Abstract
Endothelial nitric oxide synthase (eNOS) is the catalyst of endothelial nitric oxide (NO) synthesis. Polymorphisms in the eNOS gene may influence the risk of intracranial aneurysm (IA), but the results of existing researches are still inconsistent. Thus, we performed the present meta-analysis to derive a more precise estimation between eNOS polymorphisms (T786C, G894T, 27-bp-variable number of tandem repeat [VNTR]) and IA risk.
Case–control studies evaluating the association between the eNOS polymorphisms and IA risk were searched in PubMed, Ovid & Embase, Web of Science, and Chinese Wanfang datasets with the last search up to July 15, 2014. The pooled odds ratios (ORs) for the association between eNOS polymorphisms and IA and their corresponding 95% confidence intervals (CIs) were estimated using the random or fixed-effects model.
Finally, 10 studies for T786C polymorphism (1819 cases and 1893 controls), 9 studies for G894T polymorphism (1393 cases and 1508 controls), and 7 studies for 27-bp-VNTR polymorphism (1281 cases and 1406 controls) were included in the meta-analyses. In the overall analysis, no evidence of association between eNOS polymorphisms and susceptibility of IA was found. When subgrouped by race descent, significantly increased risk was detected among Asians for T786C polymorphism (heterozygous comparison of codominant model: OR = 1.294, 95% CI = 1.025–1.634; dominant model: OR = 1.277, 95% CI = 1.019–1.600), but not in Caucasians or the other 2 polymorphisms.
Our meta-analysis suggested that T786C polymorphism was associated with increased risk of IA among Asians, whereas G894T and 27-bp-VNTR polymorphisms might have no influence on the susceptibility of IA.
INTRODUCTION
The incidence of intracranial aneurysm (IA) in general population has been reported to be approximately 2% and the annual risk of IA rupture causing subarachnoid hemorrhage (SAH) was 1.9%.1,2 Aneurysmal subarachnoid hemorrhage (aSAH) constitutes about 85% of overall SAH, which accounts for 5% of all cases of stroke.3 Despite the development of intensive care and neurosurgical therapy, mortality of aSAH remains 27% to 50%3–5 and only around 60% of survival can be cured without disability.6 The high morbidity and mortality of aSAH make it a major public health problem. Yet, the main cause of IA remains obscure. Previous epidemiological studies have revealed innate (heritable connective tissue disorders, familial predisposition, and female gender) and postnatal (smoking and hypertension) factors of IA formation.7,8 At the same time, pathophysiology researches also showed that hemodynamic factors, inflammatory factors, and elevated arterial blood pressure played important roles in the pathogenesis of IAs.8–10 In the past decades, with the improvement of genetics and molecular biology, gene factors of IA were intensively investigated and polymorphisms of eNOS were one of the focuses.
Nitric oxide (NO), also known as “endothelial-derived relaxing factor,”11 is mostly produced by the catalyzing action of the 3 nitric oxide synthase (NOS) family enzymes via the conversion of L-arginine.12 It is a multifunctional molecule and participates in a large number of biological reactions, for example, active biological mediator in relaxing vascular smooth muscle in response to vasoactive substances and shear stress,11 vasodilatation maintenance the structure of the vessel wall,13 inhibiting vascular smooth muscle cell proliferation,14 and platelet and monocyte adhesion.15,16 A substantial part of NO functions may participate in the mechanism of aneurysms formation and downregulation of NO level has been reported to be associated with several vascular diseases.17
The 3 members of NOS family are neuronal (nNOS/NOS1), inducible (iNOS/NOS2), and endothelial (eNOS/NOS3).18 eNOS that is found primarily in the endothelium continuously generating NO serves to maintain basal vascular tone and cerebral blood flow, and its dysregulation may participate in the early development of aneurysms.19 eNOS is encoded by gene located on chromosome 7q35–36 that has 26 exons that span >21 kb of the genome (GenBank D26607).20 There are many functional polymorphisms in different regions of eNOS gene and several studies have been done to elucidate the relationships between polymorphisms and susceptibility of IAs. The T786C (rs2070744) is an important point mutation of thymine to cytosine at coden-786 in the 5′-flanking region of the eNOS gene, which could significantly reduce eNOS gene promoter activity and serum NO level21; G894T (Glu298Asp, rs1799983) corresponds to a Glu-Asp change at nucleotide 298 in exon 7 that demonstrated a trend for a reduced eNOS enzyme activity,22 and 27-bp-VNTR that is a variable number of tandem repeats (VNTRs, 27 bp) in intron 4 accounts influencing basal plasma NO generation.23 T786C, G894T, and 27-bp-VNTR were the 3 most clinically relevant IA-associated polymorphisms in the eNOS gene that have been reported. From 2003, these 3 polymorphisms have been reported to be associated with IA risk in a number of case–control studies with conflicting conclusions.24–36 In this study, we performed meta-analyses and corresponding stratified analyses using currently available data to clarify the effects of eNOS T786C, G894T, and 27-bp-VNTR polymorphisms on the risk of IAs.
MATERIALS AND METHODS
Publication Search
We searched the articles using the following terms: “intracranial aneurysm,” “cerebral aneurysm,” “brain aneurysm,” “SAH,” “subarachnoid hemorrhage” in combination with “polymorphism,” “variant” in combination with “eNOS,” and “endothelial nitric oxide synthase” in PubMed, Web of Science, Ovid & Embase, and Chinese Wanfang databases from the established date to July 15, 2014. The reference lists of relevant articles were also retrieved to find additional articles. There was no limitation on language or publication year.
Selection Criteria
Studies selected for further meta-analysis must meet the following criteria: case–control studies, reports about associations between eNOS T786C, G894T, or 27-bp-VNTR polymorphism and risk of IA, and inclusion of genotype frequencies of case and control subjects to perform the related statistical analysis. Duplicated reports, reviews, meta-analysis articles, and meeting abstracts without adequate information were excluded. If multiple studies involved same subjects, only the complete one was used in the analysis. Results of article selection were compared and discrepancies were resolved by consensus.
Data Extraction
The following data was extracted carefully and independently by 2 authors from each eligible study: first author's last name, publication year, country, size of the study population (case/control), race, source of the control subjects, genotyping method, endpoint of IAs, percentage of female gender, mean age, and related genotype numbers of cases and controls. Any disagreements were resolved by discussion between the 2 authors.
Statistical Analysis
The Hardy–Weinberg equilibrium (HWE) for control subjects of each study was evaluated by Pearson goodness-of-fit χ2 test. To evaluate the relationship between eNOS polymorphisms (T786C, G894T, 27-bp-VNTR) and risk of IAs, crude odds ratios (ORs) and their 95% confidence intervals (CIs) were applied. Three genetic models, for example, codominant, dominant, and recessive models, were used to determine the pooled OR. For T786C polymorphism, the codominant model was represented by heterozygous comparison of TC vs TT and homozygous comparison of CC vs TT, the dominant model was CC + TC vs TT and the recessive model was CC vs TC + TT. For G894T, the codominant model was heterozygous comparison of TG vs GG and homozygous comparison of TT vs GG, the dominant model was TT + TG vs GG and the recessive model was TT vs TG + GG. For 27-bp-VNTR polymorphism, the codominant model was represented by heterozygous comparison of ab vs bb and homozygous comparison of aa vs bb, the dominant model was aa + ab vs bb and the recessive model was aa vs ab + bb. Since the frequency of mutation homozygous genotype was equal to zero, studies by Song et al29 for T786C polymorphism and Kim et al32 for G894T polymorphism were excluded in recessive model and homozygous comparison of codominant model.37
Heterogeneity between studies was evaluated by Q test and I2 statistic. Heterogeneity was considered significant when P < 0.05 in Q test.38 Low heterogeneity is considered when I2 < 25%, moderate heterogeneity when I2 = 25% to 50%, and high heterogeneity I2 > 50%.39
The fixed-effects model was subsequently used to calculate the pooled ORs when P > 0.05. Otherwise, the random-effects model was applied. Subgroup analyses were performed by endpoint of IAs (RIA and mixed), racial descent (Asian and Caucasian), and control source (hospital-based [HCC] and population-based case–control [PCC] study). Sensitivity analysis was conducted by sequential removing each individual study and possible publication bias was calculated by the Begg40 and the Egger tests.41 The STATA software version 12.0 (STATA Corporation, College Station, TX) was used to carry out all statistical analysis.
RESULTS
Study Selection and Subject Characteristics
The flow diagram of study selection procedure was shown in Figure 1. A total of 88 articles were originally identified, including 22 from PubMed, 26 from Web of Science, 36 from Ovid & Embase, and 4 from Chinese Wanfang database. No additional finding through screening article reference was retrieved. After evaluating articles according to selection criteria, 13 articles including 11 published journal articles and 2 unpublished academic dissertations were eligible for this meta-analysis. Two studies by Khurana et al24,25 shared some common subjects. The study24 only concerning T786C polymorphism contains more cases than the other about T786C, G894T, and 27-bp-VNTR polymorphisms. Therefore, the former study was included in the meta-analysis of T786C polymorphism and the later was employed in G894T and 27-bp-VNTR polymorphisms. One article by Akagawa et al26 reported 2 studies in Japan and Korea and both of the studies met the selection criteria; therefore, we included them as single study.
FIGURE 1.
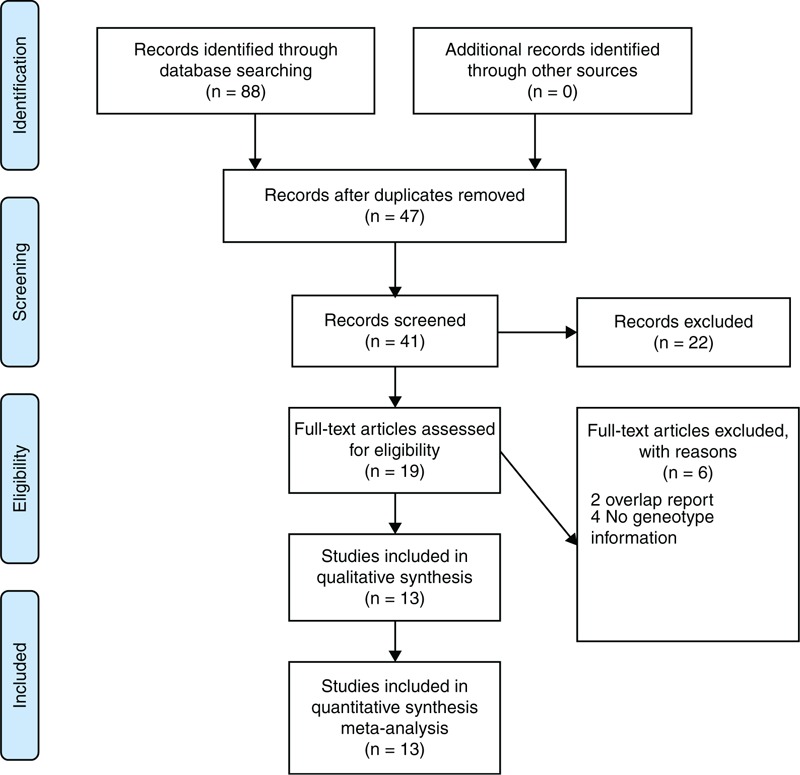
Flowchart showing the different phases of the meta-analysis.
Characteristics of the Studies
Table 1 summarizes the characteristics of included studies. The ruptured IA (RIA) in all studies was verified during surgery and the unruptured IA (UIA) was diagnosed by angiography or surgical findings. Completed genotype information was obtained and HWE tests in the controls were calculated (Table 2). Genotype distribution in most control groups was consistent with HWE with some exceptions.25,28,31,35 There were 10 studies in 9 articles including 1819 cases and 1893 controls for the eNOS T786C polymorphism. Five studies were carried out in Asians and 5 in Caucasians. Four studies used population-based control subjects and 6 used hospital-based control subjects (Tables 1 and 2). Furthermore, 5 studies used RIA patients as case subjects and the other 5 used both RIA and UIA patients.
TABLE 1.
Study Characteristics of Individual Studies Included in the Meta-Analysis
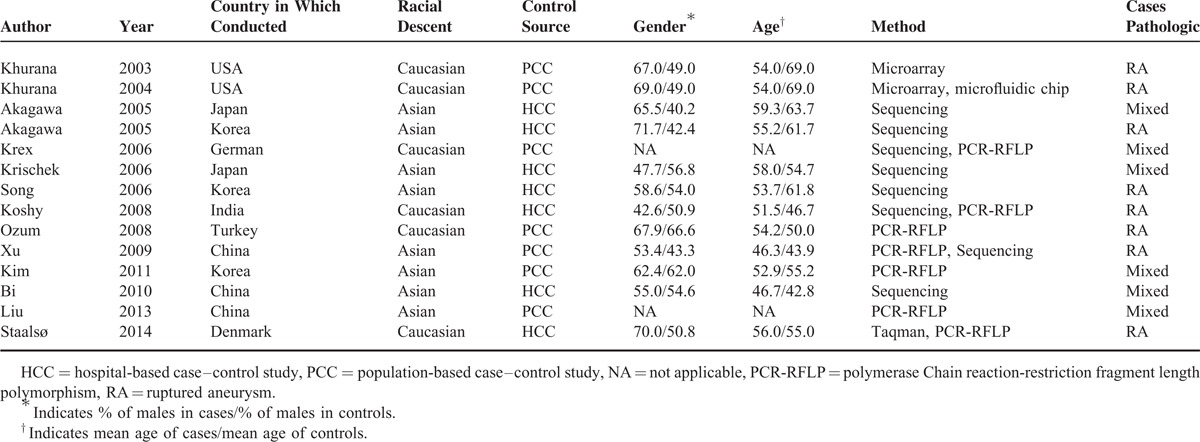
TABLE 2.
Genotype Information for Main Meta-Analysis
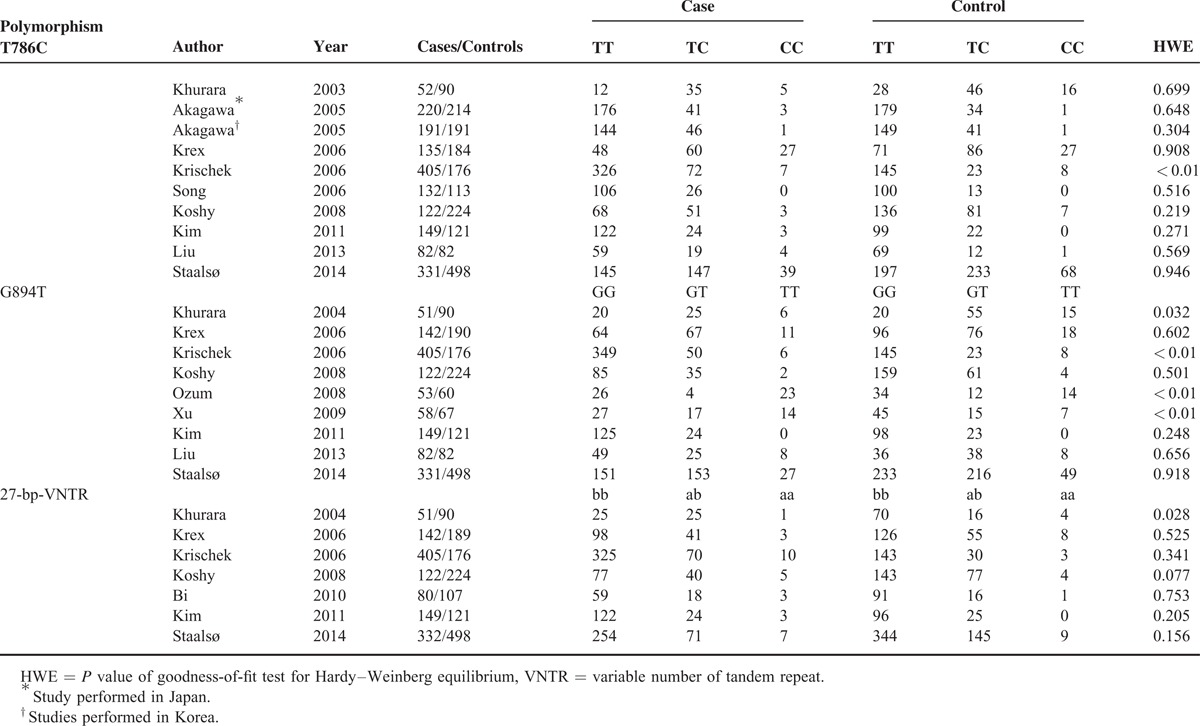
For eNOS G894T polymorphism IAs analysis, 9 studies involving 1393 cases and 1508 controls were identified for our meta-analysis. In the 9 studies, 4 were Asians and 5 Caucasians. Three of these studies were of PCC design, whereas the other 6 studies were of HCC design. Cases in the 5 studies came from RIA patients and 4 were from RIA and UIA patients (Tables 1 and 2).
eNOS 27-bp-VNTR polymorphism was conducted for 1281 cases and 1406 controls in 7 studies. Subjects of 3 studies originated from Asian populations, whereas the other 4 were from Caucasian populations. Controls were recruited randomly from hospitals in 4 studies or the general population in 3 studies. RIA patients were used as cases in 4 studies, and RIA and UIA patients were used in 3 studies (Tables 1 and 2).
Meta-Analysis
Table 3 shows the main results of this meta-analysis and the heterogeneity test of the eNOS T786C, G894T, and 27-bp-VNTR polymorphisms and IAs. For the 3 polymorphisms, the combined results based on all studies did not show any significant associations between the polymorphisms and IAs risk for all genetic models (Table 3). As stratified by ethnicity, our results showed that T786C polymorphism was associated with increased risk of IA in dominant model (CC + TC vs TT; OR = 1.277, 95% CI = 1.019–1.600) and heterozygous comparison of codominant model (TC vs TT; OR = 1.294, 95% CI = 1.025–1.634) among Asians (Figures 2 and 3), but the association did not emerge in the other genetic models of Asians or among Caucasians (Table 3). Furthermore, there was no evidence for the association between the other 2 polymorphisms and IA risk in stratified analysis based on the source of controls, ethnicity, or endpoint of IA (Table 3).
TABLE 3.
Pooled ORs and 95% CIs of Meta-Analysis
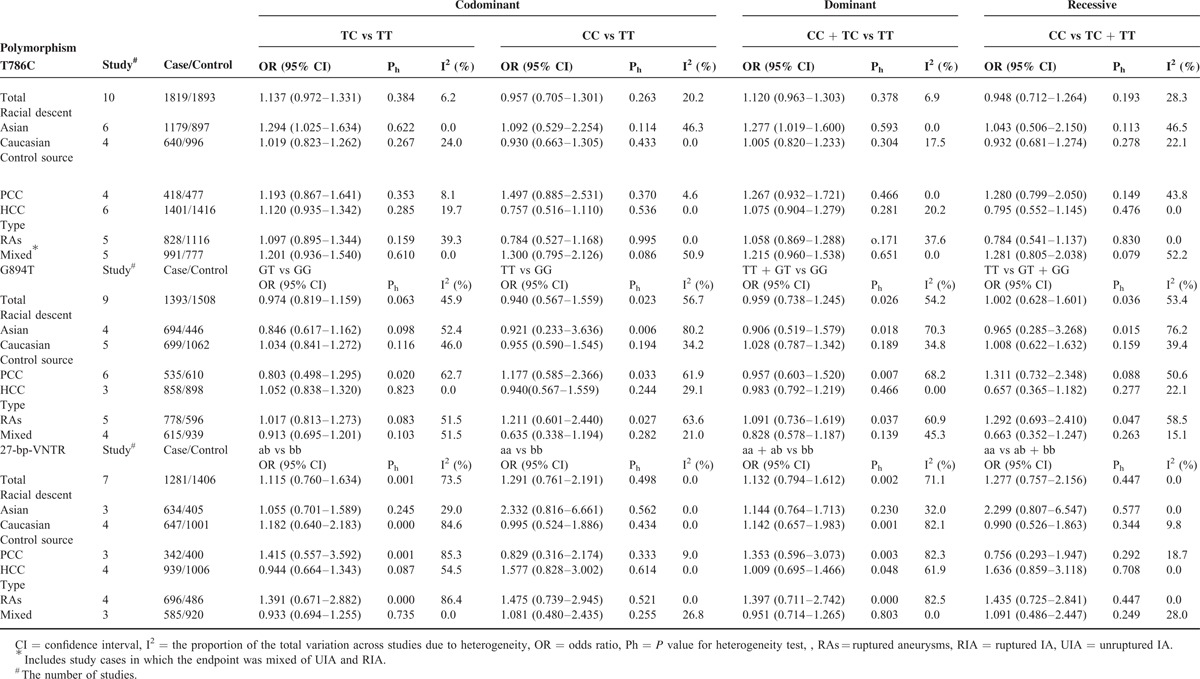
FIGURE 2.
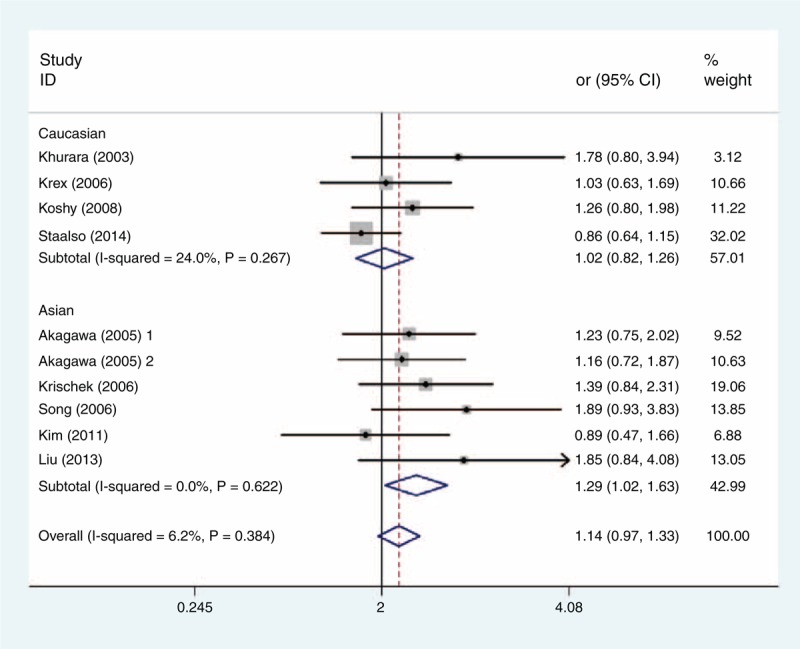
Forest plot of ORs with a fixed-effect model for association between the eNOS T786C polymorphism and subgroup IA risk under codominant model (TC vs TT). eNOS = endothelial nitric oxide synthase, OR = odds ratio.
FIGURE 3.
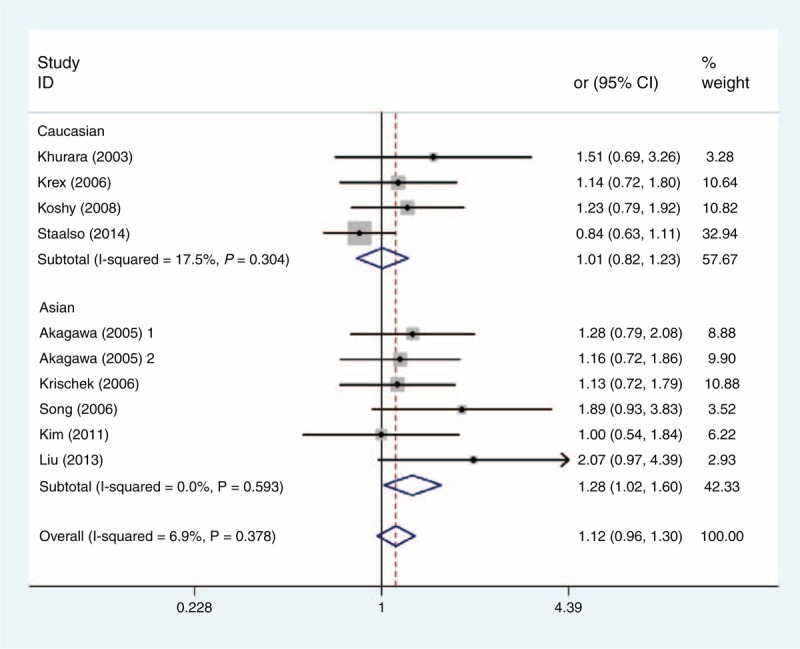
Forest plot of ORs with a fixed-effect model for association between the eNOS T786C polymorphism and subgroup IA risk under dominant model (TC + CC vs TT). eNOS = endothelial nitric oxide synthase, OR = odds ratio.
Test of Heterogeneity and Sensitivity Analyses
The heterogeneity test showed that there was no significant between-study heterogeneity in terms of the eNOS T786C polymorphism (Table 3). However, significant heterogeneity in G894T polymorphism (homozygous comparison of codominant model: Ph = 0.023, I2 = 56.7%; dominant model: Ph = 0.026, I2 = 54.2%; and recessive model: Ph = 0.036, I2 = 53.4%), and 27-bp-VNTR polymorphism (homozygous comparison of codominant: Ph = 0.001, I2 = 73.5%; dominant model: Ph = 0.001, I2 = 71.1%) (Table 3) was observed. To explore the potential sources of heterogeneity across studies, we assessed the pooled ORs under all comparisons via subgroup and sensitivity analyses. In the subgroup analysis by race, the heterogeneity of G894T was significant in the Asian studies. When stratified by source of control and endpoint of cases, heterogeneity of G894T in population-based studies and RIA cases group was significant in all models except heterozygous comparison of codominant models (Table 3). It is interesting that when we excluded the Asian population-based controls study with RIA cases by Xu,35 the heterogeneity was significantly decreased in all genetic models. So we can say that the study by Xu35 contributed to substantial heterogeneity of G894T polymorphism. In the stratified study of 27-bp-VNTR polymorphism, heterogeneity was significant in Caucasian and population-based studies when subgrouped by racial and source of control. Similarly, when we excluded a study with Caucasian subjects and population-based controls by Khurana et al,25 the heterogeneity of homozygous comparison of codominant and dominant models for 27-bp-VNTR polymorphism was obviously reduced. So the study by Khurana et al25 may contribute to heterogeneity of 27-bp-VNTR polymorphism. Furthermore, sensitivity analysis was performed to evaluate the stability of the results by excluding 1 single study at a time. The overall association between T786C polymorphism and IA risk was significantly influenced by the study of Staalsø et al.34 When excluding the study by Staalsø et al, an increased risk of significance was associated with T786C polymorphism in heterozygous comparison of codominant model (OR = 1.266, 95% CI = 1.050–1.526) and dominant model (OR = 1.255, 95% CI = 1.049–1.503) (Figures 4 and 5). The pooled ORs and corresponding 95% CIs were not significantly altered when any individual study was excluded for the other models of T786C and all models of G894T and 27-bp-VNTR polymorphisms (data not shown).
FIGURE 4.
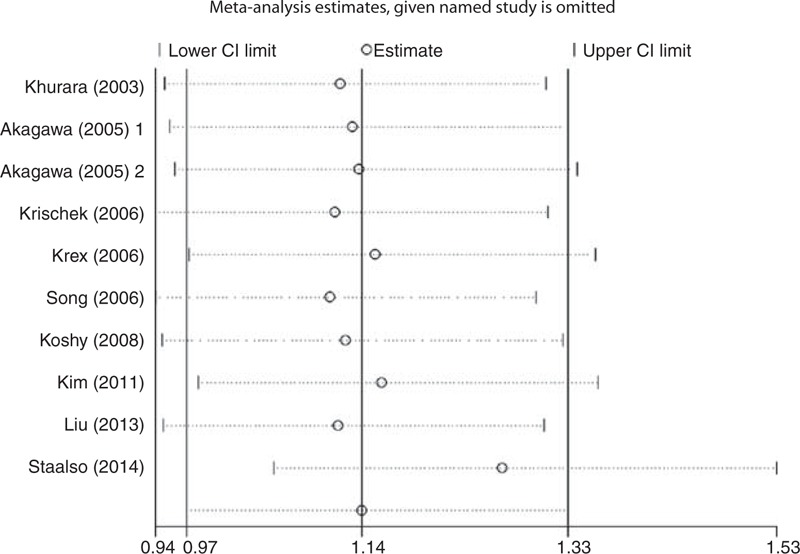
Sensitivity analyses of eNOS T786C polymorphism in TC vs TT model. eNOS = endothelial nitric oxide synthase.
FIGURE 5.
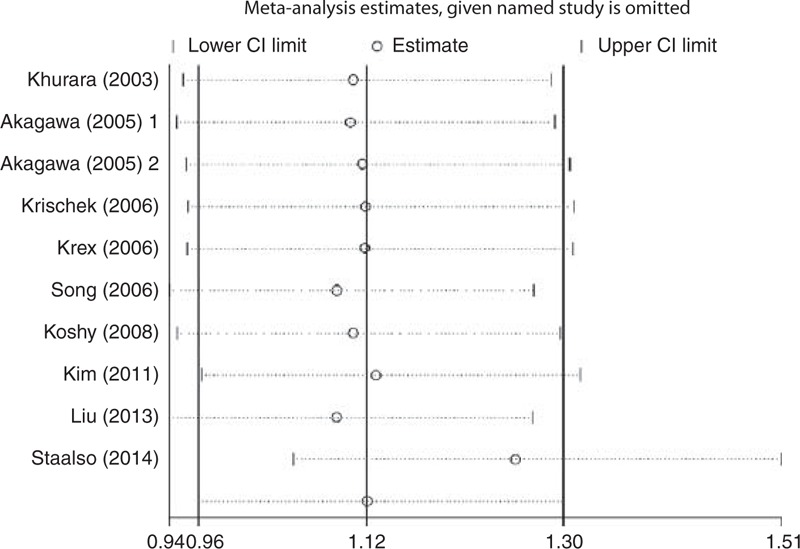
Sensitivity analyses of eNOS T786C polymorphism in TC + CC vs TT model. eNOS = endothelial nitric oxide synthase.
Publication Bias
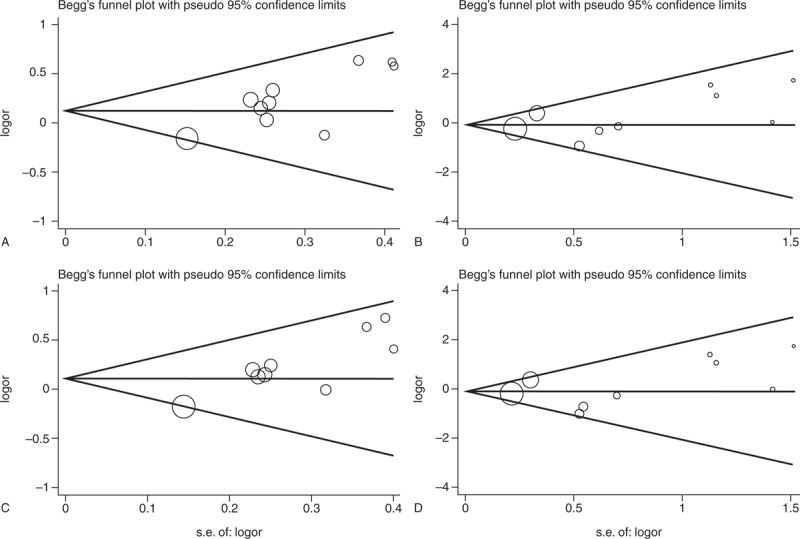
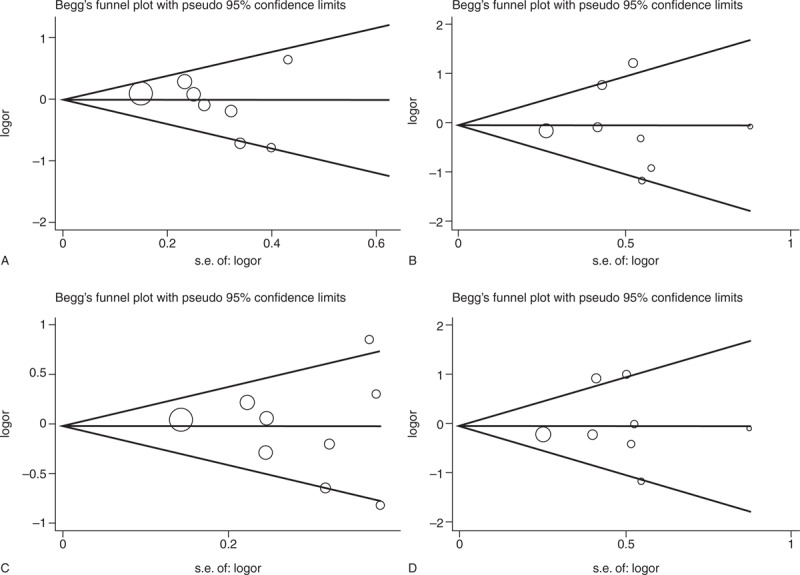
The Begg and the Egger tests were applied to evaluate the publication bias. The results of the Begg and the Egger tests suggest possible evidence of publication bias in the meta-analysis of T786C codominant model TC vs TT (Begg P = 0.074, Egger P = 0.005) and dominant model (Begg P = 0.032, Egger P = 0.001), also in 27-bp-VNTR codominant model ba vs bb (Begg P = 0.230, Egger P = 0.024) and dominant model (Begg P = 0.230, Egger P = 0.015). But no evidence of publication bias in all genetic models of G894T polymorphism was observed (Table 4). The Begg funnel plot was also constructed and the shape of the plot is consistent with the calculation results (Figures 6–8).
TABLE 4.
P Values for Publication Bias Tests
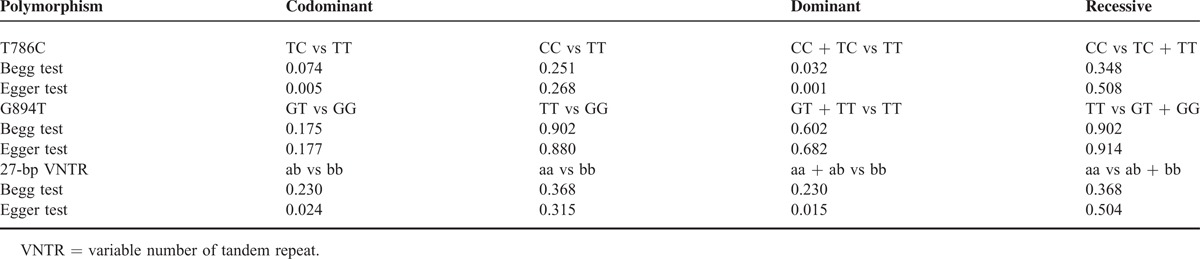
FIGURE 6.
Begg funnel plot for publication bias test of T786C. (A) TC vs TT. (B) CC vs TT. (C) CC + TC vs TT. (D) CC vs TC + TT, SE = Standard Error.
FIGURE 8.
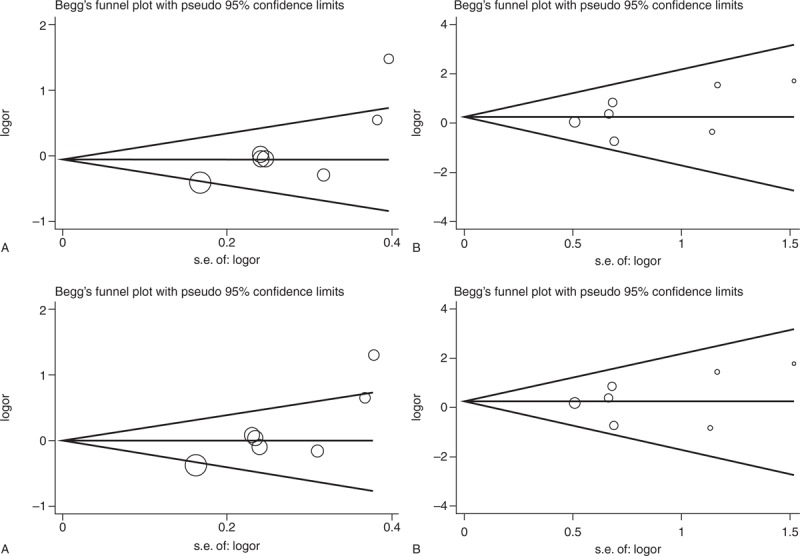
Begg funnel plot for publication bias test of 27-bp-VNTR. (A) ab vs bb. (B) aa vs bb. (C) aa + ab vs bb. (D) aa vs ab + bb. VNTR = variable number of tandem repeat, SE = Standard Error.
FIGURE 7.
Begg funnel plot for publication bias test of G894T. (A) GT vs GG. (B) TT vs GG. (C) TT + GT vs GG. (D) TT vs GT + GG, SE = Standard Error.
DISCUSSION
Because of the versatile NO that has a role in the regulation of vascular tone, hemodynamic changes, and remolding vessel wall, a lot of studies have been done to investigate the contribution made by eNOS polymorphisms to the formation and progression of various vascular diseases.18 Khurana et al24 first investigated the relationship between the T786C polymorphism and IA susceptibility and no significant association was found. After that, 9 studies from 8 articles had been done,26–30,32–34 and the result of 5 studies was negative.27,30,32–34 The other 4 Asian studies have analyzed genotype of cases and controls but did not conclude the association.26,28,29 For G894T, 3 of 9 studies showed significant associations with IA risk,31,33,35 and the other 6 got inverse result.25,27,28,30,32,34 Seven studies investigated 27-bp-VNTR and the risk of IA, and significant association was found in 3 of them,25,34,36 but was not found in the remaining 4.27,28,30,32
A meta-analysis study focusing on the relationship between the 3 eNOS polymorphisms and risk of IA was performed in 2010.42 In that meta-analysis, T786C polymorphism was significantly associated with IA risk, but neither G894T nor 27-bp-VNTR showed significant association. After that, several articles holding different viewpoints about that topic have been published. Here, we conducted a comprehensive meta-analysis to shed light on the role of eNOS polymorphisms in IA risk.
One thousand eight hundred nineteen cases and 1893 controls from 10 studies in 9 articles were eligible for the meta-analysis and no evidence for the association between eNOS T786C polymorphism and IA susceptibility was found in the overall result. However, in subgroup analysis by ethnicity, significant association was found in Asians in heterozygote comparison (OR = 1.294, 95% CI = 1.025–1.634) and the dominant model (OR = 1.277, 95% CI = 1.019–1.600), but not in Caucasians. Given that people in different region share little in environmental backgrounds and lifestyles, this discrepancy in the results between Asians and Caucasians suggested that eNOS T786C polymorphism may play a penetrance role in IA susceptibility in an ethnicity-specific manner. In fact, differences in the distribution of eNOS polymorphisms in Asians and Caucasians have been reported by Tanus-Santos et al.44 In sensitivity analysis, when we deleted 1 Caucasian study by Staalsø et al,34 significant association was observed in heterozygous comparison (OR = 1.266, 95% CI = 1.050–1.526) and dominant model (OR = 1.255, 95% CI = 1.049–1.503). The results of sensitivity analysis indicated that the main meta-analysis may be influenced powerfully by the study of Staalsø et al for its Caucasian subjects and biggest sample size (case = 331, control = 498), as well as the relatively small simple size of the whole study. Therefore, this finding make the result of overall analysis should be explained carefully and confirmed in future studies.
For eNOS G894T and 27-bp-VNTR polymorphisms, there were 9 studies containing 1393 cases vs 1508 controls and 7 studies about 1281 cases vs 1406 controls eligible. We found no significant association between both of the polymorphisms and IA risk, which were consistent with the majority but not all previous studies.25,27,28,30–36 The studies by Özüm et al,31 Xu,35 and Liu et al33 found association between increased risk of IA and G894T polymorphism. Studies by Khurana et al25 and Kim et al32 found association between increased risk of IA and 27-VNTR polymorphism. The inconsistency of these studies may be explained by differences in population background, source of controls, sample size, and also by chance. In the subgroup according to study design, race, and endpoint of IAs, similar trends with overall results were observed. In sensitivity analysis, our results were not meaningfully influenced by any individual study in all models of G894T and 27-bp-VNTR polymorphisms.
For the 3 polymorphisms of eNOS, 2 studies28,43 tried to investigate their effect to the rupture risk of IAs. The study by Khurana et al43 found evidence of association between all of the 3 polymorphisms and rupture risk of IAs but inverse result was found by Krischek et al.28 In our present meta-analysis, RIA was applied in 7 studies and mixed RIA and UIA in 6 studies (Table 1), and no study involved the UIA. To explore the potential influence of different endpoint, subgroup analysis by different endpoint was performed, and significant association was found in neither of the group in different models of the 3 eNOS polymorphisms.
Heterogeneity was observed in G894T polymorphism (homozygous comparison of codominant model: Ph = 0.023, I2 = 56.7%; dominant model: Ph = 0.026, I2 = 54.2%; and recessive model: Ph = 0.036, I2 = 53.4%), and 27-bp-VNTR polymorphism (homozygous comparison of codominant: Ph = 0.001, I2 = 73.5%; dominant model: Ph = 0.001, I2 = 71.1%). The heterogeneity might arise from different characteristics of selected studies, such as study design, sample sizes, inclusion criteria, ethnicity, endpoint of IAs, and different genotyping methodologies. In the stratification and sensitivity analyses, we found that the study by Xu35 did contribute to potential heterogeneity of G894T polymorphism and the study by Khurana et al25 produced heterogeneity of 27-bp-VNTR polymorphism, whereas influence analysis suggested that the pooled ORs for the G894T and 27-bp-VNTR polymorphisms were not influenced by the 2 studies. In this view, the results of our meta-analysis were reliable.
Evidence of publication bias was observed in codominant model heterozygous comparison and dominant model for both T786C and 27-bp-VNTR polymorphisms in the Begg and the Egger tests. To some extent, the publication bias was inevitable for this study because of the limited databases searched, the little number of eligible studies, and only English and Chinese publications included. Therefore, some unpublished and published studies in other databases or in other languages that may meet the inclusion criteria were likely to be missed.
Some limitations should be admitted when explaining the results of our meta-analysis. First, the number of eligible studies and subjects of studies was not large enough for an integrated analysis, especially for subgroup analyses. Therefore, our results should be explained with caution. Second, some inclusion studies25,28,31,34 whose genotype distribution in control group was not consistent with HWE may do contribute to the bias of the meta-analysis, although the results were not affected by these studies in sensitivity analysis. Finally, our results were based on single-factor estimates without adjustments for other risk factors. Further evaluation of IA risk should pay more attention to the potential interactions among gene–gene, gene–environment, and even different polymorphism loci of the same gene.
In conclusion, our meta-analysis indicates that eNOS T786C polymorphism is associated with elevated IA risk among Asians but not in Caucasians, whereas G894T and 27-bp-VNTR polymorphisms might have no influence on the susceptibility of IA. However, large well-designed studies are needed to be performed using standardized genotyping methods, homogeneous cases, and well-matched controls. In addition, further studies investigating the effect of gene–gene and gene–environment interactions may eventually lead to our better, comprehensive understanding of the association between the eNOS polymorphisms and IA risk.
Footnotes
Abbreviations: aSAH = aneurysmal subarachnoid hemorrhage, eNOS = endothelial nitric oxide synthase, IA = intracranial aneurysm, SAH = subarachnoid hemorrhage.
The authors have no funding and conflicts of interest to disclose.
REFERENCES
- 1.Rinkel GJ, Djibuti M, Algra A, et al. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke 1998; 29:251–256. [DOI] [PubMed] [Google Scholar]
- 2.Pakarinen S. Incidence, aetiology, and prognosis of primary subarachnoid haemorrhage: a study based on 589 cases diagnosed in a defined urban population during a defined period. Acta Neurol Scand 1967; 43:1–28. [PubMed] [Google Scholar]
- 3.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007; 369:306–318. [DOI] [PubMed] [Google Scholar]
- 4.Nieuwkamp DJ, Setz LE, Algra A, et al. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol 2009; 8:635–642. [DOI] [PubMed] [Google Scholar]
- 5.Karamanakos PN, von Und Zu Fraunberg M, Bendel S, et al. Risk factors for three phases of 12-month mortality in 1657 patients from a defined population after acute aneurysmal subarachnoid hemorrhage. World Neurosurg 2012; 78:631–639. [DOI] [PubMed] [Google Scholar]
- 6.Malmivaara K, Juvela S, Hernesniemi J, et al. Health-related quality of life and cost-effectiveness of treatment in subarachnoid haemorrhage. Eur J Neurol 2012; 19:1455–1461. [DOI] [PubMed] [Google Scholar]
- 7.Wiebers DO, Whisnant JP, Huston J, 3rd, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet 2003; 362:103–110. [DOI] [PubMed] [Google Scholar]
- 8.Caranci F, Briganti F, Cirillo L, et al. Epidemiology and genetics of intracranial aneurysms. Eur J Radiol 2013; 82:1598–1605. [DOI] [PubMed] [Google Scholar]
- 9.Turjman AS, Turjman F, Edelman ER. Role of fluid dynamics and inflammation in intracranial aneurysm formation. Circulation 2014; 129 3:373–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalouhi N, Hoh BL, Hasan D. Review of cerebral aneurysm formation, growth, and rupture. Stroke 2013; 44:3613–3622. [DOI] [PubMed] [Google Scholar]
- 11.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature 1987; 327:524–526. [DOI] [PubMed] [Google Scholar]
- 12.Knowles RG, Palacios M, Palmer RM, et al. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci USA 1989; 86:5159–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudic RD, Shesely EG, Maeda N, et al. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest 1998; 101:731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Napoli C, Paolisso G, Casamassimi A, et al. Effects of nitric oxide on cell proliferation: novel insights. J Am Coll Cardiol 2013; 62:89–95. [DOI] [PubMed] [Google Scholar]
- 15.Wolf A, Zalpour C, Theilmeier G, et al. Dietary larginine supplementation normalizes platelet aggregation in hypercholesterolemic humans. J Am Coll Cardiol 1997; 29:479–485. [DOI] [PubMed] [Google Scholar]
- 16.Kubes P, Suzuki M, Granger DN. Nitric oxide: an endogenousmodulator of leukocyte adhesion. Proc Natl Acad Sci USA 1991; 88:4651–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oemar BS, Tschudi MR, Godoy N, et al. Reduced endothelial nitric oxide synthase expression and production in human atherosclerosis. Circulation 1998; 97:2494–2498. [DOI] [PubMed] [Google Scholar]
- 18.Wang XL, Wang J. Endothelial nitric oxide synthase gene sequence variations and vascular disease. Mol Genet Metab 2000; 70:241–251. [DOI] [PubMed] [Google Scholar]
- 19.Faraci FM, Brian JE. Nitric oxide and the cerebral circulation. Stroke 1994; 25:692–703. [DOI] [PubMed] [Google Scholar]
- 20.Marsden PA, Heng HH, Scherer SW, et al. Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene. J Biol Chem 1993; 268:17478–17488. [PubMed] [Google Scholar]
- 21.Nakayama M, Yasue H, Yoshimura M, et al. T-786C mutation in the 5-flanking region of the endothelial nitric oxidesynthase gene is associated with coronary vasospasm. Circulation 1999; 99:2864–2870. [DOI] [PubMed] [Google Scholar]
- 22.Wang XL, Sim AS, Wang MX, et al. Genotype dependent and cigarette specific effects on endothelial nitric oxide synthase gene expression and enzyme activity. FEBS Lett 2000; 471:45–50. [DOI] [PubMed] [Google Scholar]
- 23.Wang XL, Mahaney MC, Sim AS, et al. Genetic contribution of the endothelial constitutive nitric oxide synthase gene to plasma nitric oxide levels. Arterioscler Thromb Vasc Biol 1997; 17:3147–3153. [DOI] [PubMed] [Google Scholar]
- 24.Khurana VG, Sohni YR, Mangrum WI, et al. Endothelial nitric oxide synthase T-786C single nucleotide polymorphism: a putative genetic marker differentiating small versus large ruptured intracranial aneurysms. Stroke 2003; 34:2555–2559. [DOI] [PubMed] [Google Scholar]
- 25.Khurana VG, Sohni YR, Mangrum WI, et al. Endothelial nitric oxide synthase gene polymorphisms predict susceptibility to aneurysmal subarachnoid hemorrhage and cerebral vasospasm. J Cereb Blood Flow Metab 2004; 24:291–297. [DOI] [PubMed] [Google Scholar]
- 26.Akagawa H, Kasuya H, Onda H. et al Influence of endothelial nitric oxide synthase T-786C single nucleotide polymorphism on aneurysm size. J Neurosurg 2005; 102:68–71. [DOI] [PubMed] [Google Scholar]
- 27.Krex D, Fortun S, Kuhlisch E, et al. The role of endothelial nitric oxide synthase (eNOS) genetic variants in European patients with intracranial aneurysms. J Cereb Blood Flow Metab 2006; 26:1250–1255. [DOI] [PubMed] [Google Scholar]
- 28.Krischek B, Kasuya H, Akagawa H, et al. Inoue I. Using endothelial nitric oxide synthase gene polymorphisms to identify intracranial aneurysms more prone to rupture in Japanese patients. J Neurosurg 2006; 105:717–722. [DOI] [PubMed] [Google Scholar]
- 29.Song MK, Kim MK, Kim TS, et al. Endothelial nitric oxide gene T-786C polymorphism and subarachnoid hemorrhage in Korean population. J Korean Med Sci 2006; 21:922–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koshy L, Easwer HV, Neetha NV, et al. Role of endothelial nitric oxide synthase gene polymorphisms in predicting aneurysmal subarachnoid hemorrhage in South Indian patients. Dis Markers 2008; 24:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Özüm Ü, Bolat N, Gül E, et al. Endothelial nitric oxide synthase gene [G894T.] polymorphism as a possible risk factor in aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien) 2008; 15:57–61. [DOI] [PubMed] [Google Scholar]
- 32.Kim TG, Kim NK, Baek MJ, et al. The relationships between endothelial nitric oxide synthase polymorphisms and the formation of intracranial aneurysms in the Korean population. Neurosurg Focus 2011; 30:E23. [DOI] [PubMed] [Google Scholar]
- 33.Liu L, Huang XS, Cai YL, et al. Association of G-894T and T-786C polymorphisms of endothelial nitric oxide synthase gene with sporadic intracranial aneurysms. Nan Fang Yi Ke Da Xue Xue Bao 2013; 33:1733–1737. [PubMed] [Google Scholar]
- 34.Staalsø JM, Edsen T, Kotinis A, et al. Association of the NOS3 intron-4 VNTR polymorphism with aneurysmal subarachnoid hemorrhage: clinical article. J Neurosurg 2014; 121:587–592. [DOI] [PubMed] [Google Scholar]
- 35.Xu L. The Relationship Between G894T Polymorphism of Endothelial Nitric Oxide Synthase Gene and Spontaneous Aneurismal Subarachnoid Hemorrhage [D]. 2009; Suzhou, Jiangsu, China: Soochow University, 1–42. [Google Scholar]
- 36.Bi Z. Study of the Relationship Between the 27-bp Repeat Polymorphism in Intron 4 of the eNOS Gene, the Plasminogen Activator Inhibitor-1 (PAI-1) promotor 4G/5G Insersion/Deletion Polymorphism and Intracrianal Aneurysm [D]. 2006; Beijing, China: Capital University of Medical Science, 1–103. [Google Scholar]
- 37.Friedrich JO, Adhikari NKJ, Beyene J. Inclusion of zero total event trials in meta-analyses maintains analytic consistency and incorporates all available data. BMC Med Res Methodol 2007; 7:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zintzaras E, Ioannidis J. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol 2005; 28:123–137. [DOI] [PubMed] [Google Scholar]
- 39.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50:1088–1101. [PubMed] [Google Scholar]
- 41.Egger M, Davey Smith G, Schneider M, et al. Bias in metaanalysis detected by a simple, graphical test. BMJ 1997; 315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McColgan P, Thant KZ, Sharma P. The genetics of sporadic ruptured and unruptured intracranial aneurysms: a genetic meta-analysis of 8 genes and 13 polymorphisms in approximately 20,000 individuals: clinical article. J Neurosurg 2010; 112:714–721. [DOI] [PubMed] [Google Scholar]
- 43.Khurana VG, Meissner I, Sohni YR, et al. The presence of tandem endothelial nitric oxide synthase gene polymorphisms identifying brain aneurysms more prone to rupture. J Neurosurg 2005; 102:526–531. [DOI] [PubMed] [Google Scholar]
- 44.Tanus-Santos JE, Desai M, Flockhart DA. Effects of ethnicity on the distribution of clinically relevant endothelial nitric oxide variants. Pharmacogenetics 2001; 11:719–725. [DOI] [PubMed] [Google Scholar]


