Supplemental Digital Content is available in the text
Abstract
Previous studies have reported that patients with bipolar disorders (BDs) exhibit increased physical comorbidity and psychological distress. Studies have shown that schizophrenia and anxiety increase the risk of peptic ulcer diseases (PUDs). Therefore, we conducted this study to determine the association between these 2 diseases and examine the possible risk factors.
We used patients diagnosed with BDs from the Taiwan National Health Insurance Research Database. A comparison cohort comprising patients without BDs was frequency matched by age, sex, and comorbidities, and the occurrence of PUDs was evaluated in both the cohorts.
The BD and non-BD cohort consisted of 21,060 patients with BDs and 84,240 frequency-matched patients without BDs, respectively. The incidence of PUDs (hazard ratio, 1.51; 95% confidence interval, 1.43–1.59; P < 0.001) was higher among the patients with BDs than the control patients. Cox models showed that irrespective of comorbidities, BDs were an independent risk factor for PUDs.
Patients with BDs exhibit a substantially higher risk for developing PUDs. According to our data, we suggest that, following a diagnosis of BD, practitioners could notice the occurrence of PUD and associated prevention. Further prospective clinical studies investigating the relationship between BDs and PUDs are warranted.
INTRODUCTION
Bipolar disorders (BDs) have been reported to lead to impaired physical function and multiple physical comorbidities, which might entail an increased medical burden for patients with BDs.1 Peptic ulcer diseases (PUDs) are associated with high morbidity and mortality.2 Although physical illness and psychological distress are associated with PUDs,3,4 recent studies have indicated a relationship between mental disorders and PUDs. People affected by PUDs are more likely to exhibit anxiety disorder.5,6 Cross-sectional studies on community-dwelling people have shown that people with mental illnesses such as schizophrenia,7 anxiety,5,6 and panic disorders8 exhibit high risks for subsequent PUDs.
However, little evidence regarding the relationship between BDs and PUDs has been presented. We hypothesized that a history of BDs increases the risk of PUDs. To prove our hypothesis, we designed a nationwide population-based study and investigated the incidence of PUDs among patients with BDs.
PATIENTS AND METHODS
Data Source
We used the claims data of Taiwan residents obtained from the Taiwan National Health Insurance program, which is a single-payer compulsory insurance program that was established in 1995. Until 2007, it covered nearly 99% of the population of Taiwan (23.74 million people). We designed this study as a population-based retrospective cohort study based on the Longitudinal Health Insurance Database 2000 (LHID2000) and the Registry for Catastrophic Illness Patients (RCIP) released by the National Health Research Institutes. The LHID2000 is subset of the National Health Insurance Research Database (NHIRD) and contains outpatient and inpatient department treatment and payment data, which are available for research. The LHID2000 contains original claims data of 1 million enrollees randomly sampled from the patients in the NHIRD between 1996 and 2011. The RCIP is a separate subset that includes patients with severe diseases, including mental disease, autoimmune disease, and cancer. Catastrophic illnesses are defined by the Taiwan government, and patients with a catastrophic illness card receive free health care for their illness and related conditions. Usage of the catastrophic illness card is reviewed using medical records and a process of equal reviews. Therefore, using the diagnoses in the RCIP database in combination with the clinical diagnoses increases the reliability. We used the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) to determine patient diagnoses. All data were deidentified, and therefore, this study was approved to exempt from full ethical review by the institutional review board of China Medical University (CMU-REC-101-012).
Study Patients
We selected as the study population patients who had a catastrophic illness card because of BD (ICD-9-CM: 296) according to the RCIP database. We identified patients with BDs who had been newly diagnosed between 2001 and 2008 (N = 21,060). The date of BD diagnosis was used as the index date. For the non-BD cohort, we randomly selected 84,240 patients without BDs from the LHID2000 database and frequency matched them with the patients with BDs by sex, age (every 5 years), and index year in a 4:1 ratio. Our major outcome in this study was PUDs (ICD-9-CM: 531–535). The exclusion criteria were the date of diagnosis of PUDs being before the index date and incomplete age or sex information. The follow-up person-years were calculated from the index date until the diagnosis of PUDs, withdrawal from the insurance system, or the end of 2011.
The following confounding factors were included for adjustment: diabetes mellitus (DM; ICD-9-CM: 250), hyperlipidemia (ICD-9-CM: 272), hypertension (HTN; ICD-9-CM: 401–405), cirrhosis (ICD-9-CM: 571), rheumatoid arthritis (RA; ICD-9-CM: 714), chronic renal disease (CRD; ICD-9-CM: 585), heart disease (HD; ICD-9-CM: 420-429), alcohol-related illness (ICD-9-CM: 291, 303, 305, 571.0, 571.1, 571.2, 571.3, 790.3), chronic obstructive pulmonary disease (COPD; ICD-9-CM: 585), anxiety (ICD-9-CM: 300), and schizophrenia (ICD-9-CM: 295). We selected anxiety as the comorbidities in this study because of the fact that BDs and anxiety share high levels of comorbidity in the clinic.9,10 We have also tried to analyze the panic disorders and schizophrenia; however, only schizophrenia (ICD-9-CM 295) could have enough patient numbers to be analyzed in the multivariable Cox proportional hazard regression model for adjustment. We also considered the antidepressants treatment in BD and non-BD cohorts, when subjects used the antidepressants drug before the end date was estimated. We chose these comorbidities because patients with BDs are usually treated by atypical antipsychotics, which may result in metabolic syndromes, cardiovascular diseases and HDs.11,12 It has been suggested that BDs are associated with chronic inflammatory diseases and neuroinflammation; therefore, we listed common chronic inflammatory diseases as the comorbidities, such as RA,13 ischemic stroke, pneumonia, bronchitis, COPD, type-2 DM, and HTN.14
Statistical Analysis
We performed all statistical analyses by using SAS 9.4 software for Windows (SAS Institute, Cary, NC). A 2-sided P value <0.05 was considered statistically significant. Summary statistics are presented as numbers and percentages for categorical data and means ± standard deviations for continuous variables. Chi-squared and Student t tests were used to compare categorical and continuous variables between the BD and non-BD cohorts. The incidence of PUDs was estimated according to age, sex, and various types of comorbidity for both the cohorts. Poisson regression analysis was performed to estimate the incidence rate ratio (IRR) in the BD and non-BD cohorts. The adjusted hazard ratios (HRs) and 95% confidence interval (95% CI) obtained through a multivariable Cox proportional hazards model analysis reflect the risk of developing PUDs in the 2 cohorts after adjustment for age, sex, and history of comorbidity.
We further analyze risk of gastric ulcer (ICD-9-CM: 531), duodenal ulcer (ICD-9-CM: 532), peptic ulcer site unspecified (ICD-9-CM: 533), gastrojejunal ulcer (ICD-9-CM: 534), and gastritis and duodenitis (ICD-9-CM: 535) between BD and non-BD cohorts. Those outcomes are separated by ICD-9-CM, when subjects had the peptic ulcer event.
R software (R Foundation for Statistical Computing, Vienna, Austria) was used to create Kaplan–Meier curves and calculate the cumulative incidence of the PUDs in the control and study groups. In addition, the log-rank test was used to measure the differences of the 2 cumulative incidence curves.
RESULTS
Our initial sample comprised 105,300 patients. After we excluded ineligible patients, 84,240 patients without BDs and 21,060 patients with BDs remained. Table 1 shows the demographic characteristics and comorbidities among the BD and non-BD cohorts at the baseline. After the sample was frequency matched, the distribution of age and sex between the 2 cohorts was similar. Table 1 shows that most patients were women (54.4%) and 35 to 64 years of age (53.0%). The mean age of the patients was 40 years. Patients in the BD cohort exhibited a significantly higher prevalence of DM, HTN, hyperlipidemia, cirrhosis, anxiety, HD, COPD, alcohol-related illness, CRD, RA, and schizophrenic at the baseline (P < 0.05). The BD cohort had more common used the antidepressants treatment than the non-BD cohort (90% vs 14%, P < 0.0001).
TABLE 1.
Demographics Factors and Comorbidity Between Bipolar and Nonbipolar Cohorts
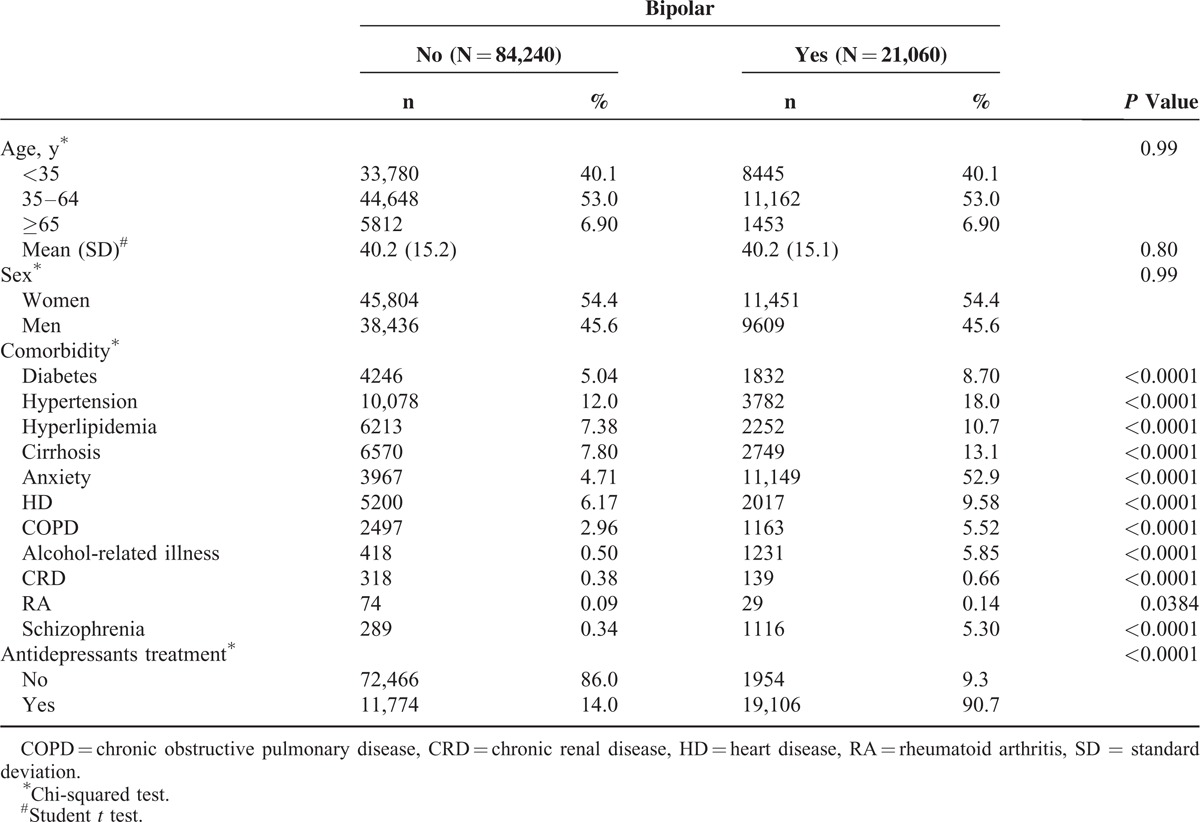
Table 2 shows the overall IRR of PUDs that was increased 1.59-fold in the BD cohort compared with the non-BD cohort (35.9 vs 22.6 per 1000 person-years). The overall adjusted HR of PUDs in patients with BDs was 1.51 (95% CI: 1.43–1.59) after we controlled for sex, age, and comorbidities. Notably, PUDs is the outcome of several complications, such as bleeding, perforation, and obstruction that result from local ulcer and gastritis is local inflammation. We further presented that BD patient had higher risk of gastrojejunal ulcer and gastric ulcer than non-BD patient (adjusted HR: 2.49, 95% CI: 1.69–3.66; adjusted HR: 1.90, 95% CI: 1.65–2.18). The incidence of gastritis and duodenitis was higher in BD cohort (18.6 per 1000 person-years vs 13.3 per 1000 person-years) and the adjusted HR was 1.40 (95% CI: 1.31–1.51) compared with non-BD cohort. BD patient had 1.64-fold (95% CI: 1.30–2.07) and 1.49-fold (95% CI: 1.33–1.65) risk to develop duodenal ulcer and peptic ulcer site unspecified than non-BD patient, respectively. Sex-specific analysis revealed BD incidence rates of 39.0 and 32.2 per 1000 person-years among women and men, respectively; these values are higher than those in the non-BD cohort (24.4 and 20.4 per 1000 person-years, respectively). Regardless of sex, the adjusted HR of PUDs was higher in the BD cohort than in the non-BD cohort (adjusted HR: 1.48, 95% CI: 1.38–1.59 for women; adjusted HR: 1.55, 95% CI: 1.43–1.69 for men). The incidence of PUDs increased with age in both the cohorts. Age-specific analysis showed that patients in the BD cohort exhibited a significantly higher risk of PUD development than that of the patients in the non-BD cohort at ages <35 and between 35 and 64 years. For patients with comorbidities, the incidence was increased. Patients with BDs and comorbidities exhibited a 1.40-fold increased risk (95% CI: 1.31–1.49) of PUDs compared with non-BD patients with comorbidities. Subject with antidepressants treatment had higher incidence of PUDs in both the cohorts (IRR: 1.56, 95% CI: 1.47–1.65). After adjusted for age, sex, and comorbidities history, BD patient with antidepressants treatment had 1.53-fold (95% CI: 1.44–1.63) risk of PUDs than non-BD patient with antidepressants treatment.
TABLE 2.
Incidence and Adjusted HR of Peptic Ulcer in Bipolar and Nonbipolar Cohorts Stratified by Sex, Age, Comorbidity, and Antidepressants Treatment
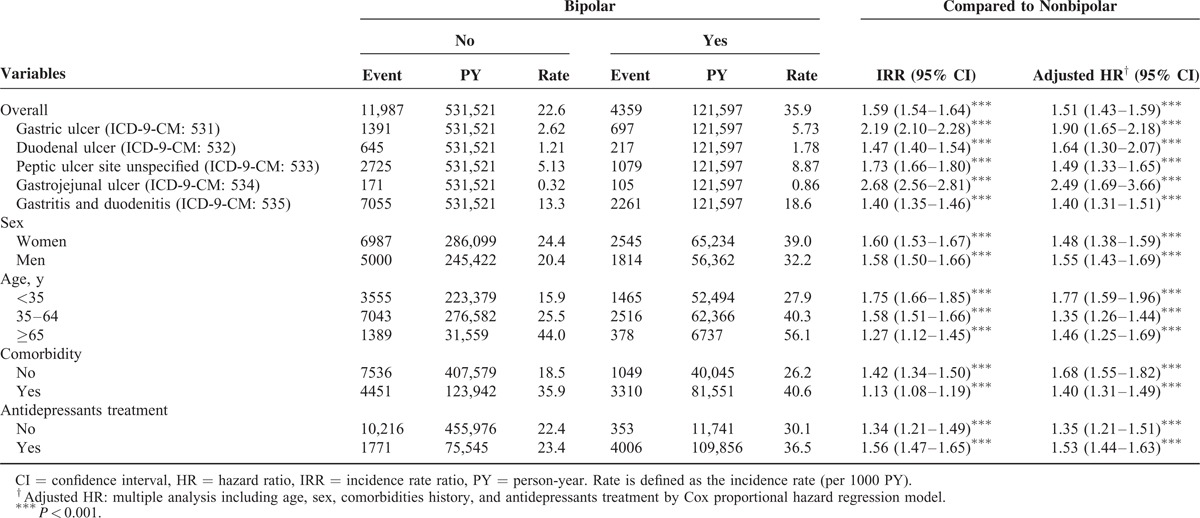
Table 3 shows a comparison between the BD and non-BD cohorts stratified by various types of comorbidity and presents the risk of PUDs in patients with BDs and comorbidities, namely, DM (adjusted HR: 1.38, 95% CI: 1.19–1.61), HTN (adjusted HR: 1.34, 95% CI: 1.21–1.49), hyperlipidemia (adjusted HR: 1.50, 95% CI: 1.31–1.72), cirrhosis (adjusted HR: 1.40, 95% CI: 1.22–1.60), anxiety (adjusted HR: 1.25, 95% CI: 1.13–1.38), HD (adjusted HR: 1.44, 95% CI: 1.25–1.66), COPD (adjusted HR: 1.35, 95% CI: 1.11–1.64), alcohol-related illness (adjusted HR: 1.66, 95% CI: 1.15–2.41), and CRD (adjusted HR: 2.17, 95% CI: 1.19–3.96). Regardless of comorbidities, the patients with BDs exhibited a higher risk of PUDs than that of the non-BD patients. We measured the association between the average number of hospital care services used because of BD exacerbation and the development of PUDs (Table 4). The adjusted HR increased with an increasing number of used hospital care services. Compared with the non-BD cohort, the adjusted HR of PUDs increased from 1.16 (95% CI: 1.08–1.24) for patients with ≤5 visits to 3.61 (95% CI: 3.34–3.91) for patients with >17 visits (P value for trend <0.0001). By the end of the follow-up period, the cumulative incidence of PUDs was 6.81% higher in the BD cohort than the non-BD cohort (25.5% vs 18.4%; Figure 1).
TABLE 3.
Incidence and Adjusted HR of Peptic Ulcer Stratified by Different Types of Comorbidities, Compared With Nonbipolar Cohort
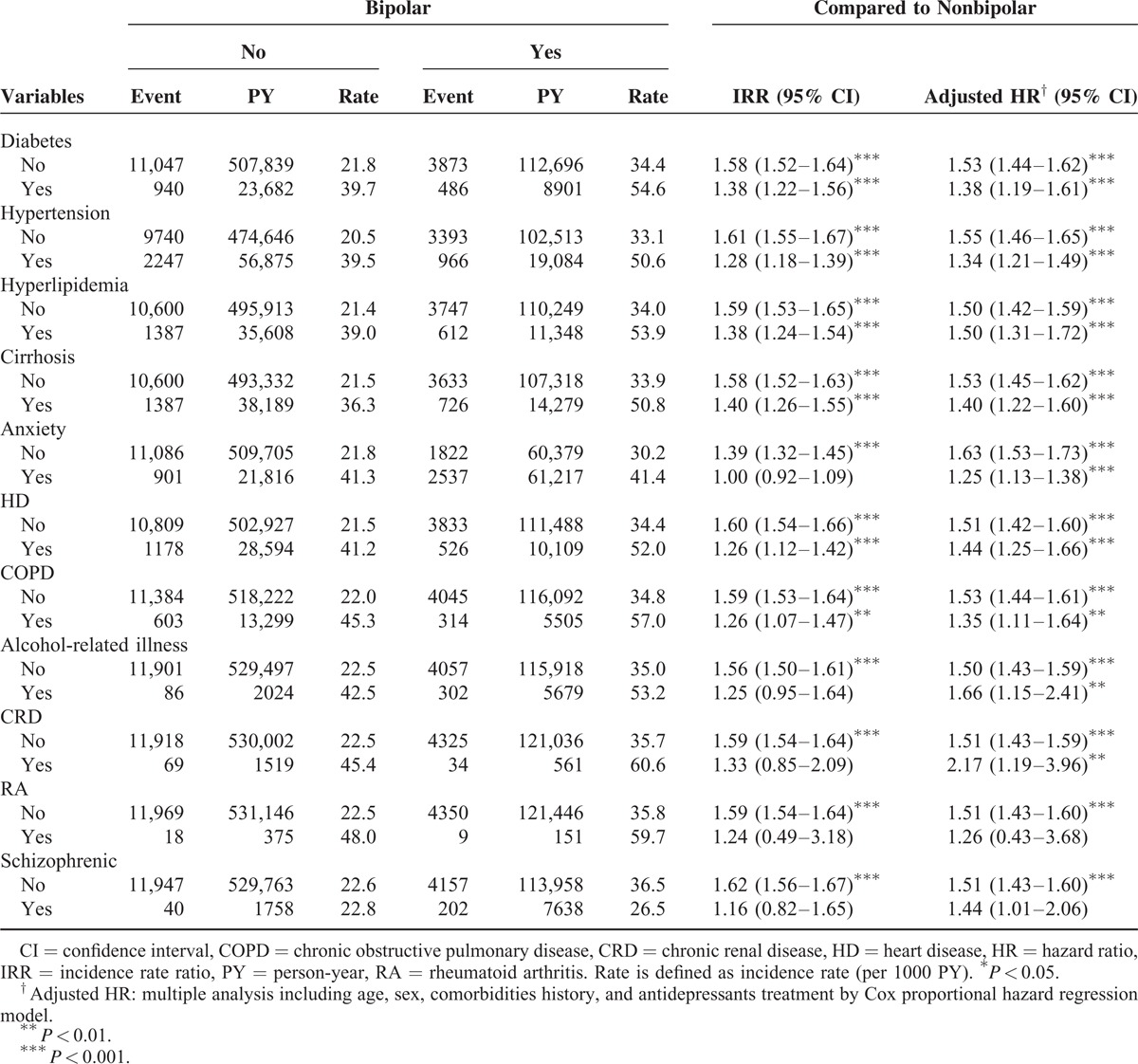
TABLE 4.
Adjusted HR of Peptic Ulcer Associated With Number of Used Hospital Care Service Per Year Due to Bipolar in Study Period
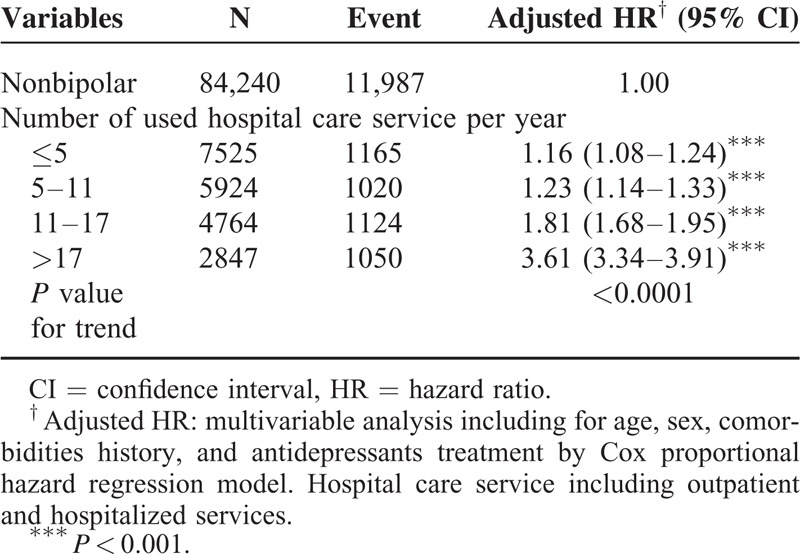
FIGURE 1.
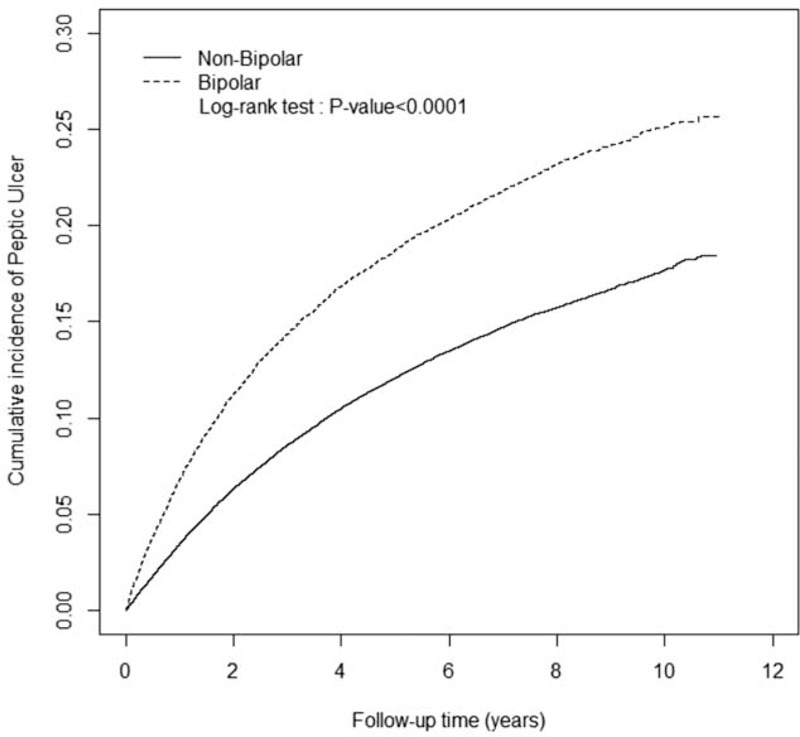
Cumulative incidence of peptic ulcer diseases among non-BD (solid line) and BD (dashed line) cohorts. BD = bipolar disorder.
DISCUSSION
This population-based study specifically examined BDs as a risk factor for PUDs by using a matched cohort and an 8-year follow-up period. The major finding of our study is the discovery of a higher incidence of subsequent PUDs among patients with BDs. Furthermore, regardless of whether the patients had DM, HTN, hyperlipidemia, cirrhosis, anxiety, HD, CRD, COPD, alcohol-related illness, CRD, or RA, BDs appear to be an independent risk factor for PUDs. Anxiety has been suggested as the risk factor of PUD.15 Although the data we showed in Table 3 were not very significant before adjustment (IRR: 1.00, 95% CI: 0.92–1.09), it showed significant difference after adjustment (HR: 1.25, 95% CI: 1.13–1.38, P < 0.001).
According to our analysis of the risk factors associated with subsequent PUDs in patients with BDs, we suggest that the mechanism is associated with the interaction between BDs and PUDs. Our findings reveal that patients with BDs were at a significantly increased risk for subsequent PUDs. Possible mechanisms may involve the hypothalamus–pituitary–adrenal (HPA) axis16 and glucocorticoid resistance.17–20 A study showed that manic states in patients with BDs are associated with enhanced dopaminergic transmission and experimental stress enhances dopamine neurotransmission and impairs cognition.16 Furthermore, stress activates the HPA axis, and the disturbed axis impairs neurocognitive function, as demonstrated in patients with BDs.16 A disturbed HPA axis caused by hippocampal damage and disinhibition was reported in patients with BDs.21 In addition, BDs have been associated with chronic neuroinflammation,22–24 which may induce glucocorticoid resistance under a chronic condition.25 Although glucocorticoids have exhibited gastroprotective effects under conditions of acute stress,25 an animal model showed the opposite effect under conditions of chronic stress.26 Consequently, BDs can increase the risk of PUDs. Studies have demonstrated chronic inflammation among BDs,22–24 DM,27,28 HTN,29 hyperlipidemia,30,31 cirrhosis,32 anxiety,33,34 COPD,35 and cerebrovascular diseases.36,37 The immune reaction associated with proinflammatory cytokines could induce neuroinflammation.38 In addition, chronic inflammation, a type of chronic stress, may disturb the HPA axis and induce hypercortisolemia and neuroinflammation through a proinflammatory response.39–41 HPA axis dysfunction has been reported to increase the risk of PUDs under conditions of chronic stress.26
Goodwin et al42 reported an association between anxiety disorders and PUDs, but this association weakened after adjustment for nicotine and alcohol dependence, suggesting that comorbid dependence on nicotine and alcohol may partially explain their observations. A study showed that high levels of alcohol consumption can induce adverse systemic effects such as reduced immune defense.43 Furthermore, heavy alcohol intake causes damage to the stomach lining, and alcohol-related illnesses mostly occur in people who drink substantial amounts of alcohol. However, our data suggest that regardless of alcohol-related illnesses, the patients with BDs exhibited a higher risk of PUDs than that of the non-BD patients (Table 3), suggesting that comorbid dependence on alcohol does not explain why patients with BDs exhibited a higher risk for subsequent PUDs.42 We have used COPD adjustment for replacing nicotine abuse. There were several reasons for COPD adjustment. First, COPD is a chronic inflammatory disease. Second, cigarette smoke is the most important risk factor of COPD, and it also induces the chronic inflammation. Because of the lack of information on healthy behaviors in NHIRD, we considered COPD instead of cigarette smoke in the Cox proportional hazard regression.44,45
We checked if BDs are the risk factor to develop PUDs. Therefore, we used the case–control study including the population-based cohort of patients having BDs with matched controls of comorbidity. It is the strength of this study. Nonetheless, some limitations of this study could be judged to read these findings in this study: first, we diagnosed BDs according to ICD-9-CM codes only using the NHIRD. Therefore, the data of BDs’ severity could not be measured to judge the risk factor for the subsequent PUDs. Second, the causal–effect association between the 2 diseases could not be evaluated by the chronological order. But we should consider the possible relationship between PUDs and BDs. Third, the NHIRD lacks a lot of possible confounding factors (such as socioeconomic status, lifestyle, and family history), which might be regarded to be associated with BDs and PUD. Fourth, it is very difficult to accurately divide the subgroups of these depression patients from the BDs cohort by ICD-9 codes only (supplementary table, http://links.lww.com/MD/A339). Fifth, severe PUD is usually combined with many complications (such as bleeding, obstruction, and perforation). In order to perform differentiated diagnoses between gastritis and PUD, we have to use the endoscopic examinations to check gastric tissues. But these invasive procedures usually were refused by these patients or resulted in severe complication (such as bleeding and perforation).46
In conclusion, we propose that patients with BDs exhibit a significantly increased risk for developing PUDs. According to our data, we suggest that following a diagnosis of BD, clinical practitioners could notice the occurrence of PUDs and associated prevention. Further prospective clinical studies investigating the relationship between BDs and PUDs are warranted.
Footnotes
Abbreviations: BDs = bipolar disorders, CI = confidence interval, HRs = hazard ratios, ICD-9-CM = International Classification of Diseases, 9th Revision, Clinical Modification, IRR = incidence rate ratio, NHIRD = National Health Insurance Research Database, PUDs = peptic ulcer diseases.
Y-CH and Ch-CH contributed equally to this work.
Author contributions—Conception/Design: Y-CH, C-CH, C-HK; Provision of study materials: C-HK; Collection and/or assembly of data: Y-CH, C-CH, Y-CW, C-HK; Data analysis and interpretation: all authors; Manuscript writing: all authors; Final approval of manuscript: all authors.
This study is supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002); China Medical University Hospital, Academia Sinica Taiwan Biobank, Stroke Biosignature Project (BM104010092); NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039-006); Taiwan Ministry of Science and Technology (MOST103-2314-B-715-001-MY2); Mackay Medical College Project (RD1030076); Tseng-Lien Lin Foundation, Taichung, Taiwan; Taiwan Brain Disease Foundation, Taipei, Taiwan; Katsuzo and Kiyo Aoshima Memorial Funds, Japan; and CMU under the Aim for Top University Plan of the Ministry of Education, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
The authors have no conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Maina G, Bechon E, Rigardetto S, et al. General medical conditions are associated with delay to treatment in patients with bipolar disorder. Psychosomatics 2013; 54:437–442. [DOI] [PubMed] [Google Scholar]
- 2.Prabhu V, Shivani A. An overview of history, pathogenesis and treatment of perforated peptic ulcer disease with evaluation of prognostic scoring in adults. Ann Med Health Sci Res 2014; 4:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levenstein S, Rosenstock S, Jacobsen RK, et al. Psychological stress increases risk for peptic ulcer, regardless of Helicobacter pylori infection or use of nonsteroidal anti-inflammatory drugs. Clin Gastroenterol Hepatol 2015; 13:498–501. [DOI] [PubMed] [Google Scholar]
- 4.Krag M, Perner A, Wetterslev J, et al. Stress ulcer prophylaxis in the intensive care unit: is it indicated? A topical systematic review. Acta Anaesthesiol Scand 2013; 57:835–847. [DOI] [PubMed] [Google Scholar]
- 5.Lim WY, Subramaniam M, Abdin E, et al. Peptic ulcer disease and mental illnesses. Gen Hosp Psychiatry 2014; 36:63–67. [DOI] [PubMed] [Google Scholar]
- 6.Taha F, Lipsitz JD, Galea S, et al. Anxiety disorders and risk of self-reported ulcer: a 10-year longitudinal study among US adults. Gen Hosp Psychiatry 2014; 36:674–679. [DOI] [PubMed] [Google Scholar]
- 7.Ozdemir V, Jamal MM, Osapay K, et al. Cosegregation of gastrointestinal ulcers and schizophrenia in a large national inpatient discharge database: revisiting the “brain-gut axis” hypothesis in ulcer pathogenesis. J Investig Med 2007; 55:315–320. [DOI] [PubMed] [Google Scholar]
- 8.Goodwin RD, Talley NJ, Hotopf M, et al. A link between physician-diagnosed ulcer and anxiety disorders among adults. Ann Epidemiol 2013; 23:189–192. [DOI] [PubMed] [Google Scholar]
- 9.Hawke LD, Velyvis V, Parikh SV. Bipolar disorder with comorbid anxiety disorders: impact of comorbidity on treatment outcome in cognitive-behavioral therapy and psychoeducation. Int J Bipolar Disord 2013; 1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Garro-Moore JK, Adams AM, Abramson LY, et al. Anxiety comorbidity in bipolar spectrum disorders: the mediational role of perfectionism in prospective depressive symptoms. J Affect Disord 2015; 174:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ellingrod VL, Taylor SF, Dalack G, et al. Risk factors associated with metabolic syndrome in bipolar and schizophrenia subjects treated with antipsychotics: the role of folate pharmacogenetics. J Clin Psychopharmacol 2012; 32:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baptista T, Serrano A, Uzcategui E, et al. The metabolic syndrome and its constituting variables in atypical antipsychotic-treated subjects: comparison with other drug treatments, drug-free psychiatric patients, first-degree relatives and the general population in Venezuela. Schizophr Res 2011; 126:93–102. [DOI] [PubMed] [Google Scholar]
- 13.Hsu CC, Chen SC, Liu CJ, et al. Rheumatoid arthritis and the risk of bipolar disorder: a nationwide population-based study. PLoS One 2014; 9:e107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoepf D, Heun R. Bipolar disorder and comorbidity: increased prevalence and increased relevance of comorbidity for hospital-based mortality during a 12.5-year observation period in general hospital admissions. J Affect Disord 2014; 169:170–178. [DOI] [PubMed] [Google Scholar]
- 15.Goodwin RD, Stein MB. Generalized anxiety disorder and peptic ulcer disease among adults in the United States. Psychosom Med 2002; 64:862–866. [DOI] [PubMed] [Google Scholar]
- 16.Young AH. The effects of HPA axis function on cognition and its implications for the pathophysiology of bipolar disorder. Harv Rev Psychiatry 2014; 22:331–333. [DOI] [PubMed] [Google Scholar]
- 17.Spiliotaki M, Salpeas V, Malitas P, et al. Altered glucocorticoid receptor signaling cascade in lymphocytes of bipolar disorder patients. Psychoneuroendocrinology 2006; 31:748–760. [DOI] [PubMed] [Google Scholar]
- 18.Watson S, Thompson JM, Ritchie JC, et al. Neuropsychological impairment in bipolar disorder: the relationship with glucocorticoid receptor function. Bipolar Disord 2006; 8:85–90. [DOI] [PubMed] [Google Scholar]
- 19.Spijker AT, Giltay EJ, van Rossum EF, et al. Glucocorticoid and mineralocorticoid receptor polymorphisms and clinical characteristics in bipolar disorder patients. Psychoneuroendocrinology 2011; 36:1460–1469. [DOI] [PubMed] [Google Scholar]
- 20.Szczepankiewicz A, Leszczynska-Rodziewicz A, Pawlak J, et al. Glucocorticoid receptor polymorphism is associated with major depression and predominance of depression in the course of bipolar disorder. J Affect Disord 2011; 134:138–144. [DOI] [PubMed] [Google Scholar]
- 21.Leszczynska-Rodziewicz A, Maciukiewicz M, Szczepankiewicz A, et al. Association between OPCRIT dimensions and polymorphisms of HPA axis genes in bipolar disorder. J Affect Disord 2013; 151:744–747. [DOI] [PubMed] [Google Scholar]
- 22.Barbosa IG, Rocha NP, Assis F, et al. Monocyte and lymphocyte activation in bipolar disorder: a new piece in the puzzle of immune dysfunction in mood disorders. Int J Neuropsychopharmacol 2014; 18:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frey BN, Andreazza AC, Houenou J, et al. Biomarkers in bipolar disorder: a positional paper from the International Society for Bipolar Disorders Biomarkers Task Force. Aust N Z J Psychiatry 2013; 47:321–332. [DOI] [PubMed] [Google Scholar]
- 24.Hope S, Dieset I, Agartz I, et al. Affective symptoms are associated with markers of inflammation and immune activation in bipolar disorders but not in schizophrenia. J Psychiatr Res 2011; 45:1608–1616. [DOI] [PubMed] [Google Scholar]
- 25.Filaretova L. The hypothalamic-pituitary-adrenocortical system: hormonal brain-gut interaction and gastroprotection. AutonNeurosci 2006; 125:86–93. [DOI] [PubMed] [Google Scholar]
- 26.Zhang S, Xu Z, Gao Y, et al. Bidirectional crosstalk between stress-induced gastric ulcer and depression under chronic stress. PloS One 2012; 7:e51148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Esser N, Paquot N, Scheen AJ. Anti-inflammatory agents to treat or prevent type 2 diabetes, metabolic syndrome and cardiovascular disease. Expert Opin Investig Drugs 2015; 24:283–307. [DOI] [PubMed] [Google Scholar]
- 28.Mushtaq G, Khan JA, Kumosani TA, et al. Alzheimer's disease and type 2 diabetes via chronic inflammatory mechanisms. Saudi J Biol Sci 2015; 22:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez GE, Rhaleb NE, D’Ambrosio MA, et al. Deletion of interleukin-6 prevents cardiac inflammation, fibrosis and dysfunction without affecting blood pressure in angiotensin II-high salt-induced hypertension. J Hypertens 2015; 33:144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Getz GS, Reardon CA. The mutual interplay of lipid metabolism and the cells of the immune system in relation to atherosclerosis. Clin Lipidol 2014; 9:657–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rafieian-Kopaei M, Setorki M, Doudi M, et al. Atherosclerosis: process, indicators, risk factors and new hopes. Int J Prev Med 2014; 5:927–946. [PMC free article] [PubMed] [Google Scholar]
- 32.Sasaki M, Miyakoshi M, Sato Y, et al. Chemokine-chemokine receptor CCL2-CCR2 and CX3CL1-CX3CR1 axis may play a role in the aggravated inflammation in primary biliary cirrhosis. Dig Dis Sci 2014; 59:358–364. [DOI] [PubMed] [Google Scholar]
- 33.Chen J, Winston JH, Fu Y, et al. Genesis of anxiety, depression, and ongoing abdominal discomfort in ulcerative colitis-like colon inflammation. Am J Physiol Regul Integr Comp Physiol 2015; 308:R18–R27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suarez EC, Sundy JS, Erkanli A. Depressogenic vulnerability and gender-specific patterns of neuro-immune dysregulation: what the ratio of cortisol to C-reactive protein can tell us about loss of normal regulatory control. Brain Behav Immun 2015; 44:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barouchos N, Papazafiropoulou A, Iacovidou N, et al. Comparison of tumor markers and inflammatory biomarkers in chronic obstructive pulmonary disease (COPD) exacerbations. Scand J Clin Lab Invest 2015; 75:126–132. [DOI] [PubMed] [Google Scholar]
- 36.Westman J, Hallgren J, Wahlbeck K, et al. Cardiovascular mortality in bipolar disorder: a population-based cohort study in Sweden. BMJ Open 2013; 3:e002373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Toledo Ferraz Alves TC, Ferreira LK, Busatto GF. Vascular diseases and old age mental disorders: an update of neuroimaging findings. Curr Opin Psychiatry 2010; 23:491–497. [DOI] [PubMed] [Google Scholar]
- 38.Singhal G, Jaehne EJ, Corrigan F, et al. Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front Neurosci 2014; 8:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Bueno B, Caso JR, Leza JC. Stress as a neuroinflammatory condition in brain: damaging and protective mechanisms. Neurosci Biobehav Rev 2008; 32:1136–1151. [DOI] [PubMed] [Google Scholar]
- 40.Choi DC, Furay AR, Evanson NK, et al. The role of the posterior medial bed nucleus of the stria terminalis in modulating hypothalamic-pituitary-adrenocortical axis responsiveness to acute and chronic stress. Psychoneuroendocrinology 2008; 33:659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hueston CM, Deak T. The inflamed axis: the interaction between stress, hormones, and the expression of inflammatory-related genes within key structures comprising the hypothalamic-pituitary-adrenal axis. Physiol Behav 2014; 124:77–91. [DOI] [PubMed] [Google Scholar]
- 42.Goodwin RD, Keyes KM, Stein MB, et al. Peptic ulcer and mental disorders among adults in the community: the role of nicotine and alcohol use disorders. Psychosom Med 2009; 71:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brenner H, Bode G, Adler G, et al. Alcohol as a gastric disinfectant? The complex relationship between alcohol consumption and current Helicobacter pylori infection. Epidemiology 2001; 12:209–214. [DOI] [PubMed] [Google Scholar]
- 44.Chang KH, Chang MY, Muo CH, et al. Increased risk of dementia in patients exposed to nitrogen dioxide and carbon monoxide: a population-based retrospective cohort study. PLoS One 2014; 9:e103078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang KH, Chung CJ, Lin CL, et al. Increased risk of dementia in patients with osteoporosis: a population-based retrospective cohort analysis. Age 2014; 36:967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gurung RB, Joshi G, Gautam N, et al. Upper gastro-intestinal bleeding: aetiology and demographic profile based on endoscopic examination at Dhulikhel Hospital, Kathmandu University Hospital. Kathmandu Univ Med J (KUMJ) 2010; 8:208–211. [DOI] [PubMed] [Google Scholar]


