Supplemental Digital Content is available in the text
Abstract
The prognostic information about CKD progression, particularly for GFR categories 1 and 2, is still limited. This cohort was therefore conducted to determine the CKD progression using a competing risk approach.
We conducted a retrospective cohort study linking community health screening with hospitals and death registry data in a province of Thailand, from 1997 to 2011. A competing risk model was applied by treating death as a competing risk factor to estimate 2-, 5-, and 10-year probability of kidney failure and median time for CKD progression from lower to higher GFR category.
There were 17,074 non-diabetic and 15,032 diabetic CKD subjects. Diabetic subjects progressed more rapidly through GFR categories with the median times for CKD progression from GFR categories G1 to G2, G2 to G3a, G3a to G3b, G3b to G4, and G4 to G5 of 4.4, 6.1, 4.9, 6.3, and 9.0 years, respectively. Non-diabetic subjects took longer to progress with the corresponding median time of 9.4, 14.0, 11.0, 13.8, and >14.3 years. After adjusting for confounders, diabetic subjects were 49% (cause-specific hazard ratio (cHR) = 1.49, 95% CI: 1.37, 1.62) more likely to develop kidney failure than non-diabetic subjects. Albuminuria categories A3 and A2 were, respectively, 3.40 (95% CI: 3.07, 3.76) and 1.71 (95% CI: 1.53, 1.92) higher risk of kidney failure when compared to A1.
For each albumin category, death rate increased as albuminuria increased particularly in diabetic subjects, which was approximately 2 times higher in A3 compared to A1. Considering GFR category, it gradually increased from G1 to G4 and sharply increased from G4 to G5 in both non-diabetic and diabetic subjects.
This study has quantified CKD progression in an Asian population within ordinary practice. Diabetic subjects progress through GFR and albuminuria categories and reach kidney failure about twice as rapidly as non-diabetic subjects.
1. INTRODUCTION
Chronic kidney disease (CKD) is now recognized as one of the leading causes of disease burden globally. The prevalence of CKD with glomerular filtration rate (GFR) categories 1 (G1) to 4 (G4) was 13.1% to 17.5% in the adult US and Thai population, respectively.1,2 Although several previous community-based studies3–10 have assessed the prognosis of CKD in general populations, none has yet estimated the time for changing kidney function and the probability of kidney failure or death according to each GFR category, particularly for early stages of G1 and G2. Although some studies had large overall sample sizes,4,7 most of them had relatively low numbers of subjects for each GFR category, lacked information on progression through GFR categories,3,4,6–10 had short follow-up times,4–6,8 and were thus unable to assess the probability of kidney failure or death. In addition, a high proportion of CKD subjects died before reaching kidney failure, therefore estimating the rate of kidney failure occurrence without taking into account death as a competing risk would yield biased results.11 We therefore conducted a large retrospective cohort study considering death as a competing risk, which aimed to quantify CKD progression starting forward from G1 to kidney failure, separately in diabetic and non-diabetic patients.
2. METHODS
2.1. Setting and Participants
The design of this study was a retrospective cohort of CKD subjects living in Ubon Ratchathani province, Thailand. Subjects were enrolled to our cohort since they were first diagnosed as CKD. The province consists of 20 districts with a population of 1.8 million. The Ubon Ratchathani Public Health Office (UBPHO) has provided medical services under a universal coverage scheme launched by the Thai government since 2002. This scheme provides a health promotion service called a “core package,” which includes annual screening for hypertension, diabetes, dyslipidemia, cardiovascular diseases (CVDs), and includes core laboratory tests (ie, complete blood count (CBC), fasting plasma glucose (FPG), lipid profile, blood urea nitrogen (BUN), serum creatinine, and urine analysis (UA)). The UBPHO computerized databases were retrieved between 2002 and 2011 and then were merged with the hospital databases from 20 district hospitals (covering ∼98% of records for both out- and in-patients) from 1997 to 2011. The 2 databases were then linked to the Thailand death registry using individuals’ unique personal identification numbers, see Figure 1.
FIGURE 1.

Flow of cohort study and data retrieval.
Subjects were eligible if they had the following criteria: aged 18 years or older, had been diagnosed as having CKD at the time of screening or later at follow up of diseases/conditions identified at screening, and had at least 3 months of follow-up. Those who had received maintenance dialysis and/or transplantation were excluded.
2.2. Studied Variables and Measurements
2.2.1. CKD and CKD Progression
We used the current CKD nomenclature recommended by the Kidney Disease: Improving Global Outcomes (KDIGO) 2012 guideline to classify CKD and its progression.12 CKD, persistent abnormalities of kidney structure or function for longer than 3 months, were classified based on cause, GFR, and albuminuria category (CGA) as follows: cause included diabetes and non-diabetes, GFR category consisted of normal or high (≥90 ml/min/1.73 m2, G1), mildly decreased (60–89 ml/min/1.73 m2, G2), mildly to moderately decreased (45–59 ml/min/1.73 m2, G3a), moderately to severely decreased (30–44 ml/min/1.73 m2, G3b), severely decreased (15–29 ml/min/1.73 m2, G4), and kidney failure (<15 ml/min/1.73 m2, G5). Albuminuria category was classified as normal to mild (albumin-to-creatinine ration (ACR) <30 mg/g, or protein reagent strip negative to trace, A1), moderate (ACR 30–300 mg/g, or protein reagent strip +, A2), and severe (ACR >300 mg/g, or protein reagent strip ≥ + +, A3). GFR and albuminuria were repeatedly assessed every 3–6 months depending on patients’ conditions. CKD progression was defined as a change of GFR category with 25% or greater drop in e-GFR from baseline.
The serum creatinine was measured using the Modified Jaffe method at each hospital's laboratory unit. The automated clinical analyzers used in district hospitals were ABX Pentra 400®, Cobas®, and Erba Mannheim®. The laboratory tests of these hospitals were standardized and calibrated every 3 months by the Department of Medical Science, Ministry of Public Health, Thailand. The modified Jaffe method was then converted to the isotope dilution mass spectrometry (IDMS) equivalent using the calibration equation from the Thai SEEK study.2 The e-GFR was then calculated using the CKD-EPI equation.13 To avoid acute changes in creatinine due to intercurrent illness, only out-patient determinations of serum creatinine and UA were used. The UA was done using a urine dipstick for testing urine protein (CYBOW™, Gyung-Num, Republic of Korea) and microscopic examinations. The result was reported as negative, trace, or ≥1+.
2.2.2. Diabetes Mellitus
Diabetes was identified from the databases according to the ICD10 code, which codes E10–E14. Diagnosis of diabetes was made by physicians based on elevated fasting plasma glucose (≥126 mg% on 2 consecutive occasions) along with clinical presentations of diabetes. In addition, this diagnosis was verified by the evidence of repeated determinations of fasting plasma glucose or prescriptions of anti-diabetic medications.
2.2.3. All-Cause Mortality
All-cause mortality was retrieved from the Bureau of Strategy and Statistics, Ministry of Public Health database from January 01, 1997 to December 31, 2011. The data were validated by verifying with death certificate information from the Ministry of the Interior. It is mandatory that all deaths are registered in Thailand, so death registries were considered complete and no loss to follow up was assumed.
2.3. Statistical Analysis
Among 32,106 eligible subjects, the data for body weight, height, albuminuria at baseline were missing in 0.5%, 12.4% and 25% of subjects, respectively. The missing data were thus imputed using multivariate chain equation assuming that the data were missing at random.14,15 Twenty imputations were constructed using linear regression to obtain the summarized estimates for further analysis.16
Time from diagnosis of CKD to CKD progression or death, whichever occurred first, was calculated for each subject. Subjects were censored if they were free from interested events and competing risk event at the end of the study period (December, 2011). Our outcomes of interest were CKD progressions, which were changes forward from G1 to G2, G2 to G3a, G3a to G3b, G3b to G4, and G4 to G5, whereas higher GFR category for each change and death were considered as competing risk events. For instance, G3a, G3b, G4, G5, and death were considered as competing risk events for a change from G1 to G2. The subdistribution hazard function17–19 was used to estimate the cumulative incidence function (CIF) and the median times of CKD progression for each change separately by diabetic and non-diabetic subjects. GFR category was treated as time varying covariates.
The cause specific hazard function17–19 was used to estimate the cause specific hazard ratio (csHR) of diabetes on kidney failure (ie, interested event) and death (ie, competing risk event) adjusting for baseline characteristics and co-morbidities, that is, sex, body mass index (BMI), hypertension, CVDs and albuminuria category. Age was not included in the multivariate cause specific hazard model because it had already been taken into account in the GFR estimation. In addition, the naïve Kaplan–Meier (KM) method was also applied to estimate the probability of interested events without accounting for competing risk of death. All analyses were performed using STATA version 13.0, and P-value less than 0.05 was taken as the threshold for statistical significance.
2.4. Ethical Considerations
The study protocol was reviewed and approved by the Ramathibodi Hospital Ethical Committee and the Ethical Committee of Ubon Ratchathani Public Health Office. Permissions for obtaining and describing data were approved. The patient records/information was anonymized and de-identified prior to analysis.
3. RESULTS
A flow chart of data retrieval for our cohort has been demonstrated in Figure 1. Among approximately 1.3 million adult population, 646,618, 434,493, and 216,151 were in age groups of 18 to 39, 40 to 59, and 60 years or older, respectively. Of these, 51,384 (7.9%), 87,662 (20.2%), and 80,319 (37.2%) in the corresponding age groups were screened for CKD between 1997 and 2011, respectively. A total of 32,106 subjects were classified as CKD according to the criteria of GFR and albuminuria. Among them, 17,074 (53.2%) and 15,032 (46.8%) subjects were respectively non-diabetes and type 2 diabetes, respectively.
Their baseline characteristics have been described in Table 1. Among diabetic subjects, the mean age was 60.6 years, 27% were males, and the median follow-up time was 4.7 years (range: 0.3, 14.2) with 70,414 person-years of observation. The median duration of diabetes was 2.0 years (range: 0–11.8 years), most subjects (70.5%) received oral hypoglycemic drugs, followed by insulin (14.6%), both oral hypoglycemic drugs and insulin (8.0%), and diet control only (6.9%).
TABLE 1.
Baseline Characteristics of Subjects by Non-Diabetic and Diabetic Groups

Among non-diabetic subjects, the mean age was 65.3 years, 45% were males, and the median follow-up time was 4.2 years (range: 0.3, 14.3) with 72,879 person-years of observation. All covariate distributions were statistically different between diabetic and non-diabetic groups, except the mean systolic blood pressure and diastolic blood pressure, respectively. Notably, the proportion of subjects on renin–angiotensin system blockade agents was significantly higher in the diabetic than non-diabetic groups (26.8% vs 13.3%, P < 0.001), see Table 1.
3.1. CKD Progression
The numbers of GFR category G1, G2, G3a, G3b, G4, and G5 at baseline enrollment have been described in Table 1. Additional baseline characteristics of subjects by GFR category have also been described in Supplementary Table 1 http://links.lww.com/MD/A170. The median time for CKD progression was estimated separately by diabetic and non-diabetic groups, see Figure 2. This suggested that diabetic subjects progressed more rapidly through GFR categories with the median times for CKD progression from GFR category G1 to G2, G2 to G3a, G3a to G3b, G3b to G4, and G4 to G5 of 4.4, 6.1, 4.9, 6.3, and 9.0 years, respectively. Non-diabetic subjects took longer to progress with the corresponding median times of 9.4, 14.0, 11.0, 13.8, and >14.3 years.
FIGURE 2.
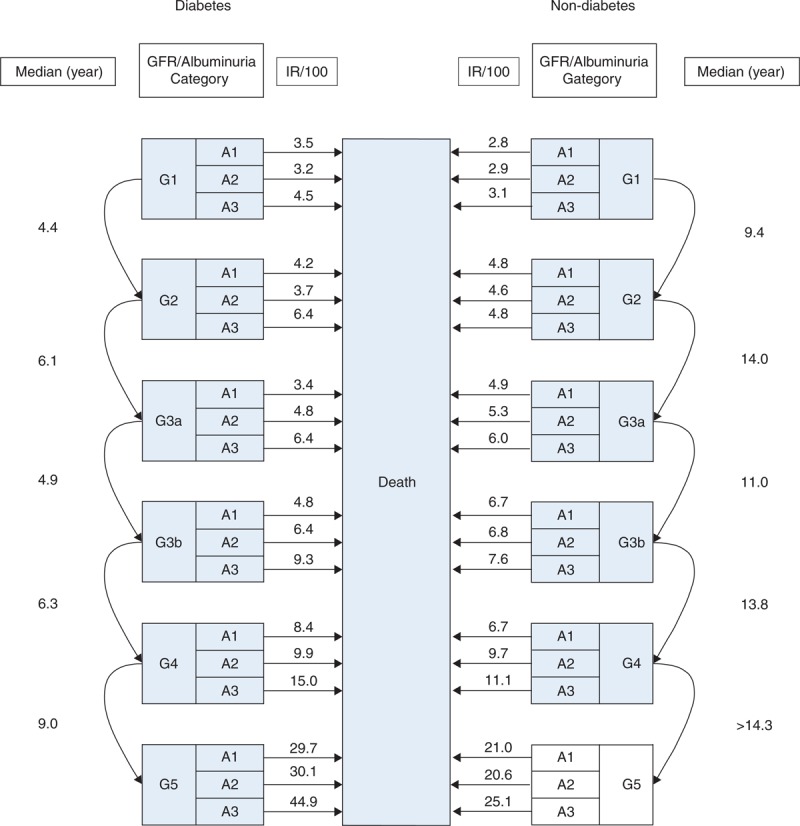
Estimation of median time of CKD progression from lower to higher GFR category and death rate by non-diabetic and diabetic subjects.
3.2. Kidney Failure
The overall kidney failure rates were 2.8% (95% CI: 2.7, 2.9) and 1.8% (95% CI: 1.7, 1.9) in diabetic and non-diabetic subjects, respectively. The CIF curves of kidney failure were plotted against the KM-methods, see Figure 3. This suggested that the probabilities of kidney failure by CIFs at 2, 5, and 10 years from index date were, respectively 3.7%, 11.0%, and 25.3% for diabetic subjects; and 3.1%, 7.5%, and 13.8% for non-diabetic subjects. The naïve KM method tended to overestimate the probabilities of kidney failure when compared to the CIFs, ie, the probabilities at 2, 5, and 10 years were 3.8%, 11.8%, and 30.8% for diabetic subjects and 3.2%, 8.2%, and 17.3% for non-diabetic subjects, respectively.
FIGURE 3.
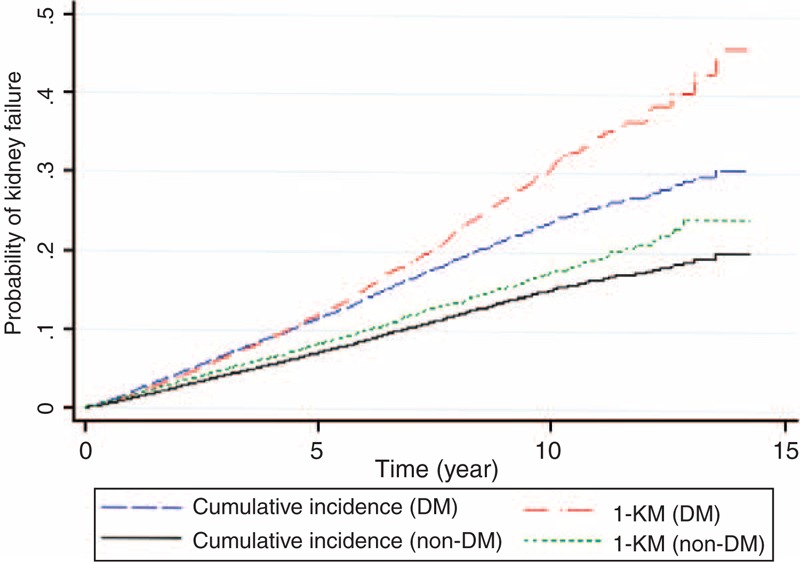
Estimation of probability of kidney failure by diabetic groups, subdistribution hazard versus KM method.
After adjusting for sex, BMI, hypertension, CVDs, and albuminuria at baseline, the csHR of diabetes was 1.49 (95% CI: 1.37, 1.62), indicating diabetic subjects were 49% significantly higher risk to develop kidney failure than non-diabetic subjects, see Table 2. Albuminuria at baseline was strongly associated with kidney failure with the csHRs of 1.71 (95% CI: 1.53, 1.92) and 3.40 (95% CI: 3.07, 3.76) for albuminuria category A2 and A3 compared to A1, respectively. In addition, having hypertension and CVDs at baseline were also at higher risk to develop kidney failure with the csHRs of 1.47 (95% CI: 1.35, 1.60) and 1.49 (95% CI: 1.37, 1.63), respectively. Conversely, higher BMI was approximately 28% (95% CI: 21%, 34%) and 55% (52%, 59%) lower risk of kidney failure for the BMIs of 22 to 24.9 and ≥25 relative to BMI <22 kg/m2, respectively.
TABLE 2.
Risk Effect of Diabetes on Kidney Failure and Death: A Cause Specific Hazard Competing Risk Model

3.3. Death Rate
The overall death rates were 5.3% (95% CI: 5.1%, 5.5%) and 5.9% (95% CI: 5.8%, 6.1%) in diabetic and non-diabetic subjects, respectively. The overall probabilities of death at 2, 5, and 10 years from index date were, respectively, 6.0%, 21.1%, and 48.9% for diabetic subjects and 8.3%, 24.2%, and 48.1% for non-diabetic subjects. The death rates of these 2 groups were stratified by GFR and albuminuria category, see Figure 2. This suggested that, for each albuminuria category, the death rates gradually increased from G1 to G4 and sharply increased from G4 to G5 in both non-diabetic and diabetic subjects. For each GFR category, the death rate increased as albuminuria increased particularly in diabetic subjects, which was approximately 2 times higher in A3 compared to A1. The death rates were slightly different from G1 to G4 in both groups, but were substantially higher in diabetic than non-diabetic groups in G5.
After adjusting for age, sex, BMI, hypertension, CVDs, and albuminuria at baseline, the risks of death was 6% (csHR = 1.06: 95% CI: 1.01, 1.12) significantly higher for diabetic subjects when compared to non-diabetic subjects, see Table 2. CVDs had the strongest effect on death with the csHR of 3.11 (95% CI: 2.84, 3.41). Albuminuria categories A2 and A3 were 1.28 (95% CI: 1.18, 1.39) and 2.01 (95% CI: 1.87, 2.17) times more likely to die relative to albumin category A1. Older age was higher risk of death with the csHRs of 1.38 (95% CI: 1.11, 1.72) and 2.41 (95% CI: 1.93, 2.00) for age groups 40–59 and ≥60 compared to age group <39 years, respectively. Hypertension was approximately 17% (95% CI: 5%, 30%) higher risk of death than non-hypertension. Female and higher BMI were preventive factors of death, ie, females were 22% (95% CI: 16%, 28%) lower risk than males, whereas BMIs of 22 to 24.9 and ≥25 kg/m2 were 35% (95% CI: 28%, 42%) and 55% (95% CI: 52%, 59%), respectively, lower risk of death than BMI of <22 kg/m2.
4. DISCUSSION
We conducted a large cohort study of CKD patients with a median follow-up time of 4.5 years in the context of normal primary care. This allowed us to clearly quantify the progression of CKD in both general and diabetic populations. CKD progressed more rapidly through kidney failure in diabetic subjects, and on average, the rate of progression was about double, while the median times for progression were, respectively, about 5 to more than 8 years shorter for changing GFR category in diabetic subjects compared to non-diabetic subjects. Albuminuria, CVD, and hypertension were associated with kidney failure progression, but conversely BMI was found to reduce such risk.
Huge numbers of studies have assessed the risk of CKD occurrence in general and high-risk populations. Contrastingly, not many studies have determined the progression of disease after CKD occurrence, and our systematic search in Medline database (up to July 22, 2012) could only identify 6 studies of CKD progression.6–8,20–22 None of them provided times for changing GFR and albuminuria categories, so our study should be able to fill in this remaining gap of knowledge. Like other previous prognostic studies,6–9,21,22 our study also showed consistent association between increased GFR and albuminuria categories and rate of death. In addition to these studies, the prognosis of death among diabetic subjects were slightly higher than non-diabetic subjects, but paradoxically the overall rate of dying at each GFR category were higher in non-diabetic subjects, except for GFR category G4 and G5 (data not shown). This may be because diabetic subjects progressed through the GFR category more rapidly which resulted in opportunities for other unobserved competing events (ie, death in our case) to play more roles in non-diabetic than diabetic subjects. However, such competing event effects were blunted by the presence of severely increased albuminuria.
The association between BMI and CKD among general population has been clear. However, association between BMI and kidney failure progression among established CKDs has been controversial. This paradoxical inverse association was consistent to findings obtained from previous studies,23–27 which probably reflected better nutritional status among subjects with higher BMI and this may contribute to the delayed kidney failure progression.
The impacts of CKD on clinical outcomes have received less concern given that globally more people are living with CKD than at any previous time.28 The cost of renal replacement therapy in Thailand has risen steadily, that is, 53, 467, 900, 1066, and 1300 million US$ in 2008, 2009, 2010, 2011, and 2012, respectively.29 Unfortunately, policy makers and communities have paid insufficient attention to CKD, that is, limited resources have been allocated to CKD and to risk factors such as diabetes. To decrease disease and economic burden of the country, the governments, policy makers, and health care providers should implement effective treatment managements, not only aiming at delaying CKD progression, but also implement effective health promotion programs aiming at prevention of diabetes. In addition, intensive treatments and health promotion programs should be urgently launched and these should be included in the Universal Health Coverage program in Thailand.
Our study has some strengths. This is a large cohort of CKD subjects recruited from real life practice that should be able to reflect the CKD progression of Asian population. The sources of the studied populations were from both general and high-risk populations, thus making the comparison of CKD prognosis in general and high-risk CKD populations feasible. The follow-up time was as long as 14 years with a median of 4.5 years, which allowed us to estimate the median time of disease progression from lower to higher GFR categories. The CKD misclassification was less likely because the diagnosis of CKD required evidence of persistent abnormal urine findings or decreased e-GFR on several occasions at least 3 months apart. Our study investigated the outcomes of routine clinical practice currently provided for people with CKD in real-world conditions.
Finally, we properly applied the subdistribution hazard model to predict the disease prognosis (ie, CIF of CKD progression) and the cause-specific hazard model to evaluate the prognostic effects (ie csHRs) on kidney failure/death separately by non-diabetic and diabetic subjects.19,30 The probability of CKD progression was estimated in presence of competing risks (ie, higher GFR category and death), which yielded more accurate estimates than the naïve KM method.17–19 The KM method treats death as censoring, given the basic assumption of constant hazard that censored subjects are independent and thus are good representatives for those subjects who are still observed. In other words, the censored event should not alter or preclude (as in our case) kidney failure occurrence. In a real situation of chronic illness such as CKD, subjects may die from other causes before reaching kidney failure leaving only surviving subjects in the cohort. As such, the remaining subjects are prone to survival bias, and thus they are not good representatives for the whole cohort.11 Performing a proportional hazard assumption test for our data found highly significant violation of this assumption (P < 0.001, data have not been shown). If such assumption is not met, applying the naïve KM method would lead to overestimation of kidney failure rate. This is like what we observed in our data, the KM method tended to yield higher probability of kidney failure than the CIF method because it treated dead subjects as if they were still at risk of having kidney failure.31 In this case, the KM method overestimated the cumulative probabilities of kidney failure as a result of high rate of competing risk.32,33 Although the competing risk models have been applied and acknowledged more in cardiovascular and oncology research, their methodological issues have only recently been discussed in nephrology.11,30,32,33
Our study also had some limitations. This study was a retrospective cohort in which the data were retrieved from databases of routine practice. Data quality controls, standardized laboratory tests, and completeness of data were not as good as a prospective cohort with research purpose. Information on treatments of co-morbidity (ie, DM, HT, CVD, dyslipidemia) such as types of drug, drug dosage, drug compliance, achievement of treatment targets or treatments of CKD itself were lacking. This may result in biased prognostic effects of the studied co-variables. This cohort was conducted using data from only one province located in the North Eastern Thailand, where the CKD prevalence was highest compared to other regions, except Bangkok.2 The healthcare resources, in terms of density of nephrologists, clinicians, nurses, and other health professionals were low compared to other regions of the country. Consequently, the pattern of CKD progression obtained under these conditions may not be similar to other developed countries, but may be generalizable to developing countries with similar structures.
In conclusion, our study has described CKD progression in a Thai population with current clinical practice. The CKD progressed more rapidly and was more likely to reach to kidney failure in diabetic than non-diabetic subjects. These subjects may need more aggressive assessments and treatments in order to delay their disease progression and increase survival.
Acknowledgments
We deeply thank the Ubon Ratchathani Public Health Office and the Bureau of Strategy and Statistics, Ministry of Public Health, Thailand for data collection and management.
Footnotes
Abbreviations: ACR = albumin-to-creatinine ratio, BMI = body mass index, CBC = complete blood count, CIF = cumulative incidence function, CKD = chronic kidney disease, cHR = cause-specific hazard ratio, CI = confidence interval, FPG = fasting plasma glucose, GFR = glomerular filtration rate, IDMS = isotope dilution mass spectrometry, KM = Kaplan–Meier, KDIGO = kidney disease: improving global outcomes, UA = urinary analysis, UBPHO = Ubon Ratchathani Public Health Office.
Funding source: This study was funded by a grant from the Health Systems Research Institute of Thailand. The funding organization had no role in the design or conduct of the study.
All authors declared no conflict of interests.
Contribution statement: AT had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Acquisition of data: PV, Analysis of data: AT, AI, PV, Study concept and design: PV, AT, AI, Drafting of the manuscript: PV, AT, AI, JA. Critical revision of the manuscript for important intellectual content: JA, AI, AT. Final approval of the version to be published: all authors.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007; 298:2038–2047. [DOI] [PubMed] [Google Scholar]
- 2.Ingsathit A, Thakkinstian A, Chaiprasert A, et al. Prevalence and risk factors of chronic kidney disease in the Thai adult population: Thai SEEK study. Nephrol Dial Transplant 2010; 25:1567–1575. [DOI] [PubMed] [Google Scholar]
- 3.Drey N, Roderick P, Mullee M, Rogerson M. A population-based study of the incidence and outcomes of diagnosed chronic kidney disease. Am J Kidney Dis 2003; 42:677–684. [DOI] [PubMed] [Google Scholar]
- 4.Foley RN, Murray AM, Li S, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol: JASN 2005; 16:489–495. [DOI] [PubMed] [Google Scholar]
- 5.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351:1296–1305. [DOI] [PubMed] [Google Scholar]
- 6.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA 2010; 303:423–429. [DOI] [PubMed] [Google Scholar]
- 7.Keith DS, Nichols GA, Gullion CM, et al. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 2004; 164:659–663. [DOI] [PubMed] [Google Scholar]
- 8.Conley J, Tonelli M, Quan H, et al. Association between GFR, proteinuria, and adverse outcomes among White, Chinese, and South Asian individuals in Canada. Am J Kidney Dis 2012; 59:390–399. [DOI] [PubMed] [Google Scholar]
- 9.Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008; 371:2173–2182. [DOI] [PubMed] [Google Scholar]
- 10.Derose SF, Rutkowski MP, Levin NW, et al. Incidence of end-stage renal disease and death among insured African Americans with chronic kidney disease. Kidney Int 2009; 76:629–637. [DOI] [PubMed] [Google Scholar]
- 11.Jager KJ, Stel VS, Zoccali C, Wanner C, Dekker FW. The issue of studying the effect of interventions in renal replacement therapy – to what extent may we be deceived by selection and competing risk? Nephrol Dial Transplant 2010; 25:3836–3839. [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3:1–150. [Google Scholar]
- 13.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubin DB, Schenker N. Multiple imputation in health-care databases: an overview and some applications. Statist Med 1991; 10:585–598. [DOI] [PubMed] [Google Scholar]
- 15.White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Statist Med 2011; 30:377–399. [DOI] [PubMed] [Google Scholar]
- 16.van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Statist Med 1999; 18:681–694. [DOI] [PubMed] [Google Scholar]
- 17.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 2012; 41:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009; 170:244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pintilie M. Analysing and interpreting competing risk data. Statist Med 2007; 26:1360–1367. [DOI] [PubMed] [Google Scholar]
- 20.Bash LD, Astor BC, Coresh J. Risk of incident ESRD: a comprehensive look at cardiovascular risk factors and 17 years of follow-up in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis 2010; 55:31–41. [DOI] [PubMed] [Google Scholar]
- 21.Di Angelantonio E, Chowdhury R, Sarwar N, et al. Chronic kidney disease and risk of major cardiovascular disease and non-vascular mortality: prospective population based cohort study. BMJ (Clin Res Ed) 2010; 341:c4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Hare AM, Bertenthal D, Covinsky KE, et al. Mortality risk stratification in chronic kidney disease: one size for all ages? J Am Soc Nephrol: JASN 2006; 17:846–853. [DOI] [PubMed] [Google Scholar]
- 23.Huang WH, Chen CY, Lin JL, et al. High body mass index reduces glomerular filtration rate decline in type II diabetes mellitus patients with stage 3 or 4 chronic kidney disease. Medicine 2014; 93:e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bentata Y, Latrech H, Abouqal R. Does body mass index influence the decline of glomerular filtration rate in diabetic type 2 patients with diabetic nephropathy in a developing country? Ren Fail 2014; 36:838–846. [DOI] [PubMed] [Google Scholar]
- 25.Lu JL, Kalantar-Zadeh K, Ma JZ, et al. Association of body mass index with outcomes in patients with CKD. J Am Soc Nephrol: JASN 2014; 25:2088–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iseki K, Tokashiki K, Iseki C, et al. Proteinuria and decreased body mass index as a significant risk factor in developing end-stage renal disease. Clin Exp Nephrol 2008; 12:363–369. [DOI] [PubMed] [Google Scholar]
- 27.Lawson JA, Lazarus R, Kelly JJ. Prevalence and prognostic significance of malnutrition in chronic renal insufficiency. J Ren Nutr 2001; 11:16–22. [DOI] [PubMed] [Google Scholar]
- 28.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives – a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 2007; 72:247–259. [DOI] [PubMed] [Google Scholar]
- 29.Tantivess S, Werayingyong P, Chuengsaman P, Teerawattananon Y. Universal coverage of renal dialysis in Thailand: promise, progress, and prospects. BMJ (Clin Res Ed) 2013; 346:f462. [DOI] [PubMed] [Google Scholar]
- 30.Noordzij M, Leffondre K, van Stralen KJ, et al. When do we need competing risks methods for survival analysis in nephrology? Nephrol Dial Transplant 2013; 28:2670–2677. [DOI] [PubMed] [Google Scholar]
- 31.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Statist Med 2007; 26:2389–2390. [DOI] [PubMed] [Google Scholar]
- 32.Lim HJ, Zhang X, Dyck R, Osgood N. Methods of competing risks analysis of end-stage renal disease and mortality among people with diabetes. BMC Med Res Methodol 2010; 10:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teixeira L, Rodrigues A, Carvalho MJ, et al. Modelling competing risks in nephrology research: an example in peritoneal dialysis. BMC Nephrol 2013; 14:110. [DOI] [PMC free article] [PubMed] [Google Scholar]


