Supplemental Digital Content is available in the text
Abstract
The association between tuberculosis (TB) and lung cancer is well known. However, carcinogenesis of TB and its connection with epidermal growth factor receptor (EGFR) mutation remains unclear. This study aimed to determine this connection to see if TB can affect the outcome of patients with lung cancer.
This is a retrospective cohort study of patients with lung cancer receiving EGFR-tyrosine kinase inhibitors (TKIs) between 1996 and 2010 using the National Health Insurance Research Database of Taiwan. Because therapeutic response was required to apply EGFR-TKIs for >90 days, only patients with a follow-up of >120 days were studied and a responder was defined as intake of EGFR-TKIs >90 days. Predictors of EGFR-TKI response and survival were identified using logistic and Cox regression analyses, respectively.
There were 8265 patients analyzed, including 6073 (73.5%) EGFR-TKI responder and 2192 (26.5%) nonresponder. A history of TB was found in 1.2% and 1.8% of the 2 groups, respectively. Comparing to male with pulmonary TB history, female with or without pulmonary TB history and male without pulmonary TB history all had a better EGFR-TKI response and 1-year progression-free survival (PFS). Gender and TB history were not independent prognostic factors of 2-year overall survival. The findings were similar in the subpopulation without chronic obstructive pulmonary disease, malignancies other than lung cancer, and low-income status.
TB has a gender-dependent impact, with better EGFR-TKI response and 1-year PFS in female patients with lung cancer. The carcinogenesis and inflammation of TB may be different between genders.
INTRODUCTION
Lung cancer is the leading cause of mortality in all patients with cancer, accounting for 14% of newly diagnosed cancer cases and 26% to 28% of all cancer deaths in the United States.1 One meta-analysis shows that epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors (TKIs) prolong progression-free survival (PFS) (hazard ratio [HR] 0.43 [0.38–0.49]), but not overall survival (OS) (HR 1.01 [0.87–1.18]), in patients with sensitive EGFR mutation.2 The frequency of sensitive EGFR mutation is found in approximately 10% of Caucasians with nonsmall cell lung cancer (NSCLC) and >50% of Asian patients.3,4 Patients with female sex, adenocarcinoma histology, never-smoking status, and Asian ethnicity are considered to have a high prevalence of EGFR mutation according to the Iressa Pan-Asia Study (IPASS) trial and the molecular epidemiology study of EGFR mutations in Asian patients with advanced NSCLC of adenocarcinoma (PIONEER) trial.4,5
Tuberculosis (TB) is a global disease affecting one third of the world's population.6 Several prospective and retrospective studies have demonstrated the association between lung cancer and pulmonary TB.7–10 One possible mechanism of carcinogenesis in patients with pulmonary TB is chronic inflammation, as proposed by Virchow in 1863.11,12 Chronic inflammation may lead to scar formation, resulting in dysplasia and scar carcinoma of the lungs.13 An experimental study in mice has not only shown a causal link between pulmonary TB and lung carcinogenesis but also established a genetic model for further analysis of the carcinogenic mechanisms activated by Mycobacterium tuberculosis.14
Recent reports regarding the impact of TB on the outcome of patients with lung cancer are contradictory: some show increased mortality in patients with lung cancer,15,16 whereas others reveal improved survival in patients with NSCLC.17 In addition, little is known about the impact of pulmonary TB on EGFR mutation and PFS. A recent report from Taiwan focusing on a small number of patients with lung adenocarcinoma (n = 275) reveals that patients with radiographic evidence compatible with old pulmonary TB have a higher incidence of EGFR mutation than those without (OR [odds ratio]: 1.83 [0.92–3.62]).18 However, the association is not found in patients with a clinical history of pulmonary TB. Large cohort studies are needed to clarify these issues.
As a mandatory universal health insurance program offering comprehensive medical care coverage, the National Health Insurance (NHI) of Taiwan covers up to 99% of residents in Taiwan.19 With a longitudinal follow-up of >22 million patients, the National Health Insurance Research Database (NHIRD) provides a very suitable research material to explore the long-term interaction between communicable and noncommunicable diseases. Moreover, the incidences of sensitive EGFR mutation (51.4%–58.4%)3,4,20 and pulmonary TB are high in Taiwan.21 Thus, this study was conducted using the NHIRD to investigate the correlation between pulmonary TB history and sensitive EGFR mutation, and clarify the impact of pulmonary TB history on PFS and OS of patients with lung cancer. Because the smoking status was not available in the NHIRD, smoking-associated comorbidities including chronic obstructive pulmonary disease (COPD),22 malignancies other than lung cancer,23 and low-income status24 were used as a proxy of smoking.
MATERIALS AND METHODS
Ethical Approval
The Institutional Review Board of the National Taiwan University Hospital, Taipei, Taiwan, approved the study (NTUH REC: 201212001W) and waived the need for informed consent because of the retrospective design and use of an encrypted database.
Case Selection
Patients with lung cancer were identified by a compatible diagnosis (International Classification of Disease, 9th Revision, Clinical Modification [ICD-9-CM] code 162) from the Registry of Catastrophic Illness Patients Database, a subset of the NHIRD. To apply for this registry, histological confirmation is obligated. The index date was defined as the initial date the patient applying for this registry for lung cancer.
The EGFR-TKIs included gefitinib and erlotinib. The prescription duration of individual EGFR-TKI was converted from the claims data according to the defined daily doses.25 These drugs required preaudit approved by the NHI administration and as second-line therapy, only benefited patients with lung adenocarcinoma after the first-line treatment with chemotherapy, except for a third-line indication of erlotinib for patients with NSCLC. The approval was reaudited every 90 days and reissued only to those with favorable response to EGFR-TKIs, that is, stable disease or partial or complete response. Thus, patients who died within 120 days after starting the EGFR-TKIs were excluded to ensure that EGFR-TKIs response was determined. Patients who discontinued EGFR-TKIs within 90 days were classified as EGFR-TKI nonresponders; others were classified as EGFR-TKI responders. The selection processes were shown in Figure 1.
Figure 1.
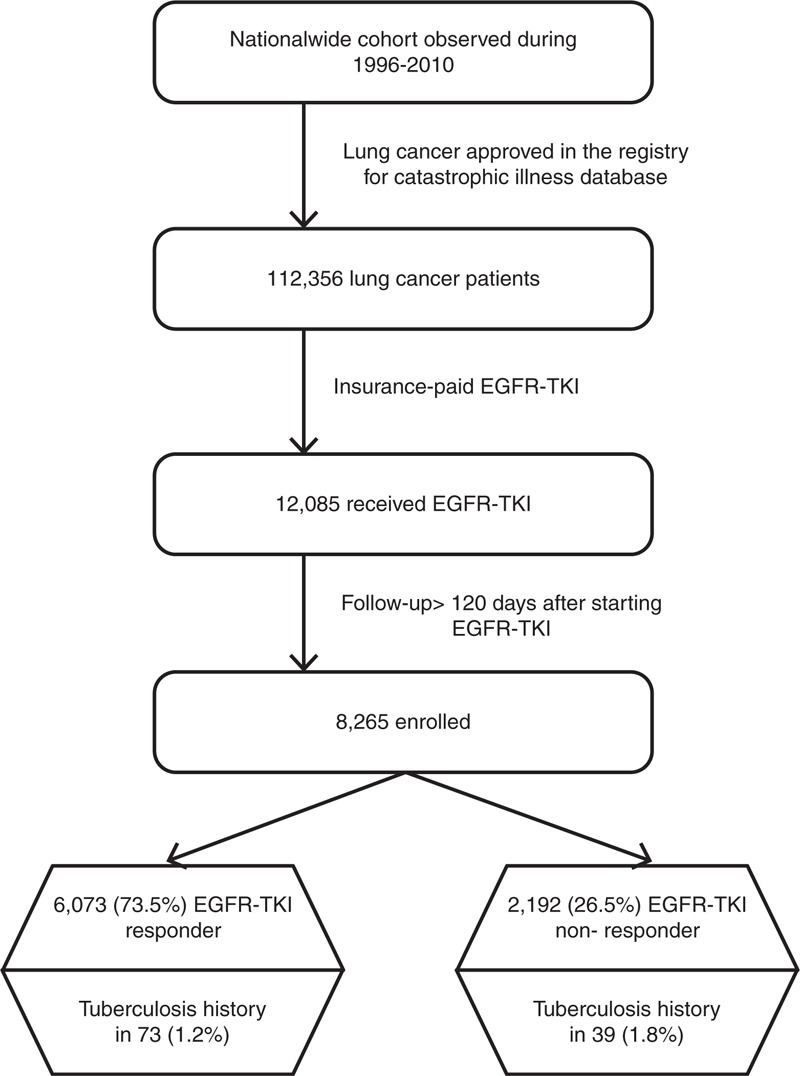
Selection and disposition of the study subjects. EGFR-TKI = epidermal growth factor receptor-tyrosine kinase inhibitor.
The association of pulmonary TB history and other comorbidities on EGFR-TKI response were studied among patients with lung cancer. All of the selected cases were followed up until December 31, 2010, announced death, or loss to follow-up (canceled health insurance prior to December 31, 2010).
Definition of Pulmonary TB
Pulmonary TB was identified according to a previous publication.26 Briefly, there was at least 2 ambulatory visits or 1 inpatient record with a compatible diagnosis (ICD-9-CM codes 010–012 and 018, and A-codes A020, A021), with at least 1 prescription consisting of ≥3 anti-TB drugs (isoniazid, rifampicin, rifabutin, ethambutol, pyrazinamide, and second-line drugs, including prothionamide, terizidone, streptomycin, kanamycin, cycloserine, aminosalicylic acid, and fluoroquinolones). Moreover, there were prescriptions of at least 2 anti-TB drugs simultaneously for not <120 days within a period of 180 days.27
Comorbidities
COPD, diabetes mellitus, end-stage renal disease (ESRD), liver cirrhosis, pneumoconiosis, autoimmune diseases, organ transplantation, acquired-immunodeficiency syndrome, and other malignancies were identified according to a previous publication.26 The low-income group was identified from the insurance status and required an annual household income of <4500 US dollars.28
Statistical Analysis
Intergroup difference was compared using the t test or Mann–Whitney U test for continuous variables based on their normality, and the χ2 test or Fisher exact test for categorical variables, as appropriate. Logistic regression analyses were performed to evaluate the impact of age, gender, comorbidities, income status, and a history of pulmonary TB on EGFR mutation.
On the basis of a previous study in Asia showing that the median PFS and OS in patients receiving gefitinib were 5.7 and 18.6 months, respectively,5 curves of 1-year PFS and 2-year OS (from the date of registry of lung cancer) for each variable were generated using the Kaplan–Meier method and compared by using the log-rank test. PFS was defined as the interval between the commencement and discontinuation of EGFR-TKIs under the assumption that patients without progression of disease would keep on EGFR-TKI therapy. Cox proportional hazards regression analysis was then applied to identify the independent prognostic factors. While conducting multivariate analyses, potential interactions between variables were checked and all variables with P ≤ 0.1 in univariate analysis were included. Statistical significance was set as P < 0.05. All analyses were conducted using the Statistical Package for the Social Sciences (SPSS, v. 18.0; SPSS Inc, Chicago, IL).
Sensitivity Analysis
Because smoking is associated with both TB and lung cancer,29,30 sensitivity analysis focusing on a predominantly nonsmoking subpopulation, patients without COPD, malignancies other than lung cancer, and low-income status, was performed to minimize the confounding effect of smoking.
RESULTS
Patient Characteristics
From 1996 to 2010, a total of 112,356 patients with lung cancer were identified with 12,085 who received erlotinib and/or gefitinib (Figure 1). Among them, 8265 patients, including 3180 (38.5%) who received erlotinib and 5085 (61.5%) who received gefitinib, had a follow-up duration of >120 days after the start of EGFR-TKI therapy. Among them, 6073 (73.5%) received EGFR-TKIs for >90 days and were classified as EGFR-TKI responders. The remaining 2192 (26.5%) were classified as nonresponders.
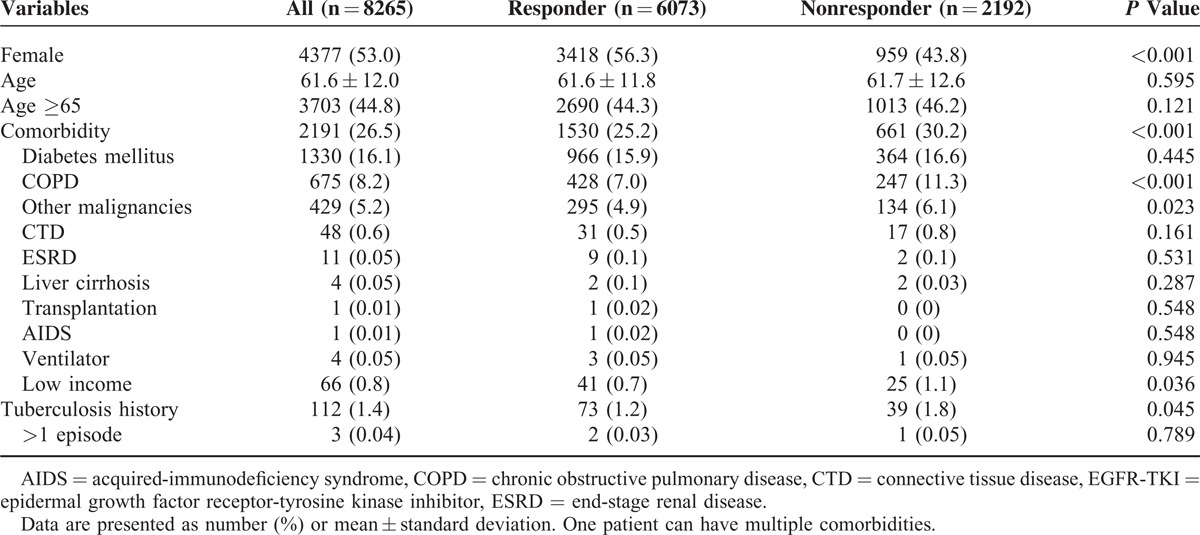
The comparisons of clinical characteristics between the EGFR-TKI responders and nonresponders were shown in Table 1. The mean age was 61.6 ± 12.0, with 44.8% aged >65 years. There was no age difference between the 2 groups. By gender, 53.0% were female. The most common underlying comorbidities were diabetes mellitus (16.1%), COPD (8.2%), and malignancies other than lung cancer (5.2%). The EGFR-TKI responders were more likely to be female (56.3% vs 43.8%, P < 0.001), less likely to have a history of pulmonary TB (1.2% vs 1.8%, P = 0.045), COPD (7.0% vs 11.3%, P < 0.001), and malignancies other than lung cancer (4.9% vs 6.1%, P = 0.023), and of low-income status (0.7% vs 1.1%, P = 0.036).
Table 1.
Patient Characteristics Based on EGFR-TKI Treatment Response
Predictors of Response to EGFR-TKI
Univariate analysis revealed that female gender was associated with good response to EGFR-TKI, whereas COPD, malignancies other than lung cancer, pulmonary TB history, and low-income status were associated with poor response (Table 2). In male patients with lung cancer, the proportion of good EGFR-TKI response was 52.7% in patients with pulmonary TB history and 68.6% in those without pulmonary TB history, and 89.5% and 78.0% in female patients with lung cancer, respectively. Because of the presence of interaction between gender and pulmonary TB history, a new variable, gender and TB history, was introduced to replace the 2 variables—gender and pulmonary TB history.
Table 2.
Univariate and Multivariate Logistic Regression Analysis for Predicting Response to ESRD-TKI
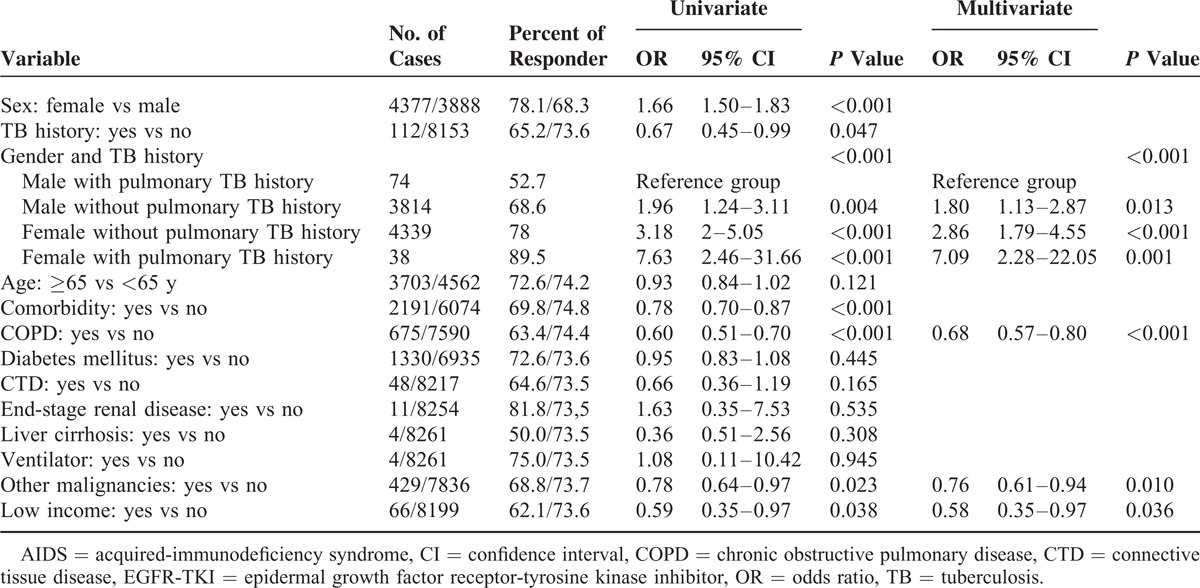
In a comparison of male with pulmonary TB history in multivariate logistic regression analysis, female with pulmonary TB history (OR: 7.09 [2.28–22.05]), female without pulmonary TB history (OR: 2.86 [1.79–4.55]), and male without pulmonary TB history (OR: 1.80 [1.13–2.87]), all had a significantly better EGFR-TKIs response (Table 2). Other independent predictors of EGFR-TKI response included COPD (OR: 0.68 [0.57–0.80]), malignancies other than lung cancer (OR: 0.76 [0.61–0.94]), and low income (OR: 0.58 [0.35–0.97]).
Prognostic Factors of 1-Year PFS
Univariate Cox regression analysis revealed that prognostic factors of 1-year PFS included gender and TB history, comorbidity (including COPD and diabetes mellitus), and low-income status (Table 3). The 1-year PFS was best in female with pulmonary TB history, followed by female without pulmonary TB history and male without pulmonary TB history, and was worst in male with pulmonary TB history. (P < 0.001, by log-rank test) (Figure 2A).
Table 3.
Multivariate Cox Proportional Hazard Regression Analysis for 1-y PFS
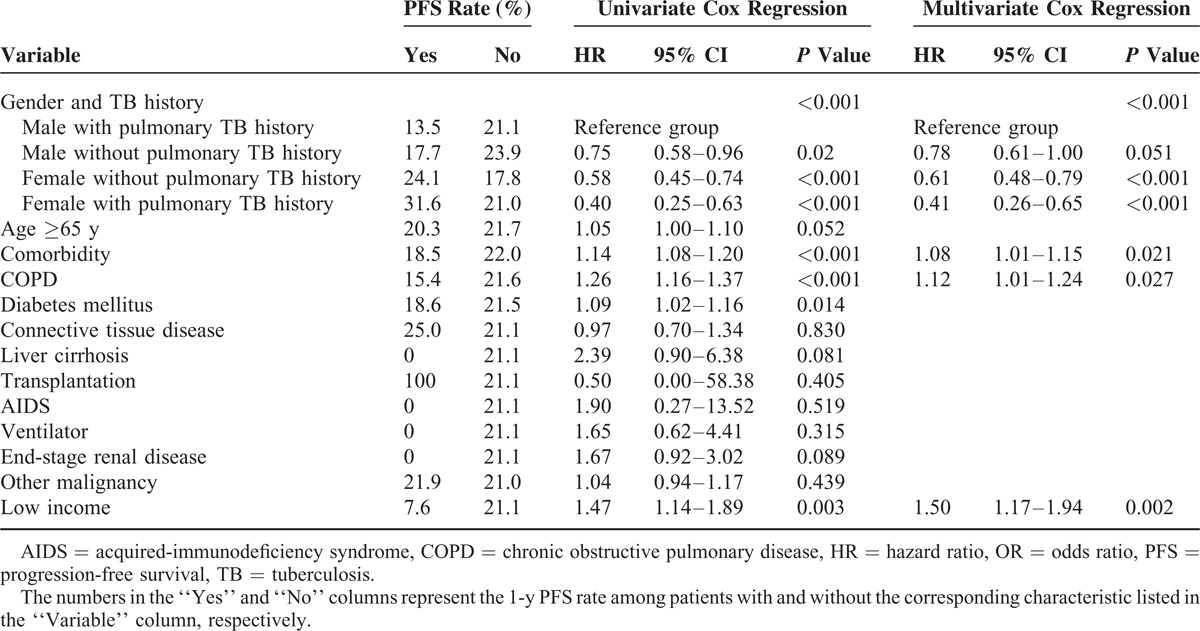
Figure 2.
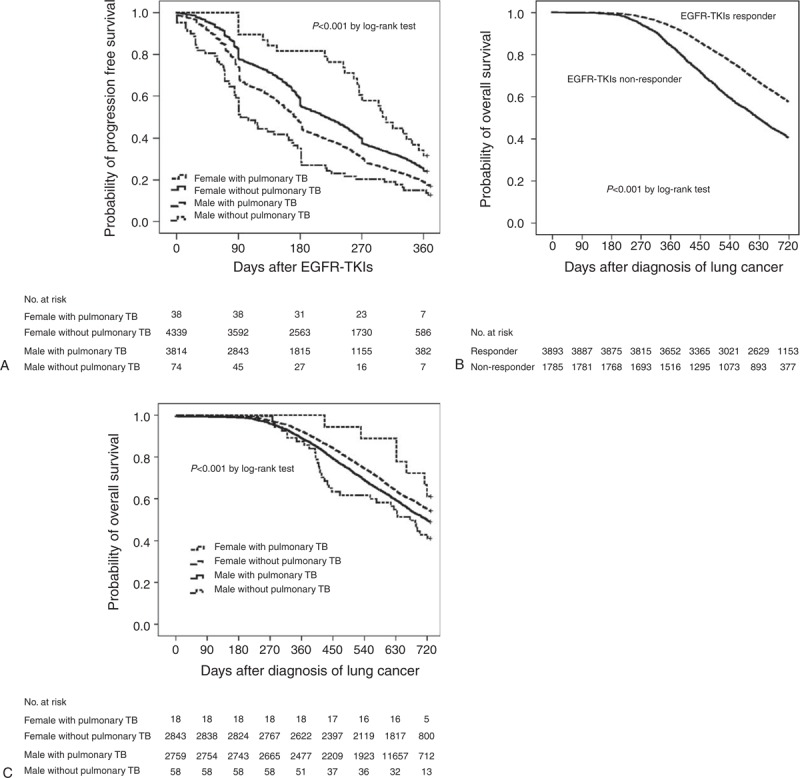
Kaplan–Meier curves for (A) 1-year progression-free survival in different genders with and without a history of pulmonary TB, (B) 2-year overall survival in EGFR-TKI nonresponder versus EGFR-TKI responder, and (C) 2-year overall survival in different genders with and without a history of pulmonary TB. EGFR-TKI = epidermal growth factor receptor-tyrosine kinase inhibitor, TB = tuberculosis.
In multivariate Cox regression analysis, female with pulmonary TB history (HR: 0.41 [0.26–0.65]), female without pulmonary TB history (HR: 0.61 [0.48–0.79]), and male without pulmonary TB history (HR: 0.78 [0.61–1.00]) all had a significantly better 1-year PFS than male with pulmonary TB history. The presence of comorbidity (HR: 1.08 [1.01–1.15]), COPD (HR: 1.13 [1.02–1.24]), and low income (HR: 1.50 [1.17–1.93]) were independent prognostic factors of 1-year PFS.
Prognostic Factors of 2-Year OS
Univariate Cox regression analysis revealed that EGFR-TKI response, gender and TB history, age ≥65 years, presence of comorbidity, presence of COPD or ESRD, and low-income status were associated with 2-year OS (Table 4; Figure 2B and C).
Table 4.
Multivariate Cox Proportional Hazard Regression Analysis for 2-y OS
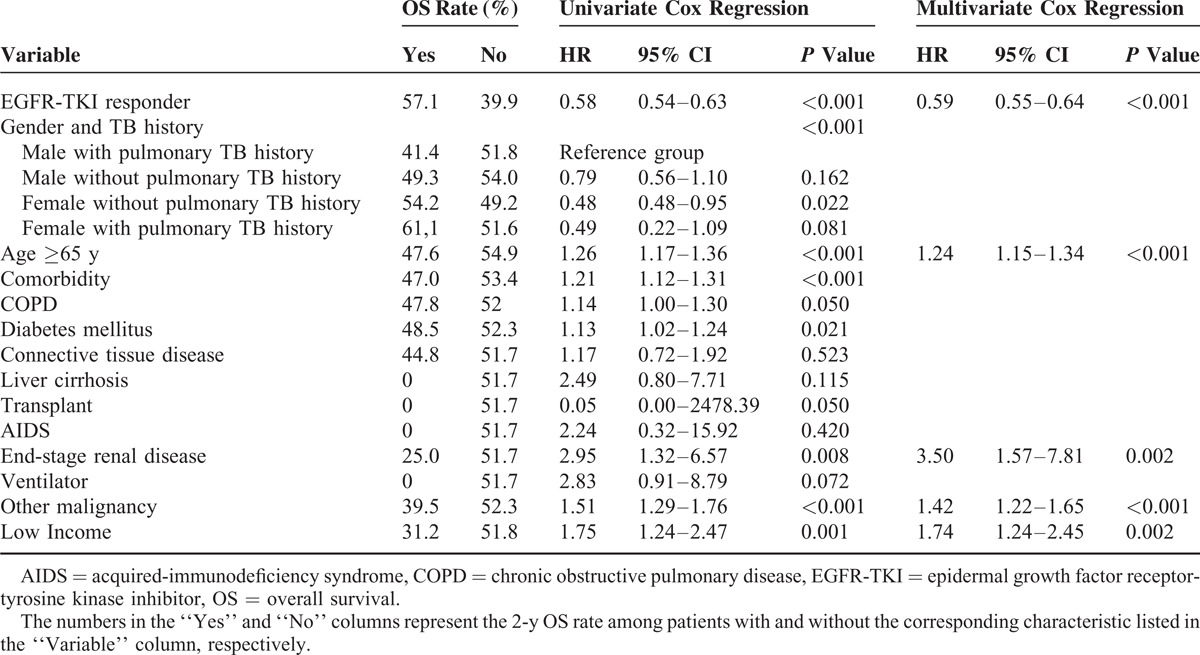
In multivariate Cox regression analysis, independent prognostic factors of 2-year OS included EGFR-TKI response (HR: 0.59 [0.55–0.64]), age ≥65 years (HR: 1.23 [1.14–1.33]), ESRD (HR: 3.48 [1.56–7.75]), malignancies other than lung cancer (HR: 1.43 [1.22–1.66]), and low-income status (HR: 1.75 [1.24–2.46]). Although being a significant predictor in univariate analysis, gender and TB history was not an independent prognostic factor for 2-year OS (Table 4).
Sensitivity Analysis
Sensitivity analysis was performed on 7140 (86.4%) patients without COPD, malignancies other than lung cancer, and low income. Interaction between gender and pulmonary TB history was still present in the subpopulation. Gender and TB history remained an independent predictor of EGFR-TKI response in logistic regression analysis (Table S1, Supplemental Content 1, http://links.lww.com/MD/A190, predicting response to EGFR-TKI in sensitivity analysis) and 1-year PFS in Cox regression analysis (Table S2, Supplemental Content 1, http://links.lww.com/MD/A190, 1-year PFS in sensitivity analysis; Figure S1, Supplemental Content 2, http://links.lww.com/MD/A188, Kaplan–Meier curves for 1-year PFS in sensitivity analysis). For the 2-year OS, again EGFR-TKI response (HR: 0.58 [0.53–0.63]), but not gender and TB history, was independent prognostic factor (Table S3, Supplemental Content 1, http://links.lww.com/MD/A190, 2-year OS in sensitivity analysis; Figure S2, Supplemental Content 3, http://links.lww.com/MD/A189, Kaplan–Meier curves for 2-year OS in sensitivity analysis).
DISCUSSION
This is the first nationwide population-based cohort study to investigate the associations between sensitive EGFR mutation and a history of pulmonary TB in patients with lung cancer. The present study has 3 major findings. First, similar to the previous studies,4,5,31 the clinical response to EGFR-TKIs is higher in female gender and patients without COPD. Other factors related to smoking include malignancies other than lung cancer23 and low income,24 which are associated with poor response to EGFR-TKIs. Second, a history of pulmonary TB is associated with poor clinical response to EGFR-TKIs in male patients but better response in female patients. The different impacts of pulmonary TB history in different genders are also observed on the 1-year PFS. Third, sensitivity analysis reveals that the impact of pulmonary TB history on EGFR-TKI response and 1-year PFS was even greater in the predominantly nonsmoking subpopulation. Thus, the different effects of pulmonary TB on lung cancer patients with different genders are unlikely because of smoking.
The process of carcinogenesis of lung cancer is multifactorial. The risk factors include smoking,30 occupational exposure to radon or asbestos, genetic factors of single-nucleotide polymorphism at 13q31.3,32 and chronic inflammation.13,14 Inflammation is a critical component of tumor progression. It is now becoming clear that the tumor microenvironment, which is largely manipulated by inflammatory cells via cytokines and chemokines, fosters proliferation, survival, and migration.12
The most common chronic pulmonary infection is TB. Two retrospective studies from China show that pulmonary TB increases the risk of lung cancer, with a HR of 6.7 to 13 in the first 5 years after TB,9,33 and remains significant after adjustments for smoking and other confounding factors. Another 2 population cohort studies in Taiwan that includes >4000 patients have similar findings.9,10 TB-related chronic inflammation leads to granuloma formation, fibrosis, and even dysplasia with scar formation. Uncontrolled inflammations with cell dysplasia and scar cancer are considered as 2 possible mechanisms in carcinogenesis from TB.13
There are several reports about the connection between chronic inflammation and EGFR mutation. One report mentions that oxidant-induced goblet cell metaplasia in human airway epithelium leads to EGFR activation.34 Another report shows nitric oxide-induced epidermal growth factor-dependent phosphorylation in A431 tumor cells.35 It has also been reported that TB increases the expression of epiregulin, which correlates with the invasive properties on EGFR-mutant cells.14,36 A recent retrospective report from Taiwan has found a possible association between old pulmonary TB and sensitive EGFR mutation, especially exon 19 deletions.18 However, in this study, the diagnosis of pulmonary TB is simply based on radiographic findings rather than mycobacteriology or a history of anti-TB treatment. Nevertheless, these reports all emphasize that inflammation may cause EGFR mutations.
Using a large number of lung cancer patients with confirmed history of pulmonary TB, the present analyses demonstrate that pulmonary TB has a different impact on EGFR mutation and outcomes of lung cancer in female and male patients, resulting in a higher incidence of TKI-sensitive EGFR mutation and better 1-year PFS in female patients. Unlike in a previous study,18 these findings imply that pulmonary TB may result in different carcinogenesis processes between different genders. Possible explanations come from several studies showing different lung cancer presentation between males and females in the carcinogenesis of smoking, incidence of mutation in K-ras, c-erbB-2, or EGFR, and mortality.37 The differences may be related to genetic difference, decreased DNA repair capacity in female patients, and hormones.38,39 Women have been shown to have a greater risk of contracting tobacco-induced lung cancer for their lack of DNA repair ability compared with men.40 Nonetheless, estrogen simulates angiogenesis and increased angiogenesis is associated with tumor metastatic potential in lung cancer.41
TB also presents as different inflammations between male and female patients.42,43 Studies show that estrogen appears to act in synergy with interferon-γ to impair mycobacterial growth in mice by regulating interferon-γ promoter.44,45 It can be hypothesized that because of the lack of DNA repairing ability and estrogen-enhanced inflammation and angiogenesis, women are prone to more damages from inflammation that is induced by Mycobacterium tuberculosis, leading to a higher incidence of EGFR mutation. However, the detailed pathophysiology remains to be studied.
Apart from the association with EGFR mutation,5 tobacco smoking increases susceptibility to TB and is associated with treatment failure of TB.46,47 Since a history of smoking and other environmental factors such as exposure to biofuels are not available in the NHIRD of Taiwan, it is possible that some of the effect of pulmonary TB on EGFR mutation observed in present study is through the effect of those toxic substances. However, results of the sensitivity analysis focusing on lung cancer patients without COPD, malignancies other than lung cancer, and low income reveal an even greater impact of pulmonary TB history on EGFR-TKI response and 1-year PFS in the predominantly nonsmoking population. These findings suggest that the different effects of pulmonary TB on lung cancer patients with different genders are unlikely to be explained by smoking.
The results are compatible with findings of the IPASS and PIONEER studies, as well as other reports,2,4,5,48 showing that female gender and nonsmoking status are associated with better response to EGFR-TKIs, which, in turn, is associated with better OS. These findings also imply that the NHIRD of Taiwan can be a useful resource material for studying the long-term impact of pulmonary TB on the carcinogenesis and outcome of lung cancer.
This study has some limitations. First, results of molecular testing for the EGFR gene mutation are not available in this cohort. Although approval of EGFR-TKI use for >90 days require reaudit based on therapeutic response, using prescription duration of TKI >90 days as the surrogate of sensitive EGFR gene mutation may still have some bias. Second, even with similar findings in sensitivity analysis, the results of the present analyses may still be confounded by smoking. Third, the cohort may include some patients with squamous cell lung cancer, because erlotinib has a third-line indication in these patients in Taiwan. However, the number is limited because <40% of the study patients receive erlotinib and the most common cell type of lung cancer in Taiwan is adenocarcinoma, especially among female patients.49 In addition, few patients with squamous cell lung cancer can tolerate previous chemotherapy and go on third-line therapy.50
CONCLUSION
This study demonstrates that pulmonary TB has a gender-dependent impact, with better EGFR-TKI response and 1-year PFS in female, but worse in male patients. These results are different from those of the previous reports. The carcinogenesis and inflammation of TB may be different between male and female patients with lung cancer, but further large clinical trials are warranted to provide more evidence.
Acknowledgment
The authors would like to thank the National Health University Health Data Research Center for providing and analyzing the National Health Insurance Research Database.
Footnotes
Abbreviations: EGFR = epidermal growth factor receptor, HR = hazard ratio, TB = tuberculosis, TKI = tyrosine kinase inhibitor.
C-HC, C-HL, C-CH, J-YW: study concept and design, analysis of data, and drafting of manuscript; C-HL, C-CH: statistical analysis and interpretation of data; C-HC, J-YW: interpretation of data and review of manuscript; and C-JY: review of manuscript. All of the authors have seen and approved the final version of the manuscript.
This work was supported by the National Science Council, Taiwan (grant NSC 99-2314-B-002-088-MY2; http://web1.nsc.gov.tw/) and the Centers for Disease Control, Taiwan (grant DOH-102-DC-1301 and MOHW-103-CDC-C-114-112302). The funding sources had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. This is not a National Institutes of Health-sponsored study.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013; 63:11–30. [DOI] [PubMed] [Google Scholar]
- 2.Lee CK, Brown C, Gralla RJ, et al. Impact of EGFR inhibitor in non-small cell lung cancer on progression-free and overall survival: a meta-analysis. J Natl Cancer Inst 2013; 105:595–605. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch FR, Bunn PA., Jr EGFR testing in lung cancer is ready for prime time. Lancet Oncol 2009; 10:432–433. [DOI] [PubMed] [Google Scholar]
- 4.Shi Y, Au JS, Thongprasert S, et al. A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014; 9:154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361:947–957. [DOI] [PubMed] [Google Scholar]
- 6.Lonnroth K, Raviglione M. Global epidemiology of tuberculosis: prospects for control. Semin Respir Crit Care Med 2008; 29:481–491. [DOI] [PubMed] [Google Scholar]
- 7.Liang HY, Li XL, Yu XS, et al. Facts and fiction of the relationship between preexisting tuberculosis and lung cancer risk: a systematic review. Int J Cancer 2009; 125:2936–2944. [DOI] [PubMed] [Google Scholar]
- 8.Nechuta S, Lu W, Chen Z, et al. Vitamin supplement use during breast cancer treatment and survival: a prospective cohort study. Cancer Epidemiol Biomarkers Prev 2011; 20:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu CY, Hu HY, Pu CY, et al. Pulmonary tuberculosis increases the risk of lung cancer: a population-based cohort study. Cancer 2011; 117:618–624. [DOI] [PubMed] [Google Scholar]
- 10.Yu YH, Liao CC, Hsu WH, et al. Increased lung cancer risk among patients with pulmonary tuberculosis: a population cohort study. J Thorac Oncol 2011; 6:32–37. [DOI] [PubMed] [Google Scholar]
- 11.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001; 357:539–545. [DOI] [PubMed] [Google Scholar]
- 12.Coussens LM, Werb Z. Inflammation and cancer. Nature 2002; 420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bobba RK, Holly JS, Loy T, et al. Scar carcinoma of the lung: a historical perspective. Clin Lung Cancer 2011; 12:148–154. [DOI] [PubMed] [Google Scholar]
- 14.Nalbandian A, Yan BS, Pichugin A, et al. Lung carcinogenesis induced by chronic tuberculosis infection: the experimental model and genetic control. Oncogene 2009; 28:1928–1938. [DOI] [PubMed] [Google Scholar]
- 15.Shieh SH, Probst JC, Sung FC, et al. Decreased survival among lung cancer patients with co-morbid tuberculosis and diabetes. BMC Cancer 2012; 12:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heuvers ME, Aerts JG, Hegmans JP, et al. History of tuberculosis as an independent prognostic factor for lung cancer survival. Lung Cancer 2012; 76:452–456. [DOI] [PubMed] [Google Scholar]
- 17.Kuo CH, Lo CY, Chung FT, et al. Concomitant active tuberculosis prolongs survival in non-small cell lung cancer: a study in a tuberculosis-endemic country. PLoS One 2012; 7:e33226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo YH, Wu CH, Wu WS, et al. Association between tumor epidermal growth factor receptor mutation and pulmonary tuberculosis in patients with adenocarcinoma of the lungs. J Thorac Oncol 2012; 7:299–305. [DOI] [PubMed] [Google Scholar]
- 19.Bureau of National Health Insurance. The National Health Insurance Statistics, 2011. http://www.nhi.gov.tw/English/webdata/webdata.aspx?menu=11&menu_id=296&webdata_id=1942&WD_ID=296. Updated December 14, 2012 Accessed May 10, 2014. [Google Scholar]
- 20.Wu JY, Shih JY, Chen KY, et al. Gefitinib therapy in patients with advanced non-small cell lung cancer with or without testing for epidermal growth factor receptor (EGFR) mutations. Medicine (Baltimore) 2011; 90:159–167. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organization. Global Tuberculosis Control: WHO, 2013. http://apps.who.int/iris/bitstream/10665/91355/1/9789241564656_eng.pdf. Updated November, 2012 Accessed April 12, 2014. [Google Scholar]
- 22.Lokke A, Lange P, Scharling H, et al. Developing COPD: a 25 year follow up study of the general population. Thorax 2006; 61:935–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabuchi T, Ito Y, Ioka A, et al. Tobacco smoking and the risk of subsequent primary cancer among cancer survivors: a retrospective cohort study. Ann Oncol 2013; 24:2699–2704. [DOI] [PubMed] [Google Scholar]
- 24.Hiscock R, Bauld L, Amos A, et al. Socioeconomic status and smoking: a review. Ann N Y Acad Sci 2012; 1248:107–123. [DOI] [PubMed] [Google Scholar]
- 25.Methodology WCCfDS. WHO International Working Group for Drug Statistics Methodology: Guidelines for ATC Classification and DDD Assignment. Oslo, Norway: WHO Collaborating Centre for Drug Statistics Methodology; 2011. [Google Scholar]
- 26.Lee CH, Lee MC, Lin HH, et al. Pulmonary tuberculosis and delay in anti-tuberculous treatment are important risk factors for chronic obstructive pulmonary disease. PLoS One 2012; 7:e37978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pirkis JE, Speed BR, Yung AP, et al. Time to initiation of anti-tuberculosis treatment. Tuber Lung Dis 1996; 77:401–406. [DOI] [PubMed] [Google Scholar]
- 28.Taiwan MotI. Public Assistance Act. Taipei, Taiwan (R.O.C.): Ministry of the Interior; 2010. [Google Scholar]
- 29.Lin HH, Ezzati M, Chang HY, et al. Association between tobacco smoking and active tuberculosis in Taiwan: prospective cohort study. Am J Respir Crit Care Med 2009; 180:475–480. [DOI] [PubMed] [Google Scholar]
- 30.Godtfredsen NS, Prescott E, Osler M. Effect of smoking reduction on lung cancer risk. J Am Med Assoc 2005; 294:1505–1510. [DOI] [PubMed] [Google Scholar]
- 31.Marchetti A, Martella C, Felicioni L, et al. EGFR mutations in non-small-cell lung cancer: analysis of a large series of cases and development of a rapid and sensitive method for diagnostic screening with potential implications on pharmacologic treatment. J Clin Oncol 2005; 23:857–865. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Sheu CC, Ye Y, et al. Genetic variants and risk of lung cancer in never smokers: a genome-wide association study. Lancet Oncol 2010; 11:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engels EA, Shen M, Chapman RS, et al. Tuberculosis and subsequent risk of lung cancer in Xuanwei. China Int J Cancer 2009; 124:1183–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Casalino-Matsuda SM, Monzon ME, Forteza RM. Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium. Am J Respir Cell Mol Biol 2006; 34:581–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruano MJ, Hernandez-Hernando S, Jimenez A, et al. Nitric oxide-induced epidermal growth factor-dependent phosphorylations in A431 tumour cells. Eur J Biochem 2003; 270:1828–1837. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Iwanaga K, Choi KC, et al. Intratumoral epiregulin is a marker of advanced disease in non-small cell lung cancer patients and confers invasive properties on EGFR-mutant cells. Cancer Prev Res (Phila) 2008; 1:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiyohara C, Ohno Y. Sex differences in lung cancer susceptibility: a review. Gend Med 2010; 7:381–401. [DOI] [PubMed] [Google Scholar]
- 38.Thomas L, Doyle LA, Edelman MJ. Lung cancer in women: emerging differences in epidemiology, biology, and therapy. Chest 2005; 128:370–381. [DOI] [PubMed] [Google Scholar]
- 39.North CM, Christiani DC. Women and lung cancer: what is new? Semin Thorac Cardiovasc Surg 2013; 25:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kure EH, Ryberg D, Hewer A, et al. p53 mutations in lung tumours: relationship to gender and lung DNA adduct levels. Carcinogenesis 1996; 17:2201–2205. [DOI] [PubMed] [Google Scholar]
- 41.Calvo A, Catena R, Noble MS, et al. Identification of VEGF-regulated genes associated with increased lung metastatic potential: functional involvement of tenascin-C in tumor growth and lung metastasis. Oncogene 2008; 27:5373–5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neyrolles O, Quintana-Murci L. Sexual inequality in tuberculosis. PLoS Med 2009; 6:e1000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feng JY, Huang SF, Ting WY, et al. Gender differences in treatment outcomes of tuberculosis patients in Taiwan: a prospective observational study. Clin Microbiol Infect 2012; 18:E331–337. [DOI] [PubMed] [Google Scholar]
- 44.Tsuyuguchi K, Suzuki K, Matsumoto H, et al. Effect of oestrogen on Mycobacterium avium complex pulmonary infection in mice. Clin Exp Immunol 2001; 123:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fox HS, Bond BL, Parslow TG. Estrogen regulates the IFN-gamma promoter. J Immunol 1991; 146:4362–4367. [PubMed] [Google Scholar]
- 46.Shang S, Ordway D, Henao-Tamayo M, et al. Cigarette smoke increases susceptibility to tuberculosis-evidence from in vivo and in vitro models. J Infect Dis 2011; 203:1240–1248. [DOI] [PubMed] [Google Scholar]
- 47.Tachfouti N, Nejjari C, Benjelloun MC, et al. Association between smoking status, other factors and tuberculosis treatment failure in Morocco. Int J Tuberc Lung Dis 2011; 15:838–843. [DOI] [PubMed] [Google Scholar]
- 48.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol 2011; 29:2866–2874. [DOI] [PubMed] [Google Scholar]
- 49.Bureau of Health Promotion, Taiwan. Cancer Registry Annual Report, 2010. http://tcr.cph.ntu.edu.tw/uploadimages/CA15_LF100_20140415.pdf. Updated Feb, 2014 Accessed June 10, 2014. [Google Scholar]
- 50.Perng RP, Yang CH, Chen YM, et al. High efficacy of erlotinib in Taiwanese NSCLC patients in an expanded access program study previously treated with chemotherapy. Lung Cancer 2008; 62:78–84. [DOI] [PubMed] [Google Scholar]


