Abstract
Nonapnea sleep disorders (NASDs) are associated with an increased risk of stroke, diabetes, and hypertension. No longitudinal study has yet examined the association between NASD and chronic kidney disease (CKD) by using epidemiologic study methods. To test this hypothesis, we examined the effect of NASD on the incidence of CKD in a large population-based retrospective cohort study.
Based on a retrospective cohort study of a general population sample of 128 to 436 patients in the Taiwan National Health Insurance Research Database from January 1, 1998 to December 31, 2001, 42 to 812 NASD patients were followed up for 10.2 ± 3.12 years, and additional 85 to 624 individuals had no NASD at baseline. The International Classification of Diseases, Ninth Revision, Clinical Modification was used to identify the diagnosis of disease. Cox proportional hazard regression models were used to assess the association between NASD and subsequent CKD risk.
The incidence rate of CKD was significantly higher in the NASD cohort than in the comparison cohort (2.68 vs 1.88 per 1000 person-years, respectively). After we adjusted for age, sex, and comorbidities, the risk of developing CKD was significant for patients with NASD (adjusted hazard ratio [HR] = 1.13; 95% confidence interval [CI] = 1.05–1.22; P < 0.01). Among different types of NASDs, patients with sleep disturbance associated disorders had a 14% increased risk of developing CKD (95% CI = 1.03–1.26; P < 0.01), whereas patients with insomnia had a 13% increased risk of subsequent CKD (95% CI = 1.02–1.25; P < 0.05) compared with the non-NASD cohort. Kaplan–Meier survival analysis indicated that the CKD-free rate was 1% lower in the NASD cohort than in the comparison cohort (log-rank test, P < 0.0001).
Our study provides evidence that patients with NASD have an increased risk of developing subsequent CKD compared with patients without NASD; men, elderly people, and patients with concomitant comorbidities are at the greatest risk.
INTRODUCTION
Chronic kidney disease (CKD) constitutes a group of disorders characterized by alterations in renal structure and function, which result in hormonal and metabolic complications according to the severity of the disease. Based on current definitions, the prevalence of CKD is rising in both Western and Eastern countries, ranging from 11.5% to 11.9% in national surveys, compared with that of diabetes.1–3 Patients with CKD carry high risks of major complications, such as progression to end-stage renal disease (ESRD),4 cardiovascular disease,5–7 and death8 in both diabetes mellitus (DM) and non-DM populations. Competing risks between ESRD and mortality in the CKD population vary with age and the presence of DM nephropathy.9,10 In addition to genetic or sociodemographic predisposition, risk factors for CKD include the presence of certain diseases and statuses, such as hypertension, DM, smoking, and obesity.11,12 Recently, hyperuricemia and lower serum bicarbonate have been considered progression factors.13,14 Public health strategies to prevent the progression of CKD have achieved evident outcomes, based on the declining incident renal replacement therapy (RRT) rate and the improved modality of initiating RRT.15
The relationship between sleep disorders and other diseases has been emphasized in recent years, particularly regarding obstructive sleep apnea (OSA) and insomnia. OSA relates to an increased risk of metabolic syndrome and cardiovascular diseases such as hypertension, stroke, and obesity.16–18 It may also mediate renal damage through increased oxidative stress, induced glomerular hyperfiltration, and hypertension.19 Insomnia, a major disease in nonapnea sleep disorders (NASDs), is associated with sympathetic nervous system activation and elevated cardiovascular risk, including hypertension, diabetes, stroke, myocardial infarction, and mortality.20–26 Several studies have shown a high prevalence of OSA and insomnia in the CKD population: 35.5%–73% for insomnia27–29 compared with 3.4%–10% for the general population.30,31
However, whether bidirectional causality exists between NASD and CKD, as implicated between sleep apnea and obesity, remains unknown. With increasing prevalence and poor outcomes in the CKD population, identifying the relationship between NASD and CKD is crucial for preventing the development and progression of CKD. The aim of our study was to determine the subsequent risk of CKD in patients with NASDs. We conducted a retrospective cohort study using data from the Taiwan National Health Insurance Research Database (NHIRD). We excluded patients with OSA to control for associated metabolic disorders.
MATERIALS AND METHODS
Data Source
The source of our data was the NHIRD, which covered claims of ambulatory care, inpatient services, dental services, and prescriptions during 1996 to 2011. A universal National Health Insurance (NHI) program was implemented in Taiwan in March 1995. Ninety-nine percent of the total population in Taiwan, approximately 23 million people, was enrolled in this NHI program (http://www.nhi.gov.tw/english/index.aspx). The National Health Research Institute (NHRI), which manages the NHIRD, has released comprehensive NHI-related administrative claims data for research. In 2000, the NHRI randomly selected 1 million participants from the NHIRD longitudinally linked data available from 1996 to 2011 to form the Longitudinal Health Insurance Database 2000 (LHID 2000) for study purposes. LHID 2000 presented a subpopulation with identical sex and age distribution related to the entire population in Taiwan. The NHRI states that no statistical differences in age, sex, and health care costs exist between LHID 2000 and all enrollees.32 Diagnosis of disease was coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The accuracy and validity of using diagnosis codes in the NHIRD have been documented.33 This study analyzed de-identified secondary data; therefore, no informed consent was required. This study was approved by a full ethical review (institutional review board (IRB) permit number: CMU-REC-101-012).
Sample Participants
Based on the LHID 2000, we identified a cohort of NASDs, consisting of patients newly diagnosed with sleep disorders other than sleep apnea (ICD-9-CM codes 307.4 and 780.5) from January 1, 1998 to December 31, 2001. The index date was defined as the first date of NASD diagnosis. We excluded patients who had sleep apnea syndrome (ICD-9-CM codes 780.51, 780.53, and 780.57) and patients with a history of CKD (ICD-9-CM codes 580–589), ESRD (ICD-9-CM code 585) before the index date, aged less than 18 years, or with incomplete demographic information. Comparison control patients were selected from people without a history of sleep disorders, CKD, or ESRD, based on the LHID 2000. For each identified NASD patient, 2 comparisons were frequency matched with age (each 5-year span), sex, and year of index date as the non-sleep disordered cohort (non-SD). Figure 1 demonstrates a study flowchart for the selection process.
Figure 1.
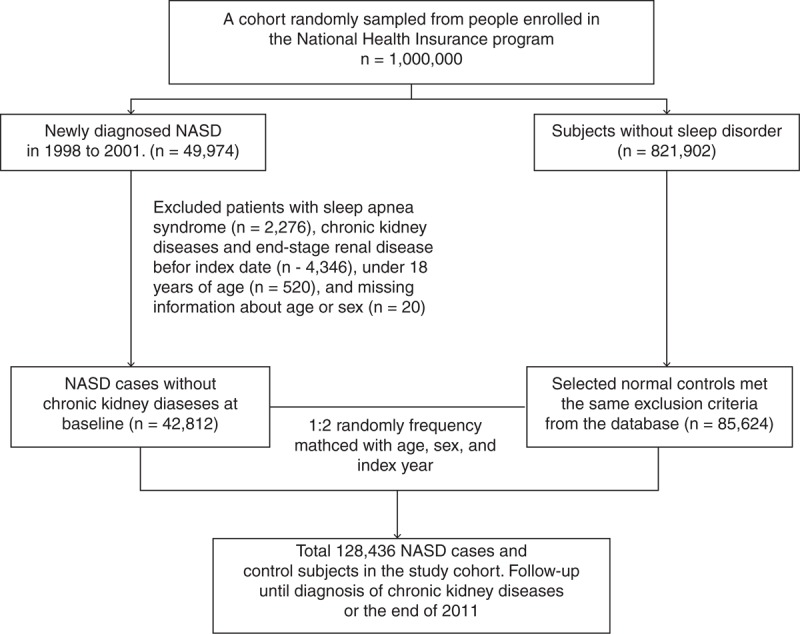
Patient selection flowchart. NASD = nonapnea sleep disorder.
Outcomes and Relevant Variables
Both the NASD and non-SD cohorts were followed from the index date to the date when CKD (ICD-9-CM code 585) occurred, when they withdrew from the insurance system, or the end of 2011. To compare the effect of different types of NASDs on CKD incidence, we further classified NASD into 3 categories: insomnia (ICD-9-CM code 780.52); sleep disturbance associated disorders including sleep disturbance (ICD-9-CM code 780.5), sleep disturbance unspecified (ICD-9-CM code 780.50), hypersomnia, unspecified (ICD-9-CM code 780.54), dysfunctions associated with sleep stages or arousal from sleep (ICD-9-CM code 780.56), sleep-related movement disorder, unspecified (ICD-9-CM code 780.58), and sleep disturbance, and other associates (ICD-9-CM code 780.59); and other sleep disorders (ICD-9-CM code 307.4). The baseline comorbidity history for each patient was determined based on claims data including hypertension (ICD-9-CM codes 401–405), DM (ICD-9-CM code 250), hyperlipidemia (ICD-9-CM code 272), coronary artery disease (CAD) (ICD-9-CM codes 410–414), stroke (ICD-9-CM codes 430–438), and heart failure (ICD-9-CM code 428).
Statistical Analysis
The baseline characteristics and comorbidity between the NASD cohort and the non-SD cohort were compared. Chi-square and t tests were used to evaluate the distributions of discrete and continuous variables, respectively. The incidence densities of CKD were calculated according to sex, age, and comorbidity for each cohort. Univariable and multivariable Cox proportional hazard regression models were used to assess the risk of CKD in the NASD cohort compared with that in the non-NASD cohort. Baseline characteristic variables, such as age, sex, and comorbidities, were included in the multivariable model for adjustment. Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using the Cox model. We used multiplicative analysis to evaluate the interaction effect of NASD and comorbidities on CKD risk. To assess the difference in the CKD-free rates between the 2 cohorts, we applied Kaplan–Meier analysis and the log-rank test. All statistical analyses were performed using SAS 9.3 (SAS Institute Inc, Cary, NC), with P < 0.05 in 2-tailed tests considered significant.
RESULTS
From January 1, 1998 to December 31, 2001, 42 to 812 patients with NASD were identified as the study cohort, and 85 to 624 non-SD matched persons without NASD were identified as the comparison cohort. The mean follow-up time was 10.2 ± 3.12 and 10.5 ± 2.74 years for the NASD and comparison cohort, respectively. The distribution of sex and age was similar in both cohorts (Table 1). Women dominated the study cohorts (64.2%) and about one-half of the individuals were younger than age 50 years. Compared with the comparison cohort, NASD patients were more likely to have comorbidities including hypertension, diabetes, hyperlipidemia, CAD, stroke, and heart failure (all P < 0.001).
Table 1.
Comparisons in Demographic Characteristics and Comorbidities in Patients With and Without NASD
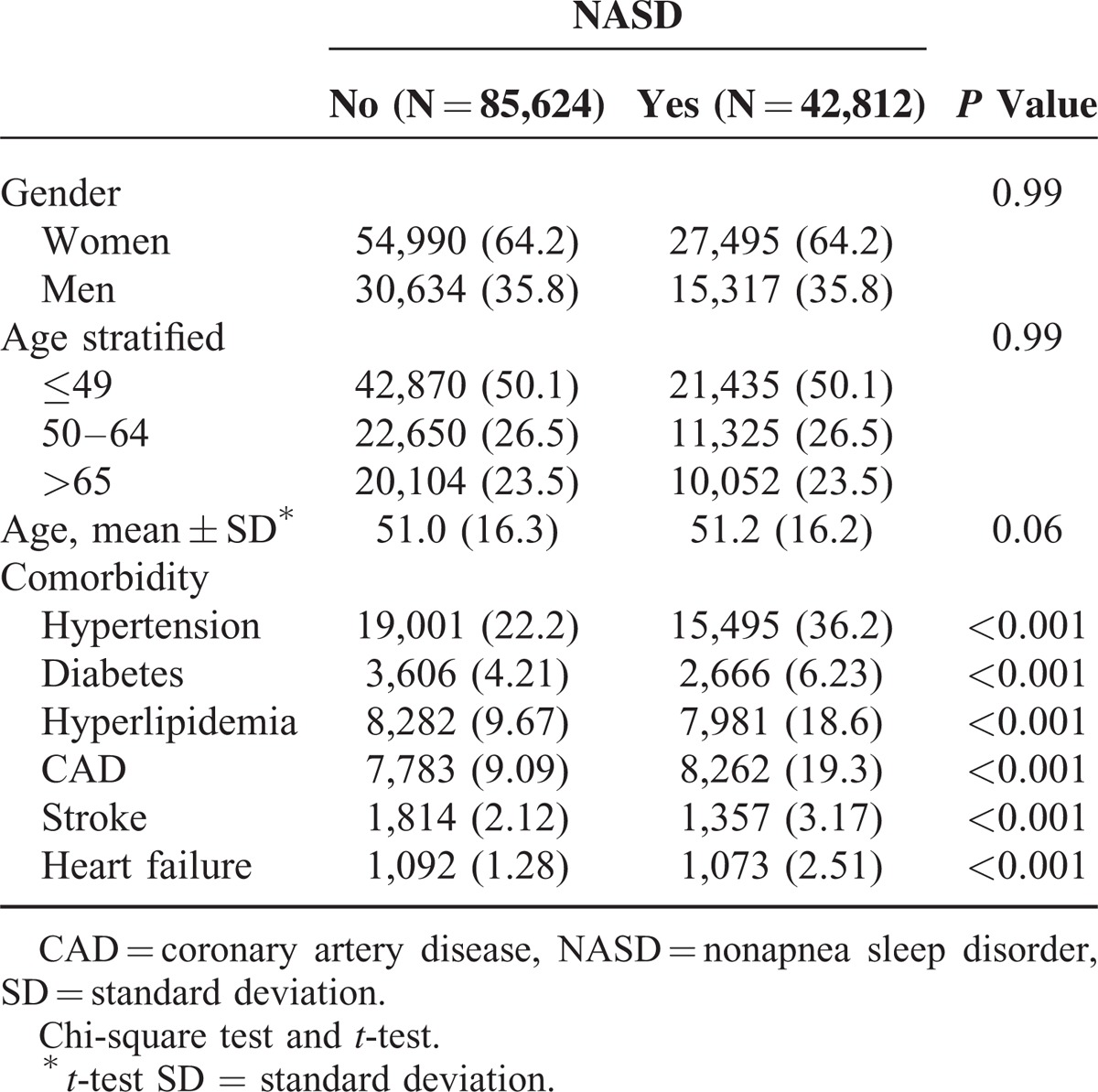
The incidence rate of CKD was significantly higher in the NASD cohort than in the comparison cohort (2.68 vs 1.88 per 1000 person-years, respectively). Patients with NASD had a higher risk of CKD compared with the comparison cohort (crude HR = 1.42 [95% CI, 1.32–1.53]; P < 0.001) (Table 2). After we adjusted for the covariates, the risk of developing CKD remained significant for patients with NASD (adjusted HR = 1.13; 95% CI = 1.05–1.22; P < 0.01). The incidence of CKD was higher in men than in women in both cohorts. Men in the NASD cohort had a significantly higher risk of CKD compared with men without NASD (adjusted HR = 1.16 [95% CI = 1.04–1.30]; P < 0.01). The incidence rate of CKD increased with age in both cohorts. Patients younger than age 49 years in the NASD cohort had a 32% increased risk of CKD compared with those in the comparison cohort (adjusted HR = 1.32 [95% CI = 1.09–1061]; P < 0.01). Patients free from comorbidities in the NASD cohort had a 33% increased risk of CKD compared with those in the comparison cohort (adjusted HR = 1.32 [95% CI = 1.09–1.61]; P < 0.001). However, patients with concomitant comorbidities in the NASD cohort carried a risk comparable with that of the comparison cohort (adjusted HR = 1.06 [95% CI = 0.97–1.16]).
Table 2.
Incidence and HRs of Chronic Kidney Diseases for NASD Cohort Compared With Non-NASD Cohort by Demographic Characteristics and Comorbidity
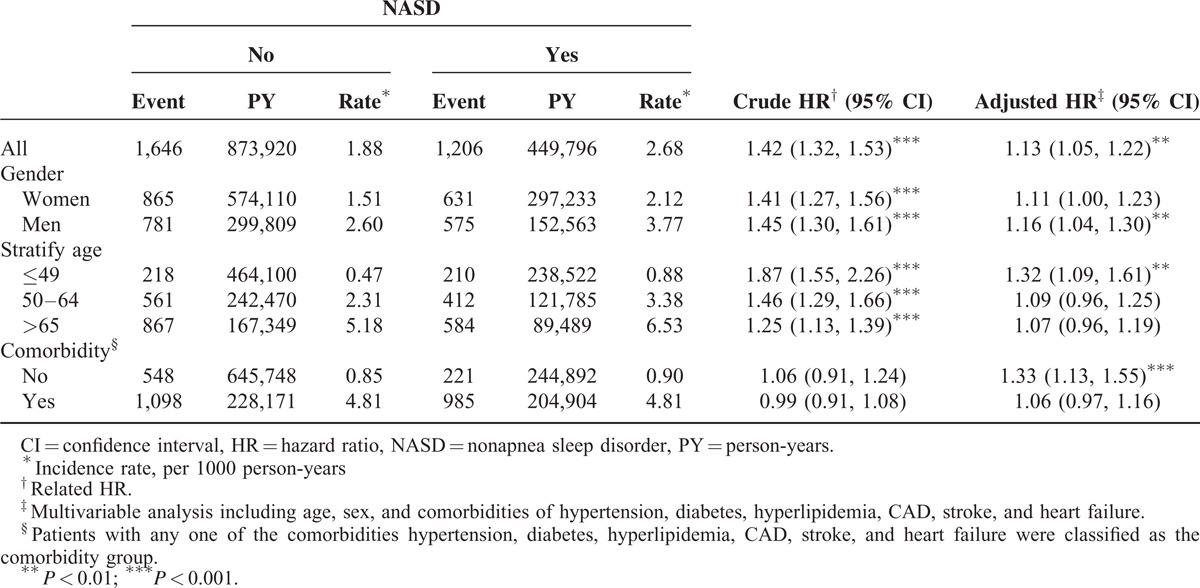
Table 3 shows that the risks of developing CKD in NASD patients increased significantly with concomitant comorbidities. Cases with NASD and diabetes had a higher risk of CKD than did the group without NASD and diabetes (adjusted HR = 4.89; 95% CI = 4.28–5.57; P < 0.001). Subgroup patients with interaction conditions were associated with an increased risk of CKD regarding NASD and hypertension (adjusted HR = 2.90; 95% CI = 2.61–3.22; P = 0.03), NASD and hyperlipidemia (adjusted HR = 2.37; 95% CI = 2.13–2.65; P = 0.001), NASD and CAD (adjusted HR = 1.84; 95% CI = 1.64–2.05; P < 0.001), NASD and stroke (adjusted HR = 1.87; 95% CI = 1.48–2.36; P = 0.007), and NASD and heart failure (adjusted HR = 2.08; 95% CI = 1.64–2.65; P = 0.002). Interactions between NASD and comorbidities were significant (all interaction P < 0.05).
Table 3.
Cox Proportional Hazard Regression Analysis for the Risk of CKD-Associated NASD With Interaction of Comorbidity
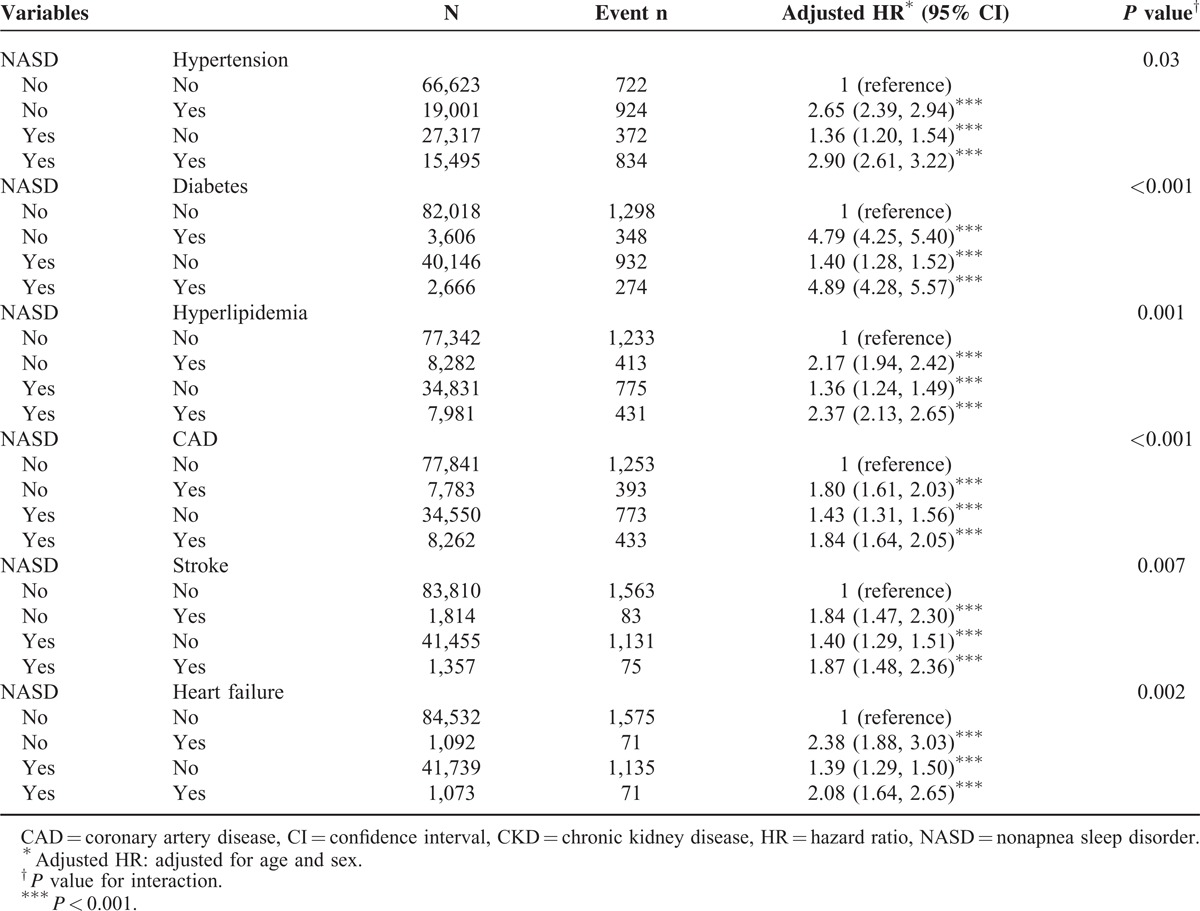
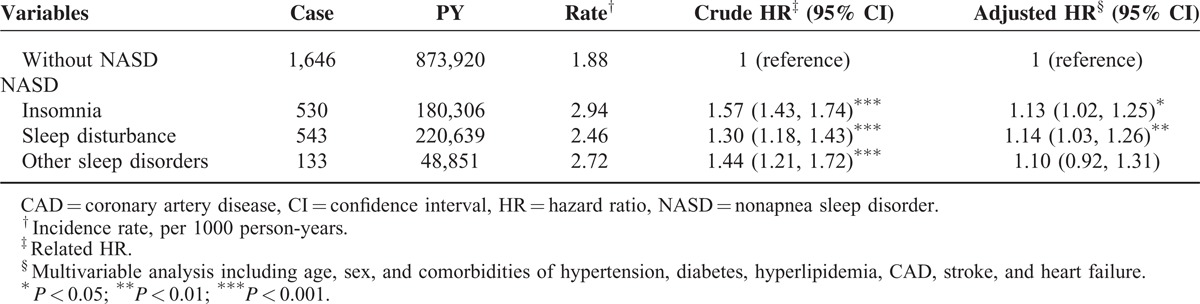
Table 4 shows different types of NASDs associated with the relative risks and hazards of CKD. Compared with the non-NASD cohort, patients with sleep disturbance associates had a 14% increased risk of developing CKD (95% CI = 1.03–1.26; P < 0.01). Patients with insomnia had a 13% increased risk of subsequent CKD (95% CI = 1.02–1.25; P < 0.05) compared with those in the non-NASD cohort.
Table 4.
Incidence Rates and HRs of Chronic Kidney Diseases in Patients With Different Types of NASDs
Kaplan–Meier survival analysis indicated that the CKD-free rate was 1% lower in the NASD cohort than in the comparison cohort (log-rank test, P < 0.0001) (Figure 2).
Figure 2.
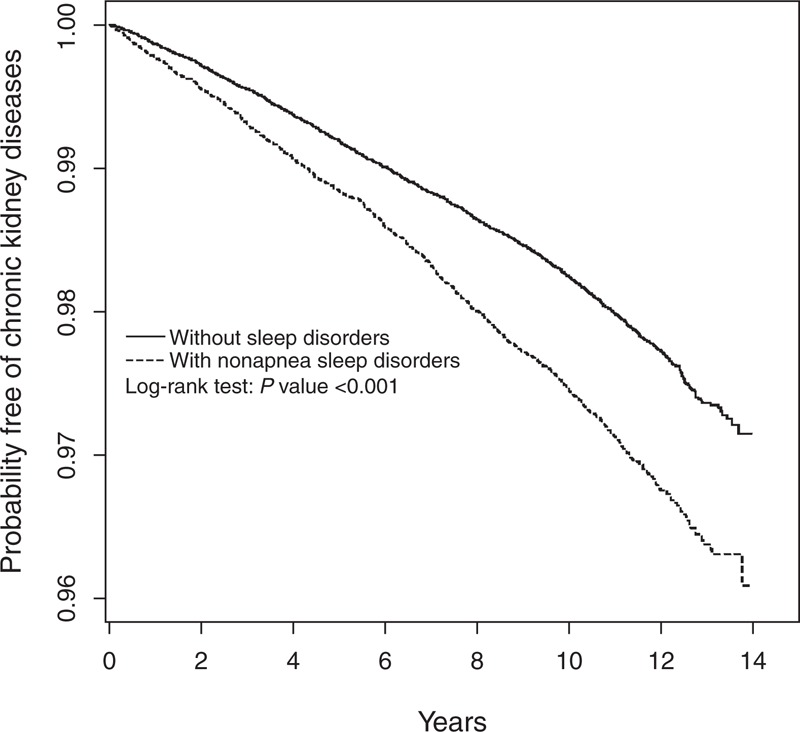
Probability free of chronic kidney diseases for patients with (dashed line) or without (solid line) nonapnea sleep disorders.
DISCUSSION
This study demonstrated that the incidence rate of CKD in the NASD cohort was 2.68 per 1000 person-years. The NASD cohort had a 1.13-fold higher risk of subsequent CKD than patients without NASD, after we adjusted for age, sex, and comorbidities. Comorbidities including hypertension, diabetes, hyperlipidemia, and heart failure were significant initiating or progression factors in CKD. In our study, the prevalence of NASD in the general population is 4.2%, compared with that in another large population-based study in the United States.31 Higher prevalence rates of insomnia in women and increased risks based on age were also observed in another study.30 Although NASD was more prevalent in women, men with NASD had a higher risk of CKD compared with men without NASD (adjusted HR = 1.16). In general, younger patients (≦49 years) carried a lower risk of CKD and other comorbidities. According to our results, younger patients with NASD already had significant risks of CKD (adjusted HR = 1.32). In addition, the effect of having NASD in subgroup patients without comorbidities carried a higher risk of CKD (adjusted HR = 1.33). Whereas in NASD patients with single or multiple comorbidities, the effect of having NASD became minor, because confounding effects may exist between comorbidities and NASD (adjusted HR = 1.06) (Table 2). Therefore, we used multiplicative analysis to examine the interaction effects between risk factors. The results showed that, as expected, significant confounding effects existed between NASD and other comorbidities. However, the interaction terms contributed additional significant risks of CKD development. The risk of CKD in patients with concomitant NASD and diabetes is 10% higher than in patients with diabetes alone (adjusted HRs = 4.89 vs 4.79, respectively) (Table 3). We further examined the association between the risk of CKD and subgroups of NASD. The risks of CKD were significant in the insomnia and sleep disturbance associate subgroups, but not in patients with other sleep disorders (adjusted HRs = 1.13, 1.14 vs 4.79, respectively) (Table 4).
Sleep disorders are classified into major categories such as insomnia, sleep-related breathing disorders, sleep-related movement disorders, and other sleep disorders. Research on sleep disorders has mainly focused on OSA. A study proposed that, through hypoxemia and obesity, OSA can increase cardiovascular risks.16 In addition, OSA is associated with hypoxemia and sleep fragmentation, which activates sympathetic activities, the renin–angiotensin–aldosterone system, and endothelial dysfunction, thus predisposing patients to renal damage.19 However, insomnia, the most prevalent sleep disorder, also has several consequences including decreased quality of life, degraded performance, and increased cardiometabolic risks.24,34,35 We hypothesized that in other sleep disorders, particularly insomnia, patients may experience the phenomenon of renal dysfunction observed in OSA patients.
To explain the association between NASD and CKD, studies have evaluated the effect of sleep disorders on the development of cardiovascular diseases. Insomnia with short sleep duration is associated with inflammation, increased sympathetic arousal, and dysregulation of the neuroendocrine system,36,37 which are subsequently related to diseases such as hypertension, obesity, and diabetes.20,21,24
One study provided direct biochemical evidence that acute sleep deprivation causes endothelial dysfunction before the increase in sympathetic activity and systolic blood pressure. Markers of vascular endothelial cell activation, including soluble intercellular adhesion molecule-1, E-selectin, and interleukin-6, raised before the elevation of sympathetic activity markers such as blood pressure and heart rate in sleep deprivation.38 Another study reported that carotid intima–media thickness was greater in elderly patients with a total sleep time of ≦5 hours compared with those with a total sleep time of ≧7 hours, implying that short sleep duration was associated with atherosclerotic risk.39 Short-term sleep deprivation has been associated with a decrease in leptin levels, impaired insulin sensitivity, and an increase in hunger, thus predisposing adults to obesity.37
The activation of sympathetic activities, including the elevated level of urinary norepinephrine and increased blood level of epinephrine and norepinephrine with half a night of sleep deprivation, was observed.40 In an in vivo model, norepinephrine can stimulate production of inflammatory mediators including IL-6 and tumor necrosis factor-α.41 A recent study examined the association between sleep duration and urinary albumin–creatinine ratio (UACR), determining that both short and long sleep durations were significantly associated with high UACR levels, an early marker of renal damage in type 2 diabetic patients.42
In summary, these cardiometabolic effects of sleep disorders might confer high risks of developing CKD. Although most of these studies have been confined to small sample sizes and a short-term follow-up, they have provided indirect evidence to support our hypothesis.
LIMITATIONS
The strengths of our study are based on using longitudinal, population-based data, demonstrating demographic characteristics for CKD and insomnia validated by previous studies on general populations. In addition, the association between 2 comorbid diseases was identified, thus yielding potential prevention implications. Our findings should be interpreted in the context of the inherent limitations of using an administrative database.
First, the NHIRD contains no detailed information regarding smoking habits, socioeconomic status, body mass index, or family history of renal diseases. The relevant biochemical variables, such as the estimated glomerular filtration rate and albuminuria level, which are critical measures of CKD severity with prognostic implications, were unavailable. These variables cannot be adjusted in analysis, because bias resulting from residual confounders might have affected the results.
Second, the diagnosis of insomnia or sleep disturbance was made by physicians, and a prospective survey questionnaire for determining the objective sleep duration and quality of life of patients was lacking. Nevertheless, the claims data regarding the diagnosis of NASDs, CKD, and other comorbidity diagnoses were nonetheless reliable because of the validity of the database,33 large sample size, and longitudinal follow-up.
Finally, data derived from a retrospective cohort study are generally of lower statistical quality than those derived from randomized trials because of potential biases. However, the association observed between NASD and CKD remained significantly independent of potential confounders.
CONCLUSION
Our study is the first population-based retrospective cohort study demonstrating that NASD may be a significant risk factor for CKD. Patients with concomitant NASD and comorbidities, as well as patients who are elderly or male, should be monitored for long-term renal consequences. Because of the rising prevalence and improved public awareness of NASD and CKD, early diagnosis and behavioral or medical treatment of sleep disorders have become crucial in preventing CKD.
Footnotes
Abbreviations: CAD = coronary artery disease, CKD = chronic kidney disease, DM = diabetes mellitus, ESRD = end-stage renal disease, NASD = nonapnea sleep disorder, OSA = obstructive sleep apnea, RRT = renal replacement therapy.
This work was supported by the study projects (CMU102-BC-2) in China Medical University; Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW103-TDU-B-212-113002); and health and welfare surcharge of tobacco products, China Medical University Hospital Cancer Research Center of Excellence (MOHW103-TD-B-111-03, Taiwan).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Levey AS, Stevens LA, Schmid CH, et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Levey AS, Schoolwerth AC, Burrows NR, et al. Centers for Disease Control and Prevention Expert Panel. Comprehensive public health strategies for preventing the development, progression, and complications of CKD: report of an expert panel convened by the Centers for Disease Control and Prevention. Am J Kidney Dis 2009; 53:522–535. [DOI] [PubMed] [Google Scholar]
- 3.Wen CP, Cheng TY, Tsai MK, et al. All-cause mortality attributable to chronic kidney disease: a prospective cohort study based on 462 293 adults in Taiwan. Lancet 2008; 371:2173–2182. [DOI] [PubMed] [Google Scholar]
- 4.Tangri N, Stevens LA, Griffith J, et al. A predictive model for progression of chronic kidney disease to kidney failure. JAMA 2011; 305:1553–1559. [DOI] [PubMed] [Google Scholar]
- 5.Sarnak MJ, Levey AS, Schoolwerth AC, et al. American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003; 108:2154–2169. [DOI] [PubMed] [Google Scholar]
- 6.Weiner DE, Tighiouart H, Griffith JL, et al. Kidney disease, Framingham risk scores, and cardiac and mortality outcomes. Am J Med 2007; 120:552.e1–552.e8. [DOI] [PubMed] [Google Scholar]
- 7.Tonelli M, Muntner P, Lloyd A, et al. Alberta Kidney Disease Network. Risk of coronary events in people with chronic kidney disease compared with those with diabetes: a population-level cohort study. Lancet 2012; 380:807–814. [DOI] [PubMed] [Google Scholar]
- 8.Matsushita K, van der Velde M, Astor BC, et al. Chronic Kidney Disease Prognosis Consortium. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010; 375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menon V, Wang X, Sarnak MJ, et al. Long-term outcomes in nondiabetic chronic kidney disease. Kidney Int 2008; 73:1310–1315. [DOI] [PubMed] [Google Scholar]
- 10.Packham DK, Alves TP, Dwyer JP, et al. Relative incidence of ESRD versus cardiovascular mortality in proteinuric type 2 diabetes and nephropathy: results from the DIAMETRIC (Diabetes Mellitus Treatment for Renal Insufficiency Consortium) database. Am J Kidney Dis 2012; 59:75–83. [DOI] [PubMed] [Google Scholar]
- 11.Haroun MK, Jaar BG, Hoffman SC, et al. Risk factors for chronic kidney disease: a prospective study of 23,534 men and women in Washington County, Maryland. J Am Soc Nephrol 2003; 14:2934–2941. [DOI] [PubMed] [Google Scholar]
- 12.Hallan S, de Mutsert R, Carlsen S, et al. Obesity, smoking, and physical inactivity as risk factors for CKD: are men more vulnerable? Am J Kidney Dis 2006; 47:396–405. [DOI] [PubMed] [Google Scholar]
- 13.Zhu P, Liu Y, Han L, et al. Serum uric acid is associated with incident chronic kidney disease in middle-aged populations: a meta-analysis of 15 cohort studies. PLoS One 2014; 9:e100801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Driver TH, Shlipak MG, Katz R, et al. Low serum bicarbonate and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis 2014; 64:534–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rayner HC, Baharani J, Dasgupta I, et al. Does community-wide chronic kidney disease management improve patient outcomes? Nephrol Dial Transplant 2014; 29:644–649. [DOI] [PubMed] [Google Scholar]
- 16.Floras JS. Sleep apnea and cardiovascular risk. J Cardiol 2014; 63:3–8. [DOI] [PubMed] [Google Scholar]
- 17.Parish JM, Somers VK. Obstructive sleep apnea and cardiovascular disease. Mayo Clin Proc 2004; 79:1036–1046. [DOI] [PubMed] [Google Scholar]
- 18.Drager LF, Togeiro SM, Polotsky VY, et al. Obstructive sleep apnea: a cardiometabolic risk in obesity and the metabolic syndrome. J Am Coll Cardiol 2013; 62:569–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adeseun GA, Rosas SE. The impact of obstructive sleep apnea on chronic kidney disease. Curr Hypertens Rep 2010; 12:378–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez-Mendoza J, Vgontzas AN, Liao D, et al. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension 2012; 60:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vgontzas AN, Liao D, Pejovic S, et al. Insomnia with objective short sleep duration is associated with type 2 diabetes: a population-based study. Diabetes Care 2009; 32:1980–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lai YJ, Lin CL, Lin MC, et al. Population-based cohort study on the increase in the risk for type 2 diabetes mellitus development from nonapnea sleep disorders. Sleep Med 2013; 14:913–918. [DOI] [PubMed] [Google Scholar]
- 23.Huang WS, Tsai CH, Lin CL, et al. Nonapnea sleep disorders are associated with subsequent ischemic stroke risk: a nationwide, population-based, retrospective cohort study. Sleep Med 2013; 14:1341–1347. [DOI] [PubMed] [Google Scholar]
- 24.Sivertsen B, Lallukka T, Salo P, et al. Insomnia as a risk factor for ill health: results from the large population-based prospective HUNT Study in Norway. J Sleep Res 2014; 23:124–132. [DOI] [PubMed] [Google Scholar]
- 25.Luyster FS, Kip KE, Buysse DJ, et al. Traditional and nontraditional cardiovascular risk factors in comorbid insomnia and sleep apnea. Sleep 2014; 37:593–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Zhang X, Winkelman JW, et al. Association between insomnia symptoms and mortality: a prospective study of U.S. men. Circulation 2014; 129:737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahmad S, Gupta R, Dhyani M. Prevalence and correlates of insomnia and obstructive sleep apnea in chronic kidney disease. N Am J Med Sci 2013; 5:641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hui DS, Wong TY, Ko FW, et al. Prevalence of sleep disturbances in Chinese patients with end-stage renal failure on continuous ambulatory peritoneal dialysis. Am J Kidney Dis 2000; 36:783–788. [DOI] [PubMed] [Google Scholar]
- 29.Hirotsu C, Tufik S, Bergamaschi CT, et al. Sleep pattern in an experimental model of chronic kidney disease. Am J Physiol Renal Physiol 2010; 299:F1379–F1388. [DOI] [PubMed] [Google Scholar]
- 30.Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev 2002; 6:97–111. [DOI] [PubMed] [Google Scholar]
- 31.Hermes E, Rosenheck R. Prevalence, pharmacotherapy and clinical correlates of diagnosed insomnia among veterans health administration service users nationally. Sleep Med 2014; 15:508–514. [DOI] [PubMed] [Google Scholar]
- 32.Cheng TM. Okma KGH, Crivelli L. Taiwan's national health insurance system: high value for the dollar. Six Countries, Six Reform Models: The Health Reform Experience of Israel, the Netherlands, New Zealand, Singapore, Switzerland and Taiwan. New Jersey: World Scientific; 2009; 71–204. [Google Scholar]
- 33.Cheng CL, Kao YH, Lin SJ. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf 2011; 20:236–242. [DOI] [PubMed] [Google Scholar]
- 34.Léger D, Massuel MA, Metlaine A. SISYPHE Study Group. Professional correlates of insomnia. Sleep 2006; 29:171–178. [PubMed] [Google Scholar]
- 35.Daley M, Morin CM, LeBlanc M, et al. The economic burden of insomnia: direct and indirect costs for individuals with insomnia syndrome, insomnia symptoms, and good sleepers. Sleep 2009; 32:55–64. [PMC free article] [PubMed] [Google Scholar]
- 36.Irwin M, Clark C, Kennedy B, et al. Nocturnal catecholamines and immune function in insomniacs, depressed patients, and control subjects. Brain Behav Immun 2003; 17:365–372. [DOI] [PubMed] [Google Scholar]
- 37.Pejovic S, Vgontzas AN, Basta M, et al. Leptin and hunger levels in young healthy adults after one night of sleep loss. J Sleep Res 2010; 19:552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sauvet F, Leftheriotis G, Gomez-Merino D, et al. Effect of acute sleep deprivation on vascular function in healthy subjects. J Appl Physiol 2010; 108:68–75. [DOI] [PubMed] [Google Scholar]
- 39.Nakazaki C, Noda A, Koike Y, et al. Association of insomnia and short sleep duration with atherosclerosis risk in the elderly. Am J Hypertens 2012; 25:1149–1155. [DOI] [PubMed] [Google Scholar]
- 40.Irwin M, Thompson J, Miller C, et al. Effects of sleep and sleep deprivation on catecholamine and interleukin-2 levels in humans: clinical implications. J Clin Endocrinol Metab 1999; 84:1979–1985. [DOI] [PubMed] [Google Scholar]
- 41.van Gool J, van Vugt H, Helle M, et al. The relation among stress, adrenalin, interleukin 6 and acute phase proteins in the rat. Clin Immunol Immunopathol 1990; 57:200–210. [DOI] [PubMed] [Google Scholar]
- 42.Ohkuma T, Fujii H, Iwase M, et al. Association between sleep duration and urinary albumin excretion in patients with type 2 diabetes: the Fukuoka diabetes registry. PLoS One 2013; 8:e78968. [DOI] [PMC free article] [PubMed] [Google Scholar]


