapy evidence-based database scale to assess the methodology of randomized controlled trials of pharmacological and nonpharmacological interventions. Phys Ther 2006; 86:817–824. [PubMed] [Google Scholar]
- 80.Dalgas U, Stenager E, Ingemann-Hansen T. Multiple sclerosis and physical exercise: recommendations for the application of resistance-, endurance-and combined training. Mult Scler 2007; 14:35–53. [DOI] [PubMed] [Google Scholar]
- 81.Medicine ACoS. ACSM's guidelines for exercise testing and prescription. Baltimore, USA: Lippincott Williams & Wilkins; 2013. [Google Scholar]


