Abstract
To perform a meta-analysis and examine the use of D-dimer levels for diagnosing acute aortic dissection (AAD).
Medline, Cochrane, EMBASE, and Google Scholar were searched until April 23, 2014, using the following search terms: biomarker, acute aortic dissection, diagnosis, and D-dimer. Inclusion criteria were diagnosis of acute aortic dissection, D-dimer levels obtained, 2-armed study. Outcome measures were the accuracy, sensitivity, specificity, positive predictive value, and negative predictive value of D-dimer level for the diagnosis of AAD. Sensitivity analysis was performed using the leave-one-out approach.
Of 34 articles identified, 5 met the inclusion criteria and were included in the analysis. The age of participants was similar between treatments within studies. The number of AAD patients ranged from 16 to 107 (total = 274), and the number of control group patients ranged from 32 to 206 (total = 469). The pooled sensitivity of D-dimer levels in AAD patients was 94.5% (95% confidence interval [CI] 78.1%–98.8%, P < 0.001), and the specificity was 69.1% (95% CI 43.7%–86.5%, P = 0.136). The pooled area under the receiver-operating characteristic curve for D-dimer levels in AAD patients was 0.916 (95% CI 0.863–0.970, P < 0.001). The direction and magnitude of the combined estimates did not change markedly with the exclusion of individual studies, indicating the meta-analysis had good reliability.
D-dimer levels are best used for ruling out AAD in patients with low likelihood of the disease.
INTRODUCTION
Acute aortic dissection (AAD) is an uncommon, but rapidly fatal medical emergency for which accurate early diagnosis and management are critical. Typical symptoms of AAD are the acute onset of tearing chest, back, and/or abdominal pain, and findings can include asymmetric blood pressure and a widened mediastinum on chest x-ray.1 However, not all patients present with typical findings; in many cases, symptoms are nonspecific, and a missed diagnosis can result in significant morbidity and death.1
The 2 commonly used classifications of aortic dissections are the DeBakey and Stanford systems.1 The DeBakey system classifies dissections based on where the intimal tear originated and the extent of dissection, whereas the Stanford system divides dissections into 2 types based on whether the ascending aorta is involved. Stanford type A aortic dissections include both DeBakey I and II variants, involve the ascending aorta, and require surgical intervention. Stanford type B dissections (DeBakey III) do not involve the ascending aorta, and can often be managed medically in the absence of complications. Currently, the diagnosis of AAD relies on imaging methods such as echocardiography (transesophageal or transthoracic), contrast-enhanced computed tomography (CT), and magnetic resonance imaging.2 But alternative diagnostic tools such as biomarkers that can be easily assayed via blood test are being sought. Potential candidates for biomarkers include smooth muscle-myosin heavy chain, creatine kinase isozyme, soluble elastin fragments, matrix metalloproteinases, C-reactive protein, N-terminal pro-brain natriuretic peptide, tenascin-C, fibrin degradation products, and calponin, all of which have been reported to increase in the event of an AAD.3–9 However, measuring systems and reference ranges are not commonly available for most of these markers.
D-dimer is a small protein fragment that is the product of fibrin degradation after a blood clot is degraded by fibrinolysis. Many conditions including inflammation, trauma, recent surgery, pregnancy, and malignancy can result in elevated D-dimer levels,2,7 so its use as a positive biomarker is somewhat limited. However, it can be used as a negative marker to rule out certain diseases, such as in pulmonary embolism (PE), a low serum D-dimer level combining with low clinical pretest probability and suspicion can rule out the condition.10 Conversely, D-dimer levels have been reported to increase in AAD, and as with PE, D-dimer has the potential use to rule out AAD in patients with a low clinical probability for the condition.11–15 The test also has the potential to differentiate between AAD and acute myocardial infarction (AMI), as and AMI patients tend to have elevated but still significantly lower of D-dimer levels than AAD patients.10
The objective of this systematic review of the literature and meta-analysis is to examine the evidence for using D-dimer levels in the emergent diagnosis of AAD, to effectively reduce the chance of missing or misdiagnosing AAD.
MATERIALS AND METHODS
Search Strategy and Selection Criteria
This systematic review and meta-analysis was conducted in accordance with PRISMA (preferred reporting items for systematic reviews and meta-analyses) guidelines. The meta-analysis does not involve patients, and thus does not require Institutional Reviewer Board approval. Medline, Cochrane, EMBASE, and Google Scholar were searched until April 23, 2014, using combinations of the following search terms: biomarker, acute aortic dissection, diagnosis, and D-dimer. Reference lists of relevant studies were hand searched. Inclusion criteria were: diagnosis of acute aortic dissection, D-dimer levels were obtained, study was 2-armed. Letters, comments, editorials, case reports, proceedings, personal communications, and non-English publications were excluded.
Study Selection and Data Extraction
Studies were identified via the search strategy by 2 independent reviewers. Where there was uncertainty regarding eligibility, a third reviewer was consulted. The following information/data were extracted from studies that met the inclusion criteria: the name of the first author, year of publication, study design, number of participants in each treatment group, participants’ age and sex, type of aortic dissection, medical conditions other than aortic dissection, D-dimer levels, and accuracy, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of D-dimer levels for the diagnosis of AAD.
Quality Assessment
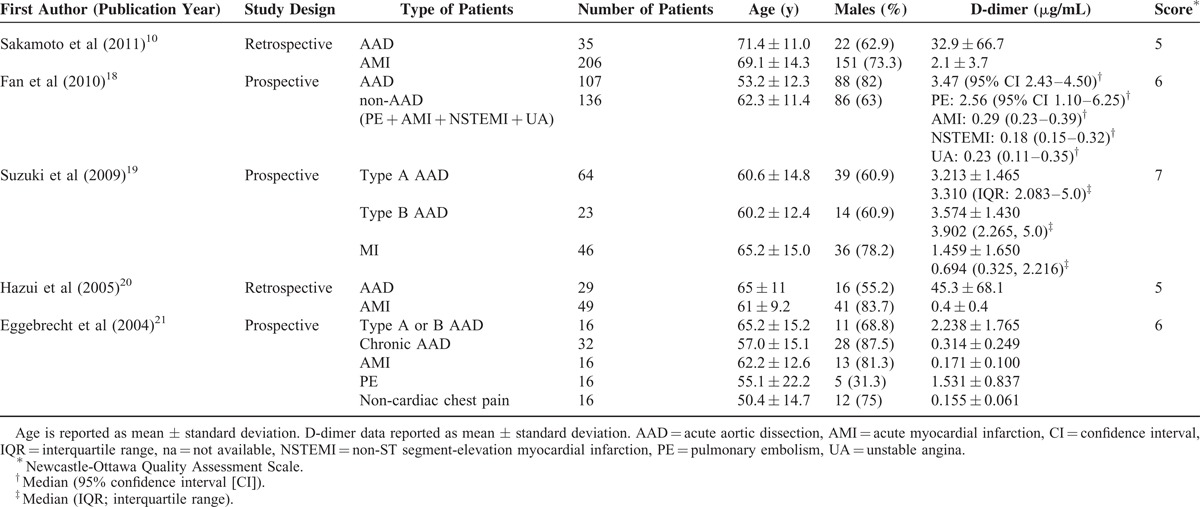
The Newcastle-Ottawa Quality Assessment Scale was used to assess the quality of the included studies.16 The Newcastle-Ottawa Scale is a valid tool for evaluating nonrandomized studies with regard to 3 criteria: patient selection, comparability of study groups, and outcome assessment. Quality assessment was also performed by the independent reviewers and a third reviewer was consulted for any uncertainties. The quality assessment score for each study was listed in Table 1, with the acceptable score being at least 5.
Table 1.
Characteristics and Outcomes of Studies Included in the Meta-analysis
Outcome Measures and Quantitative Data Synthesis
The accuracy, sensitivity, specificity, and area under the receiver-operating characteristic curve (AUC) with standard error or 95% confidence interval (CI) of D-dimer level for the diagnosis of AAD were summarized for each study. The sensitivity, specificity, and AUC were considered in AAD patients as for the effect sizes for meta-analysis. The estimated 95% CIs were calculated for each of effect sizes and for each of studies. A pooled effect size with corresponding 95% CI for total studies was calculated as well. A greater value of effect size implies better diagnostic performance of serum D-dimer level in AAD patients.
Heterogeneity among the eligible studies was assessed by determining the Cochran Q and the I2 statistic. For Cochran Q, a value of P < 0.10 indicates statistically significant heterogeneity. For the I2 statistic, which indicates the percentage of the observed between-study variability due to heterogeneity rather than chance, lack of heterogeneity is indicated by a value of 0% to 25%, moderate heterogeneity by 25% to 50%, large heterogeneity by 50% to 75%, and extreme heterogeneity by 75% to 100%. If either the Cochran Q (P < 0.10) or I2 statistics (>50%) demonstrated the existence of heterogeneity between studies, a random-effects model (DerSimonian–Laird method) of analysis was used. Otherwise, a fixed-effects model (Mantel–Haenszel method) of analysis was used.
Sensitivity analysis was carried out for the outcomes using the leave-one-out approach. Publication bias analysis was only performed if there were >5 studies as funnel plot asymmetry cannot be detected if the number of studies is ≤5.17 In all analyses, a 2-sided value of P < 0.05 was considered to indicate statistical significance. Statistical analyses were performed using Comprehensive meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ).
RESULTS
Literature Search
A flow diagram of study selection is shown in Figure 1. A total of 34 articles were initially identified, and subsequently 18 were excluded because they were duplicates or did not meet the inclusion criteria. Thus, 16 full-text articles were assessed, and of these 11 were excluded, the reasons for which were shown in Figure 1. The remaining 5 studies 10,18–21 met the requirement to be included in the meta-analysis, but only 410,18,20,21 of these 5 provided AUC data to be used in the quantitative analysis.
FIGURE 1.
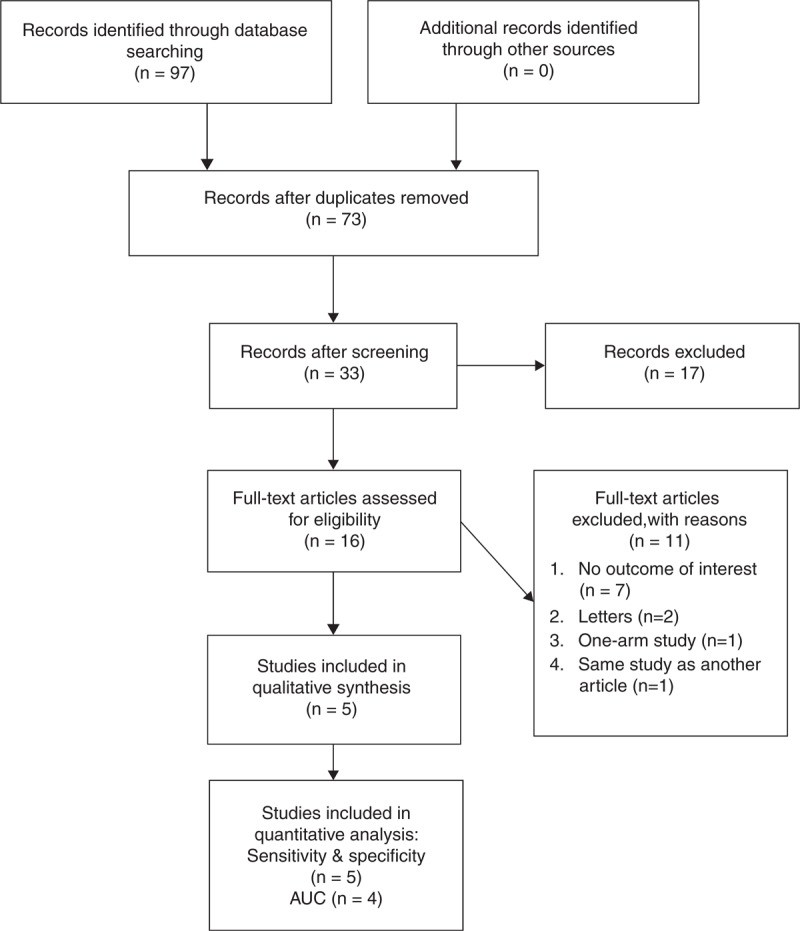
Flow diagram of study selection. AUC = area under the receiver-operating characteristic curve (adapted from PRISMA 2009 Flow Diagram template).
Study Characteristics
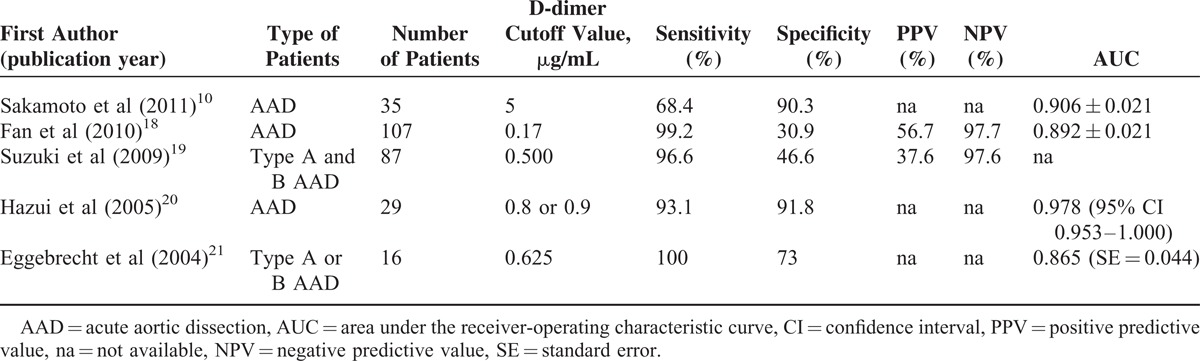
The characteristics of the 5 studies included in the meta-analysis are summarized in Table 1. The age of the participants was very similar between treatments within studies, except for that of the control group in the study by Eggebrecht et al21 wherein the mean age was 30.4 years. The number of AAD patients in the studies ranged from 16 to 107 (total = 274), and the number of patients in the control groups ranged from 32 to 206 (total = 469). D-dimer outcomes of the included studies are summarized in Table 2.
TABLE 2.
D-dimer Outcomes of the Included Studies
Outcome Measures
Sensitivity and Specificity
All 5 studies provided complete sensitivity and specificity data, and were included in the analysis. Forest plots summarizing the sensitivity and specificity of D-dimer levels are shown in Figure 2A and B, respectively. A random-effects analysis was applied because there was evidence of heterogeneity among the studies (sensitivity: Cochran Q = 24.998, I2 = 84%, P < 0.001; specificity: Cochran Q = 45.615, I2 = 91.23%, P < 0.001). The pooled sensitivity of D-dimer levels in AAD patients was 94.5% (95% CI 78.1%–98.8%, P < 0.001), and the specificity was 69.1% (95% CI 43.7%–86.5%, P = 0.136).
FIGURE 2.
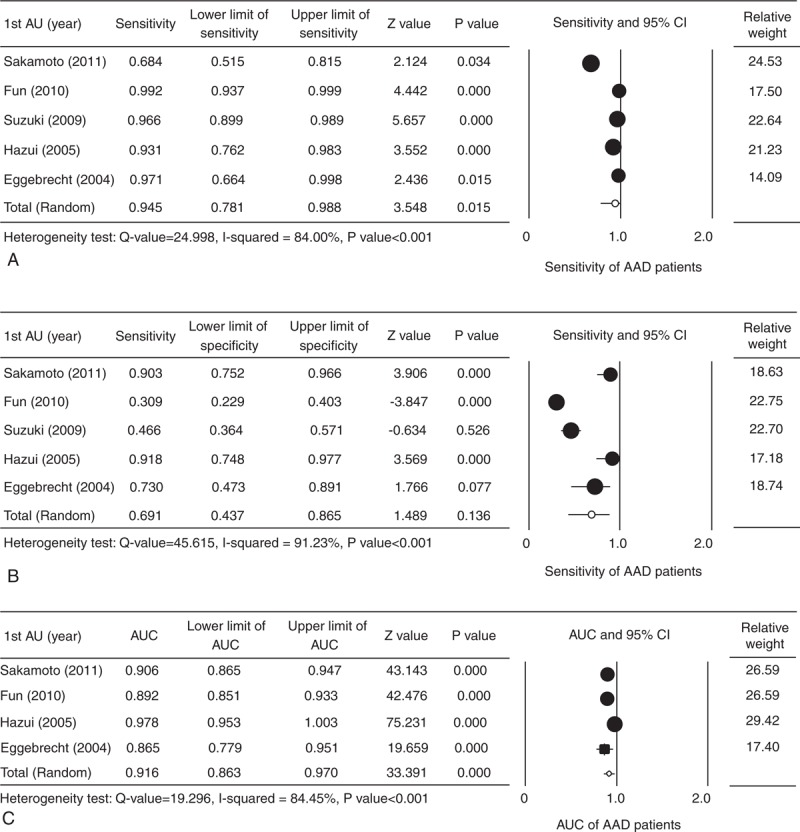
Forest plot evaluating the sensitivity (A), specificity (B), and AUC (C) of D-dimer levels in AAD patients. AAD = acute aortic dissection, AU = author, AUC = area under the receiver-operating characteristic curve, CI = confidence interval, lower limit = lower bound of the 95% CI, upper limit = upper bound of the 95% CI.
AUC
Four studies10,18,20,21 provided complete AUC data for AAD patients, and were included in the analysis. Forest plots of the pooled AUC data are shown in Figure 2C. A random-effects analysis was applied because there was an evidence of heterogeneity among the studies (Cochran Q = 19.26, I2 = 84.45%, P < 0.001). The pooled AUC for D-dimer levels in AAD patients was 0.916 (95% CI 0.863–0.970, P < 0.001). The AUC in diagnostics includes both sensitivity and specificity.
Sensitivity Analysis
Sensitivity analysis of the influence of each study on the pooled estimate for sensitivity, specificity, and AUC of D-dimer levels is shown in Figure 3A–C, respectively. The direction and magnitude of the combined estimates did not change markedly with the exclusion of individual studies, indicating that the meta-analysis had good reliability.
FIGURE 3.
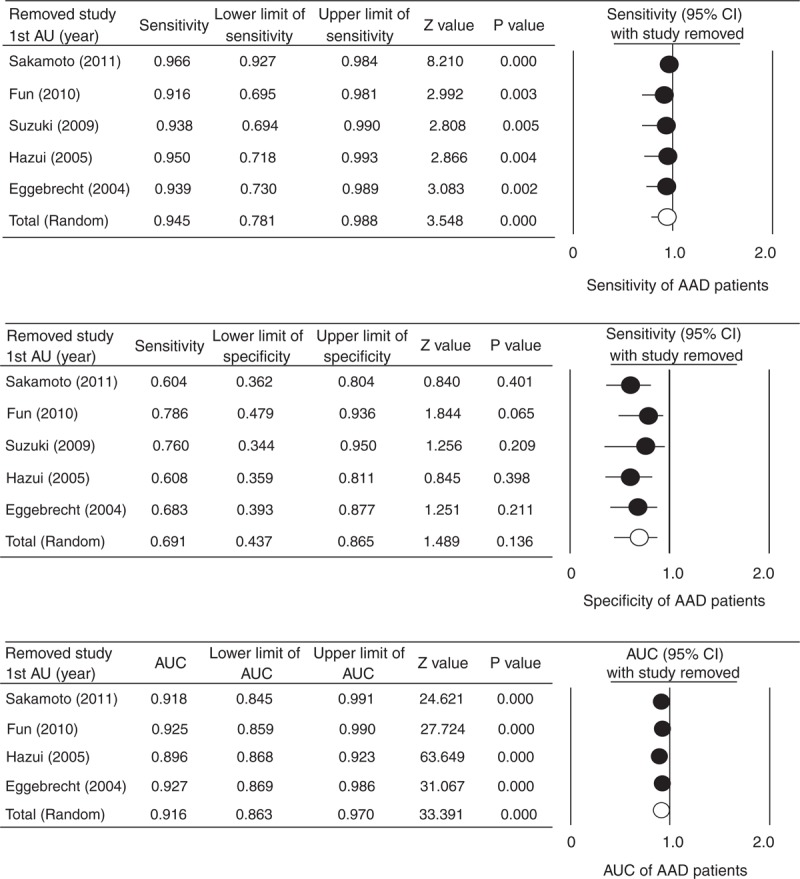
Sensitivity analysis of the influence of each study on the pooled estimate for sensitivity (A), specificity (B), and AUC (C) of D-dimer levels AAD patients using the leave-one-out approach. AAD = acute aortic dissection, AU = author, AUC = area under the receiver-operating characteristic curve, CI = confidence interval, lower limit = lower bound of the 95% CI, upper limit = upper bound of the 95% CI.
Publication Bias
Publication bias analysis was not performed because >5 studies are required to detect funnel plot asymmetry.
DISCUSSION
Acute aortic dissection (AAD) involving the ascending aorta (Stanford type A or DeBakey I and II) is highly lethal due to potential rupturing of ascending thoracic aorta, and requires emergent surgery to replace the ascending portion with a graft. It can involve the aortic valves leading to aortic incompetence. Stanford type B aortic dissection also can result in mortality and severe morbidity. The purpose of this meta-analysis was to examine the use of D-dimer levels for the diagnosis of AAD. The results indicate that D-dimer levels have very high sensitivity but only fair specificity for the diagnosis of AAD, and are best used for ruling out AAD in patients with a low likelihood of the disease.
A number of studies have examined the use of D-dimer levels to rule in or rule out a diagnosis of AAD.11–15 A large prospective multicenter study by Suzuki et al22 included that 220 patients with a suspicion of AAD found that a D-dimer cutoff level of 500 ng/mL, which is the same value for ruling out PE, was reliable for ruling out AAD with a negative likely hood ratio of 0.07 during the first 24 hours after symptom occurrence. In a retrospective review of 99 patients seen in the emergency department with a suspicion of AAD, Ersel et al23 found that using a D-dimer cutoff value of 0.246 μg/mL the sensitivity, specificity, PPV, and NPV for the diagnosis of AAD were 96.6%, 52.2%, 46.8%, and 97.3%, respectively. The AUC for excluding AAD based on a negative result was 0.764 (95% CI 0.674–0.855; P < 0.001). Nazerian et al24 combined a D-dimer cutoff value of 500 ng/mL with an aortic dissection detection risk score, and reported that a risk score of ≤1 and a negative D-dimer test could accurately rule out an aortic dissection.
The findings of this study are similar to that of a prior meta-analysis that examined D-dimer levels for the diagnosis of AAD performed in 2007 by Marill.25 The analysis included 11 studies with a total of 349 aortic dissection patients, and used the D-dimer level 0.5 μg/mL as the cutoff value. The sensitivity of the D-dimer test was 94% (95% CI 91–96%), and the specificity ranged from 40% to 100%. Another systematic review done in 2007 by Sodeck et al26 that included 16 studies with a total of 437 patients reported that D-dimer cutoff values ranged from 0.1 to 0.9 mg/mL, and that the overall sensitivity of the test was 97% (95% CI 95–98%) with a negative likelihood ratio of 0.06 (95% CI 0.02– 0.13). A more recent meta-analysis done in 2011 by Shimony et al27 that included 7 studies involving 298 patients with an AAD and 436 without reported that D-dimer level had a sensitivity of 97% (95% CI 94–99%), NPV of 96% (95% CI 93%–98%), specificity of 56% (95% CI 51%–60%), and PPV of 60% (95% CI 55%–66%). The authors concluded that a D-dimer level <500 ng/mL was useful for ruling out AAD.
The findings of our meta-analysis are comparable with those reported by Marill,25 Sodeck et al,26 and Shimony et al,27 with the pooled sensitivity of 94.5% (95% CI 78.1%–98.8%, P < 0.001) and the specificity of 69.1% (95% CI 43.7%–86.5%, P = 0.136). But our analysis includes more recently published studies, with analyses of sensitivity and specificity based on the data of AAD patients (both Stanford types A and B) and non-AAD patients primarily with cardiac or pulmonary causes of chest pain. The results show that even when comparing to patients with cardiopulmonary origins of chest pain, D-dimer level still has a high sensitivity and fairly good specificity for AAD. As AAD can be devastating if the diagnosis is missed, findings of this analysis confirm D-dimer's potential as a negative marker in saving lives, reducing cost of unnecessary testing and distress for patients with low clinical suspicion or likelihood of AAD.
Serum D-dimer level has another potential use in differentiating AAD from AMI, but the determination of an exact cutoff value requires more future studies. It is very important to differentiate between these 2 diseases in the emergency, as the standard treatment for one can lead to a fatal outcome in the other. It is also possible that some patients can present with both AMI and an acute dissection, so a quick and reliable test that can unmask the underlying dissections in such cases would be very valuable. Although all of our included studies have control group subjects composed of patients with AMI, unfortunately data of AMI patients alone could not all be extracted to perform an analysis of D-dimer in differentiating between AAD and AMI.
There are other limitations of this study that need to be considered. Only 5 studies were included in the analysis, of which only 4 provided AUC data, and numbers of subject in these studies were relatively less. Subjects also included both Stanford type A and B patients, as the number of studies was too small for an analysis of either Stanford type A or B dissection to be performed separately. Publication selection bias also could not be assessed due to the small number of studies. Furthermore, many factors affecting D-dimer levels, such as shock, systemic inflammation, age, and chronic illness, could not be accounted for in the analysis; D-dimer level may also change throughout the time course of an aortic dissection. Lastly, this study did not evaluate the types of assays used to measure D-dimer levels.
In conclusion, the results of this study confirmed findings of other studies that D-dimer levels have very high sensitivity but only fair specificity for diagnosing AAD, and are best used as a negative marker for ruling out AAD in patients with low likelihood of the disease.
Footnotes
Abbreviations: AAD = acute aortic dissection, PPV = positive predictive value, NPV = negative predictive value, CT = computed tomography, PE = pulmonary embolism, AMI = acute myocardial infarction.
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Setacci F, Sirignano P, de Donato G, et al. Acute aortic dissection: natural history and classification. J Cardiovasc Surg (Torino) 2010; 51:641–646. [PubMed] [Google Scholar]
- 2.Listijono DR, Pepper JR. Current imaging techniques and potential biomarkers in the diagnosis of acute aortic dissection. JRSM Short Rep 2012; 3:76. 10.1258/shorts.2012.012079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nagaoka K, Sadamatsu K, Yamawaki T, et al. Fibrinogen/fibrin degradation products in acute aortic dissection. Intern Med 2010; 49:1943–1947. [DOI] [PubMed] [Google Scholar]
- 4.Shinohara T, Suzuki K, Okada M, et al. Soluble elastin fragments in serum are elevated in acute aortic dissection. Arterioscler Thromb Vasc Biol 2003; 23:1839–1844. [DOI] [PubMed] [Google Scholar]
- 5.Nozato T, Sato A, Hirose S, et al. Preliminary study of serum tenascin-C levels as a diagnostic or prognostic biomarker of type B acute aortic dissection. Int J Cardiol 2013; 168:4267–4269. [DOI] [PubMed] [Google Scholar]
- 6.Hagiwara A, Shimbo T, Kimira A, et al. Using fibrin degradation products level to facilitate diagnostic evaluation of potential acute aortic dissection. J Thromb Thrombolysis 2013; 35:15–22. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki T, Distante A, Eagle K. Biomarker-assisted diagnosis of acute aortic dissection: how far we have come and what to expect. Curr Opin Cardiol 2010; 25:541–545. [DOI] [PubMed] [Google Scholar]
- 8.Yuan SM, Shi YH, Wang JJ, et al. Elevated plasma D-dimer and hypersensitive C-reactive protein levels may indicate aortic disorders. Rev Bras Cir Cardiovasc 2011; 26:573–581. [DOI] [PubMed] [Google Scholar]
- 9.Wen D, Zhou XL, Li JJ, Hui RT. Biomarkers in aortic dissection. Clin Chim Acta 2011; 412:688–695. [DOI] [PubMed] [Google Scholar]
- 10.Sakamoto K, Yamamoto Y, Okamatsu H, et al. D-dimer is helpful for differentiating acute aortic dissection and acute pulmonary embolism from acute myocardial infarction. Hellenic J Cardiol 2011; 52:123–127. [PubMed] [Google Scholar]
- 11.Ohlmann P, Faure A, Morel O, et al. Diagnostic and prognostic value of circulating D-Dimers in patients with acute aortic dissection. Crit Care Med 2006; 34:1358–1364. [DOI] [PubMed] [Google Scholar]
- 12.Weber T, Högler S, Auer J, et al. D-dimer in acute aortic dissection. Chest 2003; 123:1375–1378. [DOI] [PubMed] [Google Scholar]
- 13.Sbarouni E, Georgiadou P, Marathias A, et al. D-dimer and BNP levels in acute aortic dissection. Int J Cardiol 2007; 122:170–172. [DOI] [PubMed] [Google Scholar]
- 14.Akutsu K, Sato N, Yamamoto T, et al. A rapid bedside D-dimer assay (cardiac D-dimer) for screening of clinically suspected acute aortic dissection. Circ J 2005; 69:397–403. [DOI] [PubMed] [Google Scholar]
- 15.Perez A, Abbet P, Drescher MJ. D-dimers in the emergency department evaluation of aortic dissection. Acad Emerg Med 2004; 11:397–400. [DOI] [PubMed] [Google Scholar]
- 16.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010; 25:603–605. [DOI] [PubMed] [Google Scholar]
- 17.Sutton AJ, Duval SJ, Tweedie RL, et al. Empirical assessment of effect of publication bias on meta-analyses. BMJ 2000; 320:1574–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fan QK, Wang WW, Zhang ZL, et al. Evaluation of D-dimer in the diagnosis of suspected aortic dissection. Clin Chem Lab Med 2010; 48:1733–1737. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki T, Distante A, Zizza A, et al. IRAD-Bio Investigators. Diagnosis of acute aortic dissection by D-dimer: the International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) experience. Circulation 2009; 119:2702–2707. [DOI] [PubMed] [Google Scholar]
- 20.Hazui H, Fukumoto H, Negoro N, et al. Simple and useful tests for discriminating between acute aortic dissection of the ascending aorta and acute myocardial infarction in the emergency setting. Circ J 2005; 69:677–682. [DOI] [PubMed] [Google Scholar]
- 21.Eggebrecht H, Naber CK, Bruch C, et al. Value of plasma fibrin D-dimers for detection of acute aortic dissection. J Am Coll Cardiol 2004; 44:804–809. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki T, Distante A, Zizza A, et al. International Registry of Acute Aortic Dissection Substudy on Biomarkers (IRAD-Bio) Investigators. Preliminary experience with the smooth muscle troponin-like protein, calponin, as a novel biomarker for diagnosing acute aortic dissection. Eur Heart J 2008; 29:1439–1445. [DOI] [PubMed] [Google Scholar]
- 23.Ersel M, Aksay E, Kıyan S, et al. Can D-dimer testing help emergency department physicians to detect acute aortic dissections? Anadolu Kardiyol Derg 2010; 10:434–439. [DOI] [PubMed] [Google Scholar]
- 24.Nazerian P, Morello F, Vanni S, et al. Combined use of aortic dissection detection risk score and D-dimer in the diagnostic workup of suspected acute aortic dissection. Int J Cardiol 2014; 175:78–82. [DOI] [PubMed] [Google Scholar]
- 25.Marill KA. Serum D-dimer is a sensitive test for the detection of acute aortic dissection: a pooled meta-analysis. J Emerg Med 2008; 34:367–376. [DOI] [PubMed] [Google Scholar]
- 26.Sodeck G, Domanovits H, Schillinger M, et al. D-dimer in ruling out acute aortic dissection: a systematic review and prospective cohort study. Eur Heart J 2007; 28:3067–3075. [DOI] [PubMed] [Google Scholar]
- 27.Shimony A, Filion KB, Mottillo S, et al. Meta-analysis of usefulness of d-dimer to diagnose acute aortic dissection. Am J Cardiol 2011; 107:1227–1234. [DOI] [PubMed] [Google Scholar]


