Abstract
The aim of this article is to describe epidemiological and clinical data of patients with tuberculous lymphadenitis (TL) and evaluate the yield of the diagnostic techniques employed.
Retrospective observational study was performed at the Vall d’Hebron University Hospital, Barcelona, Spain. All adult patients with confirmed TL (microbiologically) or probable TL (suspected by clinical presentation, cyto/histopathological features, and clinical improvement after specific treatment) diagnosed from January 2001 to December 2013 were included.
One hundred twenty-two patients were included: 78 (63.9%) patients with confirmed diagnosis and 44 (36.1%) patients with probable TL. Seventy (57.4%) patients were nonnative residents. From 83 fine-needle aspiration (FNA) specimens, 54.8% (40/73) showed granulomatous inflammation, 62.5% (40/64) had positive mycobacterial culture, and 73.3% (11/15) tested positive with Xpert MTB/RIF (Cepheid, Sunnyvale, CA). From 62 biopsy samples, 96.8% (60/62) showed granulomatous inflammation, 64.6% (31/48) had positive mycobacterial culture, and 46.1% (6/13) tested positive with Xpert MTB/RIF.
TL has increasingly been diagnosed in our setting, mostly because of cases diagnosed in nonnative residents. FNA is an easy and safe technique for the diagnosis of suspected TL, and the yield regarding mycobacterial culture seems to be similar to the obtained with biopsy. The Xpert MTB/RIF test from FNA specimens may increase the accuracy of the TL diagnosis and provides quicker results.
INTRODUCTION
Tuberculous lymphadenitis (TL) is the most common extrapulmonary manifestation of tuberculosis. Although the overall rate of pulmonary tuberculosis in some countries is decreasing, the proportion of extrapulmonary cases has increased; out of 9945 diagnosed cases of tuberculosis in the United States in 2012, 846 (8.5%) were lymphadenitis.1 In Spain, 6762 cases of tuberculosis were reported in 2011 (an incidence of 14.7 cases/100,000 person-year), from which 1785 (26.4%) represented extrapulmonary tuberculosis.2 In countries where tuberculosis is not endemic, the majority of patients presenting with TL are nonnative residents (mostly from Asia) or present an immunosuppressive condition, such as human immunodeficiency virus (HIV) infection.3
Diagnosis of TL may be challenging because its nonspecific clinical presentation may overlap with other infectious and noninfectious diseases. Culture remains the gold standard for diagnosis, but results may take 3 to 4 weeks.4 A positive acid-fast bacilli (AFB) stain may indicate mycobacterial disease, although sensitivity is lower than culture, ranging from 5% to 38% depending on the studies.3,5 In AFB-negative and/or culture-negative cases, histological features, such as granulomas with or without caseous necrosis, or nonspecific lymphadenitis, may support the diagnosis of probable TL.
New molecular techniques, such as nucleic acid detection through the polymerase chain reaction (PCR) technique, are increasingly being used in the diagnosis of tuberculosis. These techniques have high sensitivity and specificity, and yield results in 24 to 48 hours. Scarce information about the role of these techniques in the diagnosis of TL has been published, and results are very different depending on the study.6,7
In this study, we present epidemiological and clinical data of patients with TL diagnosed at Vall d’Hebron University Hospital (HUVH) during the last decade, and we evaluate the yield of the different diagnostic techniques employed.
PATIENTS AND METHODS
Study Population, Data Collection, and Objectives
This is a retrospective observational study performed at the Departments of Infectious Diseases and Pneumology, HUVH, a tertiary hospital included in the International Health Program of the Catalan Health Institute (PROSICS), Barcelona, Spain. All adult patients (≥18 years old) with confirmed TL (by mycobacterial culture or PCR) or probable TL (suspected by clinical presentation, cyto/histopathological features, and clinical improvement after specific treatment) attended at HUVH from January 2001 to December 2013 were included.
The inclusion process had different steps. The first step was to obtain a list of possible cases from 3 sources: a list of patients with granulomatous lymphadenitis obtained from the registry of the Department of Pathology; a list of patients on whom a mycobacterial culture from a lymph node sample was performed, obtained from the registry of the Department of Microbiology; and a list of patients with the diagnosis of “TL” or “ganglionar tuberculosis” obtained from the general registry of the hospital. The second step included reviewing the medical records of patients from the list collated previously and retrieving those who fulfilled the inclusion criteria. Finally, the following data was collected from included patients: epidemiological data (age, gender, and country of origin), year of diagnosis, clinical features (time from the beginning of symptoms to first consultation, nodal territories affected, extranodal involvement, presence of fever and weight loss, HIV infection, or presence of other immunosuppressive conditions), tuberculin skin test, microbiological data (AFB stain, mycobacterial culture, PCR, and presence of antituberculous drugs resistance), and cyto/histopathological features.
The objectives of the study were the following: to describe the epidemiological and clinical data of patients with TL diagnosed at the HUVH during the study period, establish which diagnostic tests were performed to achieve the diagnoses, evaluate the yield of the different diagnostic techniques (AFB stain, mycobacterial culture, cyto/histopathological findings, and PCR) and the different procedures deployed to obtain the samples (biopsy and fine-needle aspiration [FNA]), and propose diagnostic strategies to improve the TL diagnostic procedure. The study protocol was approved by the Institutional Review Board of the HUVH.
Microbiological Techniques
Fresh tissues were digested and decontaminated using the method described by Kent and Kubica.8 Following decontamination and concentration, the sediment of the specimens was used for microscopic examination (Ziehl–Neelsen stain), culture, and GenXpert MTB/RIF (Cepheid, Sunnyvale, CA). For culture, samples were inoculated in MGIT medium and incubated in BACTEC MGIT 960 (Becton Dickinson Diagnostic Instrument System, Baltimore, MD). For the antimycobacterial susceptibility testing of Mycobacterium tuberculosis, we used BACTEC MGIT 960 SIRE Kit for the first-line drugs: streptomycin, isoniazid, rifampicin, ethambutol, and pyrazinamide. If there was resistance to any first-line drug, we also studied the following antibiotics: amikacin, capreomycin, ethionamide, moxifloxacin, and ofloxacin by BACTEC MGIT 960.
Statistical Analysis
Categorical data are presented as absolute numbers and proportions, and continuous variables are expressed as medians and ranges. The χ2 test or Fisher exact test, when appropriate, was used to compare the distribution of categorical variables, and the Mann–Whitney U test or Student t test was used for continuous variables (normal distribution of continuous variables was evaluated through the Kolmogorov–Smirnov test). Results were considered statistically significant if the 2-tailed P value was <0.05. SPSS software for Windows (Version 19.0; SPSS Inc, Chicago, IL) was used for statistical analyses.
RESULTS
After consulting the 3 registries, 1245 possible cases were selected. After discarding patients <18 years old, duplicated patients, and patients diagnosed in other centers from which medical records could not be reviewed (HUVH is a reference center for mycobacterial infection diagnosis, and receives samples from other centers), 897 possible cases were retrieved. Medical records of these patients were reviewed, and finally 122 patients met the inclusion criteria. A flow diagram of patients is shown in Figure 1.
FIGURE 1.
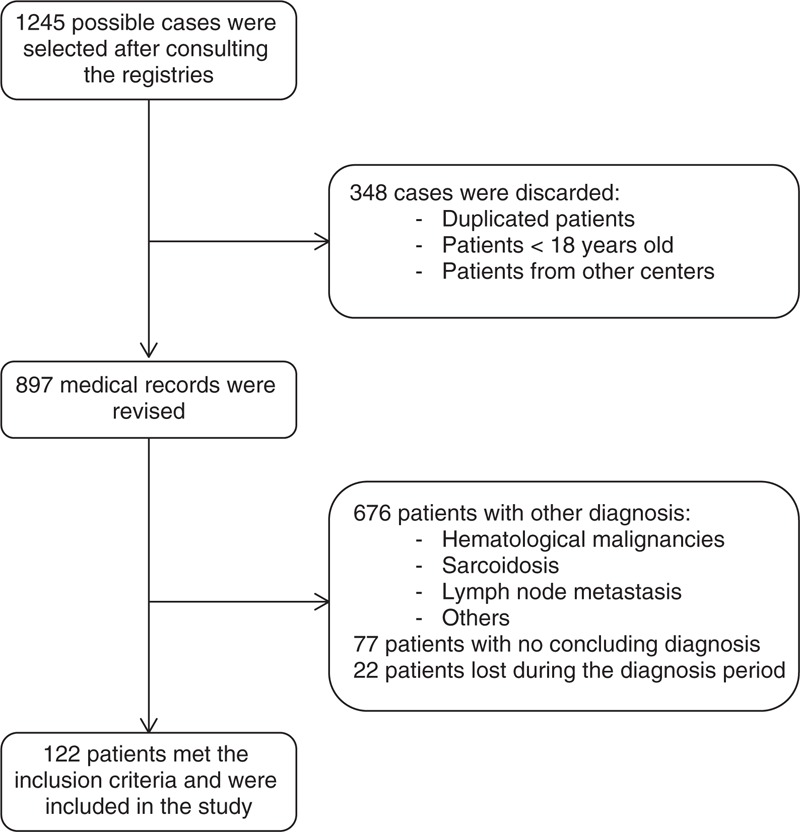
Flow diagram of patients.
Of these 122 patients, 54 (43.3%) patients were men, median age was 38 (18–83) years and median time from the beginning of symptoms to consultation was 2 (1–48) months. Figure 2 shows the number of patients with TL diagnosed by years, from 2001 to 2013. Fifty-two out of 122 (42.6%) patients were born in Spain; of the 70 (57.4%) nonnative patients, 21 (17.2%) were from Pakistan, 17 (13.9%) were from Morocco, 9 (7.4%) were from Ecuador, 6 (4.9%) were from Bolivia, and 17 (13.9%) were from other countries. An HIV test was performed on 103 patients, from whom 16 (15.5%) resulted positive. Other causes of immunosuppression were autoimmune diseases (6 patients), lymphoma (2 patients), and solid tumors (2 patients).
FIGURE 2.
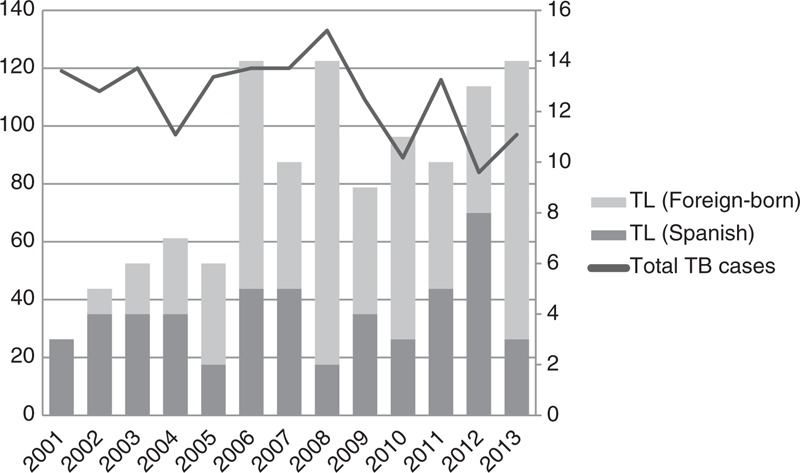
Number of patients with TL diagnosed at Vall d’Hebron University Hospital (2001–2013). Left axis refers to the total number of tuberculosis cases (line), and right axis refers to the number of TL cases (columns) diagnosed at HUVH. HUVH = Vall d’Hebron University Hospital, TB = tuberculosis, TL = tuberculous lymphadenitis.
Regarding nodal territories involved, 75 (61.5%) patients had 1 territory affected and 47 (38.5%) patients had >1 territory affected. Cervical territory was affected in 73 (59.8%) patients, supraclavicular in 34 (27.9%) patients, mediastinal in 30 (24.6%) patients, abdominal in 21 (17.2%) patients, axillary in 19 (15.6%) patients, submandibular in 16 (13.1%) patients, and inguinal in 4 (3.3%) patients. In 36 out of 122 (29.5%) patients, extranodal involvement was reported: 22 (18%) patients with pulmonary tuberculosis, 7 (5.7%) patients with disseminated tuberculosis, 2 (1.6%) patients with pleural tuberculosis, and 6 (4.9%) patients with other organs affected (choroiditis, urinary tract involvement, meningitis, spondylitis, pericarditis, and parotid involvement). The HIV-infected patients group had a higher proportion of extranodal involvement than the non-HIV-infected patients group (56.2% vs 27.6%, P = 0.023).
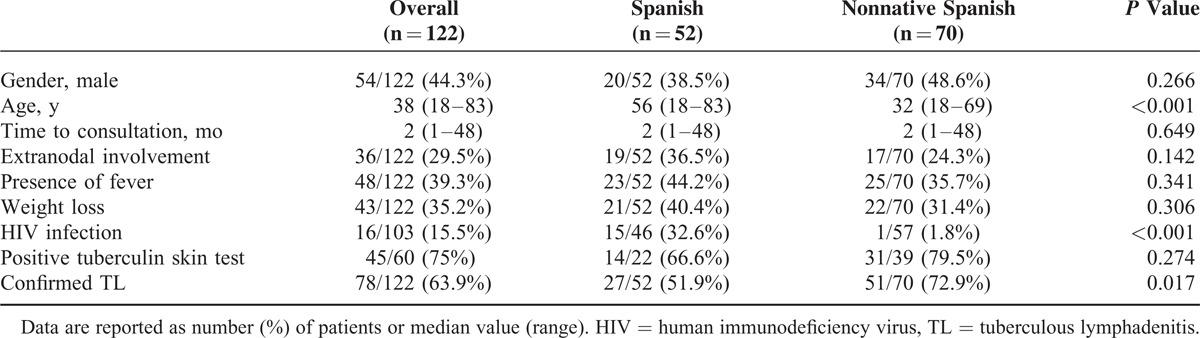
When comparing main clinical and epidemiological features between Spanish-born and nonnative patients, Spanish-born patients were older (median age 56 vs 32 years, P < 0.001) and had a higher proportion of HIV infection (32.6% vs 1.8%, P < 0.001). Moreover, lower proportion of confirmed diagnosis was found among the Spanish-born group compared with the nonnative patients (51.9% vs 72.9%, respectively, P = 0.017). More information is reported in Table 1.
TABLE 1.
Epidemiological and Clinical Data of Patients With TL Attended at the Vall d’Hebron University Hospital, Barcelona, Spain (2001–2013)
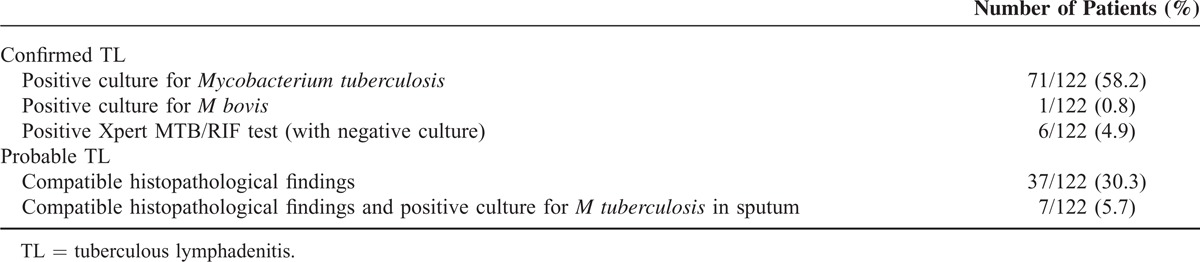
Overall, diagnosis was confirmed microbiologically in 78 (63.9%) patients, and probable TL was diagnosed in 44 (36.1%) patients (see Table 2). When M tuberculosis was isolated in culture (71 patients), resistance to isoniazid was detected in 4 (5.6%) patients, to pyrazinamide in 3 (4.2%) patients, to streptomycin in 6 (8.4%) patients, and to cycloserine in 1 (1.4%) patient; all patients presenting with any resistance were nonnative to Spain. Overall, 10 patients had resistance to at least 1 drug: 6 patients to 1 drug, and 4 patients to 2 drugs. No cases of multidrug-resistant tuberculosis were detected, and no cases of rifampicin resistance were detected by Xpert MTB/RIF test.
TABLE 2.
Diagnosis of Confirmed and Probable TL Attended at the Vall d’Hebron University Hospital, Barcelona, Spain (2001–2013)
When comparing the yield of the different techniques performed in FNA or biopsy samples, no differences were observed in the percentage of positive AFB stain (25% vs 20.8%, respectively, P = 0.605), and positive culture (62.5% vs 64.6%, respectively, P = 0.821). Although positive Xpert MTB/RIF test was more frequent in FNA samples (73.3%) than in biopsy samples (46.1%), it did not reach statistical significance (P = 0.142). Presence of granulomatous inflammation was more frequent in biopsy than in FNA samples (96.8% vs 54.8%, respectively, P < 0.001). Microbiological and histopathological findings are summarized in Table 3, and flow diagram of how the diagnostic techniques were performed is represented in Figure 3.
TABLE 3.
Yield of Different Diagnostic Techniques
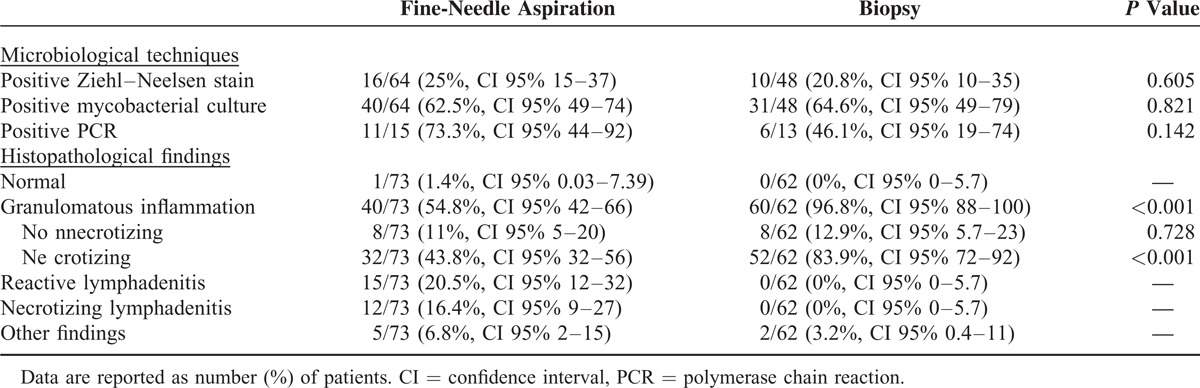
FIGURE 3.

Flow diagram of the diagnostic techniques performed (centered on mycobacterial culture results from FNA and biopsy samples). FNA = fine-needle aspiration.
Xpert MTB/RIF test was positive in 17 out of 28 (60.7%) patients. When culture was used as the reference standard, Xpert MTB/RIF test yielded a sensitivity of 68.8% and a specificity of 50%. Nevertheless, results significantly varied depending on the sample used: Xpert MTB/RIF test performed in FNA samples had a sensitivity of 71.4% and a specificity of 25%, and when performed in biopsy samples had a sensitivity of 66.7% and a specificity of 100%.
DISCUSSION
As we have mentioned previously, although the number of cases of tuberculosis is declining in countries with a low tuberculosis incidence, the proportion of extrapulmonary cases is increasing.1,2 As Figure 2 shows, the number of TL diagnosed at HUVH during the last decade has progressively increased; this increase has been due to an increase of TL in nonnative patients, as the number of TL cases in Spanish-born patients remains unchanged. A few studies have shown that M tuberculosis lineage strains are associated with the site of tuberculosis disease. The strains mostly distributed through Southeast Asia and the Indian Subcontinent (such as M tuberculosis Beijing/W strain) are more likely to cause extrapulmonary disease.9–11 This information is consistent with the results of our study, where the 37.1% of nonnative patients (26 out of 70) came from Asia, being the most represented continent of origin.
Other epidemiological and clinical data in our study are also consistent with the previously published data3,4: a higher proportion of women (55.7%) with cervical and supraclavicular nodes being the most affected (59.8% and 27.9%, respectively), and approximately a third of patients had extranodal involvement (29.5% in our study, pulmonary involvement being the most frequent). The reason for enhanced risk among women is not well understood, and possible host factors may include occupational or social practices favoring oropharyngeal exposure to mycobacterias, genetic or hormonal influences, or differences in health-seeking behavior.
Extrapulmonary tuberculosis, including TL, is more common in immunocompromised patients, such as those with HIV infection; in these patients, the disease is almost always secondary to reactivation of a latent tuberculosis infection.12 The present study shows a prevalence of HIV infection among patients with TL of 15.5%, and 10 patients had other immunosuppressive conditions. As expected in our study, HIV-infected patients had more frequently different localizations affected (TL along with extranodal involvement) than non-HIV-infected patients, reflecting a dissemination of the infection.
Regarding the diagnosis, there is no consensus on which method the sample must be obtained initially when TL is suspected. The different procedures to obtain the sample have different sensitivities, excisional biopsy being the most sensitive, as well as the most invasive. FNA is increasingly being used worldwide as a first diagnostic step because of its ease and safety profile, although sensitivity is very variable depending on the study, with positive mycobacterial culture ranging from 10% to 80%.3,4,13–16 In our study, the yield of AFB detection and mycobacterial culture in both techniques (FNA and biopsy) were similar (25% vs 20.8% of positive Ziehl–Neelsen stain, respectively, and 62.5% vs 64.6% of positive culture, respectively). These results are consistent with those published by Polesky et al3 in a study with similar characteristics (106 patients, performed in a low tuberculosis incidence setting, and a high proportion of nonnative patients): 21% of positive Ziehl–Neelsen stain and 62% of positive culture from FNA samples, and 26% of positive Ziehl–Neelsen stain and 71% of positive culture from biopsy samples. Nevertheless, given the retrospective design of our study, there is no strong evidence to conclude that the positivity of Ziehl-Neelsen stain and mycobacterial culture are equal independently of the procedure. Granulomatous inflammation was observed much more frequently in biopsy samples than in FNA samples (96.8% vs 54.8%, respectively, P < 0.001). This histological finding is helpful in AFB-negative and/or culture-negative cases. Given these results, a multistep approach in the management of suspected TL could be performed: less invasive techniques (FNA) as a first step and more invasive ones (biopsy) as a second step.
Nucleic acid detection by PCR has been widely used to diagnose pulmonary tuberculosis.17 Regarding the accuracy of these techniques in the diagnosis of TL, a systematic review was published in 2007 by Daley et al,6 showing very heterogeneous results, with a sensitivity ranging from 2% to 100% depending on the studies. Sensitivity was higher in those studies when the PCR was performed with a commercial kit instead of an in-house PCR, and in those using FNA instead of biopsy.6 Since the World Health Organization endorsed the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in HIV-infected patients from developing countries, several studies have been published to determine the accuracy of the test to diagnose extrapulmonary tuberculosis.18–21 Nevertheless, only 4 studies exclusively focused on the diagnosis of TL, all of them performed in high incidence areas for tuberculosis, showing a sensitivity of 93% to 100%.22–25 Although Xpert MTB/RIF was performed in a low number of patients in our study, it yielded a sensitivity of 73.3% when performed in FNA-obtained samples (71.4% when mycobacterial culture was the reference standard), lower than previous studies but higher than mycobacterial culture. Furthermore, Xpert MTB/RIF allowed TL diagnosis in 6 patients with negative culture; this is the reason why the specificity of Xpert MTB/RIF declined dramatically when compared with the mycobacterial culture as the reference standard. These results reinforce the possibility of using FNA and the Xpert MTB/RIF test as a first-step diagnostic approach in patients with suspected TL, and stress the idea of building on the diagnosis of TL not only in mycobacterial culture. Larger and prospective studies are needed to establish the role of Xpert MTB/RIF test in the diagnosis of suspected TL, but taking into account our results and other previously published, it could increase the sensitivity of the diagnosis and provide quicker results.
Seven patients with probable TL (with compatible histopathological findings in lymph node samples) had a positive mycobacterial culture in the sputum. This fact stresses the importance of the sputum investigation in patients with suspected TL for 2 reasons: first, because a positive mycobacterial culture in the sputum supports the nodal involvement due to tuberculosis, and second, to detect contagious patients. Another factor is that no microbiological techniques were performed in 22.8% of samples obtained from FNA and 22.5% of samples obtained from biopsy, probably because of the lack of clinical suspicion of TL and higher awareness about oncohematological malignancies. This finding highlights the importance of applying microbiological techniques in samples obtained during the study of a patient with lymphadenopathy.
This study has some limitations that are derived from its retrospective nature: information retrieved from medical records, different physicians involved, and different tests performed. A selection bias may exist, as the choice of the diagnostic technique (FNA or biopsy) by the physician could be influenced by the clinical presentation: accessibility and size of the lymphadenopathy, and greater suspicion of other etiology different from TL. Another issue is that we have included unconfirmed TL cases in the study (suspected by clinical presentation, suggestive cyto/histopathological features, and clinical improvement after specific treatment); this group may include other diseases different from tuberculosis that improved during incorrect therapy. Nevertheless, probable TL cases represented only 36.1% of the study population. Regarding the low number of cases where the Xpert MTB/RIF was performed (in 28 patients), this is because the technique has only been available since 2009.
In summary, TL is increasingly being diagnosed in our setting, mostly because of cases diagnosed in nonnative patients (especially those originating from Asia and North Africa). FNA is an easy and safe technique for the diagnosis of suspected TL, and the yield regarding mycobacterial culture and AFB detection seems to be similar to the one obtained with biopsy, but the retrospective design of the study does not allow concluding this. The Xpert MTB/RIF test from FNA specimens may increase the accuracy of the TL diagnosis and provides quicker results. Larger and prospective studies must be performed to establish better diagnosis strategies for suspected TL.
Footnotes
Abbreviations: AFB = acid-fast bacilli, FNA = fine-needle aspiration, HIV = human immunodeficiency virus, HUVH = Vall d’Hebron University Hospital, PCR = polymerase chain reaction, TL = tuberculous lymphadenitis.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.CDC (2013). Reported tuberculosis in the United States, 2012. Atlanta, GA: U.S. Department of Health and Human Services, CDC. [Google Scholar]
- 2.European Centre for Diseases Prevention and Control/WHO Regional Office for Europe (2013). Tuberculosis surveillance and monitoring in Europe 2013. Stockholm: European Centre for Disease Prevention and Control. [Google Scholar]
- 3.Polesky A, Grove W, Bhatia G. Peripheral tuberculous lymphadenitis: epidemiology, diagnosis, treatment and outcome. Medicine (Baltimore) 2005; 84:350–362. [DOI] [PubMed] [Google Scholar]
- 4.Fontanilla JM, Barnes A, Fordham von Reyn C. Current diagnosis and management of peripheral tuberculous lymphadenitis. Clin Infect Dis 2011; 53:555–562. [DOI] [PubMed] [Google Scholar]
- 5.Fain O, Lortholary O, Djouab M, et al. Lymph node tuberculosis in the suburbs of Paris: 59 cases in adults not infected by the human immunodeficiency virus. Int J Tuberc Lung Dis 1999; 3:162–165. [PubMed] [Google Scholar]
- 6.Daley P, Thomas S, Pai M. Nucleic acid amplification tests for the diagnosis of tuberculous lymphadenitis: a systematic review. Int J Tuberc Lung Dis 2007; 11:1166–1176. [PubMed] [Google Scholar]
- 7.Denkinger CM, Schumacher SG, Boehme CC, et al. Xpert MTB/RIF assay for the diagnosis of extrapulmonary tuberculosis: a systematic review and meta-analysis. Eur Respir J 2014; 44:435–446. [DOI] [PubMed] [Google Scholar]
- 8.Kent PT, Kubica GP. Public Health Mycobacteriology: A Guide for the Level III Laboratory. Atlanta, GA: Centers for Disease Control and Prevention; 1985. [Google Scholar]
- 9.Click ES, Moonan PK, Winston CA, et al. Relationship between Mycobacterium tuberculosis phylogenetic lineage and clinical site of tuberculosis. Clin Infect Dis 2012; 54:211–219. [DOI] [PubMed] [Google Scholar]
- 10.Kong Y, Cave MD, Zhang L, et al. Association between Mycobacterium tuberculosis Beijing/W lineage strain infection and extrathoracic tuberculosis: insights from epidemiologic and clinical characterization of the three principal genetic groups of M. tuberculosis clinical isolates. J Clin Microbiol 2007; 45:409–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hesseling AC, Marais BJ, Kirchner HL, et al. Mycobacterial genotype is associated with disease phenotype in children. Int J Tuberc Lung Dis 2010; 14:1252–1258. [PubMed] [Google Scholar]
- 12.Aaron L, Saadoun D, Calatroni I, et al. Tuberculosis in HIV-infected patients: a comprehensive review. Clin Microbiol Infect 2004; 10:388–398. [DOI] [PubMed] [Google Scholar]
- 13.Lau SK, Wei WI, Hsu C, et al. Efficacy of fine needle aspiration cytology in the diagnosis of tuberculous cervical lymphadenopathy. J Laryngol Otol 1990; 104:24–27. [DOI] [PubMed] [Google Scholar]
- 14.Knox J, Lane G, Wong JS, et al. Diagnosis of tuberculous lymphadenitis using fine needle aspiration biopsy. Intern Med J 2012; 42:1029–1036. [DOI] [PubMed] [Google Scholar]
- 15.Fanny ML, Beyam N, Gody JC, et al. Fine-needle aspiration for diagnosis of tuberculous lymphadenitis in children in Bangui, Central African Republic. BMC Pediatr 2012; 12:191–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Razack R, Louw M, Wright CA. Diagnostic yield of needle aspiration biopsy in HIV-infected adults with suspected mycobacterial lymphadenitis. S Afr Med J 2013; 104:27–28. [DOI] [PubMed] [Google Scholar]
- 17.Greco S, Girardi E, Navarra A, et al. Current evidence on diagnostic accuracy of commercially based nucleic acid amplification tests for the diagnosis of pulmonary tuberculosis. Thorax 2006; 61:783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Causse M, Ruiz P, Gutiérrez-Aroca JB, et al. Comparison of two molecular methods for rapid diagnosis of extrapulmonary tuberculosis. J Clin Microbiol 2011; 49:3065–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hilleman D, Rüsch-Gerdes S, Boehme C, et al. Rapid molecular detection of extrapulmonary tuberculosis by the automated GeneXpert MTB/RIF system. J Clin Microbiol 2011; 49:1202–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tortoli E, Russo C, Piersimoni C, et al. Clinical validation of Xpert MTB/RIF for the diagnosis of extrapulmonary tuberculosis. Eur Respir J 2012; 40:442–447. [DOI] [PubMed] [Google Scholar]
- 21.Vadwai V, Boehme C, Nabeta P, et al. Xpert MTB/RIF: a new pillar in diagnosis of extrapulmonary tuberculosis? J Clin Microbiol 2011; 49:2540–2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ligthelm LJ, Nicol MP, Hoek KG, et al. Xpert MTB/RIF for rapid diagnosis of tuberculous lymphadenitis from fine-needle-aspiration biopsy specimens. J Clin Microbiol 2011; 49:3967–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biadglegne F, Tesfaye W, Sack U, et al. Tuberculous lymphadenitis in northern Ethiopia: in a public health and microbiological perspectives. PLoS One 2013; 8:e81918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ablanedo-Terrazas Y, Alvarado-de la Barrera C, Hernández-Juan R, et al. Xpert MTB/RIF for diagnosis of tuberculous cervical lymphadenitis in HIV-infected patients. Laryngoscope 2013; 124:1382–1385. [DOI] [PubMed] [Google Scholar]
- 25.Van Rie A, Page-Shipp L, Mellet K, et al. Diagnostic accuracy and effectiveness of the Xpert MTB/RIF assay for the diagnosis of HIV-associated lymph node tuberculosis. Eur J Clin Microbiol Infect Dis 2013; 32:1409–1415. [DOI] [PubMed] [Google Scholar]


