Abstract
This article estimates the frequency of cardiovascular (CV) events that occurred after diagnosis in a large Spanish cohort of patients with systemic lupus erythematosus (SLE) and investigates the main risk factors for atherosclerosis.
RELESSER is a nationwide multicenter, hospital-based registry of SLE patients. This is a cross-sectional study. Demographic and clinical variables, the presence of traditional risk factors, and CV events were collected. A CV event was defined as a myocardial infarction, angina, stroke, and/or peripheral artery disease. Multiple logistic regression analysis was performed to investigate the possible risk factors for atherosclerosis.
From 2011 to 2012, 3658 SLE patients were enrolled. Of these, 374 (10.9%) patients suffered at least a CV event. In 269 (7.4%) patients, the CV events occurred after SLE diagnosis (86.2% women, median [interquartile range] age 54.9 years [43.2–66.1], and SLE duration of 212.0 months [120.8–289.0]). Strokes (5.7%) were the most frequent CV event, followed by ischemic heart disease (3.8%) and peripheral artery disease (2.2%). Multivariate analysis identified age (odds ratio [95% confidence interval], 1.03 [1.02–1.04]), hypertension (1.71 [1.20–2.44]), smoking (1.48 [1.06–2.07]), diabetes (2.2 [1.32–3.74]), dyslipidemia (2.18 [1.54–3.09]), neurolupus (2.42 [1.56–3.75]), valvulopathy (2.44 [1.34–4.26]), serositis (1.54 [1.09–2.18]), antiphospholipid antibodies (1.57 [1.13–2.17]), low complement (1.81 [1.12–2.93]), and azathioprine (1.47 [1.04–2.07]) as risk factors for CV events.
We have confirmed that SLE patients suffer a high prevalence of premature CV disease. Both traditional and nontraditional risk factors contribute to this higher prevalence. Although it needs to be verified with future studies, our study also shows—for the first time—an association between diabetes and CV events in SLE patients.
INTRODUCTION
The 10-year survival rate of systemic lupus erythematosus (SLE) patients has significantly improved during the last half century, standing at 92%.1 This improved prognosis stems from advances in the early recognition of milder cases and an improvement in general medical care (eg, antihypertensive, antibiotic, and immunosuppressive drugs, hemodialysis, transplantation, and others). However, long-term survival rates leveled off in the 1980s and since that time, 20-year survival ranges only 44% to 84%.2 Lupus patients have a risk of coronary artery disease that is 2 to 10 times that of the general population, with a greater increase in relative risk generally observed in younger patient groups.3 This reflects the fact that lupus patients have a higher risk of accelerated atherosclerosis, comparable to that of diabetes patients.4 Although it is unclear why patients with SLE are at a greater risk of accelerated atherosclerosis, both traditional and nontraditional risk factors certainly contribute to this.
Lupus patients have a higher burden of traditional risk factors compared with the general population.5 However, after controlling for traditional risk factors, individuals with SLE are at increased risk for cardiovascular disease (CVD).6,7 Measurements of the incidence and prevalence of cardiovascular (CV) events may fluctuate because of differences between studies (eg, sample size, different populations, design, or inadequate adjustment for potential confounding factors); a decreasing trend in the risk might even be occurring.6–9 This trend may be related to a better control of lupus activity and other SLE-associated risk factors for atherosclerosis such as glucocorticoids, oxidative stress, adipokines, and others.10
The risk of CV events in SLE is difficult to study given that despite the increased relative risk of CV events in this population, the absolute number of events per year in any given cohort is relatively small. Therefore, patient registries and multicentric/multiethnic cohorts are tools particularly useful for evaluating events that are relatively infrequent in chronic diseases.11–13
The aims of this study were to estimate the frequency of CV events in a large cohort of patients with SLE from Spain and investigate the main traditional and SLE-associated risk factors for atherosclerosis.
PATIENTS AND METHODS
RELESSER Registry
The RELESSER Registry is a nationwide multicenter, hospital-based registry designed by the Systemic Autoimmune Diseases Working Group of the Spanish Society of Rheumatology. The study has 2 parts: an initial cross-sectional phase (RELESSER-TRANS) and a further prospective cohort study (RELESSER-PROS) that is currently ongoing. The study included patients from 45 rheumatology university centers spread across Spain with substantial experience in the management of SLE. The Research Unit of the Spanish Society of Rheumatology was the coordinating center, providing expert methodological support at all stages of the project and carrying out study monitoring and inconsistency identification and resolution.
Written commitment was obtained from all investigators prior to participation. The study was approved by the Ethics Committee at Dr Negrín University Hospital, Gran Canaria, Spain, and then by the institutional research review boards at all participating centers. Patients signed their consent forms upon entry in RELESSER-PROS. The study was conducted in accordance with the Helsinki Declaration.
Study Design
RELESSER-TRANS is a cross-sectional, multicenter, nationwide study, and includes baseline cumulative data for every patient. A full description of the methodological issues has been published in detail elsewhere.14 Using data from RELESSER-TRANS, we assessed the prevalence of, and risk factors for, CV events among SLE patients.
Patients
Unselected consecutive adult patients with SLE, classified according to the American College of Rheumatology (ACR) 1997 criteria,15 were enrolled. All patients had been attended upon and followed at Spanish rheumatology departments. Patients were widely and homogeneously distributed across the country to avoid selection bias. The first patient was enrolled in October 2011 and the last in August 2012.
Data Collection
A specific protocol was designed to collect the study variables along with their operational definitions. The investigators were trained before beginning data collection from clinical reporting to avoiding information bias. A website for data entry was developed and implemented (RELESSER database website) to collect all information. After closing the databases, queries for missing or inconsistent data were sent to the investigators for additions and corrections. Ultimately, the percentage of missing data was <5% in 92% of the variables collected.
Definitions of Variables
Data recorded included demographic, clinical, laboratory, and treatment information. Clinical variables included comorbidities (including the Charlson comorbidity index),16 disease onset (time at which a patient met 4 ACR SLE criteria), disease duration (from diagnosis to the last visit or until death, if applicable), follow-up duration (from the first visit in the rheumatology center to the last visit or until death, if applicable), cumulative ACR criteria, Safety of Estrogens in Lupus Erythematosus National Assessment Systemic Lupus Erythematosus Disease Activity (SELENA-SLEDAI) descriptors17 and the British Isles Lupus Assessment Group (BILAG) 2004 activity18 descriptors, Sydney criteria for antiphospholipid syndrome,19 hospitalizations (and causes), current or past drug treatments and reasons for interrupting them, and nonpharmacological treatments and death (and causes). SELENA-SLEDAI,17 Systemic Lupus International Collaborating Clinics (SLICC)/ACR Damage Index (DI),20 and Katz severity index21 were all retrospectively measured at the last visit (or at death, if applicable).
Comorbidities by patient included, among others, smoking status, diabetes (previous diagnosis or ≥2 fasting serum glucose levels ≥126 mg/dL and/or antidiabetic drugs), dyslipidemia (total cholesterol ≥240 mg/dL and/or low-density lipoprotein >130 mg/dL and/or triglycerides ≥160 mg/dL and/or intake of lipid-lowering drugs), hypertension (systolic blood pressure ≥140 mm Hg and/or a diastolic blood pressure ≥90 mm Hg on ≥2 occasions and/or intake of antihypertensive drugs), peripheral artery disease, ischemic heart disease, heart failure (clinic diagnosis and/or on chest radiography), thromboembolic disease, and/or a cerebral vascular accident.
By consensus of the RELESSER Scientific Committee of RELESSER, refractory disease was defined by at least 1 of the following: failure to respond to cyclophosphamide; failure of ≥2 immunosuppressive agents other than cyclophosphamide (ie, methotrexate, azathioprine, mycophenolatemofetil/mycophenolic acid, or leflunomide); need for rituximab administration; or splenectomy.
Outcome Variable
A CV event (coronary and noncoronary) was defined as the presence of at least 1 of following: ischemic heart disease, including myocardial infarction and/or angina pectoris based on clinical diagnosis and/or ischemic changes in the electrocardiogram and/or specific changes in cardiac enzymes and/or typical findings in a coronary angiography; cerebral vascular accident based on a unequivocal previous diagnosis or on the presence of clinical manifestations and/or supported by an imaging procedure (ie, computed tomography angiography or magnetic resonance angiography); and peripheral artery disease supported on a well-established prior diagnosis or by the presence of clinical manifestations confirmed by an imaging procedure. In patients with multiple events, we counted up to 3 events/patient for myocardial infarction and for established cerebrovascular events. For the remaining events, we counted only the first that occurred since the SLE diagnosis was made. Patients with CV events that occurred before a diagnosis of SLE were excluded from the analyses.
Statistical Analysis
Data are presented as means (standard deviation [SD]), medians (interquartile range), or totals with percentages. Baseline characteristics were compared between groups using the χ2 test, the Student t test (Fisher exact test when necessary), or the Mann–Whitney U test.
Patient characteristics at onset of SLE, cumulative parameters (comorbidities, treatment with glucocorticoids, immunosuppressive drugs, and DI), and SLEDAI at last visit were taken into account as possible risk factors in a bivariate analysis. The Kruskal–Wallis test was used for comparing variables according to the location of the CV events (cerebrovascular, noncerebrovascular, or both).
Multiple logistic regression analysis with forward stepwise selection (Wald) was performed to study the association of possible risk factors with the presence of CV events that had occurred since SLE diagnosis. Factors included in the model were those that were statistically significant in the bivariate analysis. Multicollinearity of independent variables was checked using Pearson correlation coefficient. If the r-coefficient was >0.4, we entered the variables separately in the models, in order to see if any change either in associations with the dependent variable or in the explaining value of the model had occurred. Finally, age, sex, disease duration, hypertension, dyslipidemia, smoking, diabetes mellitus, serositis, renal disorder, neurological disorder, valvular dysfunction, Raynaud phenomenon, anti-double-stranded (dsDNA), low complement, antiphospholipid antibody positive, nonsteroidal anti-inflammatory drugs (NSAIDs), antimalarials, glucocorticoids, methotrexate, and azathioprine were entered in the logistic regression models to identify those factors predictive of CV events.
Two-tailed tests and a 5% significance level were used in all analyses. The analysis was performed with the statistical package SPSS 22.0 for MAC OS X (IBM Corp, Armonk, NY).
RESULTS
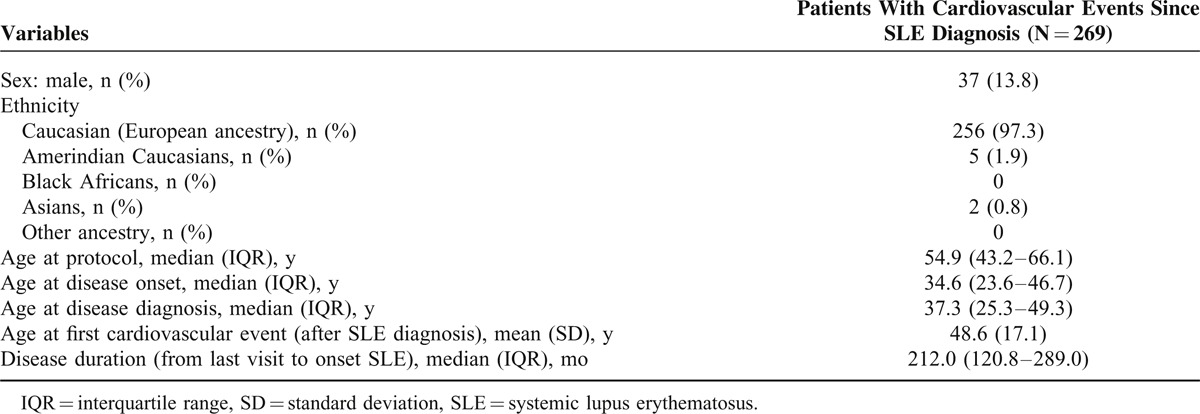
Of the 3658 eligible SLE patients enrolled in RELESSER-TRANS, 3649 (99.7%) had sufficient CV event information available. As it is shown in Table 1, most of the patients with CV events since their SLE diagnosis were White women in middle age with long-standing disease. When the percentage by decades was analyzed, we found that the frequency of immigrants from Hispanic America with CVD increased from 1996 to present day compared with the decades 1975 to 1985 and 1986 to 1995 (P < 0.001).
TABLE 1.
Demographic Characteristics of 269 Patients With Cardiovascular Events That Occurred After Diagnosis of SLE
CV Events
Three hundred seventy-four (10.9%) patients suffered 448 CV events during their lifetime. Of these, 73 patients had CV events prior to SLE diagnosis, 32 patients had missing data at the date of the event, and 269 patients (7.4% [95% confidence interval, CI, 6.6–8.3]) suffered 318 CV events after SLE diagnosis.
The average (SD) age at the first CV event since SLE diagnosis was 48.6 (17.1) years and the event occurred after a median disease duration of 10.4 years following diagnosis of SLE.
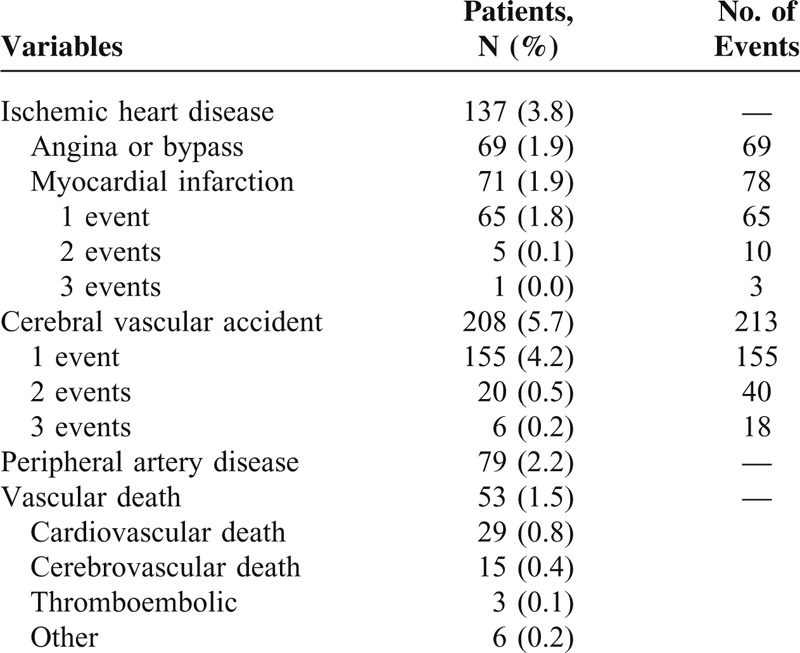
As Table 2 shows, strokes were the most frequent CV events after SLE diagnosis (5.7% [95% CI, 5.0–6.5]), followed by ischemic heart disease (3.8% [95% CI, 3.2–4.4]). One-and-a-half percent (5/318) of all events were fatal.
TABLE 2.
Frequencies of Clinical Cardiovascular Events in RELESSER-TRANS Patients That Occurred After Systemic Lupus Erythematosus Diagnosis
Factors Related to CV Events Since Diagnosis of SLE
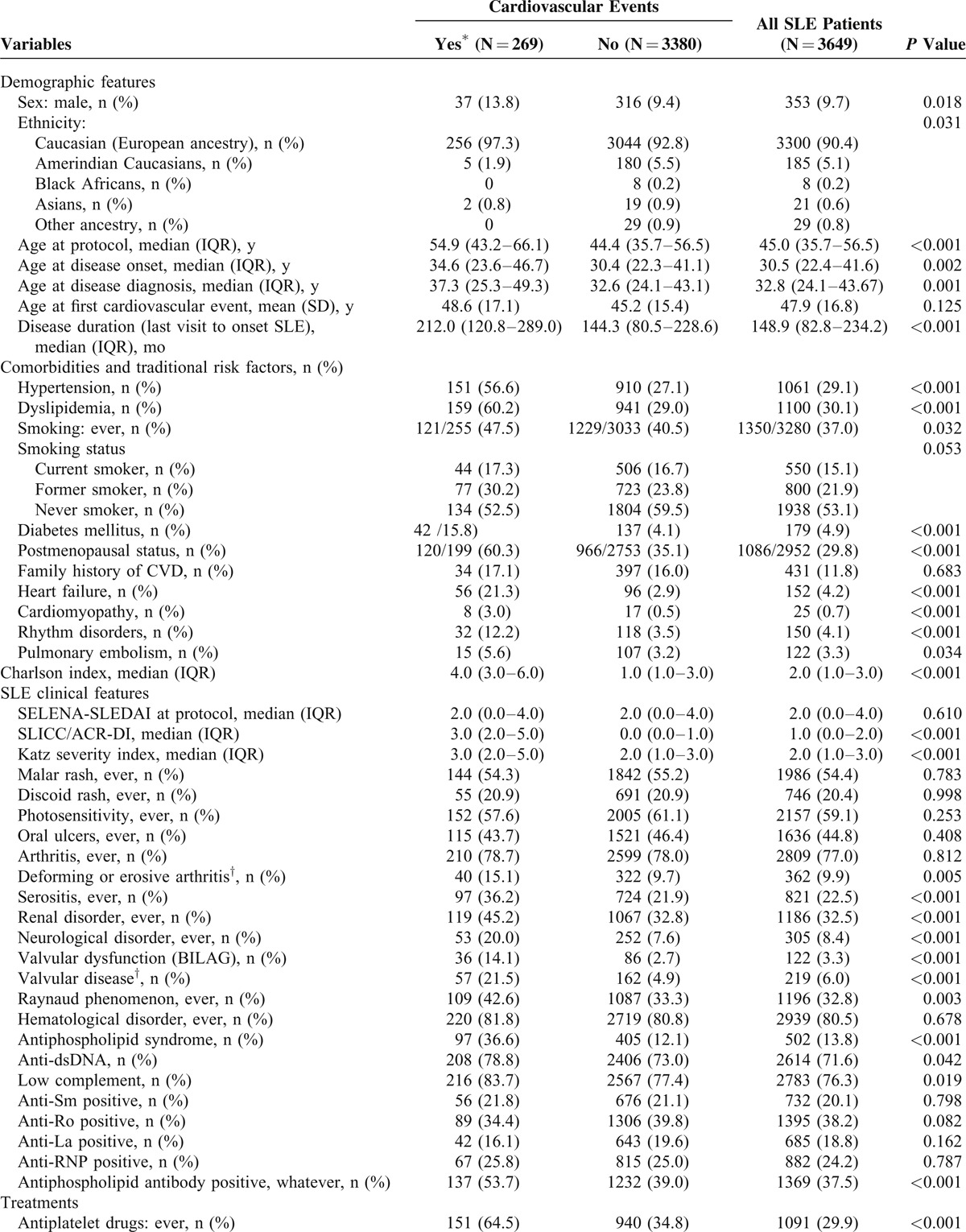
Table 3 shows the characteristics of 269 SLE patients who had CV events compared with all other patients.
TABLE 3.
Cardiovascular Events in RELESSER-TRANS Patients That Occurred Since SLE Diagnosis as a Function of Demographic, Clinical, and Treatment Characteristics
Demographic Factors
CV events were associated with male sex, older age at the last visit or at death, and longer disease duration in the univariate analysis (Table 3 ). The relatively high frequency of CV events noted in Caucasians was because of differences in age, follow-up time, and sex ratio.
The SLE patients who suffered only strokes were younger (46.2 [36.0, 60.7] vs 60.5 [52.0, 73.8] vs 59.5 [47.6, 63.3] years) and diagnosed earlier (30.3 [21.2, 43.4] vs 43.7 [31.5, 58.8] vs. 41.0 [24.9, 51.0] years) than those who suffered either noncerebrovascular events or vascular events in both areas (P < 0.001, for both comparisons).
Traditional CV Risk Factors
In the univariate analysis, an association was observed between CV events and any history of smoking, diabetes, hypertension, dyslipidemia, and postmenopausal status (Table 3 ).
SLE-Related Risk Factors
CV events were associated with certain SLE-related factors or their comorbidities. The relative frequency of CV events was higher in SLE patients with high SDI, Charlson index, and Katz index at the last evaluation. Similarly, SLE patients with either neuropsychiatric, renal, or serositis involvement, valvulopathy (defined by both BILAG and SLICC/ACR DI criteria), Raynaud phenomenon, and those with antiphospholipid syndrome had a higher frequency of CV event (Table 3 ). The presence of either anti-dsDNA, antiphospholipid antibodies (ie, lupus anticoagulant and/or anticardiolipin antibodies and/or anti-B2-glycoprotein antibodies according to the revised Sidney laboratory criteria) or low serum complement was associated with CV events as well.
By contrast, SELENA-SLEDAI index at the last visit, a history of mucocutaneous manifestations (ie, malar rash, discoid rash, photosensitivity, or oral ulcers), arthritis, hematologic manifestations, or sicca syndrome were not associated with CV events. The presence of either the positivity of anti-Sm, anti-Ro, anti-La, or anti-RNP antibodies was not associated with the occurrence of CV events.
The SLE patients who suffered only strokes had lower relative frequency of smoking (37.7% vs 52.7% vs 76.2%; P = 0.047), dyslipidemia (52.7% vs 66.7% vs 71.4%; P = 0.002), and diabetes mellitus (6.2% vs 24.3% vs 27.3%; P < 0.001) than those who had either noncerebrovascular events or vascular events in both the areas. The presence of valvular dysfunction was not associated with stroke.
Treatment-Related Risk Factors
The univariate analysis showed that the frequency of CV events was higher in patients treated with any dose of glucocorticoid in the past or at the last visit, a high dose of glucocorticoids at the last visit, immunosuppressives (at any time or at some stage/point), statins, antiplatelets, or oral anticoagulant drugs. The patients who were refractory to standard therapy were also at higher risk of CV events. However, treatment with antimalarial drugs or NSAIDs was associated with a lower frequency of CV events.
Multivariable Analyses
Table 4 shows the variables that were included in the multivariate models to determine which were independently associated with CV events since SLE diagnosis. As shown, there was a strong association between CV events and age, high blood pressure, smoking, dyslipidemia, diabetes mellitus, serositis, low complement, neuropsychiatric lupus, valvular dysfunction (SLICC/ACR DI definition), antiphospholipid antibodies, and azathioprine.
TABLE 3 (Continued).
Cardiovascular Events in RELESSER-TRANS Patients That Occurred Since SLE Diagnosis as a Function of Demographic, Clinical, and Treatment Characteristics
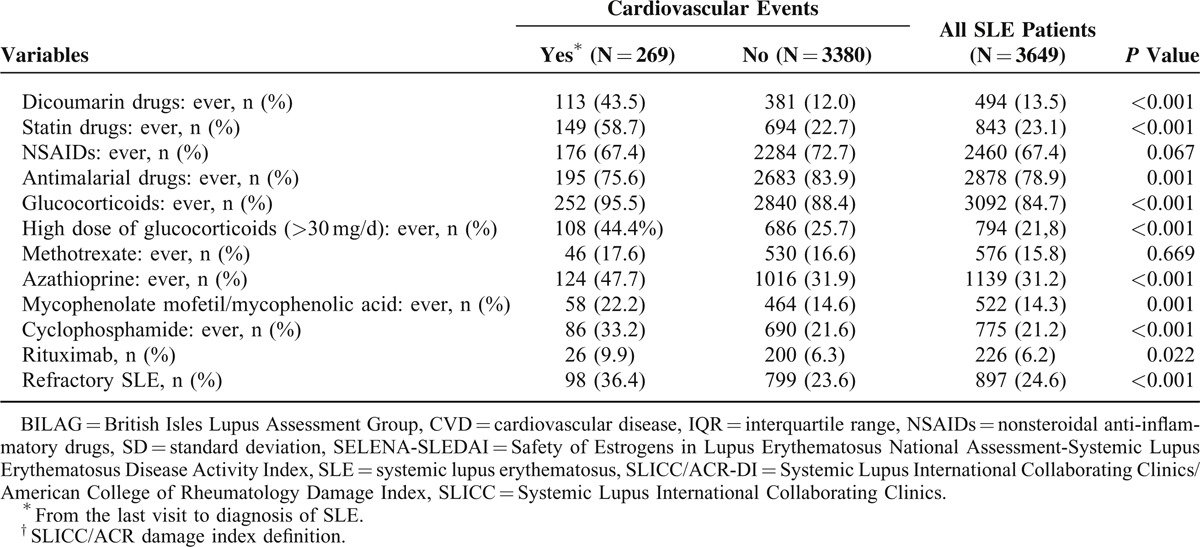
TABLE 4.
Predictor of Cardiovascular Events That Occurred Since Systemic Lupus Erythematosus Diagnosis in RELESSER Patients By Multiple Logistic Regression Analysis
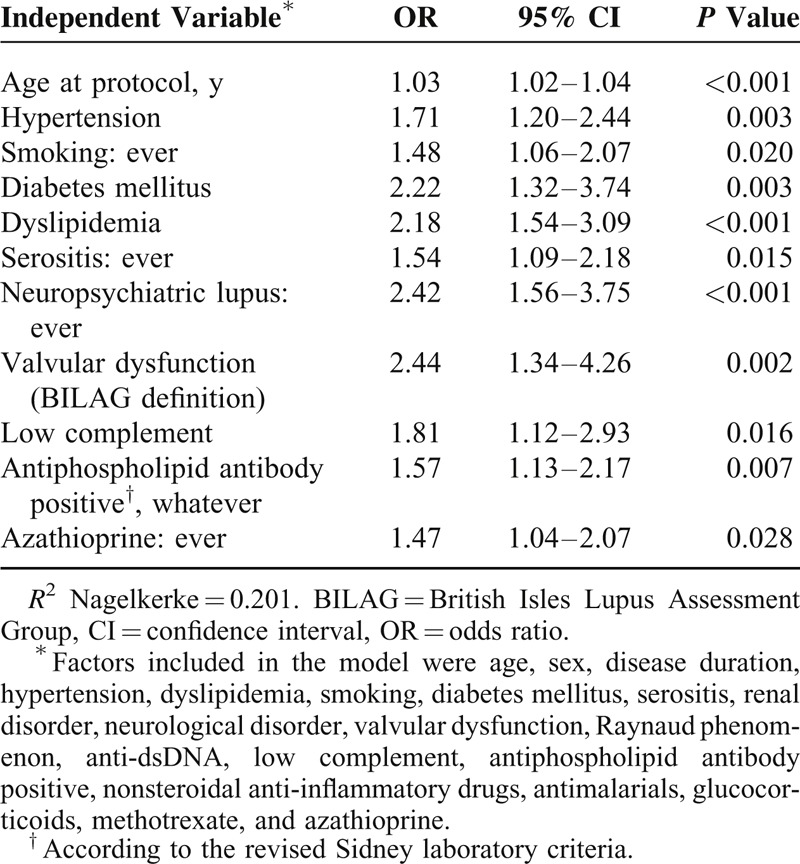
After adjusting the multivariate models, both antimalarial drugs and NSAIDs were not associated with lower frequencies of CV events. There was no association with sex, lupus nephritis, anti-dsDNA antibodies, glucocorticoids, or methotrexate.
DISCUSSION
We have studied the prevalence of CV events in a large nationwide cohort of patients with SLE from Spain. This cohort was mainly composed of patients of European ancestry. Although the proportion of Amerindian patients from Hispanic America has increased over the past 15 years, its possible influence in the cohort is still limited in comparison with the multiethnic cohorts.13,22,23
The overall prevalence of CV events in our cohort was 7.4% since SLE diagnosis. This frequency was similar to the LUMINA cohorts (7%)13 despite the fact that our patients were older and were followed-up for much longer periods. On the other hand, compared with other European4,24 and American6,7,23,25 cohorts, the frequencies of which ranged from 9.8% to 19.0%, the prevalence in our cohort was much lower. Although retrospective studies might underestimate the frequency of events, some of these differences are likely due to varying definitions of CV events and observation times, our prevalence nonetheless remained relatively low. This low frequency of CV events occurred even though our patients presented a high frequency of traditional risk factors, particularly diabetes mellitus and smoking. This phenomenon has previously been observed in a non-SLE Spanish general population.26 The “Spanish paradox” is a phenomenon observed also in some other Mediterranean countries by which the CV morbidity and mortality levels are dissociated from their CV risk factors.26 Like other groups, we have observed a higher frequency of cerebrovascular events compared with coronary or peripheral artery diseases.9,13,23,27
Although men had a higher CV risk than the women in the univariate analysis, this association was not confirmed in the multivariate models. This is due to the fact that CV risk factors promote atherosclerosis in both the sexes. Moreover, as has been mentioned earlier, our patients presented a high frequency of both diabetes mellitus and smoking, 2 CV risk factors that have a greater impact on women than men.28,29
Age contributes significantly to the development of atherosclerosis both in SLE patients and in the general population. Consistent with the previous reports,4,9,11,13,23,27 we found that the age was associated with CVD in the multivariate analysis. In fact, age increased the risk of CV events by 3% for each yearly increase in age. This figure might be somewhat higher than that observed in the general population (those between 40 and 50 years).30 Furthermore, the majority of our patients were about 49 years old when they suffered their first CV event, whereas in the non-SLE Spanish population this occurred at age ≥60.31
Diabetes, hypertension, smoking, and dyslipidemia were all independently associated with CV events in this study. Dyslipidemia (treated or not treated) has been associated with up to a 2-fold increased risk of CV events in SLE patients.7,32–34 Although these factors are strongly predictive of CVD in the general population, the results are less uniform in SLE patients. In fact, this is the first study to demonstrate that diabetes mellitus is associated with CV events in SLE. Unlike our own, none of the studies identified in a recent systematic review found that diabetes or its treatment was an independent risk factor for CVD among SLE patients.3
On the contrary, smoking increases the risk for developing SLE35 and has been associated with a risk of CV events in SLE patients in some studies.13,23,36–39 Other groups, however, have not confirmed this association.4,9,24,32 As our study is cross-sectional in nature, it cannot prove causality, only association. Thus, we decided to group smoking exposure as a single item (ie, past and active smoker) in the multivariate analyses. Frequently, patients with a high CV risk profile are more likely to quit smoking on their own initiative or when encouraged by their doctors or family. This is particularly true after a CV event has occurred. In fact, we have observed that the proportion of former smokers was significantly higher among those patients who suffered a CV event after SLE diagnosis. All these reasons suggest that in this kind of study, smokers have an otherwise better risk profile than nonsmokers, for example, they tend to be significantly younger and have a lower incidence of diabetes, hypertension, previous infarction, and severe coronary disease than nonsmokers.40 Similarly, other groups have reported an association between hypertension and CV events,32–34,41,42 particularly with strokes in SLE patients.33
Several characteristics related to SLE or treatments of it have been identified in our study that might partly explain the high risk of CV events in this patient population. These factors include serositis, low complement, neuropsychiatric lupus, valvular dysfunction (SLICC/ACR DI definition), antiphospholipid antibodies, and azathioprine but not SLENA-SLEDAI index at the last visit. Our results confirm those obtained by most, but not all, other groups.7,33,34 Although a relationship appears to exist between lupus activity and a higher frequency of CVD,43 we think that an isolated measurement of SELENA-SLEDAI is not enough to identify patients at risk. Thus, our patients present a higher frequency of clinical and laboratory manifestations related to disease activity, such as serositis, low complement, and neuropsychiatric lupus. In fact, neuropsychiatric lupus has been identified in a few studies as a strong predictor of CV events.23,37 In this sense, Urowitz et al37 suggested that neurolupus might increase the risk of CV events, both coronary and noncoronary, almost 4-fold.
On the contrary, valvulopathy in SLE can be caused by activity or damage and is a known source of cerebral emboli causing transient ischemic attacks and strokes. In our study, both definitions of valvulopathy (activity and damage) were identified as risk factors for CV events in general. However, valvulopathy was not associated with strokes alone in our SLE patients.
Interestingly, our SLE patients who were positive for antiphospholipid antibodies had a 57% greater risk of suffering a CV event. As antiphospholipid syndrome is characterized by recurrent arterial thrombosis, including ictus and myocardial infarction, it is possible that not all of the vascular events recorded in our study have an atherosclerotic origin. However, it is often impossible to accurately attribute the etiology of such events. Furthermore, these antibodies appear to be positively associated with a history of smoking in patients with a CV event history, particularly in former smokers.44 Indeed, this might represent a second-hit triggering of CV events in patients with preexisting atherosclerosis.13,38,39,43 Finally, antiphospholipid antibodies might play a role in the development of atherosclerosis via several mechanisms, such as the proinflammatory activity they directly or indirectly exert on endothelial cells,45 or by enhancing the lipid peroxidation of lipoproteins46 or reducing paraoxonase activity.47
Although there is good evidence that hydroxychloroquine prevents lupus flares and increases long-term survival in patients with SLE, it is less clear that it reduces the risk of CV events.48 Our univariate analysis suggests that it has a lowering effect on the risk of CV events risk, although this was not the case in the multivariate analysis.
Glucocorticoids have a complex effect on CV risk because of their ability to reduce inflammatory activity. However, they remain associated with hypertension, hyperglycemia, and central obesity.49 In our multivariate analysis, glucocorticoid use had no association with CVD. Only a few authors have demonstrated an association between glucocorticoids and CV events in SLE patients,32,34 probably owing to differences in the definitions of variables (eg, time of use, cumulative doses, and others).7,50 By contrast, azathioprine increased the risk of vascular events in 47% of our patients. Azathioprine correlated with more severe SLE and several studies have confirmed this association.41,51
Our study also has, however, several limitations, the main one being its retrospective design. This could affect SLICC/ACR DI calculations and lead to an underestimation of the frequency of valvular dysfunction or rhythm disorder. Nevertheless, good agreements exist between prospective and retrospective evaluation SLICC/ACR DI scores52 and SLEDAI.53 However, this limitation may be overcome in the RELESSER-PROS study that is now ongoing. Another important limitation concerns the etiologic attribution of events. CV events in SLE, particularly heart failure and rhythm disorders, can be the result of several causes, such as lupus activity, fluid overload, anemia, and others. Therefore, to reduce this limitation, none of these variables were counted as CV events. Nevertheless, in daily clinical practice, it is often not possible to establish the exact etiology of some CV event because of the concurrence of ≥1 pathogenic mechanisms in the same patient. A further limitation of our study was that we did not include important traditional risk factors, such as obesity and sedentary lifestyle. Furthermore, we did not consider 2 subgroups of diabetes (ie, types 1 and 2 diabetes), which in the context of SLE could be totally different. Although the prevalence of type 1 diabetes in SLE is unknown, it should be quite low,54 and thus its influence minimal. Finally, the strengths of the present study include the large sample size, the generalized use of validated indexes, and the mandatory clinical training of the coinvestigator.
CONCLUSION
To conclude, we have confirmed that SLE patients suffer a high prevalence of premature CVD. As with the Spanish general population, this prevalence was lower than that detected in SLE patients from either Northern Europe or Canada, despite the fact that our patients had higher prevalence of both diabetes and smoking. Our study not only emphasizes the importance of recognizing the CV risk factors in these patients, but also shows—for the first time—an association between diabetes and CV events in SLE patients. However, the latter finding needs to be verified via specifically designed studies. The main SLE manifestations associated with CV events were serositis, neuropsychiatric lupus, valvular dysfunction, low complement, positive antiphospholipid antibodies, and azathioprine.
Acknowledgments
The authors are grateful to GSK, Roche, Novartis, and UCB for sponsorship of the registry, as well as all employees of the Spanish Rheumatology Society Research Unit for their commitment and professionalism.
Footnotes
Abbreviations: ACR = American College of Rheumatology, BILAG = British Isles Lupus Assessment Group, CI = confidence interval, CV = cardiovascular, CVD = cardiovascular disease, HR = hazard ratio, LDL = low-density lipoprotein, LUMINA = lupus in minorities: nature versus nurture, NSAID = snonsteroidal anti-inflammatory drugs, SD = standard deviation, SDI = systemic lupus erythematosus damage index, SELENA-SLEDAI = Safety of Estrogens in Lupus Erythematosus National Assessment-Systemic Lupus Erythematosus Disease Activity Index, SLE = systemic lupus erythematosus, SLEDAI = Systemic Lupus Erythematosus Disease Activity Index, SLICC = Systemic Lupus International Collaborating Clinics.
JMPR is supported by the Grant 316265 (BIOCAPS) from the European Union 7th Framework Program (FP7/REGPOT-2012–2013.1).
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Cervera R, Khamashta M, Font J, et al. Morbidity and mortality in systemic lupus erythematosus during a 10-year period: a comparison of early and late manifestations in a cohort of 1,000 patients. Medicine (Baltimore) 2003; 82:299–308. [DOI] [PubMed] [Google Scholar]
- 2.Moroni G, Quaglini S, Gallelli B, et al. Progressive improvement of patient and renal survival and reduction of morbidity over time in patients with lupus nephritis (LN) followed for 20 years. Lupus 2013; 22:810–818. [DOI] [PubMed] [Google Scholar]
- 3.Schoenfeld SR, Kasturi S, Costenbader KH. The epidemiology of atherosclerotic cardiovascular disease among patients with SLE: a systematic review. Semin Arthritis Rheum 2013; 43:77–95. [DOI] [PubMed] [Google Scholar]
- 4.Koenig KF, Ribi C, Radosavac M, et al. Prevalence of vascular disease in systemic lupus erythematosus compared with type-1 diabetes mellitus: a cross-sectional study of two cohorts. Lupus 2015; 24:58–65. [DOI] [PubMed] [Google Scholar]
- 5.Karp I, Abrahamowicz M, Fortin PR, et al. Longitudinal evolution of risk of coronary heart disease in systemic lupus erythematosus. J Rheumatol 2012; 39:968–973. [DOI] [PubMed] [Google Scholar]
- 6.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum 2001; 44:2331–2337. [DOI] [PubMed] [Google Scholar]
- 7.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol 1997; 145:408–415. [DOI] [PubMed] [Google Scholar]
- 8.Mok CC, Ho LY, To CH. Annual incidence and standardized incidence ratio of cerebrovascular accidents in patients with systemic lupus erythematosus. Scand J Rheumatol 2009; 38:362–368. [DOI] [PubMed] [Google Scholar]
- 9.Magder LS, Petri M. Incidence of and risk factors for adverse cardiovascular events among patients with systemic lupus erythematosus. Am J Epidemiol 2012; 176:708–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skaggs BJ, Hahn BH, McMahon M. Accelerated atherosclerosis in patients with SLE: mechanisms and management. Nat Rev Rheumatol 2012; 8:214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urowitz MB, Gladman D, Ibanez D, et al. Atherosclerotic vascular events in a multinational inception cohort of systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2010; 62:881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pons-Estel GJ, Gonzalez LA, Zhang J, et al. Predictors of cardiovascular damage in patients with systemic lupus erythematosus: data from LUMINA (LXVIII), a multiethnic US cohort. Rheumatology (Oxford) 2009; 48:817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toloza SM, Uribe AG, McGwin G, Jr, et al. Systemic lupus erythematosus in a multiethnic US cohort (LUMINA). XXIII. Baseline predictors of vascular events. Arthritis Rheum 2004; 50:3947–3957. [DOI] [PubMed] [Google Scholar]
- 14.Rua-Figueroa I, Lopez-Longo FJ, Calvo-Alen J, et al. National registry of patients with systemic lupus erythematosus of the Spanish Society of Rheumatology: objectives and methodology. Reumatol Clin 2014; 10:17–24. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40:1725. [DOI] [PubMed] [Google Scholar]
- 16.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383. [DOI] [PubMed] [Google Scholar]
- 17.Petri M, Kim MY, Kalunian KC, et al. Combined oral contraceptives in women with systemic lupus erythematosus. N Engl J Med 2005; 353:2550–2558. [DOI] [PubMed] [Google Scholar]
- 18.Isenberg DA, Rahman A, Allen E, et al. BILAG 2004. Development and initial validation of an updated version of the British Isles Lupus Assessment Group's disease activity index for patients with systemic lupus erythematosus. Rheumatology (Oxford) 2005; 44:902–906. [DOI] [PubMed] [Google Scholar]
- 19.Miyakis S, Lockshin MD, Atsumi T, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306. [DOI] [PubMed] [Google Scholar]
- 20.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum 1996; 39:363–369. [DOI] [PubMed] [Google Scholar]
- 21.Katz JD, Senecal JL, Rivest C, et al. A simple severity of disease index for systemic lupus erythematosus. Lupus 1993; 2:119–123. [DOI] [PubMed] [Google Scholar]
- 22.Pons-Estel BA, Catoggio LJ, Cardiel MH, et al. The GLADEL multinational Latin American prospective inception cohort of 1,214 patients with systemic lupus erythematosus: ethnic and disease heterogeneity among “Hispanics”. Medicine (Baltimore) 2004; 83:1–17. [DOI] [PubMed] [Google Scholar]
- 23.Bertoli AM, Vila LM, Alarcon GS, et al. Factors associated with arterial vascular events in PROFILE: a Multiethnic Lupus Cohort. Lupus 2009; 18:958–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker-Merok A, Nossent J. Prevalence, predictors and outcome of vascular damage in systemic lupus erythematosus. Lupus 2009; 18:508–515. [DOI] [PubMed] [Google Scholar]
- 25.Petri M, Spence D, Bone LR, et al. Coronary artery disease risk factors in the Johns Hopkins Lupus Cohort: prevalence, recognition by patients, and preventive practices. Medicine (Baltimore) 1992; 71:291–302. [DOI] [PubMed] [Google Scholar]
- 26.Soriguer F, Garcia-Escobar E, Morcillo S, et al. Mediterranean diet and the Spanish paradox. A hypothesis. Med Hypotheses 2013; 80:150–155. [DOI] [PubMed] [Google Scholar]
- 27.Chang ER, Pineau CA, Bernatsky S, et al. Risk for incident arterial or venous vascular events varies over the course of systemic lupus erythematosus. J Rheumatol 2006; 33:1780–1784. [PubMed] [Google Scholar]
- 28.Fox CS, Coady S, Sorlie PD, et al. Trends in cardiovascular complications of diabetes. J Am Med Assoc 2004; 292:2495–2499. [DOI] [PubMed] [Google Scholar]
- 29.Prescott E, Hippe M, Schnohr P, et al. Smoking and risk of myocardial infarction in women and men: longitudinal population study. BMJ 1998; 316:1043–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savji N, Rockman CB, Skolnick AH, et al. Association between advanced age and vascular disease in different arterial territories: a population database of over 3.6 million subjects. J Am Coll Cardiol 2013; 61:1736–1743. [DOI] [PubMed] [Google Scholar]
- 31.Marrugat J, Sala J, Masia R, et al. Mortality differences between men and women following first myocardial infarction. RESCATE Investigators. Recursos Empleados en el Sindrome Coronario Agudo y Tiempo de Espera. J Am Med Assoc 1998; 280:1405–1409. [DOI] [PubMed] [Google Scholar]
- 32.Petri M, Perez-Gutthann S, Spence D, et al. Risk factors for coronary artery disease in patients with systemic lupus erythematosus. Am J Med 1992; 93:513–519. [DOI] [PubMed] [Google Scholar]
- 33.Mikdashi J, Handwerger B, Langenberg P, et al. Baseline disease activity, hyperlipidemia, and hypertension are predictive factors for ischemic stroke and stroke severity in systemic lupus erythematosus. Stroke 2007; 38:281–285. [DOI] [PubMed] [Google Scholar]
- 34.Nikpour M, Urowitz MB, Ibanez D, et al. Importance of cumulative exposure to elevated cholesterol and blood pressure in development of atherosclerotic coronary artery disease in systemic lupus erythematosus: a prospective proof-of-concept cohort study. Arthritis Res Ther 2011; 13:R156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takvorian SU, Merola JF, Costenbader KH. Cigarette smoking, alcohol consumption and risk of systemic lupus erythematosus. Lupus 2014; 23:537–544. [DOI] [PubMed] [Google Scholar]
- 36.Manger K, Kusus M, Forster C, et al. Factors associated with coronary artery calcification in young female patients with SLE. Ann Rheum Dis 2003; 62:846–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urowitz MB, Ibanez D, Gladman DD. Atherosclerotic vascular events in a single large lupus cohort: prevalence and risk factors. J Rheumatol 2007; 34:70–75. [PubMed] [Google Scholar]
- 38.Gustafsson J, Gunnarsson I, Borjesson O, et al. Predictors of the first cardiovascular event in patients with systemic lupus erythematosus: a prospective cohort study. Arthritis Res Ther 2009; 11:R186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gustafsson J, Simard JF, Gunnarsson I, et al. Risk factors for cardiovascular mortality in patients with systemic lupus erythematosus, a prospective cohort study. Arthritis Res Ther 2012; 14:R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruiz-Bailen M, de Hoyos EA, Reina-Toral A, et al. Paradoxical effect of smoking in the Spanish population with acute myocardial infarction or unstable angina: results of the ARIAM Register. Chest 2004; 125:831–840. [DOI] [PubMed] [Google Scholar]
- 41.Haque S, Gordon C, Isenberg D, et al. Risk factors for clinical coronary heart disease in systemic lupus erythematosus: the lupus and atherosclerosis evaluation of risk (LASER) study. J Rheumatol 2010; 37:322–329. [DOI] [PubMed] [Google Scholar]
- 42.Bessant R, Duncan R, Ambler G, et al. Prevalence of conventional and lupus-specific risk factors for cardiovascular disease in patients with systemic lupus erythematosus: a case-control study. Arthritis Rheum 2006; 55:892–899. [DOI] [PubMed] [Google Scholar]
- 43.Bengtsson C, Ohman ML, Nived O, et al. Cardiovascular event in systemic lupus erythematosus in northern Sweden: incidence and predictors in a 7-year follow-up study. Lupus 2012; 21:452–459. [DOI] [PubMed] [Google Scholar]
- 44.Gustafsson JT, Gunnarsson I, Kallberg H, et al. Cigarette smoking, antiphospholipid antibodies and vascular events in systemic lupus erythematosus. Ann Rheum Dis 2015; 74:1537–1543. [DOI] [PubMed] [Google Scholar]
- 45.Shoenfeld Y, Gerli R, Doria A, et al. Accelerated atherosclerosis in autoimmune rheumatic diseases. Circulation 2005; 112:3337–3347. [DOI] [PubMed] [Google Scholar]
- 46.Lopez LR, Salazar-Paramo M, Palafox-Sanchez C, et al. Oxidized low-density lipoprotein and beta2-glycoprotein I in patients with systemic lupus erythematosus and increased carotid intima-media thickness: implications in autoimmune-mediated atherosclerosis. Lupus 2006; 15:80–86. [DOI] [PubMed] [Google Scholar]
- 47.Delgado Alves J, Ames PR, Donohue S, et al. Antibodies to high-density lipoprotein and beta2-glycoprotein I are inversely correlated with paraoxonase activity in systemic lupus erythematosus and primary antiphospholipid syndrome. Arthritis Rheum 2002; 46:2686–2694. [DOI] [PubMed] [Google Scholar]
- 48.Ruiz-Irastorza G, Ramos-Casals M, Brito-Zeron P, et al. Clinical efficacy and side effects of antimalarials in systemic lupus erythematosus: a systematic review. Ann Rheum Dis 2010; 69:20–28. [DOI] [PubMed] [Google Scholar]
- 49.Karp I, Abrahamowicz M, Fortin PR, et al. Recent corticosteroid use and recent disease activity: independent determinants of coronary heart disease risk factors in systemic lupus erythematosus? Arthritis Rheum 2008; 59:169–175. [DOI] [PubMed] [Google Scholar]
- 50.Doria A, Shoenfeld Y, Wu R, et al. Risk factors for subclinical atherosclerosis in a prospective cohort of patients with systemic lupus erythematosus. Ann Rheum Dis 2003; 62:1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burgos PI, Vila LM, Reveille JD, et al. Peripheral vascular damage in systemic lupus erythematosus: data from LUMINA, a large multi-ethnic U.S. cohort (LXIX). Lupus 2009; 18:1303–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bernatsky S, Clarke A, Abrahamowicz M, et al. A comparison of prospective and retrospective evaluations of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index for systemic lupus erythematosus. J Rheumatol 2005; 32:820–823. [PubMed] [Google Scholar]
- 53.FitzGerald JD, Grossman JM. Validity and reliability of retrospective assessment of disease activity and flare in observational cohorts of lupus patients. Lupus 1999; 8:638–644. [DOI] [PubMed] [Google Scholar]
- 54.Kota SK, Meher LK, Jammula S, et al. Clinical profile of coexisting conditions in type 1 diabetes mellitus patients. Diabetes Metab Syndr 2012; 6:70–76. [DOI] [PubMed] [Google Scholar]


