Supplemental Digital Content is available in the text
Abstract
Olfactory outcomes as well as oronasal postoperative complications of transsphenoidal pituitary surgery have not been well studied. The objective of this study was to investigate nasal symptoms including olfactory function as well as quality of life following transsphenoidal pituitary surgery.
The study is designed as a prospective cohort study set in a single tertiary hospital.
A total of 53 patients with pituitary adenomas were included.
All patients underwent pituitary surgery with the right-sided endonasal transsphenoidal approach.
Outcomes were assessed with the Chinese version of the Medical Outcomes Study Short Form-36 (SF-36) to survey patient health, the Chinese version of the 22-item Sinonasal Outcome Test (SNOT-22), and a Toyota and Takagi (T&T) olfactometer. Assessments were carried out before surgery and at 1 week, and 1 and 4 months after surgery.
The overall SF-36 scores were significantly lower, but the SNOT-22 scores were higher at 1 week and 1 month postoperatively compared with baseline (all P < 0.001). The results of T&T olfactometer testing showed that there was a significant decline in the ability to detect odors postoperatively, even at 4 months. Multivariate linear regression analysis showed that lower education level, partial tumor removal, and longer duration of surgery were independent risk factors for a higher SNOT-22 score at 1 week after surgery.
The findings show that microscopic endonasal transsphenoidal pituitary surgery impairs olfactory function in most patients for at least 4 months after surgery.
INTRODUCTION
Pituitary adenoma is a common typically benign intracranial tumor that can cause symptoms because of hormonal dysfunction or mass effect.1 Surgical resection is the most effective treatment. The traditional approach has been the microscopic endonasal transsphenoidal approach; however, in recent years, there has been a shift to the endoscopic endonasal transsphenoidal approach.2–5 Because the surgical procedure results in varying degrees of destruction of the nasal structure, some nasal symptoms may occur postoperatively. Kilty et al6 reported prolonged nasal crusting in 10.4% and septal deviation in 3.7% of patients who underwent endoscopic transsphenoidal sellar surgery, and Petry et al7 found that oronasal postoperative complications were common in patients who had transsphenoidal surgery, in most cases by the sublabial approach. Kim et al8 found that visual analogue scale scores for nasal obstruction, sneezing, rhinorrhea, snoring, or facial pain increased significantly following surgery. Hummel and Nordin9 reviewed the literature on olfactory disorders and concluded that the data suggest that in a large proportion of patients with olfactory disorders, quality of life (QOL) as well as eating habits and nutritional intake are severely altered.
Changes in olfactory function have been reported after several types of nonpituitary nasal surgery. Min et al10 reported results suggesting that functional endoscopic surgery in patients with chronic parasinal sinusitis may significantly improve impairment of olfaction and mucociliary transport. Lee et al11 carried out endoscopic sinus surgery on patients with persistent anosmia and reported 2 patterns of histological findings in the olfactory mucosa of these patients. The findings suggested that in patients with chronic sinusitis there can be degeneration of the olfactory epithelium and that abnormalities of the olfactory epithelium may account for failure of the olfactory deficit to improve following sinus surgery. Kern12 studied pathological changes in the olfactory mucosa of chronic sinusitis patients with anosmia and concluded that inflammatory changes in the olfactory mucosa may play a role in the olfactory deficits. Soler et al13 performed endoscopic sinus surgery on patients with chronic rhinosinusitis with olfactory loss and found that specific histological inflammatory findings were not predictive of postoperative olfactory improvement. Shemshadi et al14 carried out a study to determine if patients who undergo open rhinoplasty have olfactory dysfunction after surgery. They found that such patients may have some postoperative olfactory dysfunction and require from 6 weeks to 6 months to attain full recovery of their baseline olfactory function. However, there have been only a few studies that have attempted to evaluate olfactory function after endoscopic transsphenoidal surgery, and the results have been conflicting with some studies showing profound impairment and others showing no loss or only minimal loss of function.15–18 Moreover, only a few studies have addressed QOL in patients treated surgically for pituitary adenomas.19 Karabatsou et al19 found that there was only minimal impairment of QOL in patients who underwent endoscopic surgery for pituitary adenomas. Tanemura et al20 studied patients treated surgically for nonfunctioning pituitary macroadenoma and reported that surgical treatment affected QOL physically and mentally.
Because so few studies have assessed nasal symptoms after microscopic endonasal transsphenoidal surgery, the aim of this study was to investigate the surgical outcome of this type of operation, focusing particularly on olfactory function and QOL.
MATERIALS AND METHODS
Patients
Fifty-three patients with pituitary adenomas who underwent surgical treatment in the neurosurgery department of our hospital from March 2012 to January 2013 were included in this study. Medical history was obtained before surgery. Data were recorded in detail on residence, education, work environment (indoor or outdoor), alcohol addiction, history of nasal disease, and history of nasal medication.
The inclusion criteria were no previous history of endonasal transsphenoidal surgery, able to undergo preoperative and postoperative imaging examinations, able to complete postoperative scale evaluation, and underwent endonasal transsphenoidal tumor removal after admission.
Patients were excluded if they underwent secondary endonasal transsphenoidal surgery within 4 months after the primary surgery, or underwent tumor resection by craniotomy.
Informed consent and a confidentiality agreement were obtained from the patients. The study was approved in advance by the Medical Ethics Committee of Fuzhou General Hospital, Fujian Medical University.
Imaging
All patients underwent imaging examinations before and after surgery. Imaging was carried out with a Siemens 3.0T magnetic resonance imaging (MRI) scanner (Tim Trio; Siemens Medical Solutions, Erlangen, Germany). Unenhanced and enhanced T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI) axial, coronal, and sagittal images were obtained under spin-echo sequences. Preoperative scanning was done with an enhanced coronal 3D gradient echo sequence; the parameters were repetition time/echo time = 7/2 ms, field of view = 180 × 180 mm, matrix = 448 × 305, and slice thickness = 0.8 mm. There was no interval between successive scans. Video laryngoscopy using a Pentax-EPM-3500 video system (Robin Medical Ltd, Coulsdon, England) was performed 1 week and 4 months after surgery. Computed tomography using a GE-256-slice CT scanner was performed 1 week and 4 months after surgery to obtain coronal and axial images of the nasal cavity and paranasal sinuses.
Image Analysis
The preoperative imaging data were analyzed in detail. The main types of data were tumor size, presence of tumor invasion of the cavernous sinus, sphenoid sinus, and so on; presence of fluid collection, inflammation, and so on in the postoperative nasal and paranasal sinuses on CT; presence of blood clot formation, erosion of the nasal mucosa, nasal adhesion, nasal septum perforation, and so on on postoperative video laryngoscopy.
Surgical Procedure and Standard of Identification
Surgical Procedure
Tumor resection was performed under general anesthesia. The patient was placed in supine position with 15° to 20° of neck extension. The right-sided nostril-nasal septum-transsphenoidal approach21 was used in all operations. Under the microscope, cotton slivers moistened with saline containing 0.01% norepinephrine were placed into bilateral nasal cavities for 3 minutes to shrink blood vessels in the nasal mucosa and expand the nasal cavity. A straight incision was made in the middle of the nasal septum (3 cm posterior to the anterior nostril), leaving the superior turbinate intact, and the bony part of the nasal septum was pushed away. This incision was vertical to the surgical approach, and was not a flap from the nasal septum. The cartilage and bone comprising the septum were not removed. The anterior wall of the sphenoid sinus was reached between the right mucoperiosteum and the perpendicular plate of the ethmoid bone. The perpendicular plate of ethmoid at the anterior wall of the sphenoid sinus was broken off and pushed to the left side (at the end of surgery it was repositioned in the midline). In patients with a relatively large middle turbinate, it was broken off and pushed to the lateral side. After the sellar floor was reached by opening the anterior wall of the sphenoid sinus and removing the bone of sella turcica, the dura mater was cut open in an “X” shape. The tumor was removed in pieces by using ring curettes, tumor-grasping forceps, and suction devices. The posterior part of the tumor was removed first, bilateral sides were then removed, and the anterosuperior part was removed last. A gelatin sponge was used to fill the tumor cavity that was left after collapse of the sellar diaphragm. After surgery, the bilateral nasal cavities were filled with Vaseline Gauze (Huxi Medical Materials Co., Ltd, Xinxiang,Henan, China). The tumor tissue specimen was sent to the pathology department of our hospital to confirm the immunohistochemistry-based tumor classification.
Indicators of Intraoperative Observation
The duration of intraoperative application of the nasal dilator; the tumor color, texture and blood supply; the boundary between the tumor and its peripheral tissues and the status of adhesion between them; the presence of local tumor invasion (including integrity of the bone and dura mater of the sellar floor, bilateral cavernous sinuses, etc); and the shape and status of subsidence of the sellar diaphragm were observed during surgery. The rate of tumor resection and the incidence of cerebrospinal fluid leakage were identified.
Intraoperative Identification of Invasiveness of Pituitary Adenoma
Based on the modified standard by Wolfsberger et al22 and the classification Knosp et al23, tumor invasiveness was defined when one of following indicators were found during surgery: defects in the medial wall of the cavernous sinus and tumor cells in the tissue scraped from the cavernous sinus; the tumor invasion of the sphenoid sinus and the bone and/or dura mater of the sellar floor were incomplete; the tumor invading the clivus and the clival bone was absorbed, destroyed, or uneven.
36-Item Medical Outcomes Study Short Form Evaluation
Short form-36 (SF-36) is a concise health questionnaire. In the current study, we used the Chinese version of the SF-36, which has been validated. It was authorized by the New England Medical Center in Boston and developed by the Sun Yat-sen College of Medical Science. A score of 100 points represents a perfect health status, and the health status is often shown by standardized scoring of each dimension.23,24
22-Item Sinonasal Outcome Test evaluation
The 22-item Sinonasal Outcome Test (SNOT-22) questionnaire was administered before surgery and 1 week, 1 month, and 4 months after surgery. The SNOT-22 is a disease-specific scale produced by the Royal College of Surgeons of England.25 We used the Chinese version modified by the Chinese Academy of Medical Sciences, which has been validated.26 Subjects are required to select scores in 22 items and then further choose the 5 most important problems (called “five big problems”) affecting health. The higher the score (0–5), the poorer is the health status.
T&T Olfactometer
The Toyota and Takagi (T&T) olfactometer (Daiichi Pharmaceutical Co, Ltd, Tokyo, Japan) can identify olfactory detection and each corresponding recognition threshold. The criteria for odor selection were as follows: each odor can be separated from other odors, the odor is simple and easily detected by most people, the intensity and nature of the odor are stable, and the olfactory components are simple and easy to produce. Ten candidate odorants were selected based on these criteria, and a 10-fold dilution was carried out for each odorant. Concentrations ranged from 10–1 M to 10–17 M. Each odorant is evaluated at 8 levels (−2 to 5): 0 is the normal olfactory threshold concentration, 5 is the highest, and −2 is the lowest. The olfactory function is divided into 6 grades according to the obtained recognition threshold values: mean value <−1 is defined as hyperosmia, −1 to 1 is normal olfaction, 1.1 to 2.5 is mild hyposmia, 2.6 to 4.0 is moderate hyposmia, 4.1 to 5.5 is severe hyposmia, and >5.6 is anosmia.
The test was carried out with low-concentration odorants to high-concentration odorants sequentially (from −2 to 4 or 5). When a patient can detect the odor, this concentration is the detection threshold.17,18 When a patient can identify what kind of odor it is, then this concentration is the recognition threshold17,18 Because the current study only needed to record the recognition threshold of the patients, both nostrils were tested simultaneously.
Investigation Method
Patients during hospitalization were evaluated before surgery and 1 week after surgery, and after discharge were recalled to the hospital 1 month and 4 months after surgery for sinus CT examination, SF-36 and SNOT-22 evaluation, and the T&T olfactometer test. Evaluations were carried out in person in most instances; the telephone was used when necessary. All evaluation data were recorded and verified by 2 staff members.
Statistical Analysis
Continuous variables were presented as the mean and standard deviation (SD). Categorical variables were presented by count and percentage, and the differences between categorical variables were analyzed by the χ2 test or Fisher exact test with Yate correction if any cell number was <5 or close to 0. A repeated measure analysis of variance (ANOVA) was applied to compare the means within patients for medical outcomes of the SF-36 and SNOT-22 among 4 different time points: preoperative, 1 week postoperative, 1 month postoperative, and 4 months postoperative. For repeated measures, sphericity was confirmed by using the Mauchly sphericity test and the Greenhouse Greiser correction was applied if the assumption of sphericity had been violated. The Bonferroni test was performed to compare multiple post hoc testing.
Linear regression was used to analyze any association between changed scores of SF-36 and SNOT and independent variables. The variance inflation factor (VIF) test was performed to verify the severity of multicollinearity within the multiple linear regression models. Logistic regression was used to analyze any association between changed category of the T&T olfactometer test and independent variables. The changed scores for SF-36 and SNOT were calculated as score at 1 week postoperatively minus the score preoperatively. The changed category for the T&T test was applied between before surgery and 1 week after surgery. We defined “large” change as the change from normal before surgery to loss of smell at 1 week after surgery. All other category changes were defined as “small” change.
All statistical assessments were 2-sided and evaluated at the 0.05 level of significant difference. Statistical analyses were performed with the SPSS software for Windows, version 18.0 (SPSS Inc, Chicago, IL).
RESULTS
The study included 53 patients, 20 (37.7%) men and 33 (62.3%) women. Tables 1 and 2 summarize the basic demographic and clinical characteristics, respectively.
TABLE 1.
Patient Demographic at Baseline (n = 53)

TABLE 2.
Patient Clinical Characteristics at Baseline (n = 53)
Figure 1 shows the overall change of the SF-36 score and the change in score for each of the 8 subcategories. The overall SF-36 score was significantly different among different time points (P < 0.001, Figure 1 A), and at 1 week (553 ± 88) and 1 month (574 ± 83) postoperatively the overall scores were significantly lower than that at baseline (655 ± 65) (both P < 0.001).
FIGURE 1.
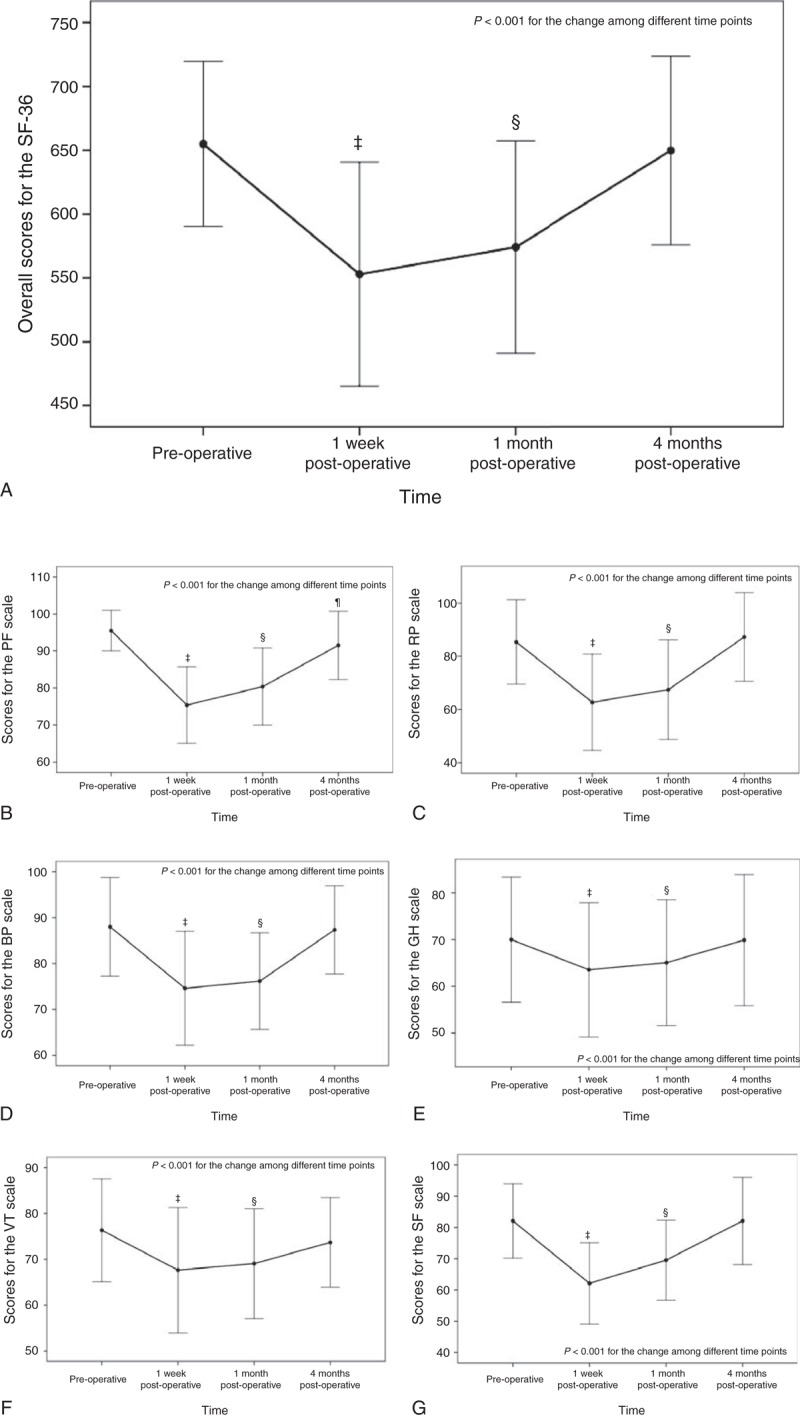
Results of the SF-36 for (A) overall, (B) PF, (C) RP, (D) BP, (E) GH, (F) VT, (G) SF, (H) RE, and (I) MH. ‡ Indicates significant difference between preoperative and 1 week postoperative using the post hoc Bonferroni test; § indicates a significant difference between preoperative and 1 month postoperative using the post hoc Bonferroni test; ¶ indicates a significant difference between preoperative and 4 months postoperative using the post hoc Bonferroni test. BP = bodily pain, GH = general health, MH = mental health, PF = physical functioning, RE = role-emotional, RP = role-physical, SF = social functioning, SF-36 = medical outcomes study short form-36, VT = vitality.
Figure 2 presents the pre- and postoperative SNOT-22 scores. The SNOT-22 score was significantly different among different time points (P < 0.001), and at 1 week (22.1 ± 6.7) and 1 month (15.5 ± 5.2) postoperatively the scores were significantly higher than at baseline (9.5 ± 4.4) (both P < 0.001).
FIGURE 1 (Continued).

Results of the SF-36 for (A) overall, (B) PF, (C) RP, (D) BP, (E) GH, (F) VT, (G) SF, (H) RE, and (I) MH. ‡ Indicates significant difference between preoperative and 1 week postoperative using the post hoc Bonferroni test; § indicates a significant difference between preoperative and 1 month postoperative using the post hoc Bonferroni test; ¶ indicates a significant difference between preoperative and 4 months postoperative using the post hoc Bonferroni test. BP = bodily pain, GH = general health, MH = mental health, PF = physical functioning, RE = role-emotional, RP = role-physical, SF = social functioning, SF-36 = medical outcomes study short form-36, VT = vitality.
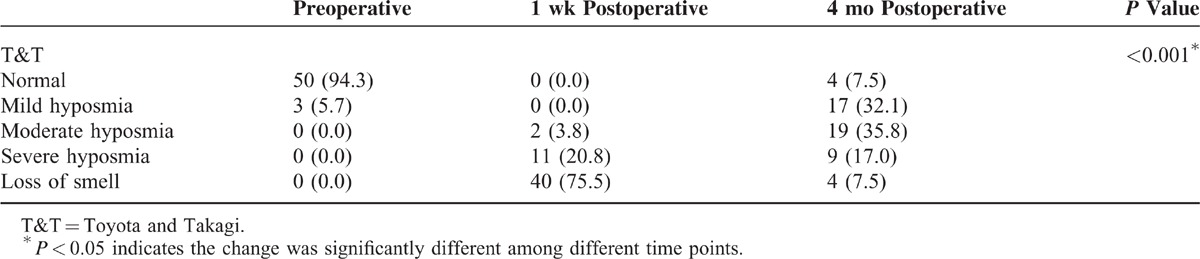
The results of the T&T olfactometer test showed that preoperatively 50 (94.3%) patients were normal and only 3 (5.7%) had mild hyposmia (Table 3). However, the test results at 1 week postoperatively showed that about three-quarters of the patients had loss of smell. The test results at 4 months postoperatively showed that one-quarter of the patients had severe hyposmia or loss of smell and 35.8% had moderate hyposmia. Four patients had complete anosmia at 4 months after surgery. Three of these patients had invasive pituitary adenomas and could not have total tumor removal. Also, for these 3 patients, the time of intraoperative application of the nasal dilation device was relatively long and exceeded the mean nasal dilation time. In addition, these 3 patients were relatively older (age range, 45–57 years) and 2 of them had low educational levels. Impairment of olfactory function of these 3 patients appears to be permanent.
TABLE 3.
Results of the T&T Olfactometer Test
The univariate and multivariate linear regression for changed scores of the SF-36 between before surgery and 1 week after surgery are shown in Table 4. No significant difference was found between demographics and changed scores of the SF-36 between before and 1 week after surgery. The results of univariate and multivariate linear regression for the SNOT-22 change in score between before surgery and 1 week after surgery are shown in Table 5. A significantly greater change in score between before surgery and 1 week after surgery was found for patients with a lower education level (P = 0.017), patients who received partial tumor resection (P = 0.026) and patients who had longer operation times (P < 0.001). Table 6 shows that the results of univariate and multivariate logistic regression for change in score of the T&T olfactometer test between before surgery and 1 week after surgery. No significant association was found between any demographic or clinical variable and change in score on the T&T olfactometer test between before surgery and 1 week after surgery.
TABLE 4.
Results of Univariate and Multivariate Linear Regression for Changes in the SF-36 Scores From Before Surgery to 1 Week After Surgery
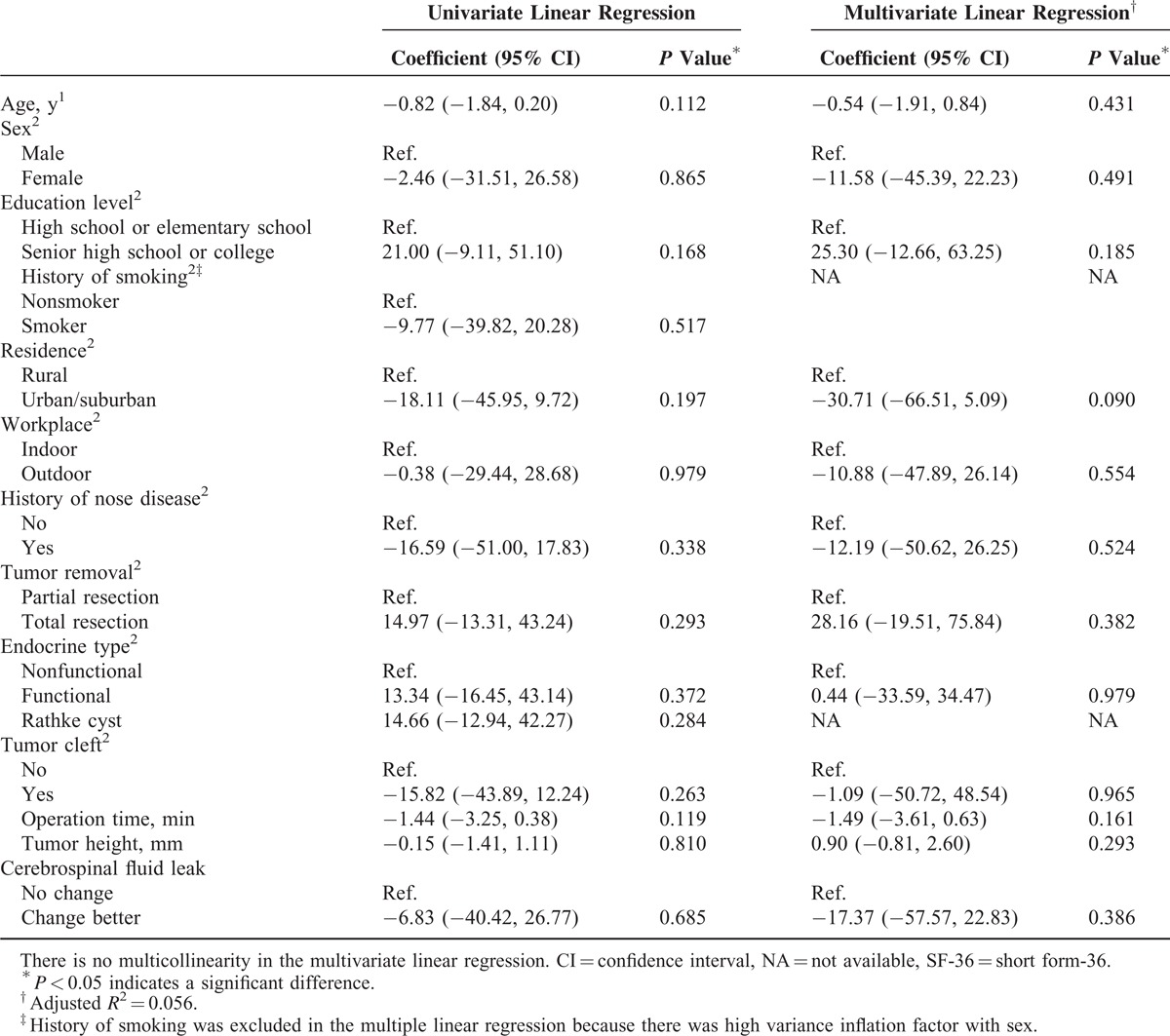
TABLE 5.
Results of Univariate and Multivariate Linear Regression for Changes in the SNOT-22 Scores From Before Surgery to 1 Week After Surgery
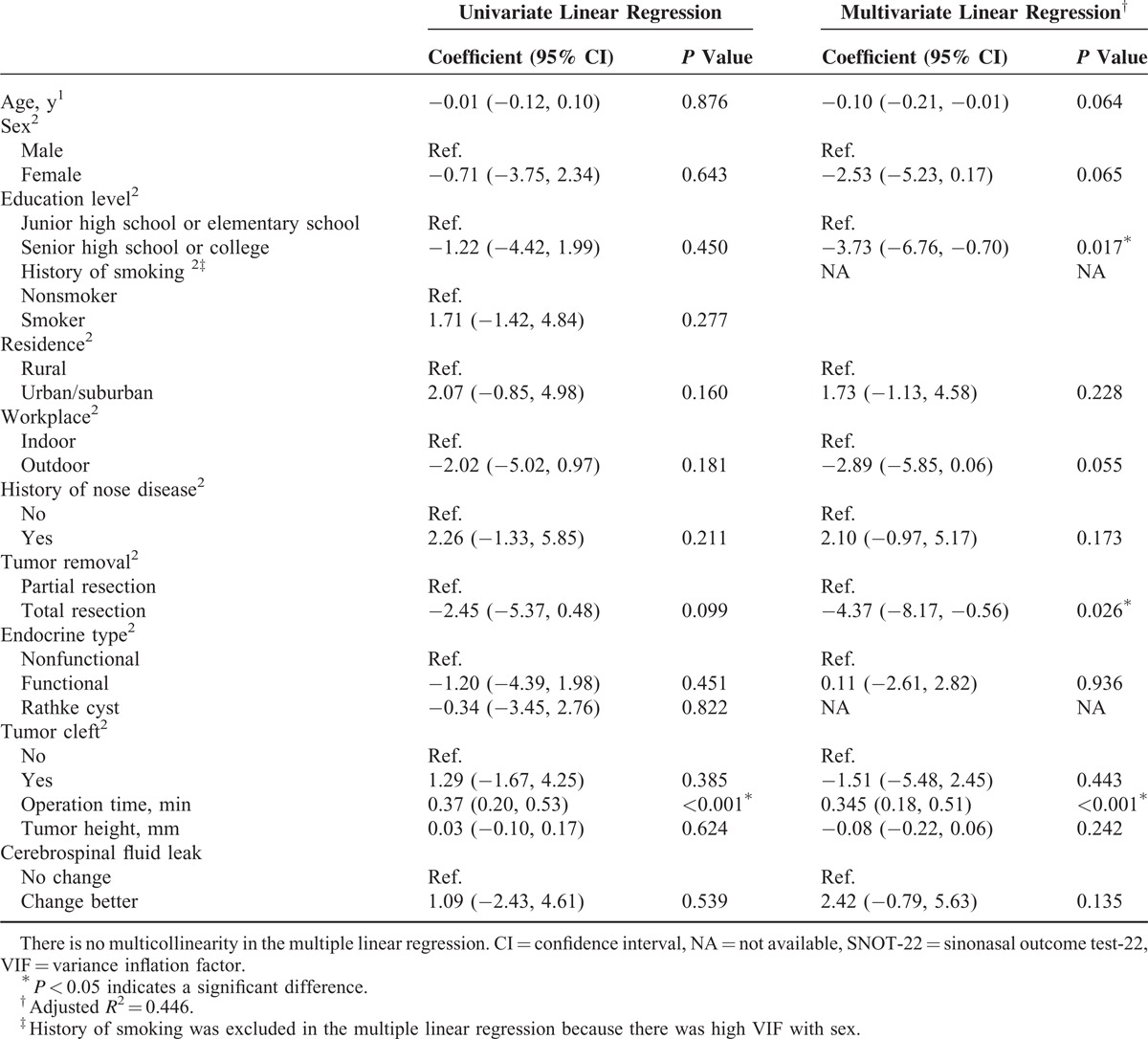
TABLE 6.
Results of Univariate and Multivariate Logistic Regression for Changes in Category of the T&T Olfactometer Test From Before Surgery to 1 Week After Surgery
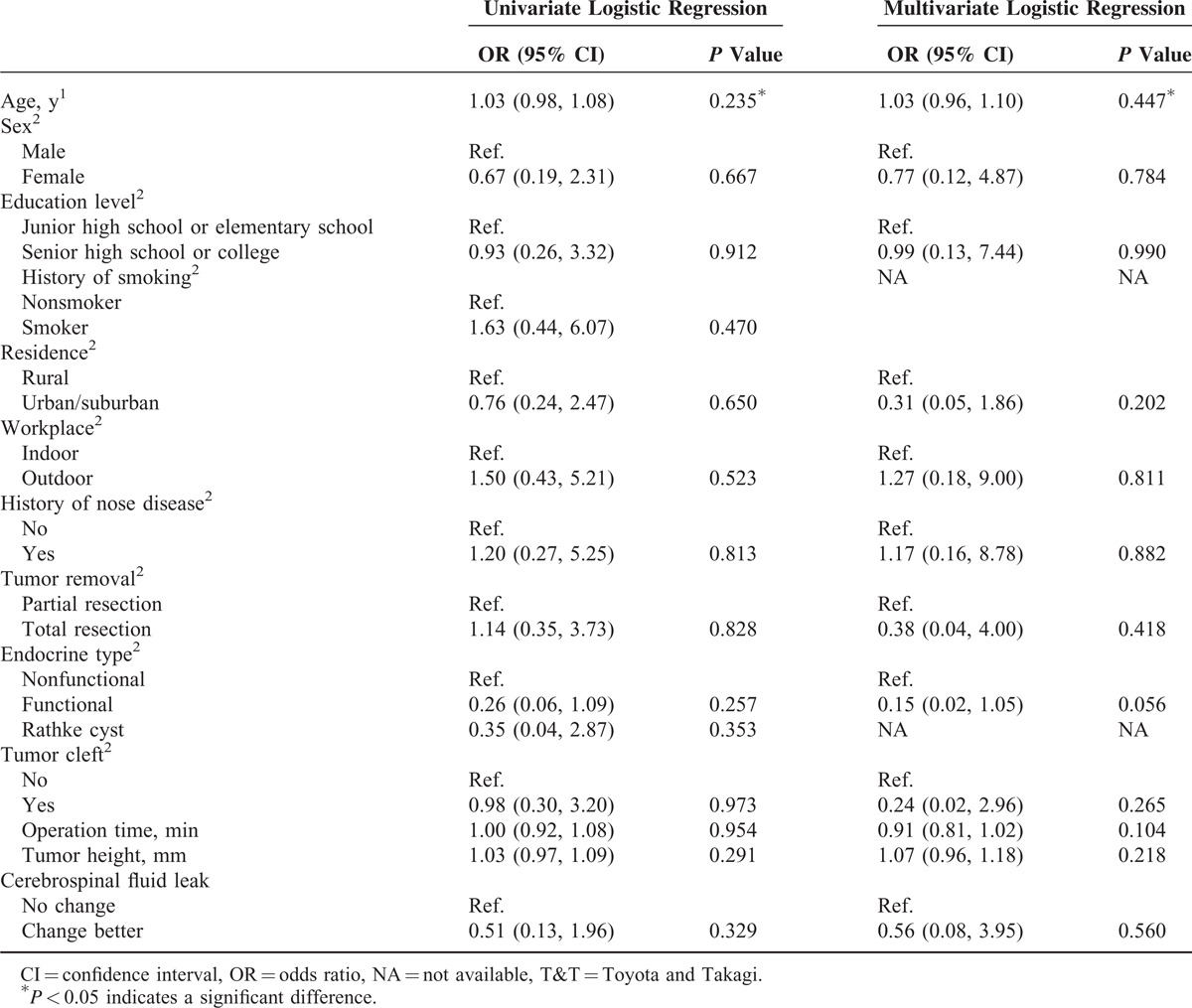
Table 7 shows neurological complications. Thirty percent of participants had no neurological complications. Over half of the participants (56.6%) had transient diabetes insipidus, 24.5% of the participants had brief headache, and 13.2% of the participants had transient dizziness. Pre- and postoperative hormone level changes are presented in the supplement http://links.lww.com/MD/A176.
TABLE 7.
Neurological Complications

An exemplary case
Figure 3 shows images obtained from a 52-year-old female patient. The patient had mild hyposmia before surgery. One week after surgery the patient had anosmia. Nasal endoscopy showed nasal cavity adhesion and blood clot attachment, and CT showed fluid collection in the nasal sinuses. Four months after surgery, the recovery of the nasal mucosa was excellent, absorption of the fluid in the nasal sinuses was incomplete, the recovery of olfactory function was poor, and the patient had severe hyposmia.
FIGURE 2.
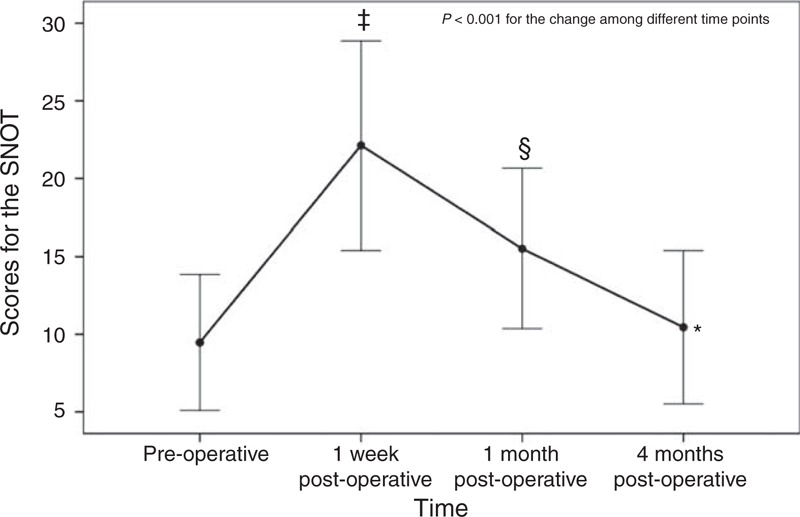
Results of the SNOT-22. ‡ Indicates a significant difference between preoperative and 1 week postoperative using the post hoc Bonferroni test; § indicates a significant difference between preoperative and 1 month postoperative using the post hoc Bonferroni test. SNOT-22 = sinonasal outcome test-22.
FIGURE 3.
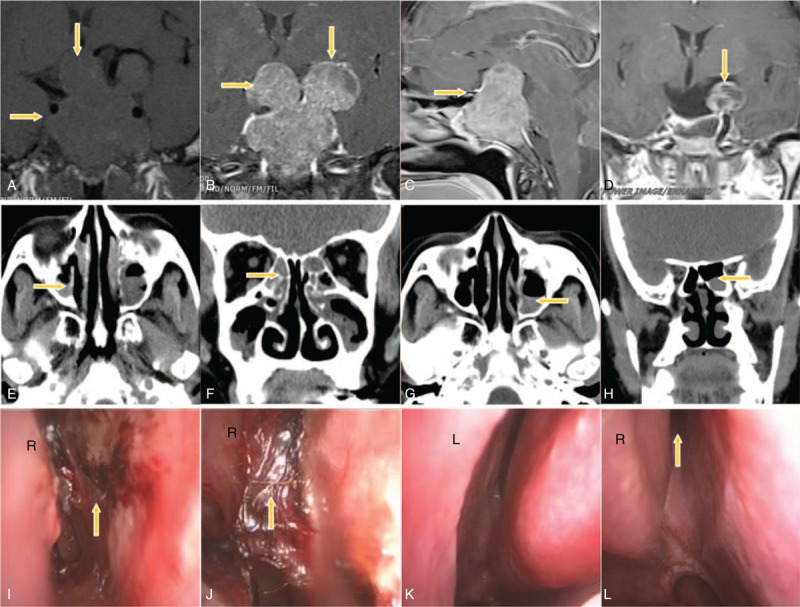
Exemplary case of a 52-year-old female patient. (A) A preoperative coronal T1WI image displays a lesion occupying the saddle area (arrow). (B) A coronal enhanced image shows that the tumor has a lobulated growth pattern and breaks through the sellar diaphragm, enters the suprasellar region, compresses the optic chiasm, squeezes the lateral ventricle, breaks through the sellar floor inferiorly, and invades the sphenoid sinus. (C) A sagittal-enhanced image shows heterogeneous enhancement of the tumor. (D) An MRI scan obtained 3 days after surgery shows partial remnants of the tumor. (E and F) CT images obtained 1 week after surgery show fluid collection in the maxillary sinus and the ethmoid sinus. (G and H) CT images obtained 4 months after surgery show incomplete absorption of fluid in the left maxillary sinus and the left sphenoid sinus. (I) Video laryngoscopy performed 1 week after surgery shows superficial erosions in the right nasal septum. (J) Bloody secretions can be observed at the medial side of the rear end of the right middle turbinate and the mucosa of the right nasal septum. (K) Video laryngoscopy performed 4 months after surgery shows excellent healing of the nasal mucosa. (L) Drainage of the region around the sphenoidal sinus ostium into the right nasal cavity is unobstructed, and the healing of the nasal mucosa is excellent.
DISCUSSION
In this study, the postoperative nasal symptoms of 53 patients who underwent microscopic endonasal transsphenoidal surgery for pituitary adenoma were fully evaluated. A significant decline in the overall SF-36 score at 1 week and 1 month after surgery, a significant increase in the SNOT-22 score at 1 week and 1 month after surgery, and all but 4 patients having mild, moderate, or severe hyposmia or loss of smell at 4 months after surgery were observed. Our results show that endonasal transsphenoidal surgery results in impaired olfactory function at least 4 months after surgery, which was the end of our follow-up period. Since olfactory function is very important for enjoying food, sensing danger, and so on, our findings should be taken into consideration in decision making when patients need surgical treatment for pituitary adenoma.
Olfactory groove obstruction, olfactory mucosal atrophy, fractures in the anterior cranial fossa, or diseases involving the olfactory pathway may all lead to decrease or lack of olfactory sensation. Rotenberg et al15 studied the olfactory function of 17 patients with pituitary adenomas before surgery and 6 months after surgery using the University of Pennsylvania Smell Identification Test (UPSIT) and found that postoperatively 9 patients had mild hyposmia, 5 had moderate hyposmia, and 1 had severe hyposmia; only 2 patients had normal olfactory function. Statistically significant recovery of olfaction was observed 7 months after surgery compared with 1 week after surgery. However, olfaction did not completely return to the preoperative level. Kahilogullari et al16 found that only 2 of the 25 patients who underwent endoscopic transsphenoidal pituitary surgery had hyposmia postoperatively based on the Smell Diskettes Olfaction Test. Hart et al17 also used the UPSIT and found that at 3 months after surgery, there was no significant difference between preoperative and postoperative UPSIT scores and concluded that the surgery had no effect on olfaction. Also, Kim et al18 who used the butanol threshold test and cross-cultural identification test, found that only 1 of the 15 patients had olfactory dysfunction 6 months after surgery. Our results are consistent with those of Rotenberg15. Kahilogullari et al, Hart et al, and Kim et al found in contrast that this type of surgery had little or no effect on olfactory function. The differences in the results could be attributable to the use of different tests for olfactory function, although both Rotenberg and Hart et al used the UPSIT. An advantage of our study is that we performed relatively objective evaluation of olfaction by using T&T olfactometry. There have been no previous reports in which postoperative olfaction after endonasal transsphenoidal surgery was evaluated using this method.
The transsphenoidal approach used in this study for all operations differs from endoscopic endonasal approaches. During transnasal–transsphenoidal endoscopic surgery, the anterior wall of the sphenoid sinus is reached through the nasal cavity after removing the posterior part of the nasal septum, and the anterior wall of the sphenoid sinus is also cut open to reach the sphenoid sinus. For the transnasal-transsphenoidal microscopic surgery used in this study, we first reached the middle part of the nasal cavity and made an incision on the mucosa of the nasal septum. We reached the anterior wall of the sphenoid sinus via this incision through a space between the bone and mucosa of the nasal septum. During endoscopic surgery, it is impossible to carry out satisfactory reposition of the mucosal flap, which is cut open and turned over at the anterior wall of the sphenoid sinus. After surgery, there is a hole left in the anterior wall of the sphenoid sinus, which makes the sphenoid sinus directly connect to the nasal cavity and destroys the original closed and moist physiological environment within the normal sphenoid sinus. With our surgical technique, we can perform excellent reposition of the mucosa after surgery, and the sphenoid sinus is as closed and moist as before.
Multivariate analysis did not identify any independent risk factors for changes in olfactory function, and therefore, this analysis did not provide information for explaining the loss of olfactory function postoperatively. Some authors have speculated about explanations for the reduction in olfactory function after transsphenoidal pituitary surgery. Kahilogullari et al16 suggested that the microscopic transsphenoidal approach may damage the mucosa and bone during nasal distraction. Berker et al27 speculated that changes in olfactory function as a result of transsphenoidal surgery may be due to excessive electrocoagulation on the olfactory epithelium. Rotenberg et al15 attributed changes in olfactory function after endoscopic transsphenoidal pituitary surgery to the use of a Hadad–Bassagasteguy vascularized septal flap. We speculate that several factors might be involved in the changes in olfactory function observed in our study. Isolation of mucosa (containing olfactory neurons) from the bone surface of the anterior wall of the sphenoid sinus and the posterior portion of the nasal septum, and electrocoagulation of bleeding on the inner surface of the mucosa may damage the blood supply to the olfactory epithelium or directly destroy the olfactory neurons. There is a possibility that olfactory nerve cells could be damaged by the application of providone-iodine (0.5% iodine content) for sterilization of both nasal cavities at the beginning of surgery and the placement of a piece of cotton containing epinephrine in the nasal cavity for 3 minutes. Another factor could be that surgery induces adhesion, bloody scab covering, and scar formation on the olfactory epithelium and its peripheral mucosa, which prevent odor molecules from reaching the olfactory receptor neurons. Also, surgery causes chronic inflammation of the olfactory epithelium and the olfactory region is covered with viscous, sticky, or purulent discharge so that olfactomedin cannot contact the cilia of the olfactory receptor neurons, which may result in decreased conductive olfactory sensation.
Complications of olfactory dysfunctions included decline and disappearance of olfactory sensation, unpleasant smelling, nasal congestion, sinusitis, headache (related to high sphenoid sinus pressure, sphenoid and ethmoid sinus inflammation, mucocele etc), diabetes insipidus (related to the neurohypophysis injury), and epistaxis (almost all from the nasal mucosa; the causes include falling off of scabs, nasal dryness, etc).
Our study was limited because the sample size, 53 patients, was relatively small. Large-sample studies should be carried out to evaluate nasal symptoms and QOL following endonasal transsphenoidal surgery. Even this study may be the first in China using a QOL scale (SF-36) for patients who underwent endonasal transsphenoidal surgery to evaluate the postoperative QOL and clinical outcomes, the eventual recovery of SF-36 and SNOT-22 to preoperative level at 4 months, making the statistically significant differences made at 1 week and 1 month become temporary. Also, the 4-month duration of follow-up was relatively short. It is necessary to carry out studies with longer follow-up duration to further understand long-term olfactory dysfunction, nasal symptoms, and QOL.
In conclusion, we found that olfactory dysfunction was relatively serious after endonasal transsphenoidal surgery and the recovery was relatively slow. In most of the patients, olfactory function did not return to the preoperative level by 4 months after surgery, and it is uncertain whether further recovery is possible or not. Since most similar studies have found little or no loss of olfactory function after endonasal transsphenoidal surgery, our findings need to be confirmed in future studies, particularly with longer follow-up periods.
Footnotes
Abbreviations: MRI = magnetic resonance imaging, QOL = quality of life, SF-36 = Medical Outcomes Study Short Form-36, SNOT-22 = 22-item Sinonasal Outcome Test, T&T = Toyota and Takagi, T1WI = T1-weighted imaging.
SW and YC contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Rotenberg B, Tam S, Ryu WH, et al. Microscopic versus endoscopic pituitary surgery: a systematic review. Laryngoscope 2010; 120:1292–1297. [DOI] [PubMed] [Google Scholar]
- 2.Haruna S, Otori N, Moriyama H, et al. Endoscopic transnasal transethmosphenoidal approach for pituitary tumors: assessment of technique and postoperative findings of nasal and paranasal cavities. Auris Nasus Larynx 2007; 34:57–63. [DOI] [PubMed] [Google Scholar]
- 3.Goudakos JK, Markou KD, Georgalas C. Endoscopic versus microscopic trans-sphenoidal pituitary surgery: a systematic review and meta-analysis. Clin Otolaryngol 2011; 36:212–220. [DOI] [PubMed] [Google Scholar]
- 4.Dusick JR, Exposito F, Mattozo CA, et al. Endonasal transsphenoidal surgery: the patient's perspective—survey results from 259 patients. Surg Neurol 2006; 65:332–342. [DOI] [PubMed] [Google Scholar]
- 5.Suberman TA, Zanation AM, Ewend MG, et al. Sinonasal quality-of-life before and after endoscopic, endonasal, minimally invasive pituitary surgery. Int Forum Allergy Rhinol 2011; 1:161–166. [DOI] [PubMed] [Google Scholar]
- 6.Kilty SJ, McLaughlin N, Bojanowski MW, et al. Extracranial complications of endoscopic transsphenoidal sellar surgery. J Otolaryngol Head Neck Surg 2010; 39:309–314. [PubMed] [Google Scholar]
- 7.Petry C, Leaes CG, Pereira-Lima JF, et al. Oronasal complications in patients after transsphenoidal hypophyseal surgery. Braz J Otorhinolaryngol 2009; 75:345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim BY, Son HL, Kang SG, et al. Postoperative nasal symptoms associated with an endoscopic endonasal transsphenoidal approach. Eur Arch Otorhinolaryngol 2013; 270:1355–1359. [DOI] [PubMed] [Google Scholar]
- 9.Hummel T, Nordin S. Olfactory disorders and their consequences for quality of life. Acta Otolaryngol 2005; 125:116–121. [DOI] [PubMed] [Google Scholar]
- 10.Min YG, Yun YS, Song BH, et al. Recovery of nasal physiology after functional endoscopic sinus surgery: olfaction and mucociliary transport. ORL J Otorhinolaryngol Relat Spec 1995; 57:264–268. [DOI] [PubMed] [Google Scholar]
- 11.Lee SH, Lim HH, Lee HM, et al. Olfactory mucosal findings in patients with persistent anosmia after endoscopic sinus surgery. Ann Otol Rhinol Laryngol 2000; 109 (8 pt 1):720–725. [DOI] [PubMed] [Google Scholar]
- 12.Kern RC. Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope 2000; 110:1071–1077. [DOI] [PubMed] [Google Scholar]
- 13.Soler ZM, Sauer DA, Mace JC, et al. Ethmoid histopathology does not predict olfactory outcomes after endoscopic sinus surgery. Am J Rhinol Allergy 2010; 24:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shemshadi H, Azimian M, Onsori MA, et al. Olfactory function following open rhinoplasty: a 6-month follow-up study. BMC Ear Nose Throat Disord 2008; 8:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rotenberg BW, Saunders S, Duggal N. Olfactory outcomes after endoscopic transsphenoidal pituitary surgery. Laryngoscope 2011; 121:1611–1613. [DOI] [PubMed] [Google Scholar]
- 16.Kahilogullari G, Beton S, Al-Beyati ES, et al. Olfactory functions after transsphenoidal pituitary surgery: endoscopic versus microscopic approach. Laryngoscope 2013; 123:2112–2119. [DOI] [PubMed] [Google Scholar]
- 17.Hart CK, Theodosopoulos PV, Zimmer LA. Olfactory changes after endoscopic pituitary tumor resection. Otolaryngol Head Neck Surg 2010; 142:95–97. [DOI] [PubMed] [Google Scholar]
- 18.Kim SW, Park KB, Khalmuratova R, et al. Clinical and histologic studies of olfactory outcomes after nasoseptal flap harvesting. Laryngoscope 2013; 123:1602–1606. [DOI] [PubMed] [Google Scholar]
- 19.Karabatsou K, O’Kelly C, Ganna A, et al. Outcomes and quality of life assessment in patients undergoing endoscopic surgery for pituitary adenomas. Br J Neurosurg 2008; 22:630–635. [DOI] [PubMed] [Google Scholar]
- 20.Tanemura E, Nagatani T, Aimi Y, et al. Quality of life in nonfunctioning pituitary macroadenoma patients before and after surgical treatment. Acta Neurochir 2012; 154:1895–1902. [DOI] [PubMed] [Google Scholar]
- 21.Wang S, Zhang J, Wei L, et al. Incision choice of endonasal transsphenoidal approach and its anatomic basis. Chin J Neurosurg Dis Res 2008; 7:111–115. [Google Scholar]
- 22.Wolfsberger S, Ba-Ssalamah A, Pinker K, et al. Application of three-tesla magnetic resonance imaging for diagnosis and surgery of sellar lesions. J Neurosurg 2004; 100:278–286. [DOI] [PubMed] [Google Scholar]
- 23.Knosp E, Steiner E, Kitz K, et al. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 1993; 33:610–617. [DOI] [PubMed] [Google Scholar]
- 24.Lü W, Qi F, Gao ZQ, et al. Quality of life survey on patients with chronic rhinosinusitis by using Chinese version of the 22-item sinonasal outcome test (SNOT-22) [in Chinese]. Chin J Otorhinolaryngol Head Neck Surg 2008; 43:18–21. [PubMed] [Google Scholar]
- 25.Morley AD, Sharp HR. A review of sinonasal outcome scoring systems—which is best. Clin Otolaryngo l 2006; 31:103–109. [DOI] [PubMed] [Google Scholar]
- 26.Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. [Guidelines for diagnosis and treatment of chronic rhinosinusitis (2012, Kunming)]. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2013. Feb;48(2):92–94. [PubMed] [Google Scholar]
- 27.Berker M, Hazer DB, Yücel T, et al. Complications of endoscopic surgery of the pituitary adenomas: analysis of 570 patients and review of the literature. Pituitary 2012; 15:288–300. [DOI] [PubMed] [Google Scholar]


