Supplemental Digital Content is available in the text
Abstract
Many meta-analyses have confirmed the technical feasibility and favorable short-term surgical outcomes of laparoscopic gastrectomy (LG) for gastric cancer patients, but the long-term survival outcome of LG remains controversial compared with open gastrectomy (OG).
This study aimed to compare the 5-year overall survival (OS), recurrence, and gastric cancer–related death of LG with OG among gastric cancer patients.
PubMed was searched to February 2014.
The resectable gastric cancer patients who underwent curative LG or OG were eligible. The studies that compared 5-year OS, recurrence, or gastric cancer–related death in the LG and OG groups were included.
A meta-analysis, meta-regression, sensitivity analysis, subgroup analysis, and stage-specific analysis were performed to estimate the survival outcome between the two groups and identify the potential confounders. Quality assessment was based on a tailored comparability scoring system.
Twenty-three studies with 7336 patients were included. The score of comparability between two groups and the extent of lymphadenectomy were two independent confounders. Based on the well-balanced studies, the 5-year OS (OR = 1.07, 95% CI 0.90–1.28, P = 0.45), recurrence (OR = 0.83, 95% CI 0.68–1.02, P = 0.08), and gastric cancer–related death (OR = 0.86, 95% CI 0.65–1.13, P = 0.28) rates were comparable in LG and OG. Several subsets such as the publication year, study region, sample size, gastrectomy pattern, extent of lymphadenectomy, number of nodes harvested, and proportion of T1–2 or N0–1 did not influence the estimates, if they were well balanced. Particularly, the stage-specific estimates obtained comparable results between the two groups.
Randomized controlled trials comparing LG with OG remain sparse to assess their long-term survival outcomes.
The major contributions of this systematic review compared with other meta-analyses are a comprehensive collection of available long-term survival outcomes within a much larger number of observations and a more precise consideration of confounders. Current knowledge indicates that the long-term survival outcome of laparoscopic gastric cancer surgery is comparable to that of open surgery among early or advanced stage gastric cancer patients, and LG is acceptable with regard to oncologic safety.
INTRODUCTION
Gastric cancer is the fourth most common cancer and the second most common cause of cancer death worldwide.1,2 Radical gastrectomy with lymphadenectomy is the essential curative approach for resectable gastric cancer patients.3,4 Laparoscopy-assisted distal gastrectomy for early gastric cancer was first introduced in 1991.5 During the latest two decades, laparoscopic gastric cancer surgery has become increasingly common in eastern Asia.6–8 In Western countries, laparoscopic gastrectomy (LG) for gastric cancer has received much attention.9,10
In the early period of the technique, LG was generally performed only for resection of early gastric cancers. A meta-analysis of randomized controlled trials concluded that laparoscopy-assisted gastrectomy (LAG) had a short-term advantage in the early recovery of gastric cancer patients such as by decreasing intraoperative blood loss and postoperative early morbidity.11 A recent report of a large Japanese nationwide cohort found similar beneficial results by laparoscopic distal gastrectomy among early gastric cancer patients.12 A growing number of reports has demonstrated the technical feasibility and safety of LG for locally advanced gastric cancer.13,14
With a high mortality-to-incidence ratio, the management of gastric cancer is challenging.2 The long-term survival effectiveness of LG is still pending considering pneumoperitoneum carbon dioxide, intra-abdominal hyperpressure, greater procedural complexity, a longer operation time, and a lower lymph node harvest.11,15 Concerning the oncological aspects, the application of LG for gastric cancer has been questioned because of early reports of port-site metastases.16 Reduced lymph node retrieval might violate the curability of a potentially radical resection.17 Pneumoperitoneum carbon dioxide and a prolonged operation time might impair the immune defense against metastasis and peritoneal seeding.18,19
The long-term survival of cancer patients is a key measure of the effectiveness of health care systems.20 This systematic review comprehensively searched available studies and performed meta-analyses to compare the 5-year survival outcomes of LG with those of conventional open gastrectomy (OG).
METHODS
Search Strategy
A comprehensive PubMed search from January 1990 to February 2014 was performed using the following strings: ”Stomach Neoplasms”[Mesh] AND ”Laparoscopy”[Mesh] AND (”1990”[PDAT]: ”2014”[PDAT]) AND ”humans”[MeSH Terms] AND English[lang]. Reference lists of systematic reviews or meta-analyses were additionally checked to identify potential eligible studies. The language of all of the publications was limited to English.
Study Eligibility
The eligible studies were selected according to the following criteria: (1) randomized or nonrandomized comparative studies were considered; (2) the patients included were diagnosed with gastric cancer; (3) early or locally advanced candidates were acceptable; (4) there were no limitations for race, age, or gender; (5) the staging system was based on the individual reports; (6) the patients in the LG and OG groups were compared; (7) the laparoscopic procedures mainly included LAG, and additionally totally laparoscopic gastrectomy (TLG) and hand-assisted laparoscopic gastrectomy (HALG) were also considered; (8) any extent of lymphadenectomy from D1 to D2+ was acceptable; (9) in the LG and OG groups, the range of follow-up length should cover 60 months; (10) all the potentially eligible studies should report at least one of the primary outcome measures, including the 5-year overall survival (OS), tumor recurrence, and gastric cancer–related death rates; and (11) the numbers of events could be extracted from the original reports.
Selection and Data Extraction
The procedures were performed in a peer-review manner by two independent reviewers. The general information that was extracted included the publication year, sample size, study design, general patient characteristics, and intervention details. The dichotomous data for the outcome measures mentioned above were extracted, including the total number of participants and events for each group. The number of events was calculated by the actual reported percentages, if possible. If the OS survival curves were presented, the number of individuals at risk was calculated by extracting the values at each inflexion. The recurrence data could be reported directly in the full text or calculated from the 5-year disease-free survival rate.
Quality Assessment
The quality of each included study was assessed by two approaches. First, the comparability (comparable, unclear, or not comparable) of 13 relevant items, including the tumor site, tumor size, histological differentiation, stage, patient age, patient sex, proportion of distal gastrectomy, proportion of D2 lymphadenectomy, number of nodes harvested, postoperative mortality, adjuvant chemotherapy received, length of follow-up, and percentage of loss. These 13 items were considered to be associated with the survival outcomes as potential confounders. Second, a cumulative quality score was calculated based on the above 13 items. If an item were comparable between the two groups, a score of “”0”’ was given. If the balance of any item were unclear or not comparable, scores of “1”’ or “‘3,” respectively, were given. A higher cumulative comparability score indicated a lower quality study. The reason why the scale was nonlinear is because of linear scale (0, 1, and 2) unable to both underline the incomparability and result in an enough wide range of accumulative scores to determine an efficient cutoff to exclude heterogeneous studies (data not shown).
Statistics
The STATA 12.0 statistical software was used for the synthesis and analysis. (1) The meta-analyses were performed initially using a fixed effects model. An odds ratio (OR) with a 95% confidence interval (CI) was calculated for the dichotomous data. The Mantel-Haenszel method was used to test the significance of the dichotomous data in a meta-analysis. Forest plots showed the results of the meta-analyses. A P value of less than 0.05 was considered statistically significant. (2) The between-study statistical heterogeneity was tested by a standard chi-square test, and a random effects model was used for a P value of less than 0.1. (3) Begg test and Egger test were performed to test for a publication bias, and the results were presented in a funnel plot. (4) To select any potential confounders, meta-regressions were performed in four models, including the study feature, tumor comparability, operation comparability, and postoperative comparability. A P value of less than 0.05 was considered to function as a confounder. (5) A sensitivity analysis was performed to determine an optimal cutoff of the quality scores and distinguish the quality of an individual study as good or poor. The meta-analyses were resynthesized from the low to high scoring subsets. A scatter plot with quadratic fit and a 95% CI area was drawn to observe the correlation between the scores and the ORs. (6) In addition, the subgroup analyses of the well-qualified studies and stage-specific analyses were performed to identify a contribution by an individual factor.
Ethics, standards of reporting, data availability
This systematic review was not submitted to any biomedical ethical committee for approval, and meanwhile no consent was required from the analyzed individuals. This systematic review was performed and reported according to the PRISMA standard. All the data are fully available from the published papers.
RESULTS
Literature
The procedures of the literature search and selection are shown in Figure 1. A total of 23 studies were selected from 1137 citations. A total of 7336 (3368 vs 3968) subjects were included in the LG and OG groups. There were two randomized controlled trials, whereas 21 studies were case-control studies. The studies were regionally distributed as follows: four from Italy, five from Japan, nine from Korea, and five from China. Twenty studies used the LAG technique, two used TLG, and one used TLG and HALG. The OS results were extracted from 22 studies, the recurrence results from 17 studies, and the gastric cancer–related death results from nine studies. The details of the studies including the quality assessment results are shown in Supplementary Table 1 (http://links.lww.com/MD/A184).21–43 The reasons for the exclusion of 10 comparative studies that reported survival outcomes are shown in Supplementary Table 2 (http://links.lww.com/MD/A185).44–53
FIGURE 1.
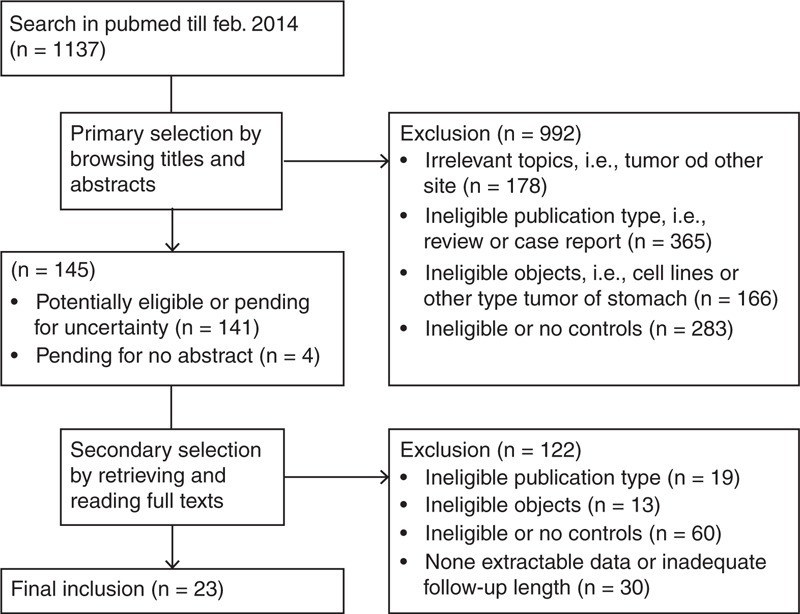
Literature search and selection procedures.
Five-year overall survival
All-pooled meta-analysis
In an all-pooled manner, 22 studies that reported the 5-year OS were synthesized in a meta-analysis (Supplementary Figure 1 http://links.lww.com/MD/A182). The LG group presented a better OS outcome than did the OG group in this initial analysis by random effect model due to significant between-study heterogeneity (I-squared = 82.7%, P < 0.01). No publication bias influenced the meta-analysis (Supplementary Figure 2 http://links.lww.com/MD/A183). Meta-regression found the cumulative comparability score and the comparability of D2 lymphadenectomy were independent confounders (Supplementary Table 3 http://links.lww.com/MD/A186). These two confounders were tested in subsequent sensitivity analyses and subgroup analyses.
Sensitivity analyses
To fix an optimal cutoff of the cumulative comparability scores, a scatter plot with quadratic fit and 95% CI area was drawn to observe the correlation between the scores and the ORs (Figure 2). A visible trend was OR increasing with a higher score. With scores higher than 5, the 95% CI area of the ORs became significantly favorable for LG.
FIGURE 2.

Scatter plot of the comparability scores and the ORs of the overall survival with quadratic fit and 95% CI area. CI = confidence interval.
The meta-analyses were performed based on different comparability score subsets from low to high (Supplementary Table 4 http://links.lww.com/MD/A187). There was no significant difference between the LG and OG groups, based on the subset 0–2 scores (OR = 1.22, 95% CI 0.85, 1.74, P = 0.29) and 3–5 scores (OR = 1.11, 95% CI 0.87, 1.41, P = 0.41). Based on the 6–10 and >10 score subsets, LG was superior to OG because of a more obvious imbalance between the two groups. In particular, the >10 score subset presented a significant heterogeneity (P < 0.01), and the random effects model was used.
A sensitivity analysis was performed by repooling the comparability scores of the studies scoring 5 or lower (Figure 3). Fourteen studies were repooled, and 1807 and 1844 patients were analyzed in the LG and OG groups, respectively. The result showed that there was no longer a significant difference between the LG and OG groups (OR = 1.07, 95% CI 0.90, 1.28, P = 0.45), and no heterogeneity was presented (P = 1.00).
FIGURE 3.
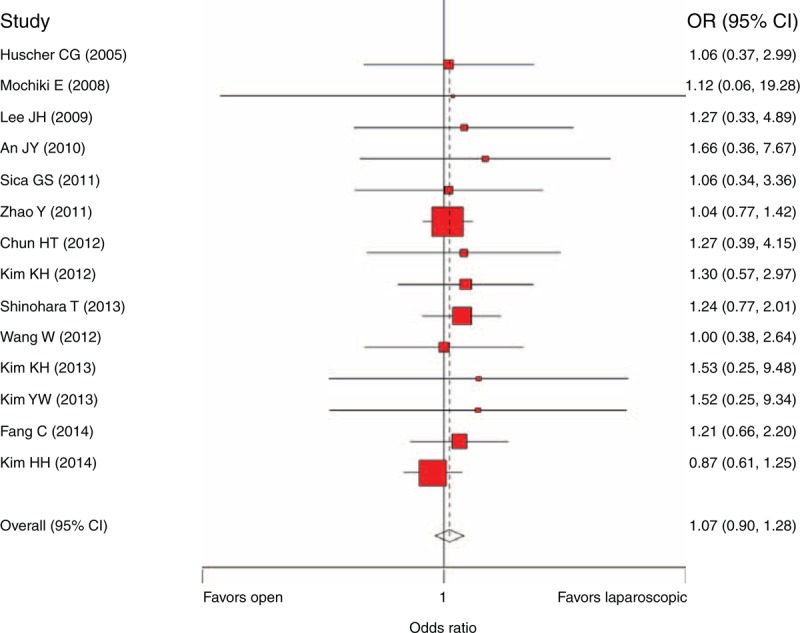
Sensitivity analysis of the 5-year overall survival comparison (including the score ≤5 studies and the matched sub-study extracted from Kim et al, 2014).43 CI = confidence interval, OR = odds ratio.
Subgroup analyses
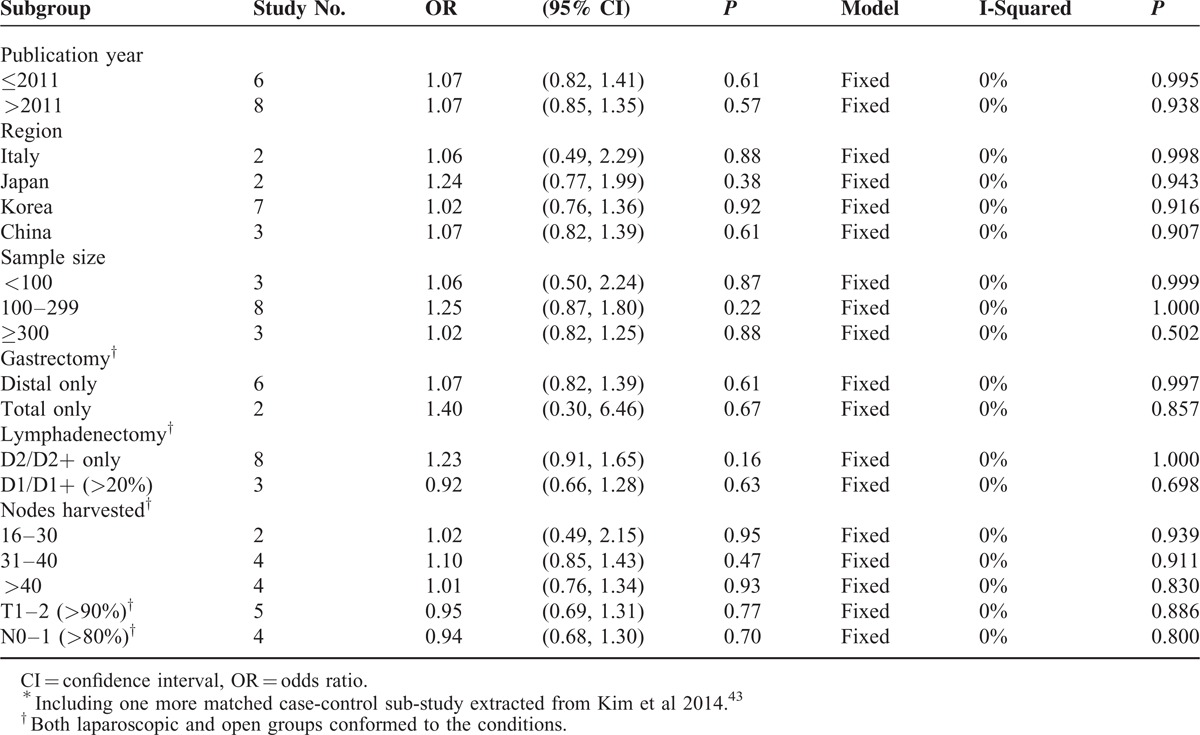
The subgroup analyses aimed to determine whether any distinguishing subset could be benefited by LG in the long-term survival (Table 1) rates. Only the 14 studies with comparability scores of 5 or lower were included in the subgroup analyses. All of the subsets did not have a preference for LG, including the publication year, region, sample size, gastrectomy pattern, lymphadenectomy extent, amount of nodes harvested, proportion of T1–2, and proportion of N0–1. In particular, regarding lymphadenectomy as an independent confounder, the D2/D2+ only subset (OR = 1.23, 95% CI 0.91, 1.65, P = 0.16) and the D1/D1+ > 20% subset (OR = 0.92, 95% CI 0.66, 1.28, P = 0.63) had equal preference to LG or OG, if well balanced.
TABLE 1.
Subgroup Analyses of the 5-Year Overall Survival Based on Score ≤5 Studies∗
Recurrence and Gastric Cancer–Related Death
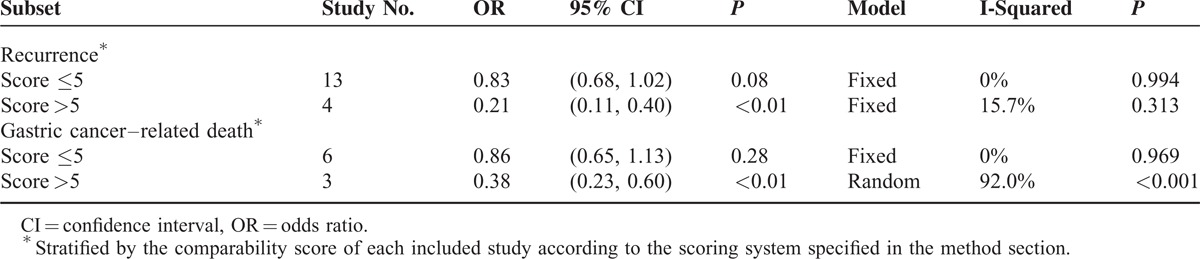
Among those studies with comparability scores of 5 or lower, 13 studies (1172 of LG vs 1209 of OG) reported recurrence results, and six studies (578 of LG vs 544 of OG) reported gastric cancer–related deaths (Table 2). The meta-analyses showed no significant difference in the recurrence (OR = 0.83, 95% CI 0.68, 1.02, P = 0.08) or gastric cancer–related death (OR = 0.86, 95% CI 0.65, 1.13, P = 0.28) rates between the two groups. The meta-analyses based on the studies with comparability scores higher than 5 demonstrated obvious preferences for LG (Supplementary Table 4).
TABLE 2.
Five-Year Recurrence and Gastric Cancer–Related Death
Stage-Specific Survival and Recurrence
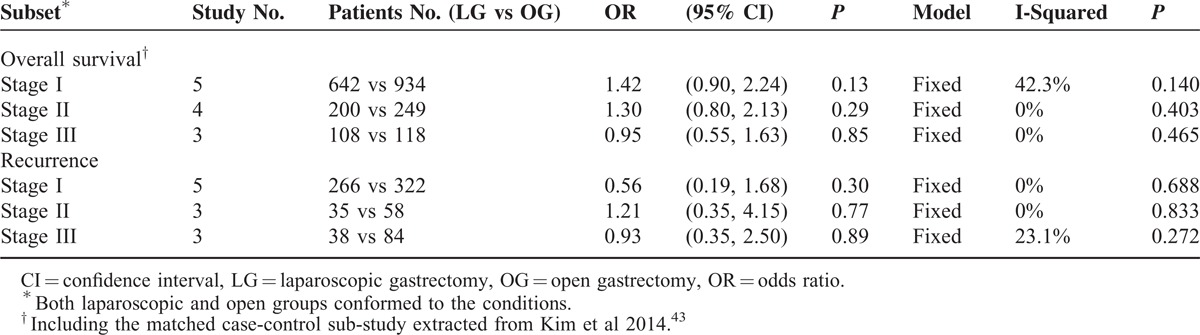
The tumor stage is a determining factor in long-term survival outcomes; the stage-specific analyses were carried out on the 5-year OS and recurrence (Table 3). The proportion of the available subjects in the stage I subset was two- to threefold higher than in stage II–III. The OS and recurrence were comparable between the laparoscopic and open groups in stage I, II, or III (P > 0.05).
TABLE 3.
Stage-Specific Analyses of the 5-Year Overall Survival and Recurrence
DISCUSSION
The major contributions of this systematic review compared with other meta-analyses are a comprehensive collection of available long-term survival outcomes within a much larger number of observations and a more precise consideration of confounders. The results indicate that the degree of comparability between the groups and the lymphadenectomy extent are two independent confounders that influence the estimates. Based on the well-balanced studies, the 5-year OS, recurrence, or gastric cancer–related death is comparable between LG and OG with narrow 95% CIs. Several factors such as the publication year, study region, sample size, gastrectomy pattern, lymphadenectomy extent, number of nodes harvested, and proportion of T1–2 or N0–1 do not influence the estimates, if the studies are well balanced. In particular, the stage-specific estimates obtain comparable results between the two groups.
This updated systematic review confirms the results of several previous meta-analyses. Qiu et al found a nonsignificant difference in the 3-year OS between laparoscopy-assisted and open distal gastrectomy in cases of advanced disease.54 Chen et al reported a similar long-term outcome between laparoscopy-assisted and open total gastrectomy.55 Wei et al and Ding et al found that among the patients who had undergone D2 lymphadenectomy, the OS and recurrence were comparable.56,57 Choi et al compared the two interventions in the advanced gastric cancer patients and found no significant difference in the long-term OS or disease-free survival.58 Zhang et al analyzed the early gastric cancer patients in a study from Asia and found that the recurrence rates were not different between LG andOG.59 Based on the randomized controlled trials, Sun et al found similar tumor recurrence rates between the laparoscopic and open groups.60
Compared to previously published meta-analyses, major improvement of the present meta-analysis is a full consideration of the multiple confounders for long-term survival outcome in the aspect of surgical oncology. The long-term survival outcome is influenced by many factors, including the tumor characteristics, operation pattern, and postoperative management. The comparability of these confounders contributes to the assessment of the survival estimate between LG and OG. The meta-regression and sensitivity analysis showed that the cumulative effects generated by increasing imbalance could lead to a false result favoring LG. Because of the complexity of the procedures and the uncertainty of LG, surgeons prefer to select candidates with relatively smaller tumor size and earlier stage disease for the LG group, which is the reason that the initial meta-analysis shows that LG has better 5-year OS than OG.
For the meta-analysis, we selected the well-balanced studies that make these no difference results have greater robustness in the various subsets. Although a lymphadenectomy was determined to be an independent confounder, no significant difference was shown in the extent of the dissection between the LG and OG groups, and the numbers of nodes harvested were well balanced between the two groups. In the early period, there was a shortage of lymph node dissection in LG because of a technical problem. LG was typically performed with a D1 or D1+ lymphadenectomy, and fewer nodes were harvested.61–63 With LG development in recent years, most surgeons are experienced in D2-LG, harvesting as many nodes as in open surgery.33,64–65 Based on current knowledge, lymphadenectomy is no longer a critical technical defect in LG.
The stage-matched meta-analyses are powerful for showing that LG is not inferior to OG for any stage of resectable disease. At the beginning, LG was only indicated in early gastric cancer patients, and the feasibility and safety of LG were widely accepted.66 The LG technique has recently been extended for use in advanced disease,67 and the controversy concerning the oncological aspects requires surgeons to pay increasing attention to the technique. This systematic review found that stage I or stage II/III diseases have comparable long-term survival and recurrence rates from LG and OG procedures. The evidence supports the use of LG in advanced resectable disease.
There are limitations to this systematic review. First, only two eligible small-sized RCTs are included in the meta-analyses, whereas the other studies are retrospective case-control studies. The quality of the original studies is an internal determining factor of evidence robustness. Because of the nature of surgical techniques, it is relatively difficult to conduct RCTs, especially double-blind studies, which are usually not feasible. There are several completed or ongoing RCTs in Japan, Korea, and China, including the JOCG-0912, JLSSG-0901, KLASS-01, KLASS-02, and CLASS-01 trials.68–73 These RCTs compare LG with OG in early or advanced stage disease, and the expectation of their long-term results is merited. Second, the entire observation is sufficient for the findings; however, the stage-specific analyses include only a small patient sample, especially of stage II and stage III patients. The strength of the stage-specific analyses is limited for reaching a convincing conclusion, and the trials mentioned above are required for more robust evidence. Third, the TNM staging systems have changed at different periods, which might cause systematic errors in the stage-specific analyses. A pooling analysis of the individual patient data should be a more effective method of resolving this problem. Fourth, the evidence in comparison of long-term survival outcome between LG and OG is sparse from Western countries. Therefore, the extrapolation of our findings might be limited to Western populations. Finally, although the publications seem to be clustered very close to the “no-effect” line of an OR of 1 when evaluating the funnel plot, there are 13 studies on the negative side as opposed to seven on the positive effect side (Supplementary Figure 2). It might indicate a small bias toward negative studies that are unfavorable to OG, despite of no significance in Begg test and Egger test. Thus, this is an argument why a novel and tailored measurement for quality assessment was needed to yield a more robust evidence based on homogeneous studies.74
The current evidence indicates that the long-term survival and recurrence rates of laparoscopic gastric cancer surgery are comparable to those of open surgery for the treatment of early or advanced stage gastric cancer, if the technical quality of the procedures is comparable. Additional high-quality RCTs are required for more confirmative conclusion.
Competing Interest: None declared.
Authors’ Contributions: Xin-Zu Chen and Lei Wen contributed equally to this research as co-first authors. X-Z Chen (chen_xz_wch_scu@126.com) analyzed and wrote the paper; L Wen (wenlei1998@sina.com), C-X Liu (chaoxuliu@yahoo.com), and Y-Y Rui (righton_123@163.com) searched the literature and extracted the data; and Q-C Zhao (zhaoqcfmmu@126.com), Z-G Zhou (zhou767@163.com), and J-K Hu (hujkwch@126.com) performed the quality control and proofread the paper.
ACKNOWLEDGMENTS
This research was granted by the National Natural Science Foundation of China (No. 81301866 and No. 81372344).
Footnotes
Abbreviations: HALG = hand-assisted laparoscopic gastrectomy, LAG = laparoscopy-assisted gastrectomy, LG = laparoscopic gastrectomy, OG = open gastrectomy, OS = overall survival, RCT = randomized controlled trial, TLG = totally laparoscopic gastrectomy.
Funding: National Natural Science Foundation of China (No. 81372344 and No. 81301866).
Dr Chen and Dr Wen contributed equally as co-first authors.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006; 24:2137–2150. [DOI] [PubMed] [Google Scholar]
- 2.Shen L, Shan YS, Hu HM, et al. Management of gastric cancer in Asia: resource-stratified guidelines. Lancet Oncol 2013; 14:e535–e547. [DOI] [PubMed] [Google Scholar]
- 3.Sasako M, Sano T, Yamamoto S, et al. Japan Clinical Oncology Group. D2 lymphadenectomy alone or with para-aortic nodal dissection for gastric cancer. N Engl J Med 2008; 359:453–462. [DOI] [PubMed] [Google Scholar]
- 4.Songun I, Putter H, Kranenbarg EM, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomized nationwide Dutch D1D2 trial. Lancet Oncol 2010; 11:439–449. [DOI] [PubMed] [Google Scholar]
- 5.Kitano S, Iso Y, Moriyama M, et al. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 1994; 4:146–148. [PubMed] [Google Scholar]
- 6.Yang HK, Suh YS, Lee HJ. Minimally invasive approaches for gastric cancer-Korean experience. J Surg Oncol 2013; 107:277–281. [DOI] [PubMed] [Google Scholar]
- 7.Etoh T, Inomata M, Shiraishi N, et al. Minimally invasive approaches for gastric cancer-Japanese experiences. J Surg Oncol 2013; 107:282–288. [DOI] [PubMed] [Google Scholar]
- 8.Chen XZ, Li YY, Hu JK, et al. Spread and development of laparoscopic surgery for gastric tumors in mainland China: initial experiences. Hepatogastroenterology 2012; 59:654–658. [DOI] [PubMed] [Google Scholar]
- 9.Bracale U, Pignata G, Lirici MM, et al. Laparoscopic gastrectomies for cancer: The ACOI-IHTSC national guidelines. Minim Invasive Ther Allied Technol 2012; 21:313–319. [DOI] [PubMed] [Google Scholar]
- 10.Strong VE. Laparoscopic resection for gastric carcinoma: Western experience. Surg Oncol Clin N Am 2012; 21:141–158. [DOI] [PubMed] [Google Scholar]
- 11.Chen XZ, Hu JK, Yang K, et al. Short-term evaluation of laparoscopy-assisted distal gastrectomy for predictive early gastric cancer: a meta-analysis of randomized controlled trials. Surg Laparosc Endosc Percutan Tech 2009; 19:277–284. [DOI] [PubMed] [Google Scholar]
- 12.Yasunaga H, Horiguchi H, Kuwabara K, et al. Outcomes after laparoscopic or open distal gastrectomy for early-stage gastric cancer: a propensity-matched analysis. Ann Surg 2013; 257:640–646. [DOI] [PubMed] [Google Scholar]
- 13.Nam BH, Kim YW, Reim D, et al. Laparoscopy assisted versus open distal gastrectomy with D2 lymph node dissection for advanced gastric cancer: design and rationale of a Phase II Randomized Controlled Multicenter Trial (COACT 1001). J Gastric Cancer 2013; 13:164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu J, Hu J, Huang C, et al. The impact of age and comorbidity on postoperative complications in patients with advanced gastric cancer after laparoscopic D2 gastrectomy: results from the Chinese laparoscropic gastrointestinal surgery study (CLASS) group. Eur J Surg Oncol 2013; 39:1144–1149. [DOI] [PubMed] [Google Scholar]
- 15.Are C, Talamini MA. Laparoscopy and malignancy. J Laparoendosc Adv Surg Tech A 2005; 15:38–47. [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara M, Kodera Y, Misawa K, et al. Longterm outcomes of early-stage gastric carcinoma patients treated with laparoscopy-assisted surgery. J Am Coll Surg 2008; 206:138–143. [DOI] [PubMed] [Google Scholar]
- 17.Chen XZ, Yang K, Zhang B, et al. Is retrieval of >25 lymph nodes a superior criterion for locally advanced gastric cancer surgery? Ann Surg 2011; 254:834–835. [DOI] [PubMed] [Google Scholar]
- 18.Gutt CN, Hollander D, Brier CH, et al. Influence of laparoscopy and laparotomy on systemic and peritoneal T lymphocytes in a rat model. Int J Colorectal Dis 2001; 16:216–220. [DOI] [PubMed] [Google Scholar]
- 19.Ure BM, Niewold TA, Bax NM, et al. Peritoneal, systemic, and distant organ inflammatory responses are reduced by a laparoscopic approach and carbon dioxide versus air. Surg Endosc 2002; 16:836–842. [DOI] [PubMed] [Google Scholar]
- 20.De Angelis R, Sant M, Coleman MP, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE-5-a population-based study. Lancet Oncol 2014; 15:23–34. [DOI] [PubMed] [Google Scholar]
- 21.Huscher CG, Mingoli A, Sgarzini G, et al. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 2005; 241:232–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mochiki E, Toyomasu Y, Ogata K, et al. Laparoscopically assisted total gastrectomy with lymph node dissection for upper and middle gastric cancer. Surg Endosc 2008; 22:1997–2002. [DOI] [PubMed] [Google Scholar]
- 23.Lee JH, Yom CK, Han HS. Comparison of long-term outcomes of laparoscopy-assisted and open distal gastrectomy for early gastric cancer. Surg Endosc 2009; 23:1759–1763. [DOI] [PubMed] [Google Scholar]
- 24.Wong SK, Tsui DK, Li MK. Laparoscopic distal gastrectomy for gastric cancer: initial experience on hand-assisted technique and totally laparoscopic technique. Surg Laparosc Endosc Percutan Tech 2009; 19:298–304. [DOI] [PubMed] [Google Scholar]
- 25.An JY, Heo GU, Cheong JH, et al. Assessment of open versus laparoscopy-assisted gastrectomy in lymph node-positive early gastric cancer: a retrospective cohort analysis. J Surg Oncol 2010; 102:77–81. [DOI] [PubMed] [Google Scholar]
- 26.Orsenigo E, Di Palo S, Tamburini A, et al. Laparoscopy-assisted gastrectomy versus open gastrectomy for gastric cancer: a monoinstitutional Western center experience. Surg Endosc 2011; 25:140–145. [DOI] [PubMed] [Google Scholar]
- 27.Sica GS, Iaculli E, Biancone L, et al. Comparative study of laparoscopic vs open gastrectomy in gastric cancer management. World J Gastroenterol 2011; 17:4602–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao Y, Yu P, Hao Y, et al. Comparison of outcomes for laparoscopically assisted and open radical distal gastrectomy with lymphadenectomy for advanced gastric cancer. Surg Endosc 2011; 25:2960–2966. [DOI] [PubMed] [Google Scholar]
- 29.Chun HT, Kim KH, Kim MC, et al. Comparative study of laparoscopy-assisted versus open subtotal gastrectomy for pT2 gastric cancer. Yonsei Med J 2012; 53:952–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eom BW, Kim YW, Lee SE, et al. Survival and surgical outcomes after laparoscopy-assisted total gastrectomy for gastric cancer: case-control study. Surg Endosc 2012; 26:3273–3281. [DOI] [PubMed] [Google Scholar]
- 31.Hamabe A, Omori T, Tanaka K, et al. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymph node dissection for advanced gastric cancer. Surg Endosc 2012; 26:1702–1709. [DOI] [PubMed] [Google Scholar]
- 32.Kim KH, Kim MC, Jung GJ, et al. Comparative analysis of five-year survival results of laparoscopy-assisted gastrectomy versus open gastrectomy for advanced gastric cancer: a case-control study using a propensity score method. Dig Surg 2012; 29:165–171. [DOI] [PubMed] [Google Scholar]
- 33.Sato H, Shimada M, Kurita N, et al. Comparison of long-term prognosis of laparoscopy-assisted gastrectomy and conventional open gastrectomy with special reference to D2 lymph node dissection. Surg Endosc 2012; 26:2240–2246. [DOI] [PubMed] [Google Scholar]
- 34.Siani LM, Ferranti F, De Carlo A, et al. Completely laparoscopic versus open total gastrectomy in stage I-III/C gastric cancer: safety, efficacy and five-year oncologic outcome. Minerva Chir 2012; 67:319–326. [PubMed] [Google Scholar]
- 35.Bo T, Peiwu Y, Feng Q, et al. Laparoscopy-assisted vs. open total gastrectomy for advanced gastric cancer: long-term outcomes and technical aspects of a case-control study. J Gastrointest Surg 2013; 17:1202–1208. [DOI] [PubMed] [Google Scholar]
- 36.Gordon AC1, Kojima K, Inokuchi M, et al. Long-term comparison of laparoscopy-assisted distal gastrectomy and open distal gastrectomy in advanced gastric cancer. Surg Endosc 2013; 27:462–470. [DOI] [PubMed] [Google Scholar]
- 37.Lee MS, Lee JH, Park do J, et al. Comparison of short- and long-term outcomes of laparoscopic-assisted total gastrectomy and open total gastrectomy in gastric cancer patients. Surg Endosc 2013; 27:2598–2605. [DOI] [PubMed] [Google Scholar]
- 38.Kim KH, Kim YM, Kim MC, et al. Is laparoscopy-assisted total gastrectomy feasible for the treatment of gastric cancer? A case-matched study. Dig Surg 2013; 30:348–354. [DOI] [PubMed] [Google Scholar]
- 39.Kim YW, Yoon HM, Yun YH, et al. Long-term outcomes of laparoscopy-assisted distal gastrectomy for early gastric cancer: result of a randomized controlled trial (COACT 0301). Surg Endosc 2013 Nov; 27:4267–4276. [DOI] [PubMed] [Google Scholar]
- 40.Shinohara T, Satoh S, Kanaya S, et al. Laparoscopic versus open D2 gastrectomy for advanced gastric cancer: a retrospective cohort study. Surg Endosc 2013; 27:286–294. [DOI] [PubMed] [Google Scholar]
- 41.Wang W, Chen K, Xu XW, et al. Case-matched comparison of laparoscopy-assisted and open distal gastrectomy for gastric cancer. World J Gastroenterol 2013; 19:3672–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fang C, Hua J, Li J, et al. Comparison of long-term results between laparoscopy-assisted gastrectomy and open gastrectomy with D2 lymphadenectomy for advanced gastric cancer. Am J Surg 2014; 208:391–396. [DOI] [PubMed] [Google Scholar]
- 43.Kim HH, Han SU, Kim MC, et al. Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol 2014; 32:627–633. [DOI] [PubMed] [Google Scholar]
- 44.Dulucq JL, Wintringer P, Stabilini C, et al. Laparoscopic and open gastric resections for malignant lesions: a prospective comparative study. Surg Endosc 2005; 19:933–938. [DOI] [PubMed] [Google Scholar]
- 45.Lee WJ, Wang W, Chen TC, et al. Totally laparoscopic radical BII gastrectomy for the treatment of gastric cancer: a comparison with open surgery. Surg Laparosc Endosc Percutan Tech 2008; 18:369–374. [DOI] [PubMed] [Google Scholar]
- 46.Francescutti V, Choy I, Biertho L, et al. Gastrectomy and esophagogastrectomy for proximal and distal gastric lesions: a comparison of open and laparoscopic procedures. Surg Innov 2009; 16:134–139. [DOI] [PubMed] [Google Scholar]
- 47.Shuang J, Qi S, Zheng J, et al. A case-control study of laparoscopy-assisted and open distal gastrectomy for advanced gastric cancer. J Gastrointest Surg 2011; 15:57–62. [DOI] [PubMed] [Google Scholar]
- 48.Cai J, Wei D, Gao CF, et al. A prospective randomized study comparing open versus laparoscopy-assisted D2 radical gastrectomy in advanced gastric cancer. Dig Surg 2011; 28:331–337. [DOI] [PubMed] [Google Scholar]
- 49.Jeong SH, Lee YJ, Park ST, et al. Risk of recurrence after laparoscopy-assisted radical gastrectomy for gastric cancer performed by a single surgeon. Surg Endosc 2011; 25:872–878. [DOI] [PubMed] [Google Scholar]
- 50.Chen WJ, Xu CY, Shen JG, et al. Outcome of laparoscopy-assisted gastrectomy vs. open gastrectomy for gastric cancer: a retrospective comparative study. Hepatogastroenterology 2012; 59:938–941. [DOI] [PubMed] [Google Scholar]
- 51.Cai J, Zhang C, Zhang H, et al. Open versus laparoscopy-assisted D2 radical gastrectomy in advanced upper gastric cancer: a retrospective cohort study. Hepatogastroenterology 2013; 60:1805–1808. [PubMed] [Google Scholar]
- 52.Zhao XF, Jeong O, Jung MR, et al. A propensity score-matched case-control comparative study of laparoscopic and open extended (D2) lymph node dissection for distal gastric carcinoma. Surg Endosc 2013; 27:2792–2800. [DOI] [PubMed] [Google Scholar]
- 53.Lin J, Huang C, Zheng C, et al. A matched cohort study of laparoscopy-assisted and open total gastrectomy for advanced proximal gastric cancer without serosa invasion. Chin Med J (Engl) 2014; 127:403–407. [PubMed] [Google Scholar]
- 54.Qiu J, Pankaj P, Jiang H, et al. Laparoscopy versus open distal gastrectomy for advanced gastric cancer: a systematic review and meta-analysis. Surg Laparosc Endosc Percutan Tech 2013; 23:1–7. [DOI] [PubMed] [Google Scholar]
- 55.Chen K, Xu XW, Zhang RC, et al. Systematic review and meta-analysis of laparoscopy-assisted and open total gastrectomy for gastric cancer. World J Gastroenterol 2013; 19:5365–5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei HB, Wei B, Qi CL, et al. Laparoscopic versus open gastrectomy with D2 lymph node dissection for gastric cancer: a meta-analysis. Surg Laparosc Endosc Percutan Tech 2011; 21:383–390. [DOI] [PubMed] [Google Scholar]
- 57.Ding J, Liao GQ, Liu HL, et al. Meta-analysis of laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer. J Surg Oncol 2012; 105:297–303. [DOI] [PubMed] [Google Scholar]
- 58.Choi YY, Bae JM, An JY, et al. Laparoscopic gastrectomy for advanced gastric cancer: are the long-term results comparable with conventional open gastrectomy? A systematic review and meta-analysis. J Surg Oncol 2013; 108:550–556. [DOI] [PubMed] [Google Scholar]
- 59.Zhang CD, Chen SC, Feng ZF, et al. Laparoscopic versus open gastrectomy for early gastric cancer in Asia: a meta-analysis. Surg Laparosc Endosc Percutan Tech 2013; 23:365–377. [DOI] [PubMed] [Google Scholar]
- 60.Sun J, Li J, Wang J, et al. Meta-analysis of randomized controlled trials on laparoscopic gastrectomy vs. open gastrectomy for distal gastric cancer. Hepatogastroenterology 2012; 59:1699–1705. [DOI] [PubMed] [Google Scholar]
- 61.Kitano S, Shiraishi N, Kakisako K, et al. Laparoscopy-assisted Billroth-I gastrectomy (LADG) for cancer: our 10 years’ experience. Surg Laparosc Endosc Percutan Tech 2002; 12:204–207. [DOI] [PubMed] [Google Scholar]
- 62.Hyodo M, Hosoya Y, Yokoyama T, et al. Gasless laparoscopy-assisted distal gastrectomy for early cancer via mini-laparotomy using an abdominal wall lift. Hepatogastroenterology 2003; 50:2279–2281. [PubMed] [Google Scholar]
- 63.Memon MA, Khan S, Yunus RM, et al. Meta-analysis of laparoscopic and open distal gastrectomy for gastric carcinoma. Surg Endosc 2008; 22:1781–1789. [DOI] [PubMed] [Google Scholar]
- 64.Miura S, Kodera Y, Fujiwara M, et al. Laparoscopy-assisted distal gastrectomy with systemic lymph node dissection: a critical reappraisal from the viewpoint of lymph node retrieval. J Am Coll Surg 2004; 198:933–938. [DOI] [PubMed] [Google Scholar]
- 65.Tokunaga M, Hiki N, Fukunaga T, et al. Laparoscopy-assisted distal gastrectomy with D2 lymph node dissection following standardization-a preliminary study. J Gastrointest Surg 2009; 13:1058–1063. [DOI] [PubMed] [Google Scholar]
- 66.Kitano S, Shiraishi N. Current status of laparoscopic gastrectomy for cancer in Japan. Surg Endosc 2004; 18:182–185. [DOI] [PubMed] [Google Scholar]
- 67.Park do J, Han SU, Hyung WJ, et al. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc 2012; 26:1548–1553. [DOI] [PubMed] [Google Scholar]
- 68.Nakamura K, Katai H, Mizusawa J, et al. A phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric Cancer (JCOG0912). Jpn J Clin Oncol 2013; 43:324–327. [DOI] [PubMed] [Google Scholar]
- 69.Etoh T, Japanese Laparoscopic Surgery Study Group (JLSSG). Randomized controlled trial to evaluate laparoscopic versus open surgery for advanced gastric cancer (JLSSG0901: Adv.GC-LAP/OPEN, P II/III). Unique trial number: UMIN000003420 https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr.cgi?function=brows&action=brows&type=summary&recptno=R000004144&language=E, accessed on 13/01/2015. [Google Scholar]
- 70.Kim HH, Hyung WJ, Cho GS, et al. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report-a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg 2010; 251:417–420. [DOI] [PubMed] [Google Scholar]
- 71.Kim HH, Han SU, Kim MC, et al. Prospective randomized controlled trial (phase III) to comparing laparoscopic distal gastrectomy with open distal gastrectomy for gastric adenocarcinoma (KLASS 01). J Korean Surg Soc 2013; 84:123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Han SU, Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group. Efficacy of laparoscopic subtotal gastrectomy with D2 lymph node dissection for locally advanced gastric cancer (KLASS-02-RCT). ClinicalTrials.gov. http://clinicaltrials.gov/ct2/show/NCT01456598, accessed on 13/01/2015. [Google Scholar]
- 73.Li G, Chinese Laparoscopic Gastrointestinal Surgical Study (CLASS) Group. Multicenter study on laparoscopic distal subtotal gastrectomy for advanced gastric cancer (CLASS-01). ClinicalTrials.gov. http://clinicaltrials.gov/show/NCT01609309, accessed on 13/01/2015. [Google Scholar]
- 74.Phillips B, Ball C, Sackett D, et al. Oxford Centre for Evidence-based Medicine – Levels of Evidence (March 2009). 2009. http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/, accessed on 13/01/2015. [Google Scholar]


