Abstract
Cardiotoxicity is a well-recognized side effect induced by chemotherapeutic drugs such as anthracycline and trastuzumab through different mechanisms. Currently, accumulating evidence supports that dexrazoxane (DZR) can minimize the risk of cardiotoxicity. In this study, we investigated whether dexrzoxane could reduce cardiotoxicity in the treatment of anthracycline combined with trastuzumab.
We randomly divided 90 experimental F344 rats into control group, chemotherapeutics and trastuzumab (doxorubicin [DOX] + herceptin [Her]) group, and chemotherapeutics, trastuzumab, and DZR (DOX + Her + DZR) group. Animal status and body weight, cardiac function, serum cardiac markers, cardiomyocyte apoptosis of the rats, and expression level of calpain-2 were evaluated. Left ventricular ejection fraction (LVEF) and fractional shortening (FS) of the left ventricle were observed. The serum levels of malondialdehyde (MDA) and cardiac troponin I (cTnI) and cardiomyocyte apoptosis were detected by enzyme linked immunosorbent assay and TdT-mediated dUTP nick end labeling assays. The mRNA and protein level of calpain-2 were measured by reverse transcriptase polymerase chain reaction and Western blot.
We observed that the LVEF and FS of the left ventricle were significantly higher in the DOX + Her + DZR group than that in the DOX + Her group (P < 0.05). The serum levels of MDA and cTnI between DOX + Her group and DOX + Her + DZR group were significantly different. In addition, cardiomyocyte apoptosis in the DOX + Her + DZR group was significantly less severe than that in the DOX + Her group (P < 0.05). After DZR treatment, the calpain-2 mRNA and protein levels in the DOX + Her + DZR group were significantly higher than the DOX + Her group (P < 0.05).
Our results suggest that DZR can effectively reduce the cardiotoxicity of combinatorial treatment of trastuzumab and anthracycline partly through upregulating calpain-2.
INTRODUCTION
Cardiotoxicity, a common lethal complication associated with intensive anticancer drugs used in chemotherapy,1,2 impacts on effectiveness of anticancer therapy and survival of patients. Since St. Gallen international experts reached the consensus that the standardized treatment regimen would be chemotherapy (anthracyclines and taxol chemotherapeutics) combined with anti-human epidermal growth factor receptor 2(HER2) therapy (trastuzumab) in luminal B (HER2+) type and HER2 overexpressed-type patients with breast cancer (BC),3,4 the presence of anthracyclines-related cardiotoxicity (including choronic congestive heart failure) and the trastuzumab-related cardiotoxicity5–8 were doomed to limit the application of chemotherapy and anti-HER2 therapy combinational treatment in BC.
Dexrazoxane (DZR) is a derivative of ethylene diamine tetraacetic acid and a potent metal ion chelater, which was developed as a potent cardioprotective agent. As its exclusively protective effect for the cardiotoxicity, it was consecutively marketed worldwide. Currently, an increasing number of clinical studies have suggested that the use of DZR before anthracyclines administration could significantly improve the cardiac lesions induced by adriamycin/epirubicin.9 Despite the mechanisms of its cardioprotective effects remained elusive,10,11 it is also the cardiac protective agent that has been confirmed in patients with cancer who have received trastuzumab treatment.
Calpains belong to a family of cell stress response proteins, which constitute a superfamily of intracellular calcium-dependent neutral cysteine proteases whose members are widely expressed in a variety of cells and tissues.12 In mammals, calpain-1 and calpain-2 are expressed ubiquitously. It is demonstrated that calpains are important in apoptosis.13 Recent studies also showed that calpain significantly contributes to diabetic cardiomyopathy in different mouse models of type-1 diabetes.14 These studies suggest that calpain activation may play a role in the progression of heart failure.15 Previous studies showed that calpain may be implicated in doxorubicin (DOX)-induced cardiomyocyte death.16,17
In this study, we aim to investigate the efficacy and mechanism of DZR on prevention of cardiotoxicity that is induced by anthracycline combined with trastuzumab in a rat model.
MATERIALS AND METHODS
Experimental Materials
Animals
The animal experiments conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health and were approved by the Institutional Review Board of the Tianjin Medical University Cancer Institute and Hospital, Tianjin, China. Ninety female F344 rats, 9 weeks old, were purchased from the Laboratory Animal Science Department of the Peking University Health Science Center (License No. SCXK (Jing) 2006–2008). Animals were bred in a natural ventilated room, with 12-hour light/dark cycle, a temperature of 25°C to 28°C, and a relative humidity of 70% to 85%.
Reagents and Instruments
DZR (Aonuoxian) was kindly donated by Jiangsu Aosaikang Pharmaceutical Co, Ltd (Jiangsu, China), trastuzumab (herceptin [Her]) was provided by Roche (Basel, Switzerland), and DOX (adriamycin) was provided by Haimen Pharmaceuticals (Zhejiang, China). Superoxide dismutase (SOD) 2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt assay kits and malondialdehyde (MDA) test kits were purchased from Nanjing Jiancheng Bioengineering Institute (Jiangsu), and the rat serum cardiac troponin I (cTnI) enzyme linked immunosorbent assay (ELISA) kit, rat serum brain natriuretic peptide (BNP) ELISA kit, and rat serum atrial natriuretic peptide (ANP) ELISA kit were purchased from R&D Co. (Minneapolis, MN). All other test agents were provided by the Key Laboratory of Tianjin Tumor Hospital, Tianjin.
Enzyme-linked immune detector, superclean bench, incubator, centrifuge, polymerase chain reaction (PCR) apparatus, and all other laboratory apparatus were provided by the Key Laboratory of Tianjin Tumor Hospital or the Institute of Hematology, Chinese Academy of Medical Science, Beijing, China.
Methods
Establishment of Animal Model and Design
Body weights of rats at the start of the experiment were 210 ± 15 g. According to the Russo method,18 90 female F344 rats were injected with N-nitroso-N-methylurea (Sigma-Aldrich, St Louis, MO) to induce and establish the BC models. Ninety rats were randomly assigned into control group, DOX + Her group, and DOX + Her + DZR group. In each group of 30 animals, intraperitoneal injection was adopted as the administration route.
Control group: injection of the same volume of saline at the same time; N = 30.
DOX + Her group: injection of 0.8 mg/kg adriamycin, once a week, for 2 weeks; then injection of 2 mg/kg trastuzumab, once a week, for 2 weeks; N = 30.
DOX + Her + DZR group: dosing procedure was the same as that in the DOX + Her group, and injecting 40 mg/kg DZR before each trastuzumab administration; N = 30.
In each group, 10 rats were randomly selected and dissected to collect the cardiac myocytes for microscopically observation and apoptosis analysis on week 4. The remaining ones were observed for 1 more month to evaluate the mortality rates.
Cardiac Indexes of Serum SOD, MDA, cTnI, BNP, and ANP
Blood samples were intravenously collected at baseline and week 4. The rats were anesthetized by intraperitoneal injection of 2% pentobarbital sodium. Blood samples were collected from the caudal veins, and serum samples were separated by centrifugation. The cTnI, BNP, and ANP levels were detected by ELISA assay. The serum SOD levels were determined by xanthine oxidase method, and the serum MDA levels were determined by thiobarbituric acid method.
Cardiac Function
Left ventricular ejection fraction (LVEF), left ventricular end-diastolic diameter (LVEDD), and the left ventricular fractional shortening (% FS) were monitored at baseline and week 4 by a Philip Sonos 7500 echocardiograph (Amsterdam, The Netherlands) with the mode selected as follows: S12 cardiac ultrasonic probe—frequency, 8 MHz; superficial organ mode—scanning speed, 150 mm/s; ultrasonic tangent plane; and parasterna left ventricle long-axis view.
Myocardial Pathology
The cardiac muscles within the left ventricular free wall were removed out and then fixed in 10% neutral formalin solution. The paraffin blocks were serially sectioned, gradient dewaxed, stained with Harris hematoxylin for 5 minutes, dipped in alcohol containing 1% hydrochloric acid for 5 to 10 seconds, washed in tap water for 25 minutes, and stained with 0.5% eosin for 2 minutes. The slides were dehydrated in gradient alcohol, cleared, and mounted.
Apoptosis
Cardiomyocyte apoptosis was detected by TdT-mediated dUTP nick end labeling (TUNEL) assay (Roche): paraffin blocks were serially sectioned, routinely dewaxed, high temperature antigen repaired (95°C) for 10 minutes, soaked in 3% hydrogen peroxide solution for 20 minutes, and digested in 20 μg/mL proteinase K for 30 minutes. The 30 mmol/L TUNEL buffer was prepared, stained with hematoxylin for 5 minutes, dipped in alcohol containing 1% hydrochloric acid for 5 to 10 seconds, washed in tap water for 25 minutes, and stained with 0.5% eosin for 2 minutes. The slides were dehydrated in gradient alcohol, cleared, and mounted. For each slide, 5 myocardial fields were randomly selected, and the number of TUNEL-positive cells and the total cells were counted for each high magnification field, respectively. Average apoptotic index (AI) was calculated for 5 fields.
Real-Time RT-PCR
Total RNA was extracted from cardiomyocytes using Trizol Reagent (Gibco-BRL, Gaithersburg, MD) following the manufacturer's instructions. Real-time reverse transcriptase (RT)-PCR was performed to analyze mRNA expression for calpain-1, calpain-2, and glyceraldehyde-3-phosphate dehydrogenase as previously described.19
Western Blotting
For Western blot analyses, 20 μg of total protein was electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide gelelectrophoresis gel, transferred onto polyvinylidene difluoride membrane, blocked, and then incubated with calpain-1 and calpain-2 antibodies (Cell Signaling Inc., Danvers, MA). Corresponding horseradish peroxidase-conjugated secondary antibody was then used on them at room temperature for 2 hours. Protein bands were detected on an Odyssey scanner (LICOR Biosciences, Lincoln, NE) using Pierce West Pico chemiluminescent detection system (Thermo Fisher Scientific Inc, Rockford, IL).
Statistics
All statistical analyses were calculated by the SPSS software (SPSS 17.0). Measurement data were presented as X (—) ± s. For the body weight, statistical significances were determined by Mann–Whitney U test. For the cardiac index, cardiac function, and apoptosis analyses, Student t test was performed. For mRNA level, 1-way ANOVA was performed. The survival rates in 1 month were conducted using Fisher exact test. The significance level (α) was 0.05.
RESULTS
Animal Status and Body Weight
Among the 90 rats who underwent the process of establishing the BC model, 1 rat died during the injection and 4 rats died before assigning into groups. The tumor initiation rate was 94.44% (85/90).
At baseline, no significant differences existed among all 3 groups of experimental animals (Figure 1). In the DOX + Her group, animals had significantly emaciated at all subsequent stages compared with those in the control group (all P < 0.05). At weeks 2 and 3, the body weights of rats were increased in the DOX + Her + DZR group (P = 0.018 and 0.031) compared with the DOX + Her group animals. At week 4, the 2 groups had similar body weight with no significance.
Figure 1.

Effect of chemotherapy and DZR treatment on body weight of animals. Animals in DOX + Her group (n = 28) had significantly reduced body weight compared with the control group (n = 29) (all P < 0.05). DOX + Her + DZR group (n = 28) animals had increased body weight at week 2 (P = 0.018) and week 3 (P = 0.031) compared with DOX + Her group. Each point represents the mean ± SD. DOX + Her = chemotherapeutics and trastuzumab, DOX + Her + DZR = chemotherapeutics, trastuzumab, and dexrazoxane, SD = standard deviation.
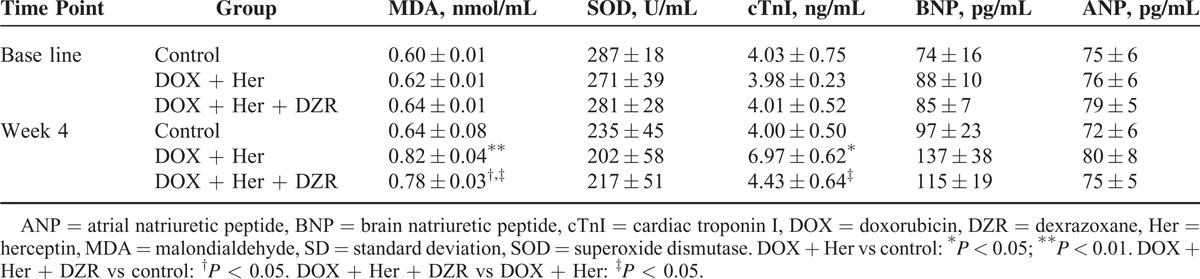
Effects of DZR on Serum SOD, MDA, cTnI, BNP, and ANP Levels
The SOD, MDA, cTnI, BNP, and ANP expressions in serum were shown in Table 1. The concentrations of MDA and cTnI in the DOX + Her group were significantly higher than those in the control group (P = 0.006 and 0.020 for MDA and cTnI, respectively). When compared with DOX + Her + DZR, these indexes in the DOX + Her group were also significantly higher at week 4 (P = 0.012 and 0.034 for MDA and cTnI, respectively). For the serum SOD, BNP, and ANP levels at baseline, no marked differences had been found among these 3 groups (Table 1).
Table 1.
Effect of Dexrazoxane on Serum SOD, MDA, cTnI, BNP, and ANP Levels (Means ± SD, n = 10 in Each Group)
Assessment of Cardiac Function
Cardiac functions were monitored at week 4 (Table 2). As compared with the control group, DOX + Her revealed significant difference in LVEF (P = 0.004), FS (P = 0.026), and LVEDD (P < 0.001). Treatment with DOX + Her + DZR increased the LVEF and FS (P = 0.027 and 0.031, respectively), and decreased the index of LVEDD (P = 0.019) compared with the DOX + Her group (Table 2).
Table 2.
Left Ventricular Function at Week 4 (Means ± SD, n = 10 in Each Group)

In order to investigate the effect of DZR on myocardial pathology, we also performed a hematoxylin–eosin staining in sectioned paraffin blocks of heart muscle samples from the rats (Figure 2). Microscopically, most of the cardiomyocytes in the DOX + Her group developed different extent of vacuolar degeneration and edema, obviously widened intercellular space, and a large amount of inflammatory cell infiltrates (Figure 2A–C). Compared with those pathological findings in the DOX + Her group, DZR pretreatment largely counteracted the severe damages induced by anthracycline combined with trastuzumab in the DOX + Her + DZR group.
Figure 2.
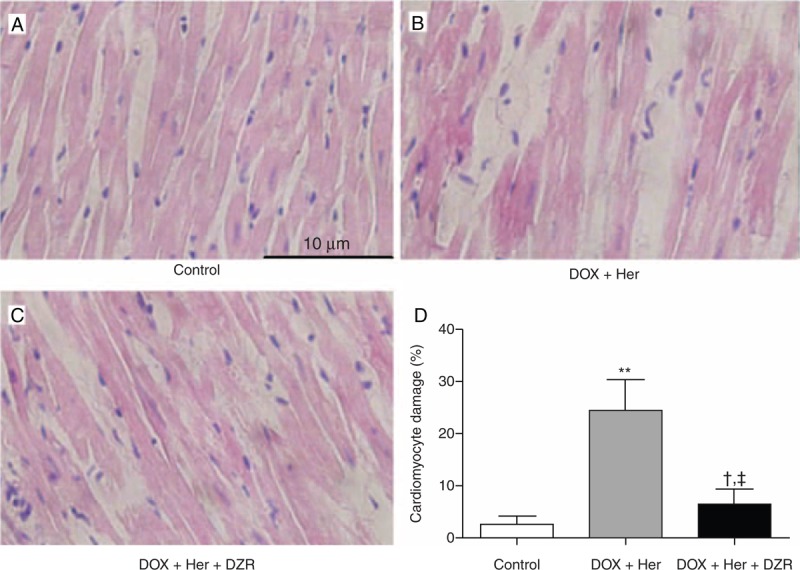
Morphology of myocardial tissues in rats of different treatment groups at week 4. (A) HE staining of myocardial tissues in the control group, original magnification 400×. (B) HE staining of myocardial tissues in DOX + Her group, original magnification 400×. (C) HE staining of myocardial tissues in DOX + Her + DZR group, original magnification 400×. (D) Comparison of cardiomyocyte damage between different treatment groups. Each column represents the mean ± SD. The statistical analysis was performed with Student t test. DOX + Her vs control: ∗∗P < 0.01; DOX + Her + DZR vs control: †P < 0.05; DOX + Her + DZR vs DOX + Her: ‡P < 0.05. DOX + Her = chemotherapeutics and trastuzumab, DOX + Her + DZR = chemotherapeutics, trastuzumab, and dexrazoxane, HE = hematoxylin–eosin, SD = standard deviation.
DZR Relieves Cardiomyocyte Apoptosis Induced by Combined Therapy
As is well established, the cardiomyocyte apoptpsis was an important cause of cardiac toxicity induced by anthracycline or Her therapy. Therefore, we investigated the incidence of cardiomyocyte apoptosis in different groups to identify whether DZR could relieve the apoptosis or not. In Figure 3, the nucleoli of apoptotic cells appeared as yellow granules. The incidence of cardiomyocyte apoptosis in control rats was 4.81% ± 1.24%. After chemotherapy as a targeted therapy, apoptotic cells significantly increased (Figure 3D). The AI of the DOX + Her group was 13.1% ± 0.87%, whereas DZR significantly decreased the AI of cardiomyocyte of rats in the DOX + Her + DZR group compared with the chemotherapy group (P = 0.014) (Figure 3).
Figure 3.
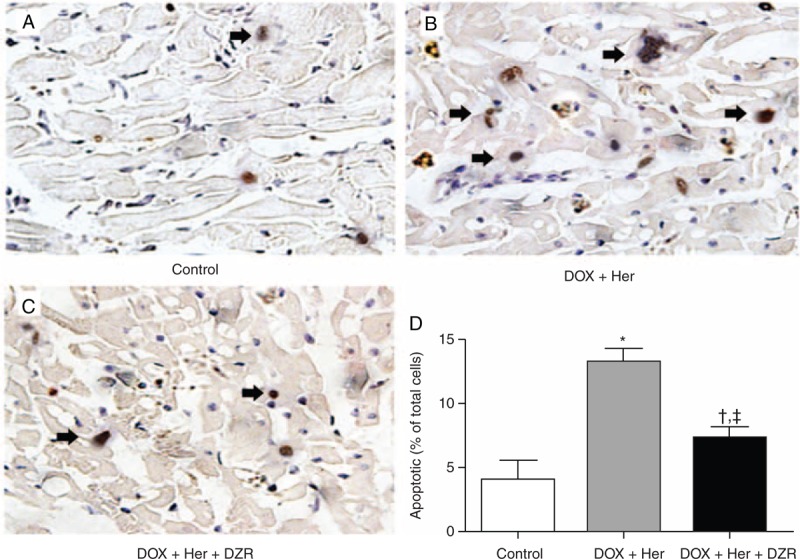
Cardiomyocyte apoptosis changes in rats of different treatment groups at week 4. (A) Cardiomyocyte apoptosis in the control group. The nucleoli of apoptotic cells appeared as yellow granules. (B) Cardiomyocyte apoptosis in DOX + Her group. (C) Cardiomyocyte apoptosis in DOX + Her + DZR group. (D) Comparison of apoptotic indexes between different treatment groups. Each column represents the mean ± SD. The statistical analysis was performed with Student t test. DOX + Her vs control: ∗P < 0.05; DOX + Her + DZR vs control: †P < 0.05; DOX + Her + DZR vs DOX + Her: ‡P < 0.05. DOX + Her = chemotherapeutics and trastuzumab, DOX + Her + DZR = chemotherapeutics, trastuzumab, and dexrazoxane.
DZR Induces Calpain-2 Expression
Calpains, a family of calcium-dependent thiol proteases, are important in apoptosis and inflammatory responses in cardiomyocytes under stress.13,20 It has been confirmed that DOX reduced calpain-1 and calpain-2 and enhanced apoptosis. To investigate whether DZR could influence the mRNA expression levels of calpain-1 and calpain-2, quantitative RT-PCR was performed on cardiomyocytes from rats in the control group, DOX + Her group, and DOX + Her + DZR group. Compared with the control group, the calpain-2 mRNA and protein levels in the DOX + Her group were significantly decreased. After DZR treatment, the expressions of calpain-2 in the DOX + Her + DZR group were significantly higher than the DOX + Her group (P < 0.05). However, there were no differences of calpain-1 mRNA and protein levels among the control group, DOX + Her group, and DOX + Her + DZR group (Figure 4).
Figure 4.

Analysis of calpain-1 and calpain-2 expression. (A) At week 4, the mRNA expression levels of calpain-1 and calpain-2 in cardiac tissue samples from different groups were analyzed by quantitative RT-PCR. (B) The expression of calpain-1 and calpain-2 proteins was analyzed by Western blot. (C) Quantification of calpain-1, relative to GAPDH expression. (D) Quantification of calpain-2, relative to GAPDH expression. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. DOX + Her = chemotherapeutics and trastuzumab, DOX + Her + DZR = chemotherapeutics, trastuzumab, and dexrazoxane, NS = not significant, RT-PCR = reverse transcriptase-polymerase chain reaction.
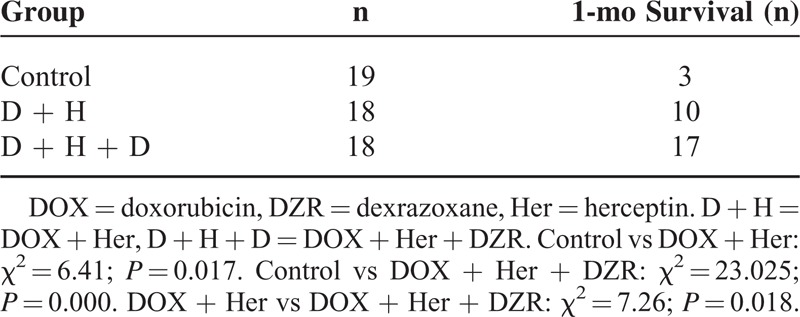
One-Month Survival
The survival rates of remaining experimental rats in 1 month in each group were calculated and analyzed in Table 3. As demonstrated in the DOX + Her group, the 1-month survival rates (11/18) were marked higher than the control group (3/19) (P = 0.017). Meanwhile, 16/18 in the DOX + Her + DZR group totally exceeded the survival rates in the previous 2 groups (P = 0.000 for control vs DOX + Her + DZR and P = 0.018 for DOX + Her vs DOX + Her + DZR).
Table 3.
One-Month Survival Rate Among Groups
DISCUSSION
In this study, the efficiency of DZR used in standardized sequential chemotherapy combined with trastuzumab was determined first. In the DOX + Her + DZR group, the use of DZR was associated with lower levels of serum MDA and cTnI, which indicated that DZR could relieve side effects of cardiotoxicity induced by anthracycline combined with trastuzumab. In addition, DZR displayed an obviously protective effect against the cardiomyocyte apoptosis. This in vivo study on rats demonstrated that combination with DZR made the standardized sequential chemotherapy with trastuzumab much safer.
HER2 protein was mainly located at myocardial transverse tubules, which were closely related with cardiac functions. In patients with severe cardiac functional failure, HER2 expression in myocardial cells was inhibited.21 As a tumor-target drug for HER2+ BC, trastuzumab, an antibody of HER2, was adopted in clinical treatments. However, trastuzumab-induced cardiotoxicity always accompany with therapies. The mechanism of trastuzumab-induced cardiotoxicity remained uncertain, which hindered the successful effect of trastuzumab on BC. Currently, substantial differences had been proved between mechanisms of trastuzumab-induced cardiac lesions and anthracyclines-induced cardiac lesions.22 The cardiotoxicity induced by anthracyclines was mainly related with free-radical production, mitochondrial lesions, and apoptosis, whereas this cardiotoxicity was mainly manifested as cell membrane lipid peroxidation, muscle fiber loss, and myocardial cell vacuolization, and all these processes would directly lead to irreversible cardiac lesions. In contrast, most of the cardiotoxicity induced by targeted drug trastuzumab were reversible responses, and no irreversible cardiac lesions had been found upon pathological examinations of patients who died from trastuzumab-induced heart diseases.23 Presently, it was reported that the inhibition of HER2 protein would affect Erk1/2 phosphorylation process, which was related with the cell stability of myocardial fibers.24 Previous studies had confirmed that the advanced age, hypertension, and pretherapy low LVEF were the high-risk factors for the occurrence of cardiotoxicity events in patients with BC who had received trastuzumab therapy.25 On the contrary, the use of chemotherapeutics, in particular anthracyclines, would lead to cardiac functional lesions, and the combination of anthracyclines and trastuzumab would significantly increase the incidence and severity of cardiotoxicity in patients.26 Based on this problem, it was a hot study topic that how to prevent the occurrence of cardiac adverse events with chemotherapy combined with trastuzumab.
As markers of oxygen-free radical injury, the serum SOD and MDA levels in different treatment groups were measured, respectively. As reported, a large number of basic medical and clinical studies had presumed that oxygen-free radicals had participated in the whole process for the development and progression of cardiovascular diseases.27 Oxygen-free radicals within the body were usually produced by enzymatic or nonenzymatic systems, and the oxygen-free radicals produced would attack biomembrane and trigger lipid peroxidation, and lipid peroxides such as MDA would be produced. In this study, no difference existed in SOD level; however, the serum MDA levels of the rats were significantly decreased in the DOX + Her + DZR group (P < 0.01) at week 4. It suggested that proper administration of DZR did not suppress the incidence of oxygen-free radical but reduced the amount of its production, such as MDA. Furthermore, as lipid peroxides, the decreased level of MDA might protect the integrity of cell membrane in cardiac myocytes. cTnI was closely related to the development and outcome of heart failure symptoms, and thus as an ideal, specific myocardial cell biomarker.28 When the cardiomyocytes were damaged, the cell membrane was disrupted, and the free cTnI would be first released into the blood. As the lesions aggravated, the bound part of cTnI would be degraded and continuously released into the blood, and resulted in a sustainable cTnI elevation. After 4 weeks, the difference between the DOX + Her group and the DOX + Her + DZR group was statistically significantly decreased (P < 0.05), which further confirmed the protective effect of DZR on cell membranes.
Polanski et al29 have constructed caveolin-1 knockout transgenic mice and found that DZR treatment could decrease cardiomyocyte apoptosis and downregulate the bcl-2 expression in the tested rats, suggesting that DZR could execute its myocardial protective effects possibly by blocking cardiomyocyte apoptosis. A study by Spallarossa et al30 has also demonstrated that anti-ErbB2 antibodies could result in cardiomyocyte apoptosis caused by adriamycin exposure, whereas DZR could effectively decrease the incidence of this kind of apoptosis. Similar results have been shown in the present study, that is, AI for DOX + Her group was 13.1% ± 0.87% and for DOX + Her + DZR group was 5.1% ± 0.8%. These AIs were significantly lower than that in the DOX + Her group (P < 0.05). These results demonstrated that DZR could effectively block the cardiomyocyte apoptosis.
The calpain system is an important group of proteases, with calpain-1 and calpain-2 being the most widely studied family members.31 These neutral cysteine proteases are responsible for the controlled proteolysis of a number of cellular substrates important in cellular migration, survival, and apoptosis.32 It is confirmed that calpain-2 is a growth-promoting protein and ubiquitous in human tissues.33 As apoptosis-related protein, calpain-2 can promote p53 unbiquitination.34 Jeon et al35 have found that apoptosis by aloe-emodin is mediated through downregulation of calpain-2 in Huh-7 cells. A study by Wang et al36 has also revealed that overexpression of calpastatin, an endogenous calpain inhibitor, enhances DOX-induced cardiac injuries through calpain inhibition and thus, calpains may protect cardiomyocytes against DOX-induced cardiotoxicity. In the present study, we evaluated the expression levels of calpain-1 and calpain-2 and found that the mRNA and protein expression levels of calpain-2 of cardiac muscle tissues from rats in the DOX + Her group were significantly decreased than those in the control group and the DOX + Her + DZR group. However, no differences of calpain-1 were observed among the control group, the DOX + Her group, and the DOX + Her + DZR group. As DZR, belonging to a class of bis (2, 6-dioxopiperazines), has been proven effective for reduction of DOX-induced cardiotoxicity, previous studies have found that the cardiac protective effect of DZR is mediated by depletion of topoismerases IIβ, which contains bisdoxopiperazine-binding site.37 Moreover, a research by Spagnuolo et al38 has showed that hypoxia inducible factor (HIF)-1α and HIF-2α were induced and activated in the DZR-mediated protection of cardiomyocytes from DOX-induced toxicity. In our study, compared with the above results, it is demonstrated that the antiapoptotic effect of DZR was partly related with upregulation of calpain-2 in the cardiac muscle. However, the molecular mechanism is not yet elucidated. As the mRNA and protein level of calpain-2 were both elevated, the regulatory mechanism of DZR on calpain-2 expression might be on the transcription level. DZR may increase the promoter activity of calpain-2 gene by affecting the DNA-binding activity of transcription factors such as nuclear transcription factor kappa B and so on. This hypothesis remains to be investigated in our further study.
When interpreting our results, several limitations should be concerned. First, size of the animal was small in this study. Second, the molecular mechanism of cardioprotective effect of DZR needs to be investigated deeply.
In conclusion, this study demonstrated that DZR could prevent the cardiotoxicity induced by chemotherapeutics combined with trastuzumab. DZR could decrease serum MDA and cTnI levels of the rat, induce the expression level of calpain-2, and relieve the cardiomyocyte apoptosis. All of these would lay a solid theoretic foundation for the clinical prevention of cardiotoxicity induced by chemotherapy combined with trastuzumab.
Acknowledgment
The authors would like to thank Biolight Tec. Company, Nanjing, China, for the technical support.
Footnotes
Abbreviations: AI = apoptotic index, ANP = atrial natriuretic peptide, BC = breast cancer, BNP = brain natriuretic peptide, cTnI = cardiac troponin I, DOX = doxorubicin, DZR = dexrazoxane, EDTA = ethylene diamine tetraacetic acid, FS = fractional shortening, Her = herceptin, LVEDD = left ventricular end-diastolic diameter, LVEF = left ventricular ejection fraction, MDA = malondialdehyde, SOD = superoxide dismutase.
TM and SZ contributed equally to this study and share first authorship.
This study was supported by the National Science and Technology Support Program (No. 2013BAI09B08) and Tianjin Municipal Major Scientific and Technological Special Project for Significant Anticancer Development (No. 12ZCDZSY15700).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Morris PG, Hudis CA. Trastuzumab-related cardiotoxicity following anthracycline-based adjuvant chemotherapy: how worried should we be? J Clin Oncol 2010; 28:3407–3410. [DOI] [PubMed] [Google Scholar]
- 2.Thavendiranathan P, Poulin F, Lin KD, et al. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol 2014; 63 (25 Pt A):2751–2768. [DOI] [PubMed] [Google Scholar]
- 3.Goldhirsch A, Wood WC, Gelber RD, et al. Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 2007; 18:1133–1144. [DOI] [PubMed] [Google Scholar]
- 4.Goldhirsch A, Wood WC, Coates AS, et al. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol 2011; 22:1736–1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst Rev 2012; 4:CD006243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001; 344:783–792. [DOI] [PubMed] [Google Scholar]
- 7.Fedele C, Riccio G, Malara AE, et al. Mechanisms of cardiotoxicity associated with ErbB2 inhibitors. Breast Cancer Res Treat 2012; 134:595–602. [DOI] [PubMed] [Google Scholar]
- 8.Procter M, Suter TM, de Azambuja E, et al. Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol 2010; 28:3422–3428. [DOI] [PubMed] [Google Scholar]
- 9.Speyer J, Wasserheit C. Strategies for reduction of anthracycline cardiac toxicity. Semin Oncol 1998; 25:525–537. [PubMed] [Google Scholar]
- 10.Wiseman LR, Spencer CM. Dexrazoxane: a review of its use as a cardioprotective agent in patients receiving anthracycline-based chemotherapy. Drugs 1998; 56:385–403. [DOI] [PubMed] [Google Scholar]
- 11.Hasinoff BB, Hellmann K, Herman EH, et al. Chemical, biological and clinical aspects of dexrazoxane and other bisdioxopiperazines. Curr Med Chem 1998; 5:1–28. [PubMed] [Google Scholar]
- 12.Saez ME, Ramirez-Lorca R, Moron FJ, et al. The therapeutic potential of the calpain family: new aspects. Drug Discov Today 2006; 11:917–923. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Li Y, Shan L, et al. Over-expression of calpastatin inhibits calpain activation and attenuates myocardial dysfunction during endotoxaemia. Cardiovasc Res 2009; 83:72–79. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Ma J, Zhu H, et al. Targeted inhibition of calpain reduces myocardial hypertrophy and fibrosis in mouse models of type 1 diabetes. Diabetes 2011; 60:2985–2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galvez AS, Diwan A, Odley AM, et al. Cardiomyocyte degeneration with calpain deficiency reveals a critical role in protein homeostasis. Circ Res 2007; 100:1071–1078. [DOI] [PubMed] [Google Scholar]
- 16.Jang YM, Kendaiah S, Drew B, et al. Doxorubicin treatment in vivo activates caspase-12 mediated cardiac apoptosis in both male and female rats. FEBS Lett 2004; 577:483–490. [DOI] [PubMed] [Google Scholar]
- 17.Lim CC, Zuppinger C, Guo X, et al. Anthracyclines induce calpain-dependent titin proteolysis and necrosis in cardiomyocytes. J Biol Chem 2004; 279:8290–8299. [DOI] [PubMed] [Google Scholar]
- 18.Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat 1982; 2:5–73. [DOI] [PubMed] [Google Scholar]
- 19.Lizama C, Lagos CF, Lagos-Cabre R, et al. Calpain inhibitors prevent p38 MAPK activation and germ cell apoptosis after heat stress in pubertal rat testes. J Cell Physiol 2009; 221:296–305. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Arnold JM, Pampillo M, et al. Taurine prevents cardiomyocyte death by inhibiting NADPH oxidase-mediated calpain activation. Free Radic Biol Med 2009; 46:51–61. [DOI] [PubMed] [Google Scholar]
- 21.Rohrbach S, Niemann B, Silber RE, et al. Neuregulin receptors erbB2 and erbB4 in failing human myocardium: depressed expression and attenuated activation. Basic Res Cardiol 2005; 100:240–249. [DOI] [PubMed] [Google Scholar]
- 22.Bria E, Cuppone F, Milella M, et al. Trastuzumab cardiotoxicity: biological hypotheses and clinical open issues. Expert Opin Biol Ther 2008; 8:1963–1971. [DOI] [PubMed] [Google Scholar]
- 23.Guarneri V, Lenihan DJ, Valero V, et al. Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: the M.D. Anderson Cancer Center experience. J Clin Oncol 2006; 24:4107–4115. [DOI] [PubMed] [Google Scholar]
- 24.Pentassuglia L, Graf M, Lane H, et al. Inhibition of ErbB2 by receptor tyrosine kinase inhibitors causes myofibrillar structural damage without cell death in adult rat cardiomyocytes. Exp Cell Res 2009; 315:1302–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen T, Xu T, Li Y, et al. Risk of cardiac dysfunction with trastuzumab in breast cancer patients: a meta-analysis. Cancer Treat Rev 2011; 37:312–320. [DOI] [PubMed] [Google Scholar]
- 26.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J Clin Oncol 2002; 20:1215–1221. [DOI] [PubMed] [Google Scholar]
- 27.Dragojevic-Simic VM, Dobric SL, Bokonjic DR, et al. Amifostine protection against doxorubicin cardiotoxicity in rats. Anticancer Drugs 2004; 15:169–178. [DOI] [PubMed] [Google Scholar]
- 28.Adamcova M, Simunek T, Kaiserova H, et al. In vitro and in vivo examination of cardiac troponins as biochemical markers of drug-induced cardiotoxicity. Toxicology 2007; 237:218–228. [DOI] [PubMed] [Google Scholar]
- 29.Polanski AK, Ebner A, Ebner B, et al. Dexrazoxane prevents the development of the impaired cardiac phenotype in caveolin-1-disrupted mice. J Cardiovasc Pharmacol 2013; 61:545–552. [DOI] [PubMed] [Google Scholar]
- 30.Spallarossa P, Altieri P, Pronzato P, et al. Sublethal doses of an anti-erbB2 antibody leads to death by apoptosis in cardiomyocytes sensitized by low prosenescent doses of epirubicin: the protective role of dexrazoxane. J Pharmacol Exp Ther 2010; 332:87–96. [DOI] [PubMed] [Google Scholar]
- 31.Perrin BJ, Huttenlocher A. Calpain. Int J Biochem Cell Biol 2002; 34:722–725. [DOI] [PubMed] [Google Scholar]
- 32.Sorimachi H, Ono Y. Regulation and physiological roles of the calpain system in muscular disorder. Cardiovasc Res 2012; 96:11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y, Wang KK. The calpain family and human disease. Trends Mol Med 2001; 7:355–362. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, Xing D, Chen WR, et al. Calpain-mediated pathway dominates cisplatin-induced apoptosis in human lung adenocarcinoma cells as determined by real-time single cell analysis. Int J Cancer 2008; 122:2210–2222. [DOI] [PubMed] [Google Scholar]
- 35.Jeon W, Jeon YK, Nam MJ. Apoptosis by aloe-emodin is mediated through down-regulation of calpain-2 and ubiquitin-protein ligase E3A in human hepatoma Huh-7 cells. Cell Biol Int 2012; 36:163–167. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Zheng D, Wei M, et al. Over-expression of calpastatin aggravates cardiotoxicity induced by doxorubicin. Cardiovasc Res 2013; 98:381–390. [DOI] [PubMed] [Google Scholar]
- 37.Deng S, Yan T, Jendrny C, et al. Dexrazoxane may prevent doxorubicin-induced DNA damage via depleting both Topoisomerase II isoforms. BMC Cancer 2014; 14:842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Spagnuolo RD, Recalcati S, Tacchini L, et al. Role of hypoxia-inducible factors in the dexrazoxane-mediated protection of cardiomyocytes from doxorubicin-induced toxicity. Br J Pharmacol 2011; 163:299–312. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


