Supplemental Digital Content is available in the text
Abstract
Enteral immunonutrition (EIN) has been established to be as a significantly important modality to prevent the postoperative infectious and noninfectious complications, enhance the immunity of host, and eventually improve the prognosis of gastrointestinal (GI) cancer patients undergoing surgery. However, different support routes, which are the optimum option, remain unclear. To evaluate the effects of different EIN support regimes for patients who underwent selective surgery for resectable GI malignancy, a Bayesian network meta-analysis (NMA) of randomized controlled trials (RCTs) was conducted.
A search of PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) was electronically searched until the end of December 2014. Moreover, we manually checked reference lists of eligible trials and review and retrieval unpublished literature. RCTs which investigated the comparative effects of EIN versus standard enteral nutrition (EN) or different EIN regimes were included if the clinical outcomes information can be extracted from it.
A total of 27 RCTs were incorporated into this study. Pair-wise meta-analyses suggested that preoperative (relative risk [RR], 0.58; 95% confidence interval [CI], 0.43–0.78), postoperative (RR, 0.63; 95% CI, 0.52–0.76), and perioperative EIN methods (RR, 0.46; 95% CI, 0.34–0.62) reduced incidence of postoperative infectious complications compared with standard EN. Moreover, perioperative EIN (RR, 0.65; 95% CI, 0.44–0.95) reduced the incidence of postoperative noninfectious complications, and the postoperative (mean difference [MD], −2.38; 95% CI, −3.4 to −1.31) and perioperative EIN (MD, −2.64; 95% CI, −3.28 to −1.99) also shortened the length of postoperative hospitalization compared with standard EN. NMA found that EIN support effectively improved the clinical outcomes of patients who underwent selective surgery for GI cancer compared with standard EN.
Our results suggest EIN support is promising alternative for operation management in comparison with standard EN, and perioperative EIN regime is the optimum option for managing clinical status of patients who underwent selective surgery for GI cancer.
INTRODUCTION
Gastrointestinal (GI) malignancy has been the leading cause of cancer death worldwide, and it cannot be radically treated resulting from the complexity of pathomechanism and mutations of drug-resistant.1 Hitherto, surgical resection is still the mainstay of curative treatment for patients with GI cancer in spite of effective alternatives have been developed.2 However, it is noted that patients who underwent the selective surgery for GI cancer are at high risk of developing postoperative adverse events (eg, postoperative infectious or noninfectious complications, immune depression, longer length of hospitalization, etc.)3–5 because of several factors such as malnourished status, absolute diet, neoplasm-induced host immunity defection, and surgery-associated stress.6–8 The postoperative clinical outcomes will be modulated by multiple factors which included anti-inflammatory agent, immunoenhancer, nutrition status, etc; however, nutrition support is the most important alternative which was used to decrease the incidence of postoperative infectious and noninfectious complications, enhance the host immunity, and eventually shorten the length of postoperative hospitalization and greatly decrease the medical expenditure, as well as improve the prognosis of the given patients.8–11
Published evidences suggested that enteral immunonutrition (EIN) diet which enriched with at least 2 of arginine (Arg), omega-3-fatty acids (ω-3-FA), glutamine (Glu), or ribonucleic acid (RNA) has the potential to decrease the infection risk and shorten the length of postoperative hospitalization.10,12–17 Multiple randomized controlled trials (RCTs) have been performed to investigate the comparative effects of EIN versus standard enteral nutrition (EN) or different deliver routes of immunonutrition.5,18–23 Several systematic reviews (SRs) and meta-analyses comparing EIN related to standard (conventional) EN or different immunonutrition support routes in the patients who underwent the selective surgery for GI cancer have also been completed.8,24–26 No study was published to evaluate which is the optimum EIN support regime. Traditional head-to-head meta-analyses can directly analyze the comparative effects of 2 individual interventions; however, it was not applicable to this case in which one expected to compare ≥3 treatments. Bayesian network meta-analysis (NMA), which was an expansion of traditional direct comparison meta-analysis, can cover the shortage by combining direct and indirect evidences simultaneously.
So, we undertook a Bayesian NMA of RCTs regarding different deliver routes of EIN compared with standard EN in order to establish the optimum immunonutrition support regime.
MATERIAL AND METHODS
The Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P)27 and the Cochrane Handbook for Systematic Reviews of Interventions28 were used to guide this study. We performed all analyses based on the published studies previously, and thus no ethical approval and informed consent were required. In addition, we critically appraised the quality of reporting of this study by using the PRISMA 2009 checklist (Table S1, http://links.lww.com/MD/A345).29
Searching Strategy
Databases which included PubMed, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) were electronically searched by independent investigators (G-MS and XT) to collect any RCTs which investigated the comparative effects of EIN versus standard EN or different deliver routes of EIN support until the end of December 2014. We used following search terms to perform procedures by using combination of medical subject heading and free word embedded in specific files involving title, keywords, and abstract: “Esophageal Neoplasms,” “Stomach Neoplasms,” “Liver Neoplasms,” “Colonic Neoplasms,” “Rectal Neoplasms,” “Pancreatic Neoplasms,” “Digestive System Neoplasms,” “Gastrointestinal Neoplasms,” “Colorectal Neoplasms,” “Bile Duct Neoplasms,” “Gallbladder Neoplasms,” “Arginine,” “Fatty Acids, Omega 3,” “Glutamine,” “RNA,” “Nutritional Support,” “Parenteral Nutrition,” “Enteral Nutrition,” “Postoperative Period,” and “General Surgery.” This search strings were constructed by using Boolean operator. We also manually checked the reference of lists of eligible studies and corresponding review to include any potential study to guarantee the precision and recall ratio. No other restrictions were imposed. The search terms and strings were presented in Supplement 1, http://links.lww.com/MD/A345.
Identification of Study
The following inclusion criteria were identified according to the PICOS acronym (participant, intervention, comparison, outcomes of interest, and study design): Population (P): all the patients who were scheduled to selective surgery for GI cancer were included in this study. Intervention (I) and Comparison (C): the trials evaluated the comparative effects of EIN diet which enriched with at least 2 of Arg, Glu, ω-3-FA, and RNA versus standard EN. EIN diet administration was performed at preoperation, postoperation, or perioperation period. Outcomes of interest (O): we assessed the following outcome measures: postoperative infectious or noninfectious complications and length of postoperative hospitalization. Study design (S): only RCTs with or without blind method were considered.
We would like to exclude the following studies: patients have unresectable GI malignancy, underlying cardiovascular pathology, active preoperative infection, administration of corticosteroids or immunosuppressive agents, and renal or hepatic function impairment; experimental data; lack of essential information and cannot acquire primary data from authors; the article with the most strict methodology and most complete data was chosen to be analyzed in terms of duplicate literature; and nonoriginal research such as review, letter and specialist comments, and non-RCTs.
Data Extraction
Two independent investigators (LZ and Y-XO) extracted the following basic information and essential continuous and binary data for expected outcome of interest from each included study by using the predesigned standard data extraction form (Tables S2, http://links.lww.com/MD/A345): study ID which included first author and publication year, country, surgery type, age of participants, sample size, nutrition status, interventions, and reported outcome of interest. The author would be contacted to acquire the complete data when necessary. Any divergences between authors concerning the eligibility of a study were resolved by consensus or consulting a third author (XT).
Assessing Quality of Methodology
Two independent investigators (L-JY and J-GZ) were assigned to critically appraise the methodology quality of all eligible studies in accordance with the Cochrane Handbook of Systematic Review of Interventions28 to pool reliable and robust estimated effect sizes which were used to improve clinical practice. Seven indexes were independently appraised accordingly, and the following evaluation results were cross-checked: randomization sequence generation, allocation concealment, blinding of participants and study personnel, blinding of outcome assessors, incomplete outcome data, selective reporting, and other biases. The risk of each incorporated study was rated as “high bias risk,” “unclear bias risk,” or “low bias risk” according to the adequate level of information extracted. A third investigator (Hong-Lin Yang) was assigned to disagreement between assessors.
Traditional Pair-Wise Meta-Analysis
We performed initially the traditional pair-wise meta-analysis to evaluate the comparative effects of 2 individual treatments which can be directly compared. The estimates of dichotomous and continuous data were expressed as relative risk (RR) and mean difference (MD), respectively. The heterogeneity between studies was tested by using χ2 test,30 and proportion of the overall variation that is attributable to between-study heterogeneity was also estimated by using I2 statistic.31 Substantial heterogeneity was considered unless the value of I2 statistic was <50%. We adopted fixed- or random-effect model to calculate the summary statistic according to the clinical diversity and methodological variation, as well as the homogeneity test.
Bayesian Network Meta-analysis
Bayesian NMA is a generalization of pair-wise meta-analysis. It is an alternative to pool direct and indirect or different indirect evidences simultaneously. A Bayesian random-effects NMA, which was based on the Markov chain Monte Carlo (MCMC) simulation from the posterior distribution, was adopted to calculate the estimates of relative effects and all model parameters.32 To gain convergence, we performed each MCMC chain with 40,000 iterations and 10,000 burn-in. We have drawn the comparison-adjusted funnel plot to assess the small study effects. The results were also presented by using the surface under the cumulative ranking curve (SUCRA) and the higher SUCRA value was correspond to better results for respective treatment.33 A Bayesian NMA can be carried out based on a key assumption which shows the consistency of results between direct and indirect comparisons. We calculate the inconsistency factor by using the loop-specific method to assess the inconsistency.34
All analyses were carried out by using the RevMan 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2013), Stata 12 (StataCorp, TX), and WinBUGS 1.4 (Imperial College School of Medicine at St Mary's, London).
RESULTS
Search Results and Characteristics of Trials
We captured 321 potential citations based on these given search terms and strings at the initial search stage. One hundred and twenty-five duplications were excluded by using EndNote 7.2 (Thomson Reuters, MI). One hundred and fifty-two citations were excluded after screening the title and abstract. We accessed the remaining full-text to further assess the eligibility. After screening the full-text, 20 ineligible trials were excluded resulting from several reasons such as lack of outcomes of interest, ineligible intervention regimes, and ineligible participants. All procedures were performed independently by 2 investigators. And eventually, 27 eligible studies4,5,10–23,35–44 were incorporated into this SR and meta-analysis. The basic characteristics of included studies were shown in Table 1. The flow chart of retrieval and selection of literature was shown in Figure 1.
TABLE 1.
Basic Characteristics of Included Studies Comparing Enteral Immunonutrition Regimes Which Included Preoperative, Postoperative, and Perioperative Versus Standard Enteral Nutrition
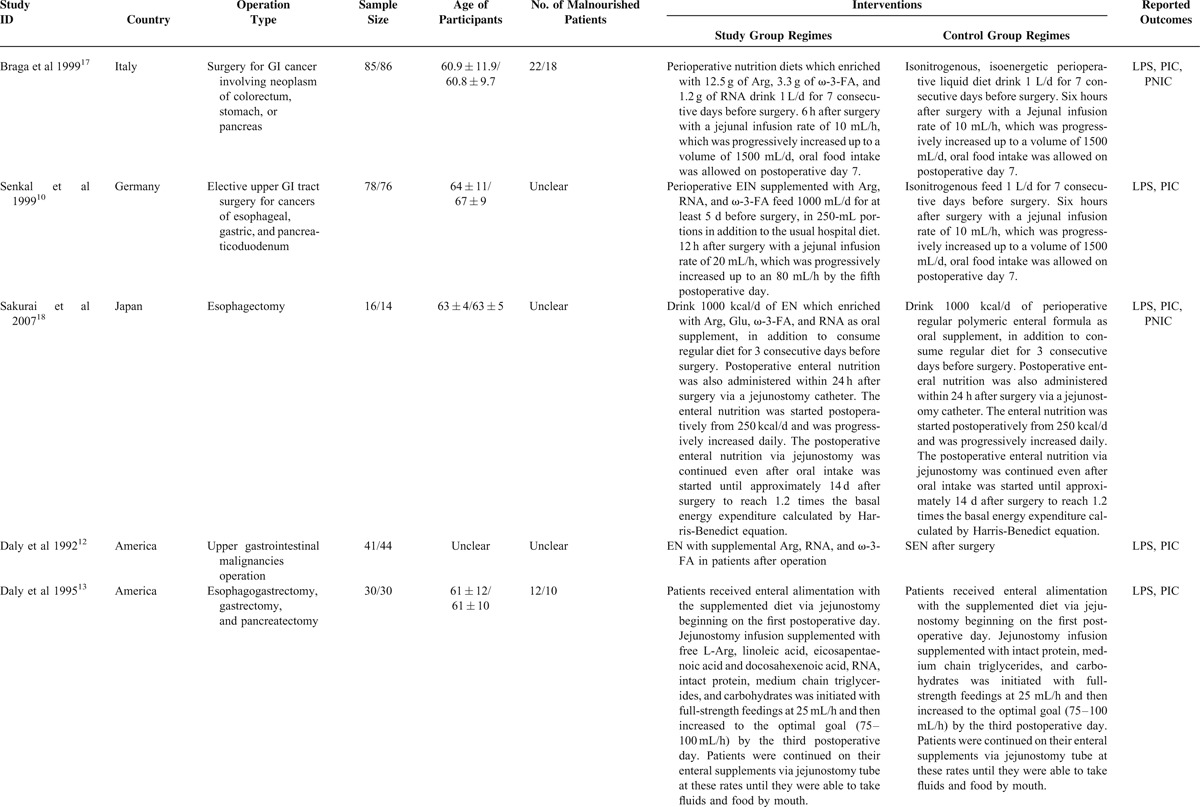
FIGURE 1.
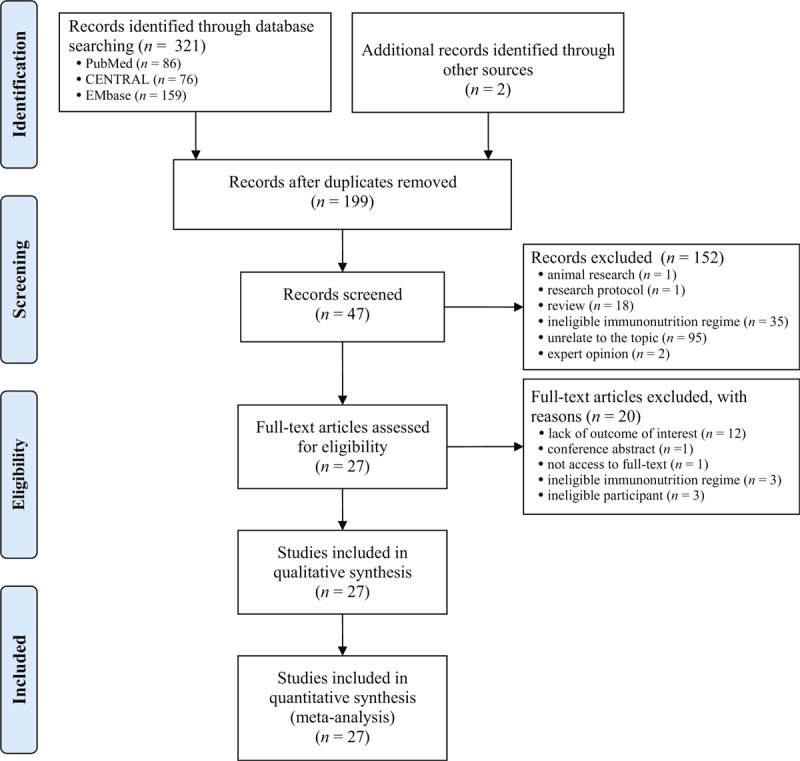
Flow diagram of retrieval and selection of literature.
TABLE 1.
Basic Characteristics of Included Studies Comparing Enteral Immunonutrition Regimes Which Included Preoperative, Postoperative, and Perioperative Versus Standard Enteral Nutrition
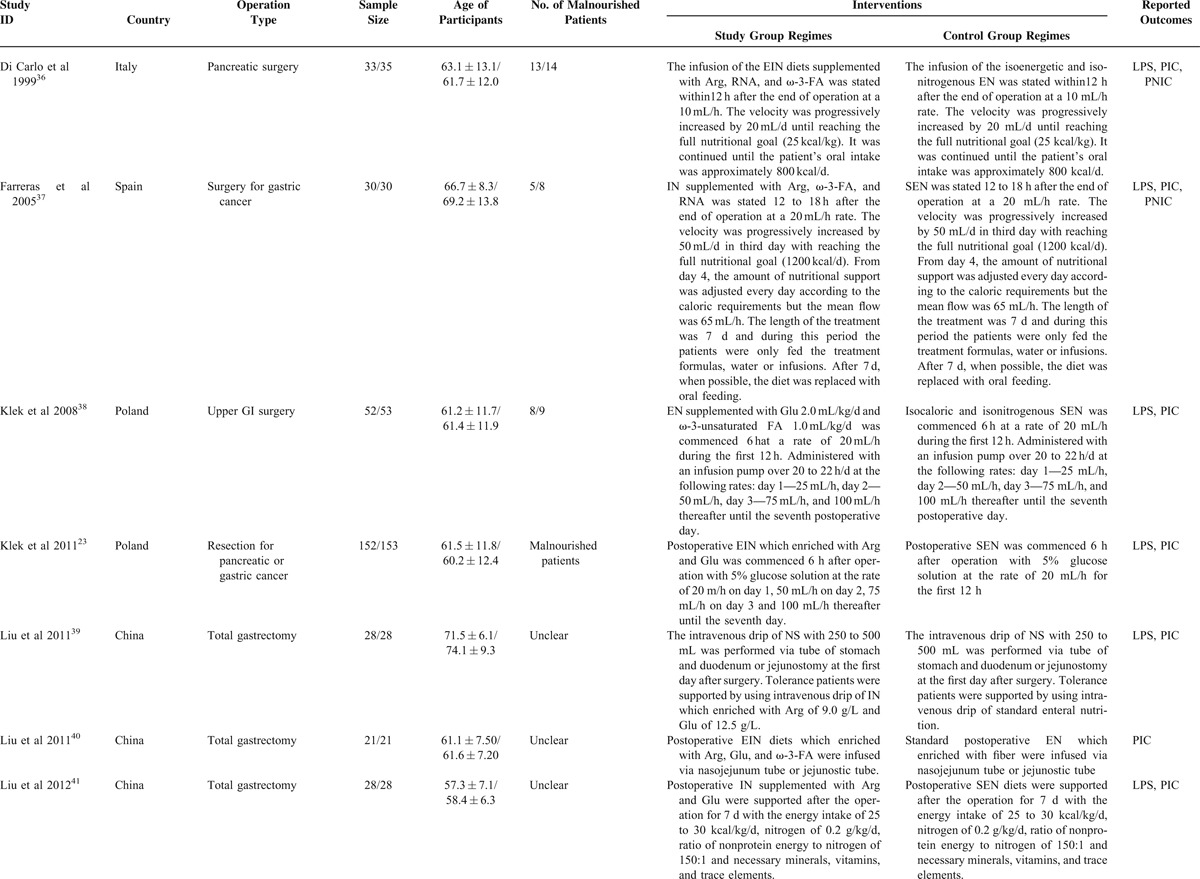
TABLE 1.
Basic Characteristics of Included Studies Comparing Enteral Immunonutrition Regimes Which Included Preoperative, Postoperative, and Perioperative Versus Standard Enteral Nutrition
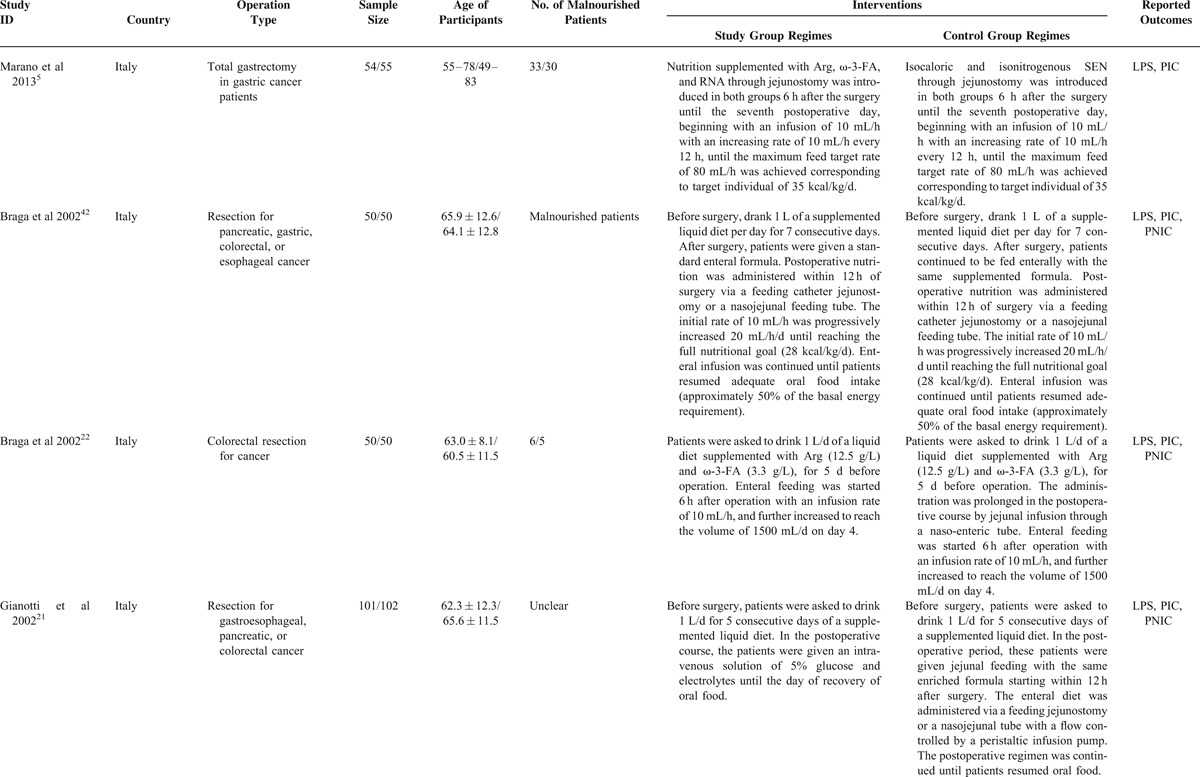
TABLE 1.
Basic Characteristics of Included Studies Comparing Enteral Immunonutrition Regimes Which Included Preoperative, Postoperative, and Perioperative Versus Standard Enteral Nutrition
Assessment of Methodological Quality
We critically appraised the methodological quality of included studies in accordance with the Cochrane Collaboration's Risk of Bias Tool.28 The proportion of appropriate description of randomization, allocation concealment, and blinding is the 48% (13/27), 37% (10/27), and 44%, respectively. All included trials were rated as low bias risk in incomplete outcome data because the authors stated the drop-out reasons in detail and used the intent-to-treat method to analyze the data. The quality of all eligible studies was graded as low bias risk because expected outcomes of interest were all reported in terms of selective reporting index. Other bias sources were not identified. The graphical result of methodological quality was shown in Figure 2.
FIGURE 2.

Assessment of risk of bias: (A) risk of bias graph; (B) risk of bias summary.
Evidence Network
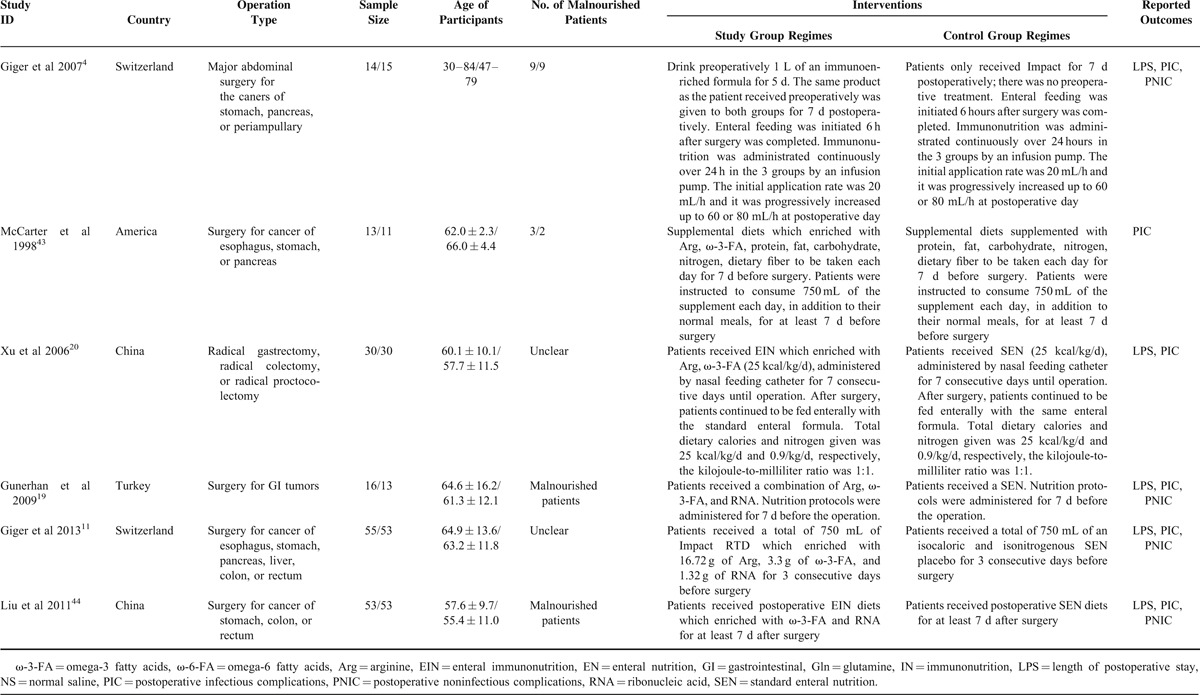
In this SR and meta-analysis, we investigated the comparative effects of EIN which included 3 support routes involving preoperative, postoperative, and perioperative periods related to standard EN. We have drawn the evidence network plot in terms of postoperative infectious complications, postoperative noninfectious complications, and length of postoperative hospitalization. Twenty-four are two-arm studies and the remaining are three-arm trials. The evidence network plot was shown in Figure 3.
FIGURE 3.
Evidence networks: (A) network for postoperative infectious complications; (B) network for postoperative noninfectious complications; (C) network for length of postoperative hospitalization. EIN = enteral immunonutrition, SEN = standard enteral nutrition.
Inconsistency Test
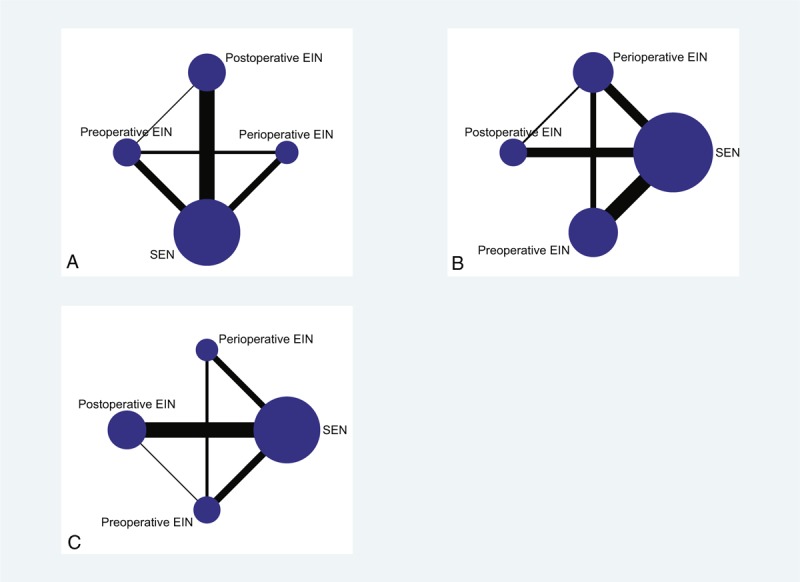
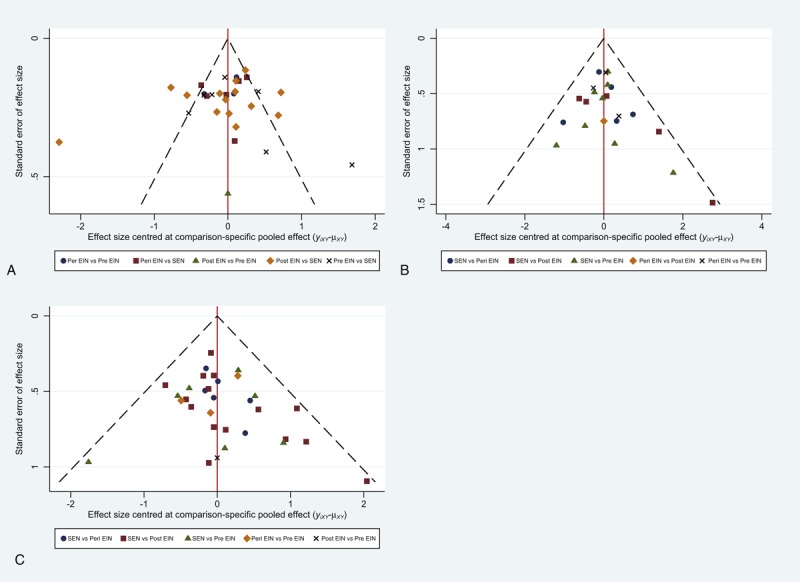
We performed the comparison-adjusted funnel plot to test the small study effect. The funnel plots indicated asymmetrical graph, and suggested that the pooled results may be negatively affected by small study effects (Figure 4). We also tested the inconsistency of results between direct and indirect comparisons. The inconsistency plots suggested that the statistical inconsistency was generally low for weight control as the corresponding confidence intervals (CIs) included zero (Figure 5).
FIGURE 4.
Comparison-adjusted funnel plots: (A) funnel plot for postoperative complication; (B) funnel plot for postoperative noninfectious complications; (C) funnel plot for length of postoperative hospitalization. Pre = preoperative, Post = postoperative, Peri = perioperative, EIN = enteral immunonutrition, SEN = standard enteral nutrition.
FIGURE 5.
Inconsistency plot: (A) inconsistency plot for postoperative infectious complications; (B) inconsistency plot for postoperative noninfectious complications; (C) inconsistency plot for length of postoperative hospitalization. EIN = enteral immunonutrition, SEN = standard enteral nutrition.
Postoperative Infectious Complications
We identified 7 eligible trials,11,19–22,42,43 which directly evaluated the comparative effects of preoperative EIN diet versus standard EN, and all were incorporated into this traditional pair-wise meta-analysis. A total of 313 and 307 patients were randomly divided into preoperative EIN group and standard EN group, respectively. All trials were considered to be homogenous (χ2 = 8.12, P = 0.23, I2 = 26%), and thus a fixed-effect model based on Mantel–Haenszel (M-H) method was used to estimate the pooled result. The meta-analysis indicated that the preoperative EIN effectively decreased the incidence of postoperative infectious complications compared with standard EN (RR, 0.58; 95% CI, 0.43–0.78) (Figure 6A). The Bayesian NMA obtained similar results (RR, 0.41; 95% CI, 0.26–0.63) (Table 2).
FIGURE 6.
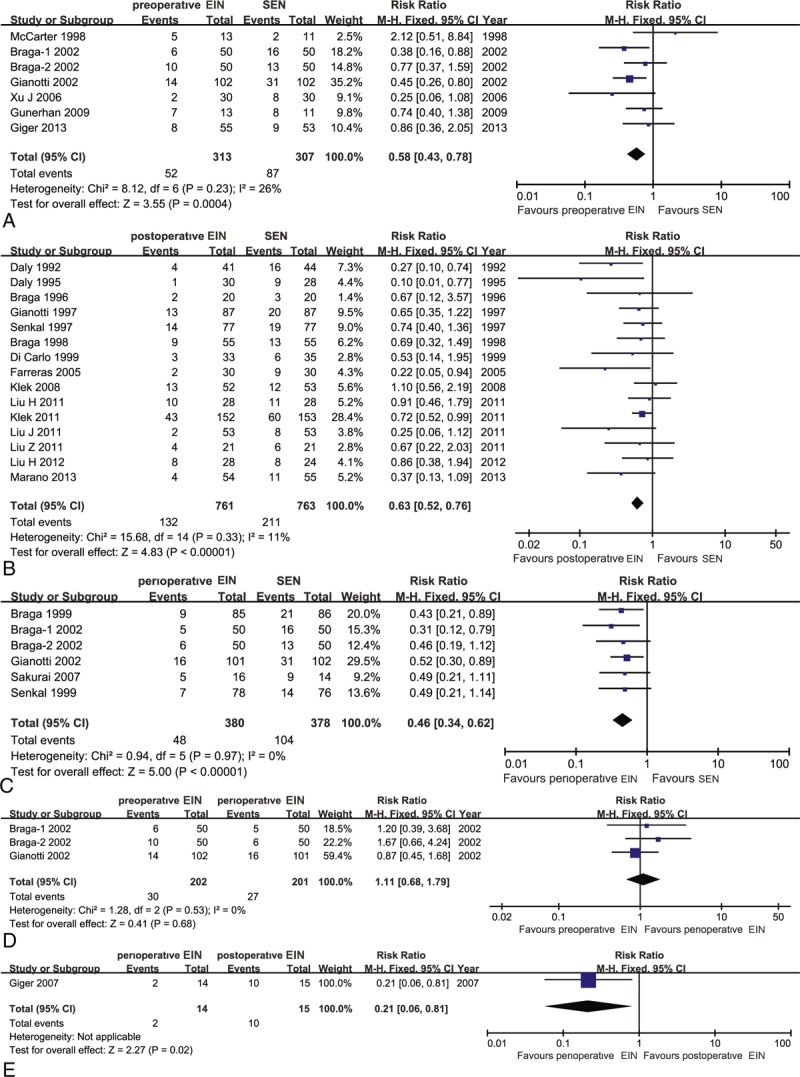
Traditional pair-wise meta-analysis on postoperative infectious complications: (A) preoperative EIN versus SEN; (B) postoperative EIN versus SEN; (C) perioperative EIN versus SEN; (D) preoperative EIN versus perioperative EIN; (E) perioperative EIN versus postoperative EIN. EIN = enteral immunonutrition.
TABLE 1.
Basic Characteristics of Included Studies Comparing Enteral Immunonutrition Regimes Which Included Preoperative, Postoperative, and Perioperative Versus Standard Enteral Nutrition
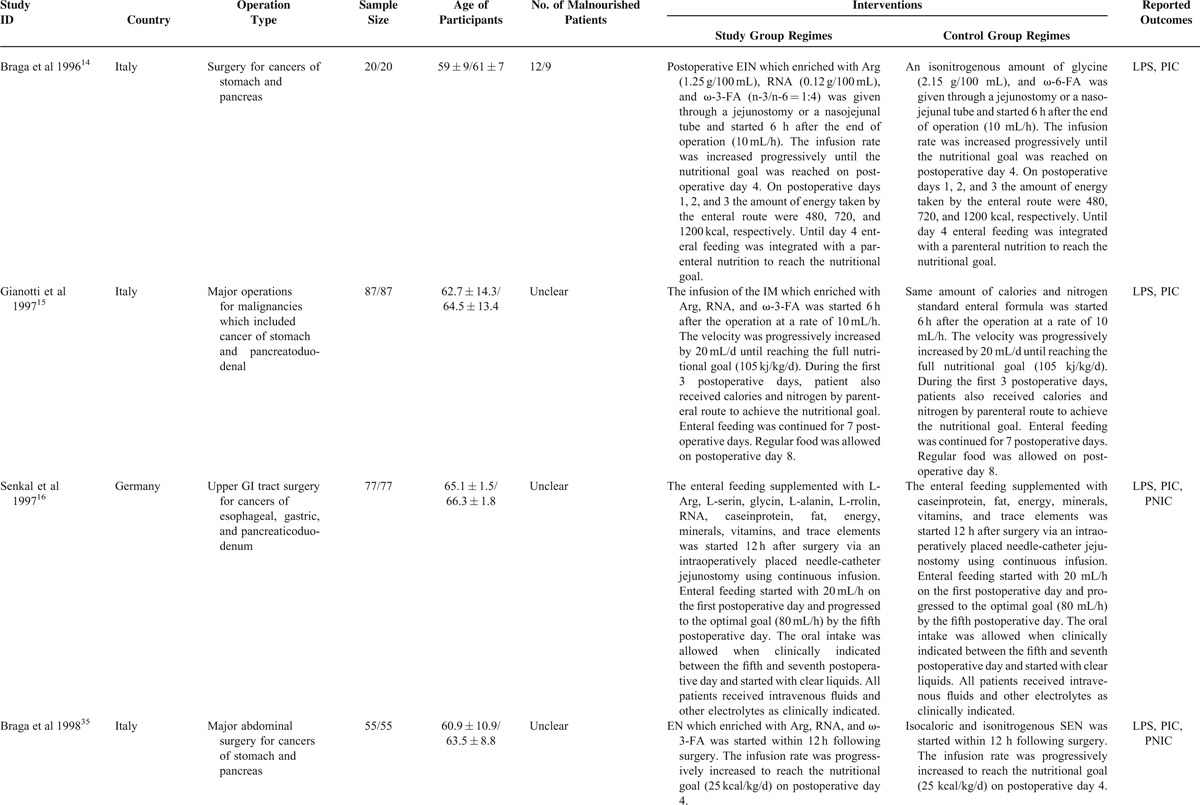
TABLE 2.
Network Meta-Analysis of Direct and Indirect Evidence Comparisons of EIN and SEN for GI Cancer
Fifteen eligible studies,5,12–16,23,35–41,44 which included 1524 participants, directly compared the effects of postoperative EIN related to standard EN in terms of postoperative infectious complications. The homogenous test did not identify the statistical heterogeneity (χ2 = 15.68, P = 0.33, I2 = 11%). So, we selected a fixed-effect model based on M-H framework to calculate the estimate. The meta-analysis indicated a significant difference, and the postoperative EIN was better than standard EN (RR, 0.63; 95% CI, 0.52–0.76) (Figure 6B). Bayesian NMA also indicated significant difference (RR, 0.55; 95% CI, 0.40–0.74) (Table 2).
Six trials,10,17,18,21,22,42 which included 380 and 378 patients, between perioperative EIN and standard EN groups reported the incidence of postoperative infectious complications. No statistical heterogeneity was detected by using homogeneous test (χ2 = 0.94, P = 0.97, I2 = 0%). Then a fixed-effect model was adopted to perform the meta-analysis. The pooled result suggested that perioperative EIN was superior to standard EN concerning effects of decreased incidence of postoperative infectious complications (RR, 0.46; 95% CI, 0.34–0.62) (Figure 6C). The result was maintained by the Bayesian NMA (RR, 0.36; 95% CI, −0.23 to 0.55) (Table 2).
The incidence of postoperative infectious complications was presented in 3 eligible studies,21,22,42 which assessed the comparative effects of preoperative versus perioperative EIN. In total, 202 and 201 patients were randomly received preoperative and perioperative EIN diet, respectively. No statistical heterogeneity was identified (χ2 = 1.28, P = 0.53, I2 = 0%), so a fixed-effect model was used to carry out the pair-wise meta-analysis. The meta-analysis indicated no significant difference (RR, 1.11; 95% CI, 0.68–1.79) (Figure 6D), and the Bayesian NMA confirmed the result (Table 2).
Only one4 involving 29 patients investigated the comparative effects of postoperative EIN compared with perioperative EIN, and the result indicated that perioperative period decreased the incidence of postoperative infectious complications compared with postoperative period method (RR, 0.21; 95% CI, 0.06–0.81) (Figure 6E). However, the result from Bayesian NMA did not indicate significant difference when the perioperative EIN compared with postoperative EIN (RR, 0.65; 95% CI, 0.39–1.12) (Table 2).
No study which directly compared the effects in decreasing postoperative infectious complications between preoperative and postoperative method was identified. However, we adopted a Bayesian NMA to evaluate the comparative effects of preoperative compared with postoperative EIN in terms of given outcome. The indirect evidence indicated no significant difference (RR, 0.76; 95% CI, 0.43–1.26) (Table 2).
Furthermore, we estimated the SCURA probabilities of different treatments for the postoperative infectious complications. The corresponding values were 72.50%, 39.57%, 87.93%, and 0.001% for preoperative, postoperative, perioperative EIN, and standard EN, respectively. The ranking of 4 treatments in terms of probability of postoperative infectious complications was shown in Figure 7A.
FIGURE 7.
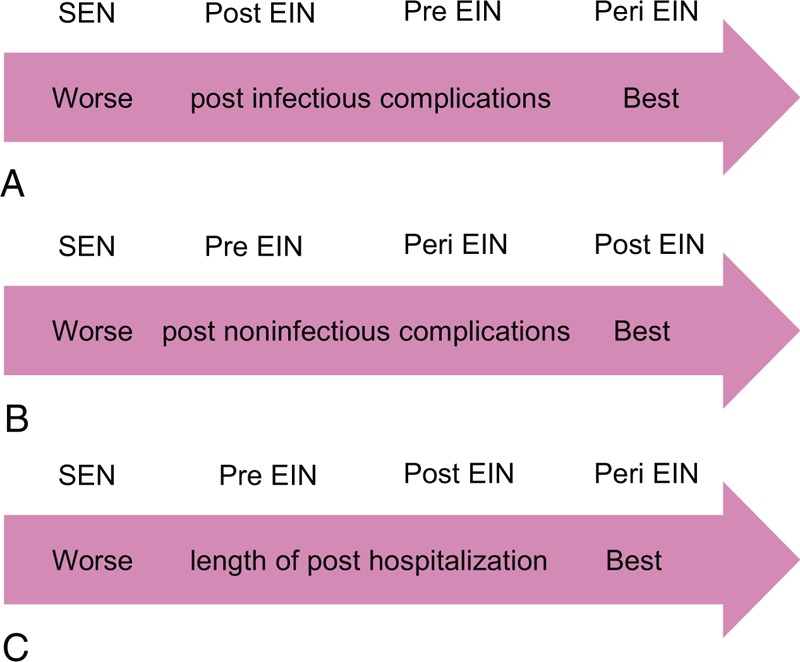
Ranking of treatments: (A) ranking of treatments in terms of postoperative infectious complications; (B) ranking of treatments in terms of postoperative noninfectious complications; (C) ranking of treatments in terms of length of postoperative hospitalization. Pre = preoperative, Post = postoperative, Peri = perioperative, EIN = enteral immunonutrition, SEN = standard enteral nutrition.
Postoperative Noninfectious Complications
Seven eligible studies,11,19–22,42,43 which included 620 patients, reported the direct effects which EIN for the decreased the incidence of postoperative noninfectious complications relative to standard EN. All studies were considered to be homogenous (χ2 = 4.06, P = 0.67, I2 = 0%). Then, we used a fixed-effect model to calculate the estimate. The meta-analysis did not indicate significant difference (RR, 0.88; 95% CI, 0.67–1.16) (Figure 8A). Meanwhile, we performed a Bayesian NMA to estimate corresponding pooled result, and it generated similar results (RR, 0.80; 95% CI, 0.48–1.33) (Table 2).
FIGURE 8.
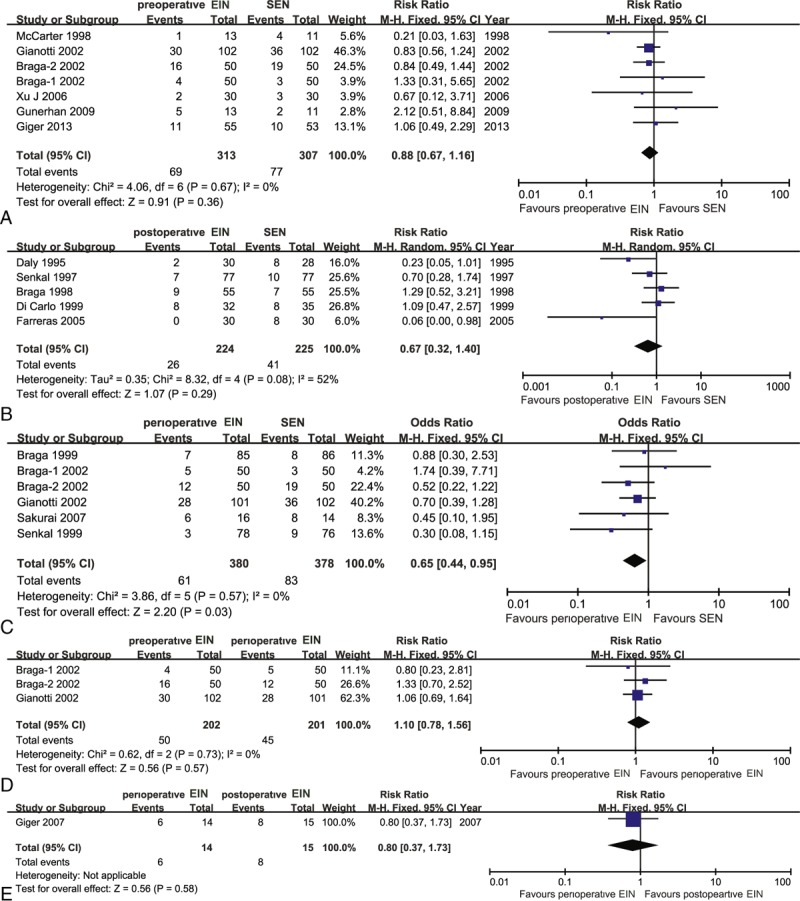
Traditional pair-wise meta-analysis on postoperative noninfectious complications: (A) preoperative EIN versus SEN; (B) postoperative EIN versus SEN; (C) perioperative EIN versus SEN; (D) preoperative EIN versus perioperative EIN; (E) perioperative EIN versus postoperative EIN. EIN = enteral immunonutrition.
We identified 5 eligible trials,13,16,35–37 which included 449 patients, to investigate the comparative effects of decreased postoperative noninfectious complications of postoperative EIN versus standard EN. The homogeneous test detected substantial statistical heterogeneity (χ2 = 8.32, P = 0.08, I2 = 52%), so a random-effect model based on inverse variance was used to calculate the estimate. The meta-analysis indicated no significant difference (RR, 0.67; 95% CI, 0.32–1.40) (Figure 8B). The reliability and robust of this result was enhanced by Bayesian NMA (RR, 0.59; 95% CI, 0.31–1.07) (Table 2).
Six eligible trials10,17,18,21,22,42 involving 758 participants evaluated the effects of direct comparison of perioperative EIN related to standard EN. No statistical heterogeneity was tested (χ2 = 3.86, P = 0.57, I2 = 0%), and then a fixed-effect model was used to perform this meta-analysis. The pair-wise meta-analysis revealed that perioperative immunonutrition method was better than the standard EN in decreasing incidence of postoperative noninfectious complications (RR, 0.65; 95% CI, 0.44–0.95) (Figure 8C). A similar trend was obtained by Bayesian analysis, where the difference between the perioperative immunonutrition method and standard EN groups almost reached significance (RR, 0.62; 95% CI, 0.37–1.00) (Table 2).
In addition, three21,22,42 and one4 reported the results of preoperative EIN versus perioperative and postoperative EIN relative to perioperative EIN method in terms of postoperative noninfectious complications, respectively. All pooled results did not reach significance (Figure 8D and E). The similar summary results were generated from corresponding Bayesian NMA (Table 2).
Study which directly established the comparative effects of preoperative versus postoperative EIN in terms of the incidence of postoperative noninfectious complications was not identified. However, we adopted a Bayesian NMA to evaluate the comparative effects of postoperative EIN compared with preoperative EIN in terms of given outcome. The indirect evidence indicated no significant difference (RR, 0.75; 95% CI, 0.33–1.59) (Table 2).
To determine the best treatment method, we calculated SUCRA probability of 4 interventions for the incidence of postoperative noninfectious complications. The SUCRA probabilities were 40.06%, 76.96%, 74.33%, and 8.09% for the preoperative, postoperative, perioperative EIN, and standard EN, respectively. The ranking of the 4 treatments for the postoperative noninfectious complications was shown in Figure 7B.
Length of Postoperative Hospitalization
Seven eligible studies,11,19–22,42,43 which included 620 patients, reported the results of preoperative EIN directly compared with standard EN in shortening the length of postoperative hospitalization. Statistical heterogeneity was detected (χ2 = 33.49, P < 0.00, I2 = 82%). So, a fixed-effect model was used to calculate the estimate. The meta-analysis indicated no significant difference (MD, −0.94; 95% CI, −1.53 to 2.33) (Figure 9A). Meanwhile, we performed a Bayesian NMA to estimate corresponding pooled result, and it generated similar result when compared standard EN with preoperative EIN (MD, 0.29; 95% CI, −0.32 to 0.89) (Table 2).
FIGURE 9.
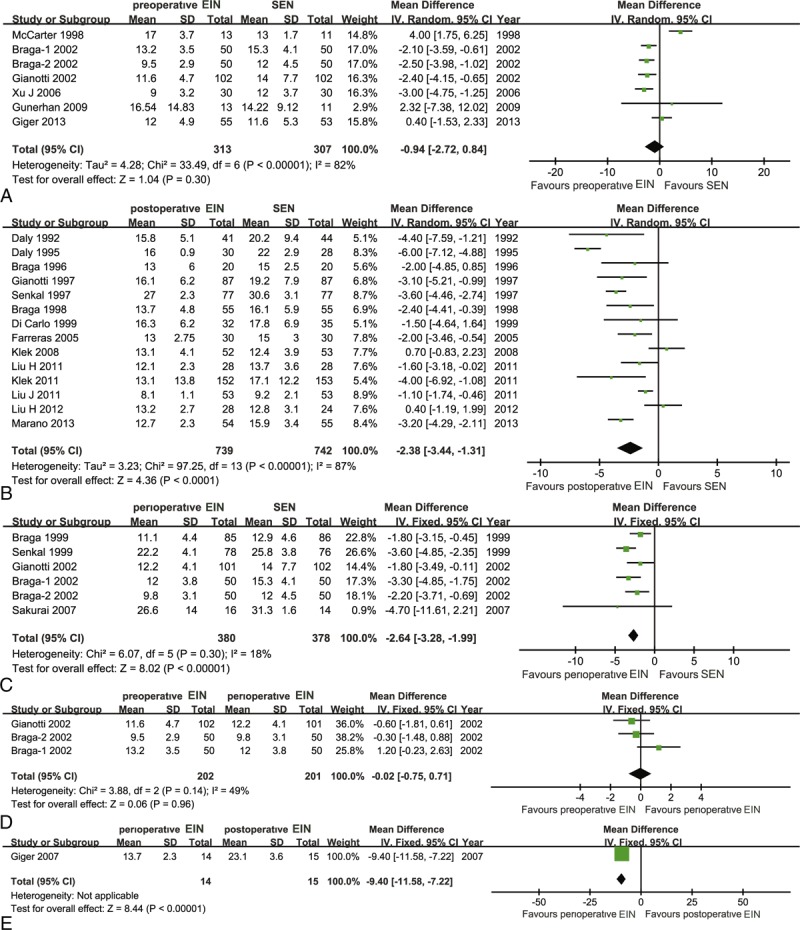
Traditional pair-wise meta-analysis on length of postoperative hospitalization: (A) preoperative EIN versus SEN; (b) postoperative EIN versus SEN; (C) perioperative EIN versus SEN; (D) preoperative EIN versus perioperative EIN; (E) perioperative EIN versus postoperative EIN. EIN = enteral immunonutrition.
Fifteen eligible studies,5,12–16,23,35–39,41,44 which included 1481 participants, directly compared the effects of postoperative EIN related to standard EN in terms of length of postoperative hospitalization. The homogenous test identified the substantial statistical heterogeneity (χ2 = 97.25, P < 0.00, I2 = 87%). Then, we used a fixed-effect model within M-H framework to calculate the estimate. The meta-analysis indicated a significant difference and the postoperative EIN was better than standard EN (MD, −2.38; 95% CI, −3.44 to 1.31) (Figure 9B). Bayesian NMA also indicated significant difference between standard EN and postoperative EIN in terms of length of postoperative hospitalization (MD, 0.48; 95% CI, 0.06–0.91) (Table 2).
Six eligible trials10,17,18,21,22,42 involving 758 participants evaluated the effects of direct comparison of perioperative EIN related to standard EN. No statistical heterogeneity was tested (χ2 = 6.07, P = 0.30, I2 = 18%). Then, we used a fixed-effect model to estimate the summary result. The pair-wise meta-analysis revealed that perioperative immunonutrition method was better than standard EN in shortening the length of postoperative hospitalization (MD, −2.64; 95% CI, −3.28 to 1.99) (Figure 9C). However, an opposite trend was obtained from Bayesian analysis, in which the difference between the standard EN and perioperative immunonutrition method reached significance (MD, 0.84; 95% CI, 0.25–1.45) (Table 2).
In addition, three21,22,42 and one4 reported the results of preoperative EIN versus perioperative and postoperative EIN relative to perioperative immunonutrition method in terms of length of postoperative hospital stay, respectively. For preoperative versus perioperative comparison, no statistical heterogeneity was detected (χ2 = 3.88, P = 0.14, I2 = 49%), so a fixed-effect model was adopted to perform this pair-wise meta-analysis. The pooled result did not indicate significant difference (MD, −0.02; 95% CI, −0.75 to 0.71) (Figure 9D), and the Bayesian NMA obtained similar result (MD, 0.56; 95% CI, −0.17 to 1.30). For postoperative versus perioperative comparison, one suggested that perioperative immunonutrition method effectively shortened the length of postoperative hospital stay relative to postoperative (MD, −9.40; 95% CI, −11.58 to −7.22) (Figure 9E). The similar summary results were generated from corresponding Bayesian NMA (MD, 0.36; 95% CI, −0.34 to 1.07) (Table 2).
No study directly compared the effects of preoperative EIN on postoperative length of hospitalization compared to postoperative EIN approach. Hence, a Bayesian NMA was performed to establish the comparative effects of preoperative EIN compared with postoperative EIN in terms of this measure of interest. The indirect evidence indicated no significant difference (MD, 0.20; 95% CI, −0.53 to 0.93) (Table 2).
We calculated SUCRA probability of 4 interventions for the length of postoperative hospitalization to quantitatively rank these treatments. The SUCRA probability was 39.73%, 61.48%, 92.53%, and 6.25% for the preoperative, postoperative, perioperative EIN, and standard EN, respectively. The ranking of the 4 treatments for the length of postoperative hospital stay was shown in Figure 7C.
DISCUSSION
EIN which enriched with some interested immunonutrients (Arg, ω-3-FA, Glu, and RNA) has been recommended to manage the postoperative clinical status since 1990.45 However, various different EIN support methods confused the clinical decision. The optimum option for patient who underwent surgery for GI malignancy remains a topic of debate. Several published RCTs investigated the comparative effects of immunonutrition versus standard EN,10,12–17 and these results consistently suggested that EIN plays a key role in managing postoperative clinical status of patients undergoing surgery for GI cancer. The timing of EIN support method involved preoperative, postoperative, and perioperative periods. Several SRs and meta-analyses which evaluated the comparative effects of EIN versus standard EN have been published.8,25,26,46–48 In addition, of these six SRs, two systematically assessed the direct evidences between preoperative or postoperative versus perioperative EIN methods.8,26 However, it is noted that these SRs and meta-analyses were performed by using traditional pair-wise meta-analysis method which cannot compare >2 treatments concerning certain topic simultaneously. Selecting optimum treatment to guide the clinical practice is a dynamic source, which drives the development of medical science. NMA within Bayesian framework can solve these problems, which cannot direct traditional comparison meta-analysis. Unfortunately, no NMA, which evaluated the comparative effects of different EIN support methods and ranked these methods, was published.
As far as we know, this is the first Bayesian NMA that established the relative effects of various different EIN methods for patients who underwent the surgery for GI cancer. We have incorporated 27 eligible trials into this study. To clearly present the relationship of treatments, we have plotted the evidence networks for each outcome of interest, and 3 included trials21,22,42 directly compared preoperative with perioperative EIN methods, as well as only one4 directly compared postoperative with perioperative EIN methods. However, no study, which directly compared preoperative with postoperative EIN methods, was captured.
Traditional pair-wise meta-analyses well demonstrated that EIN method, including preoperative, postoperative, and perioperative periods, effectively reduced the incidences of postoperative infectious. For the postoperative noninfectious complications, only perioperative EIN method effectively decreased associated incidence compared with standard EN. In terms of length of postoperative hospitalization, postoperative and perioperative EIN method are superior to standard EN. Moreover, it indicated that there was no significant difference among 3 methods of EIN support in terms of postoperative infectious and noninfectious complications and length of postoperative hospitalization. We have also performed Bayesian NMA to further assess corresponding comparative effects of different treatments. These pooled results which generated from NMA are similar to that of traditional meta-analyses.
It is noted that these results of our meta-analysis are in accordance with previous reports, in which EIN could enhance host immunity and reduce inflammatory response by modulated immune mediators, biochemical indicator, and inflammatory mediators. For example, a plenty of studies published previously revealed that early postoperative supplementation with EIN suppressed the expression of prostaglandin-2, interlukin-6, and tumor necrosis factor-α.13–15,49,50 When compared with postoperative EIN method, preoperative EIN regime significantly increased the level of transferring.20
In order to provide clinical practitioners with the optimum treatment, we also quantitatively ranked all alternatives according to the SUCRA probabilities. The results manifested that perioperative was better than preoperative and postoperative EIN regimes in terms of postoperative infectious complications (Figure 7A). For postoperative noninfectious complications, postoperative was superior to perioperative and preoperative regimes (Figure 7B). In terms of length of postoperative hospital stay, perioperative was better than postoperative and preoperative immunonutrition regimes (Figure 7C). And thus, we recommended preferentially perioperative immunonutrition regime as the nutrition support option for patients who underwent surgery for GI cancer.
We performed a comprehensive search strategy of literature so that this NMA can generate more accurate estimates of effects, whereas some limitations existed in this study which need to be acknowledged. First, the nutrition status of participants who enrolled into these eligible original trials varies from across studies. Second, conference abstract was ineligible for selection criteria of this study, and it may cause incomplete retrieval of literature. Third, the comparison-adjusted funnel plots were drawn and these graphs indicated small study effects. Last but not least, most of the results generated from NMA are in accordance with that of traditional pair-wise meta-analyses, but there were significant inconsistency existed in the loop which was consisted of standard EN, postoperative EIN, and preoperative EIN for postoperative infectious complications and one which was made of standard EN, preoperative EIN, and postoperative EIN.
CONCLUSION
We concluded that EIN support method is superior to standard EN, and the perioperative EIN regime is the optimum treatment option for patients who underwent surgery for GI cancer because of low incidence of postoperative infectious and noninfectious complications and shorter length of postoperative hospital stay.
Acknowledgments
The authors appreciate the work of editors and anonymous reviewers.
Footnotes
Abbreviations: ω-3-FA = omega-3-fatty acids, Arg = arginine, EIN = enteral immunonutrition, EN = enteral nutrition, GI = gastrointestinal, Glu = glutamine, MCMC = Markov chain Monte Carlo, MeSH = medical subject heading, NMA = network meta-analysis, RCTs = randomized controlled trials, RNA = ribonucleic acid, SCURA = surface under the cumulative ranking curve, SR = systematic review.
G-MS, XT, LZ, and Y-XO have contributed equally to this work as first author.
G-MS, XT, LZ, and Y-XO conceived the study. G-MS, XT, TS, and ZZ collected the data. G-MS, XT, LZ, and Y-XO performed statistical analyses. L-JY, J-GZ, and H-LY assessed the quality of eligible studies. G-MS, XT, and LZ drafted the manuscript. XT and Y-XO improved the language. All authors read and approved the final manuscript.
The authors have no funding and conflicts of interest to disclose.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Ardizzoni A, Tiseo M, Boni L. Validation of standard definition of sensitive versus refractory relapsed small cell lung cancer: a pooled analysis of topotecan second-line trials. Eur J Cancer 2014; 50:2211–2218. [DOI] [PubMed] [Google Scholar]
- 2.Fujitani K, Tsujinaka T, Fujita J, et al. Prospective randomized trial of preoperative enteral immunonutrition followed by elective total gastrectomy for gastric cancer. Br J Surg 2012; 99:621–629. [DOI] [PubMed] [Google Scholar]
- 3.Ates E, Yilmaz S, Erkasap S, et al. Perioperative immunonutrition ameliorates the postoperative immune depression in patients with gastrointestinal system cancer (prospective clinical study in 42 patients). Acta Gastroenterolo Belg 2004; 67:250–254. [PubMed] [Google Scholar]
- 4.Giger U, Buchler M, Farhadi J, et al. Preoperative immunonutrition suppresses perioperative inflammatory response in patients with major abdominal surgery—a randomized controlled pilot study. Ann Surg Oncol 2007; 14:2798–2806. [DOI] [PubMed] [Google Scholar]
- 5.Marano L, Porfidia R, Pezzella M, et al. Clinical and immunological impact of early postoperative enteral immunonutrition after total gastrectomy in gastric cancer patients: a prospective randomized study. Ann Surg Oncol 2013; 20:3912–3918. [DOI] [PubMed] [Google Scholar]
- 6.Tang R, Chen HH, Wang YL, et al. Risk factors for surgical site infection after elective resection of the colon and rectum: a single-center prospective study of 2,809 consecutive patients. Ann Surg 2001; 234:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tartter PI, Martinelli G, Steinberg B, et al. Changes in peripheral T-cell subsets and natural-killer cytotoxicity in relation to colorectal cancer surgery. Cancer Detect Prev 1986; 9:359–364. [PubMed] [Google Scholar]
- 8.Zheng Y, Li F, Qi B, et al. Application of perioperative immunonutrition for gastrointestinal surgery: a meta-analysis of randomized controlled trials. Asia Pac J Clin Nutr 2007; 16 suppl 1:253–257. [PubMed] [Google Scholar]
- 9.Rosa F, Bossola M, Pacelli F, et al. Malnutrition and postoperative complications in abdominal surgery. Ann Surg 2011; 254:666–667. [DOI] [PubMed] [Google Scholar]
- 10.Senkal M, Zumtobel V, Bauer KH, et al. Outcome and cost-effectiveness of perioperative enteral immunonutrition in patients undergoing elective upper gastrointestinal tract surgery: a prospective randomized study. Arch Surg 1999; 134:1309–1316. [DOI] [PubMed] [Google Scholar]
- 11.Giger-Pabst U, Lange J, Maurer C, et al. Short-term preoperative supplementation of an immunoenriched diet does not improve clinical outcome in well-nourished patients undergoing abdominal cancer surgery. Nutrition 2013; 29:724–729. [DOI] [PubMed] [Google Scholar]
- 12.Daly JM, Lieberman MD, Goldfine J, et al. Enteral nutrition with supplemental arginine, RNA, and omega-3 fatty acids in patients after operation: immunologic, metabolic, and clinical outcome awaiting upper gastrointestinal malignancies. Surgery 1992; 112:56–67. [PubMed] [Google Scholar]
- 13.Daly JM, Weintraub FN, Shou J, et al. Enteral nutrition during multimodality therapy in upper gastrointestinal cancer patients recieve or not enteral jejunostomy feedings. Ann Surg 1995; 221:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braga M, Vignali A, Gianotti L, et al. Immune and nutritional effects of early enteral nutrition after major abdominal operations. Eur J Surg 1996; 162:105–112. [PubMed] [Google Scholar]
- 15.Gianotti L, Braga M, Vignali A, et al. Effect of route of delivery and formulation of postoperative nutritional support in patients undergoing major operations for malignant neoplasms. Arch Surg 1997; 132:1222–1229. [DOI] [PubMed] [Google Scholar]
- 16.Senkal M, Mumme A, Eickhoff U, et al. Early postoperative enteral immunonutrition: clinical outcome and cost- comparison analysis in surgical patients. Crit Care Med 1997; 25:1489–1496. [DOI] [PubMed] [Google Scholar]
- 17.Braga M, Gianotti L, Radaelli G, et al. Perioperative immunonutrition in patients undergoing cancer surgery: results of a randomized double-blind phase 3 trial. Arch Surg 1999; 134:428–433. [DOI] [PubMed] [Google Scholar]
- 18.Sakurai Y, Masui T, Yoshida I, et al. Randomized clinical trial of the effects of perioperative use of immune-enhancing enteral formula on metabolic and immunological status in patients undergoing esophagectomy. World J Surg 2007; 31:2150–2157. [DOI] [PubMed] [Google Scholar]
- 19.Gunerhan Y, Koksal N, Sahin UY, et al. Effect of preoperative immunonutrition and other nutrition models on cellular immune parameters. World J Gastroenterol 2009; 15:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu J, Zhong Y, Jing D, et al. Preoperative enteral immunonutrition improves postoperative outcome in patients with gastrointestinal cancer. World J Surg 2006; 30:1284–1289. [DOI] [PubMed] [Google Scholar]
- 21.Gianotti L, Braga M, Nespoli L, et al. A randomized controlled trial of preoperative oral supplementation with a specialized diet in patients with gastrointestinal cancer. Gastroenterology 2002; 122:1763–1770. [DOI] [PubMed] [Google Scholar]
- 22.Braga M, Gianotti L, Vignali A, et al. Preoperative oral arginine and n-3 fatty acid supplementation improves the immunometabolic host response and outcome after colorectal resection for cancer. Surgery 2002; 132:805–814. [DOI] [PubMed] [Google Scholar]
- 23.Klek S, Sierzega M, Szybinski P, et al. The immunomodulating enteral nutrition in malnourished surgical patients—a prospective, randomized, double-blind clinical trial. Clin Nutr 2011; 30:282–288. [DOI] [PubMed] [Google Scholar]
- 24.Pradelli L, Mayer K, Muscaritoli M, et al. N-3 fatty acid-enriched parenteral nutrition regimens in elective surgical and ICU patients: a meta-analysis. Crit Care 2012; 16:R184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cao W, Zhang CL. Enteral immunonutrition for malignant gastrointestinal tumor during perioperative period: A meta-analysis. Chin J Evid-based Med 2013; 13:992–1000. [Google Scholar]
- 26.Zhang Y, Gu Y, Guo T, et al. Perioperative immunonutrition for gastrointestinal cancer: a systematic review of randomized controlled trials. Surg Oncol 2012; 21:e87–e95. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015; 4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiley Online Library, Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Vol. 5. 2008. [Google Scholar]
- 29.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8:336–341. [DOI] [PubMed] [Google Scholar]
- 30.Bowden J, Tierney JF, Copas AJ, et al. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol 2011; 11:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 32.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004; 23:3105–3124. [DOI] [PubMed] [Google Scholar]
- 33.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011; 64:163–171. [DOI] [PubMed] [Google Scholar]
- 34.Chaimani A, Higgins JP, Mavridis D, et al. Graphical tools for network meta-analysis in STATA. PLoS One 2013; 8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braga M, Gianotti L, Vignali A, et al. Artificial nutrition after major abdominal surgery: impact of route of administration and composition of the diet. Crit Care Med 1998; 26:24–30. [DOI] [PubMed] [Google Scholar]
- 36.Di Carlo V, Gianotti L, Balzano G, et al. Complications of pancreatic surgery and the role of perioperative nutrition. Dig Surg 1999; 16:320–326. [DOI] [PubMed] [Google Scholar]
- 37.Farreras N, Artigas V, Cardona D, et al. Effect of early postoperative enteral immunonutrition on wound healing in patients undergoing surgery for gastric cancer. Clin Nutr 2005; 24:55–65. [DOI] [PubMed] [Google Scholar]
- 38.Klek S, Kulig J, Sierzega M, et al. The impact of immunostimulating nutrition on infectious complications after upper gastrointestinal surgery: a prospective, randomized, clinical trial. Ann Surg 2008; 248:212–220. [DOI] [PubMed] [Google Scholar]
- 39.Liu H, Ling W, Cao H. Effects of immune-enhanced enteral nutrition and parenteral nutrition on immune and nutritional function in elderly patients with gastric cancer after total gastrectomy. Journal of Shanghai Jiaotong University (Medical Science) 2011; 31:1000–1004. [Google Scholar]
- 40.Liu Z, Yu J. Effect of immune enhanced enteral nutrition on postoperative immune function and inflammatory responses in gastric cancer patients with radical gastrectomy. Chinese J Cancer Prev Treat 2011; 18: 66-67+69. [Google Scholar]
- 41.Liu H, Ling W, Shen ZY, et al. Clinical application of immune-enhanced enteral nutrition in patients with advanced gastric cancer after total gastrectomy. J Dig Dis 2012; 13:401–406. [DOI] [PubMed] [Google Scholar]
- 42.Braga M, Gianotti L, Nespoli L, et al. Nutritional approach in malnourished surgical patients. A prospective randomized study. Arch Surg 2002; 137:174–180. [DOI] [PubMed] [Google Scholar]
- 43.McCarter MD, Gentilini OD, Gomez ME, et al. Preoperative oral supplement with immunonutrients in cancer patients. JPEN J Parenter Enteral Nutr 1998; 22:206–211. [DOI] [PubMed] [Google Scholar]
- 44.Liu JZ, Lan T, Zhang JS, et al. Use of postoperative enteral immunonutrition in malnutrition patients with gastrointestinal malignant tumor. Zhonghua Wei Chang Wai Ke Za Zhi 2011; 14:799–802. [PubMed] [Google Scholar]
- 45.Walker WA. Current opinion in gastroenterology. Curr Opin Gastroenterol 2012; 28:547–550. [DOI] [PubMed] [Google Scholar]
- 46.Javed MS, Taylor I. A review of recent randomized trials in colorectal disease. Colorectal Dis 2002; 4:90–96. [DOI] [PubMed] [Google Scholar]
- 47.Mabvuure NT, Roman A, Khan OA. Enteral immunonutrition versus standard enteral nutrition for patients undergoing oesophagogastric resection for cancer. Int J Surg 2013; 11:122–127. [DOI] [PubMed] [Google Scholar]
- 48.Osland E, Hossain MB, Khan S, et al. Effect of timing of pharmaconutrition (immunonutrition) administration on outcomes of elective surgery for gastrointestinal malignancies: a systematic review and meta-analysis. JPEN J Parenter Enteral Nutr 2014; 38:53–69. [DOI] [PubMed] [Google Scholar]
- 49.Wu GH, Zhang YW, Wu ZH. Modulation of postoperative immune and inflammatory response by immune-enhancing enteral diet in gastrointestinal cancer patients. World J Gastroenterol 2001; 7:357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen DW, Fei ZW, Zhang YC, et al. Role of enteral immunonutrition in patients with gastric carcinoma undergoing major surgery. Asian J Surg 2005; 28:121–124. [DOI] [PubMed] [Google Scholar]


