Abstract
There are many methods to assess liver function, but none of them has been verified as fully effective. The purpose of this study is to establish a comprehensive method evaluating perioperative liver reserve function (LRF) in patients with primary liver cancer (PLC).
In this study, 310 PLC patients who underwent liver resection were included. The cohort was divided into a training set (n = 235) and a validation set (n = 75). The factors affecting postoperative liver dysfunction (POLD) during preoperative, intraoperative, and postoperative periods were confirmed by logistic regression analysis. The equation for calculating the preoperative liver functional evaluation index (PLFEI) was established; the cutoff value of PLFEI determined through analysis by receiver-operating characteristic curve was used to predict postoperative liver function.
The data showed that body mass index, international normalized ratio, indocyanine green (ICG) retention rate at 15 minutes (ICGR15), ICG elimination rate, standard remnant liver volume (SRLV), operative bleeding volume (OBV), blood transfusion volume, and operative time were statistically different (all P < 0.05) between 2 groups of patients with and without POLD. The relationship among PLFEI, ICGR15, OBV, and SRLV is expressed as an equation of “PLFEI = 0.181 × ICGR15 + 0.001 × OBV − 0.008 × SRLV.” The cutoff value of PLFEI to predict POLD was −2.16 whose sensitivity and specificity were 90.3% and 73.5%, respectively. However, when predicting fatal liver failure (FLF), the cutoff value of PLFEI was switched to −1.97 whose sensitivity and specificity were 100% and 68.8%, respectively.
PLFEI will be a more comprehensive, sensitive, and accurate index assessing perioperative LRF in liver cancer patients who receive liver resection. And keeping PLFEI <−1.97 is a safety margin for preventing FLF in PLC patients who underwent liver resection.
INTRODUCTION
Currently, surgical resection is a preferred treatment option for patients with liver cancer except for liver transplant.1 Because of postoperative liver failure, severe complications like shock and death take place in many patients after liver resection.2–4 Liver reserve function (LRF) after resection, risk factors leading liver dysfunction or failure, methods for preventing these risk factors, and postoperational liver regeneration or repair have become the focus of research. When pursuing the elimination of the target lesion, 1 of the most critical measurements for liver cancer is to ensure the safety of the implementation of treatment programs and obtain the best effects of the eventual rehabilitation in patients. In order to achieve the desirable outcome, it is essential to have not only excellent surgical technique but also a good system that can assess the status of LRF in patients about to receive liver resection.
Indocyanine green (ICG) clearance test is a sensitive and accurate method that assesses LRF quantitatively.5–7 In the process of liver resection, many factors affect the perioperative LRF. These factors mainly include liver resection volume, hypoxia injury caused by operative bleeding, ischemia reperfusion injury, and other liver damages caused by intraoperative blood transfusion, stretch, squeeze, and anesthetic drugs. Although a single ICG clearance test is an accurate, quantitative assessment for preoperative LRF, it cannot make a comprehensive evaluation for perioperative LRF. After taking into consideration the main preoperative, intraoperative, and postoperative factors, one can achieve a comprehensive assessment for perioperative LRF.
In previous studies, many researchers use preoperative methods to evaluate LRF, and some of them already consider postoperative influencing factors, such as standard remnant liver volume (SRLV). SRLV, which is an effective, simple indicator assessing LRF in liver cancer patients about to receive liver resection, is defined as remnant liver volume (RLV) divided by body surface area (BSA). It plays an important role guiding the prediction of LRF of postoperative patients and thus helps preventing postoperative liver failure.8 Varieties of studies indicate that intraoperative factors significantly affect postoperative liver function,9–14 especially intraoperative bleeding and operating time; however, none of these intraoperative factors is brought into assessing methods. It is well known that excessive bleeding might increase perioperative mortality or complications that affect long-term survival in patients.9–11 Therefore, evaluation can be more effective if intraoperative factors are taken into consideration when assessing perioperative LRF.
In this study, we combined multiple perioperative factors to evaluate postoperative liver function in PLC patients and established an equation calculating the preoperative liver functional evaluation index (PLFEI) that combines preoperative, intraoperative, and postoperative factors influencing liver function. We confirmed that PLFEI acts as a sensitive, comprehensive, objective, and effective indicator for evaluating perioperative liver function in liver cancer patients about to receive resection.
METHODS
Study Design
First, we analyzed the clinical data of 235 patients who underwent liver resection between September 2010 and April 2014 at the Affiliated Hospital of Guilin Medical University, Guilin, China. We identified the determinants and established a logistic regression model for evaluating perioperative liver function in patients with PLC. Second, we proposed a new indicator PLFEI calculated by a logistic regression equation; the cutoff value of PLFEI was determined by receiver-operating characteristic (ROC) curve analysis. Third, we evaluated the validity and reliability of PLFEI using an independent set including 75 patients who underwent liver resection at the same institutes from March 2014 to October 2014.
Postoperative Liver Dysfunction Criteria
Postoperative liver dysfunction was defined as the total bilirubin levels ≥50 μmol/L and/or prothrombin time (PT) index <50% at fifth postoperative day.15 Patients were followed up for 1 month after liver resection. The postoperative patients were divided into 2 groups: Group A without liver dysfunction and Group B with liver dysfunction.
Patients
The local ethics committee approved this study protocol; all patients included in this study signed an informed consent. Three hundred ten consecutive PLC patients who underwent liver resection were enrolled in the study at the Affiliated Hospital of Guilin Medical University.
The training set recruited 235 cases of patients. Two hundred six patients were males and 29 were females; the average age was 50.41 ± 11.01 years (ranging from 24 to 84 years). Postoperative pathologic examination confirmed 195 (82.98%) patients as having hepatocellular carcinoma and 40 patients (17.02%) as having cholangiocarcinoma. The average diameter of tumors in these patients was 8.5 ± 5.2 cm (ranging from 3.0 to 20.2 cm); 215 (91.49%) patients had a history of hepatitis B or their HBsAg was positive, and hepatitis C antibody in 10 cases (4.26%) was positive. Three of 10 patients without viral hepatitis were diagnosed as having nonalcoholic fatty liver disease (NAFLD).
Seventy-five patients were included in the validating set; 61 patients were males and 14 cases were females, and the average age was 46.3 ± 11.4 years (ranging from 28 to 81 years). Among them, postoperative pathologic examination determined 61 (81.33%) patients as having hepatocellular carcinoma and 14 (18.67%) cases as having cholangiocarcinoma. The average diameter of these tumors was 9.1 ± 4.2 cm (ranging from 4.0 to 18.5 cm). Sixty-six (88.0%) patients had a history of hepatitis B or their HBsAg was positive, and hepatitis C antibody in 3 cases (4%) was positive.
ICG Test
All the patients in this study conducted an ICG clearance test at 1 to 3 days before operation. ICGR15 data was obtained from Pulse Dye Densito-Graph Analyzer (DDG-3300K; Nihon Kohden, Tokyo, Japan). After an optical sensor of the ICG clearance meter was attached to a patient's index finger, ICG (5 mg/mL, Dandong Medical and Pharmaceutical Co., Liaoning, China) was intravenously administered at a dose of 0.5 mg/kg body weight via central venous catheter that was immediately flushed with normal saline.
INR and BMI
PT was determined at 1 to 3 days before operation and on the postoperative days 1, 3, 5, and 7 using PT test kit (Nanjing Jiancheng technology Co., Ltd, Nanjing, China) and automatic blood coagulation instrument (Sysmex, Japan) in accordance with the manufacturer's instructions. International normalized ratio (INR) = (patient PT/control PT)ISI. Body mass index (BMI, kg/m2) = body weight (kg)/body height (m)2. The patients were divided into 4 grades according to the BMI16,17: underweight (<18.5), normal (18.5–24.9), overweight (25.0–29.9), and obesity (>30.0 kg/m2).
Liver Volume Measurement
All the patients received a computed tomography (CT) scan (64-slice spiral CT; General Electric Co., Fort Myers, FL) before surgery. A parallel 3-dimensional of the liver was reconstructed to evaluate total liver volume (TLV, mL) and tumor volume. Liver resection volume (LRV, mL) was measured by water displacement method during operation. The RLV (mL) = TLV − LRV. SRLV (mL/m2) = RLV/BSA (m2). BSA (m2) = body weight (kg)0.425 × body height (cm)0.725 × 0.007184.18
Liver Cirrhosis and NAFLD
According to the preoperative Child–Pugh score classification, liver cirrhosis was graded into 3 groups19: mild cirrhosis (Child–Pugh A), moderate cirrhosis (Child–Pugh B), and severe cirrhosis (Child–Pugh C). NAFLD was diagnosed according to the postoperative pathological examination, and segmented into steatosis, nonalcoholic steatohepatitis, and cirrhosis.16,20,21 Patients should not be diagnosed as NAFLD whose liver fat lesions were caused by other common liver diseases, particularly hepatitis C, hepatitis B, and alcoholic liver disease.16,20,21
Operative Time and Bleeding Volume
Operative time was defined as the interval between open and closure of skin. Operative bleeding volume (OBV, mL) was the amount of blood that the patient lost during operation, and was calculated from the start to the end of the operation. The volume of blood loss was the suction volume minus rinsing fluids, and adding the weight of blood absorbed by swabs (assuming that 1 mL = 1 g).22,23
Surgical Methods
All patients were given intravenous anesthesia and the selection of surgery method was based on results of operative exploration, preoperative examination-related indicators (ICGR15, CT, Child–Pugh classification, etc.), and the size and location of the tumor.
The methods of liver resection for the training set (n = 235) were summarized as follows: nonanatomic liver resection or local tumor resection (n = 109), left hemihepatectomy (n = 42, one of them with partial resection of the caudate lobe), right hemihepatectomy (n = 37, two of them with partial resection of the caudate lobe), mesohepatectomy (n = 13), left lateral liver resection (n = 19), right posterior lobe resection (n = 13), and extensive liver resection (n = 2). Surgical specimens of all the patients were routinely sent for pathological investigation. Two hundred ten patients had cirrhosis. Twenty patients had left or right branch of portal vein thrombosis and no one had trunk thrombosis. Thirty patients had liver resection without portal clamping. Thirty-seven patients had selective hemihepatic portal clamping and 40 patients had intermittent Pringle maneuver. The protocol for total portal occlusion did not exceed 15 minutes (9.2 ± 2.6 minutes per time) and the time interval of 2 consecutive blocks was >5 minutes. Thirty-two patients submitted to a liver resection received no blood transfusion.
The methods of liver resection for the validation set (n = 75) were summarized as follows: nonanatomic liver resection or local tumor resection (n = 33), left hemihepatectomy (n = 12), right hemihepatectomy (n = 7), the caudate lobe resection (n = 6), mesohepatectomy (n = 7), left lateral liver resection (n = 8), and right posterior lobe resection (n = 2). Surgical specimens of all the patients were routinely sent to pathological investigation. Sixty-seven patients had cirrhosis. Ten patients had left or right branch of portal vein thrombosis and no one had trunk thrombosis. Thirty-six patients had liver resection without portal clamping. Eighteen patients had selective hemihepatic portal clamping and 10 patients had intermittent Pringle maneuver. Twenty patients received blood transfusion during liver resection.
Statistical Analysis
The continuous variables were expressed as mean ± standard deviation, and the means comparison between the 2 groups were examined by t test. The rate comparison between the 2 groups was done by χ2 test. The model was conducted by logical regression analyses and the formula predicting the status of postoperative liver function was obtained from the logical regression model mentioned earlier.
Most of statistical analyses were completed by SPSS19.0 statistical software. The ROC curve analysis was done by the MedCalc analysis software 12.4.0.0 (Ostend, Belgium). P < 0.05 was considered statistically significance.
RESULTS
Characteristics of Postoperative Patients Surveyed
Based on the postoperative liver dysfunction criteria, 204 patients were included into Group A and 31 patients were included into Group B. The group B was further divided into 2 subgroups: subgroup I, with 27 patients, all of whom recovered from POLD, and subgroup II, with 4 patients, all of whom died of liver failure. BMI, INR, ICGK, ICGR15, SRLV, OBV, blood transfusion volume, and operative time were statistically different between the 2 groups (P < 0.05), as shown in Table 1. Liver resections in 20 patients (13 in Group A and 7 in Group B) were >60% of TLV. LRV was not significantly different between the 2 groups (P = 0.235). The average OBV in patients was 733.6 ± 772.8 mL (range 100–5500 mL). Liver dysfunction occurred in 17 of 175 patients with OBV <1000 mL, which was lower than those with OBV >1000 mL (14/60, P < 0.01). None of 225 (95.74%) patients with viral hepatitis were found with obvious fat lesions in the liver, and 3 of 10 patients without viral hepatitis were diagnosed as NAFLD. In the 3 patients, 1 occurred with POLD and 2 without POLD. NAFLD had no significant difference between Group A and Group B, as shown in Table 1. In patients without virus hepatitis, NAFLD had no significant difference between the groups with or without POLD, as shown in Table 2.
TABLE 1.
Comparison of Clinical Indexes Between Group A and Group B
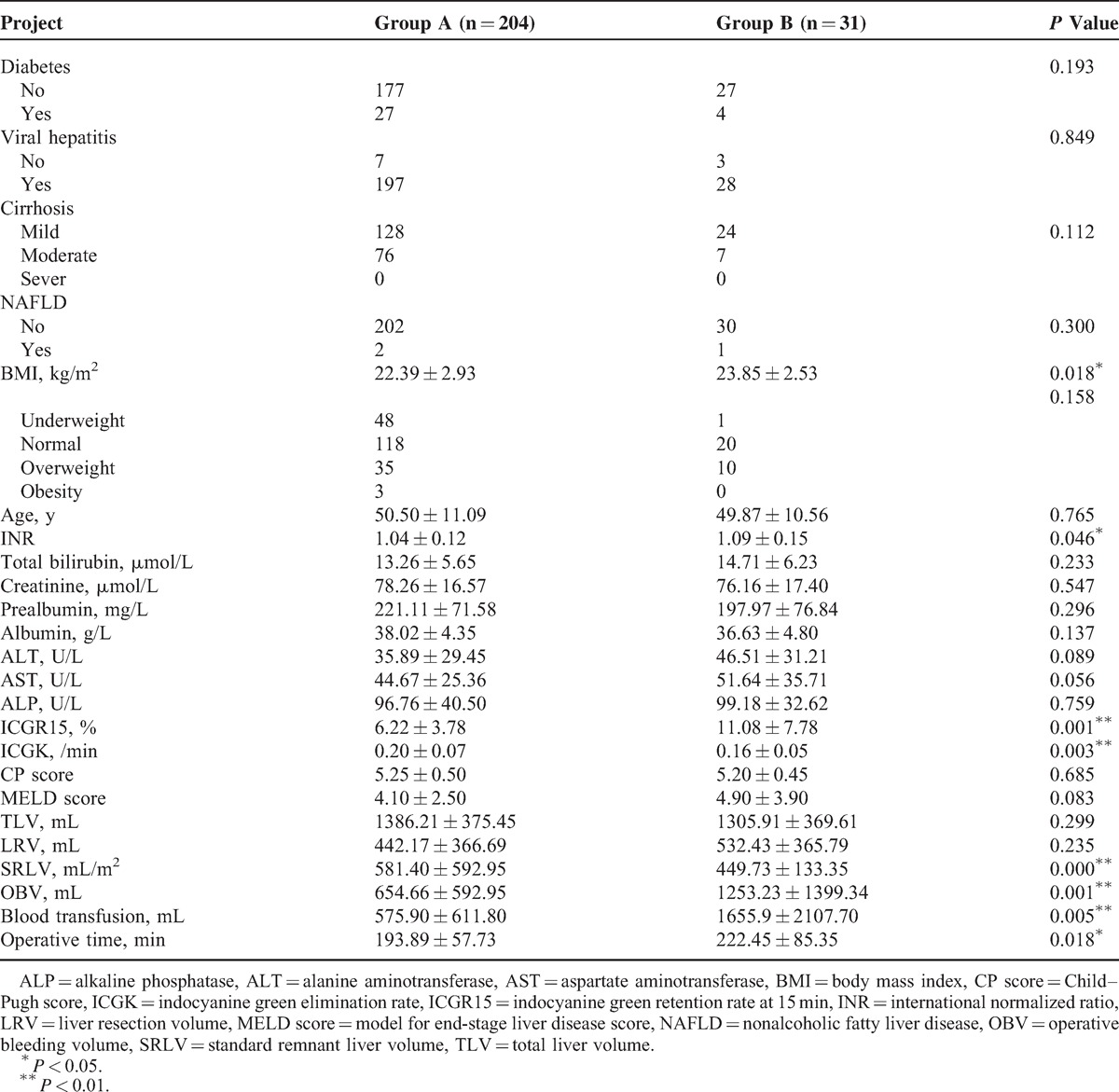
TABLE 2.
Comparison of NAFLD in Patients Without Virus Hepatitis

Determinants of Postoperative Patients With Liver Dysfunction
The binary logical regression analysis was used to identify the relationship between the status of postoperative liver function and BMI, INR, ICGR15, ICGK, SRLV, OBV, blood transfusion volume, and operative time. The introduced variables were ICGR15, OBV, and SRLV and excluded variables were ICGK, INR, blood transfusion volume, operative time, and BMI by analysis using the forward stepwise method whose probability for entry and removal were 0.05 and 0.1, respectively. Therefore, the determinants of POLD were ICGR15, OBV, and SRLV as shown in Table 3. POLD was positively correlated with ICGR15 and OBV (all P < 0.05); however, it was negatively correlated with SRLV.
TABLE 3.
Covariates Included in the Logistic Regression Model (n = 235)
PLFEI and the Cutoff
The logistic regression equation was established, namely, PLFEI = 0.181 × ICGR15 + 0.001 × OBV − 0.008 × SRLV. The PLFEI cutoff predicting POLD was −2.16 determined by ROC analysis from the training set; the sensitivity and specificity were 90.3% and 73.5%, respectively (Figure 1). The cutoff value of PLFEI to predict fatal liver failure (FLF) was −1.97 whose sensitivity and specificity were 100% and 68.8%, respectively (Figure 2). Postoperative FLF happened in all patients whose PLFEI was >−1.97 (Figure 3).
FIGURE 1.
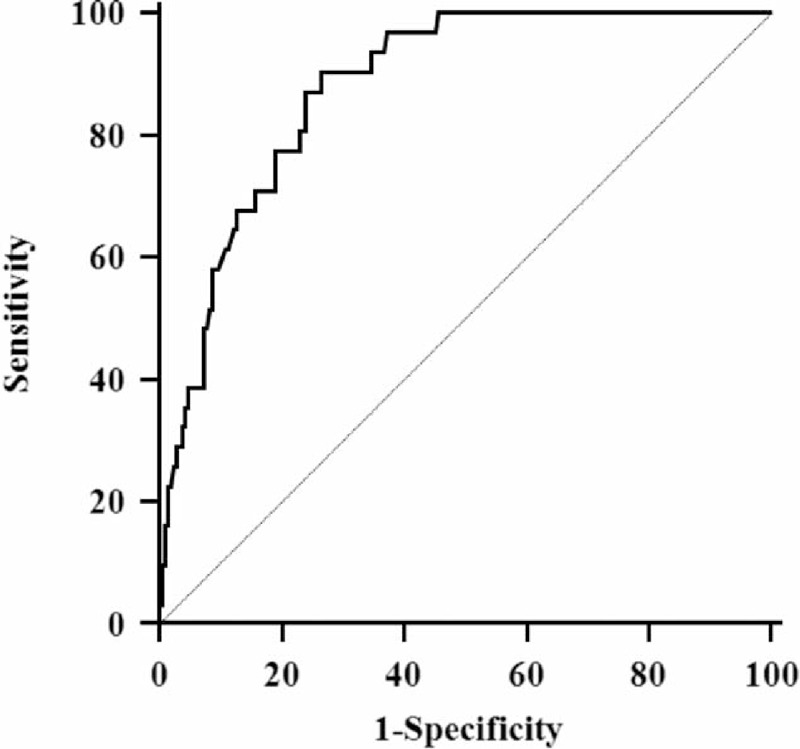
Sensitivity and specificity of a PLFEI value analyzed by ROC curve. The PLFEI value >−2.16 was used to predict POLD; its sensitivity and specificity were 90.30% and 73.5%, respectively. Area under the ROC curve was 0.879, standard error was 0.0261, and 95% confidence interval was from 0.830 to 0.918. PLFEI = preoperative liver functional evaluation index, POLD = postoperative liver dysfunction, ROC = receiver-operating characteristic.
FIGURE 2.
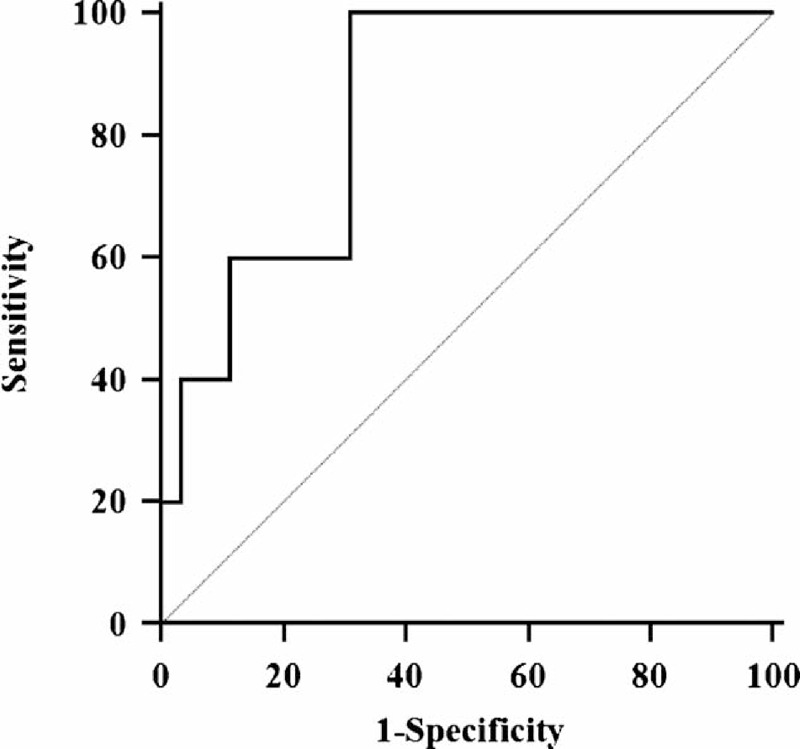
Sensitivity and specificity of a PLFEI value analyzed by ROC curve. The PLFEI value >−1.97 was used to predict FLF; its sensitivity and specificity were 100% and 68.8%, respectively. Area under the ROC curve was 0.885, standard error was 0.0709, and 95% confidence interval was from 0.837 to 0.923. FLF = fatal liver failure, PLFEI = preoperative liver functional evaluation index, ROC = receiver-operating characteristic.
FIGURE 3.
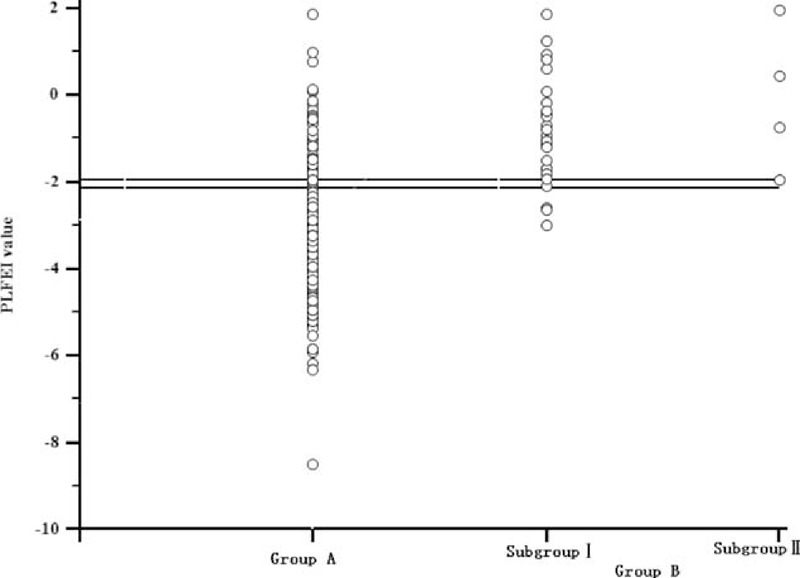
Majority of patients in Group A have a PLFEI value <−2.16, and the majority of patients in Group B have a PLFEI value >−2.16, and 4 patients who died of FLF have a PLFEI value >−1.97. FLF = fatal liver failure, PLFEI = preoperative liver functional evaluation index.
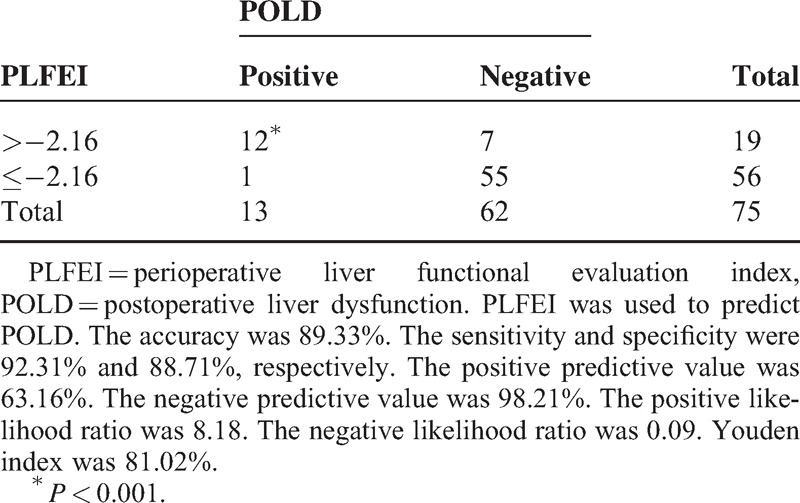
Validity and Reliability of PLFEI
The sensitivity and the negative predictive value were >90% and the accuracy and the specificity were nearly 90% among the validating samples for predicting POLD (Table 4).
TABLE 4.
Evaluation of PLFEI in Predicting POLD
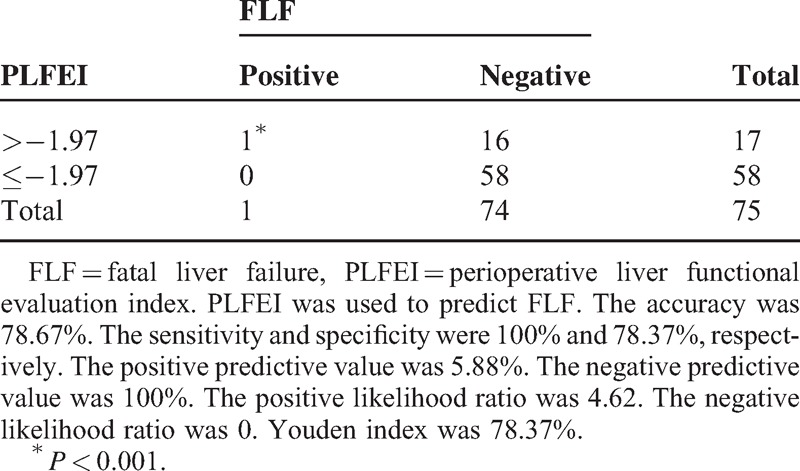
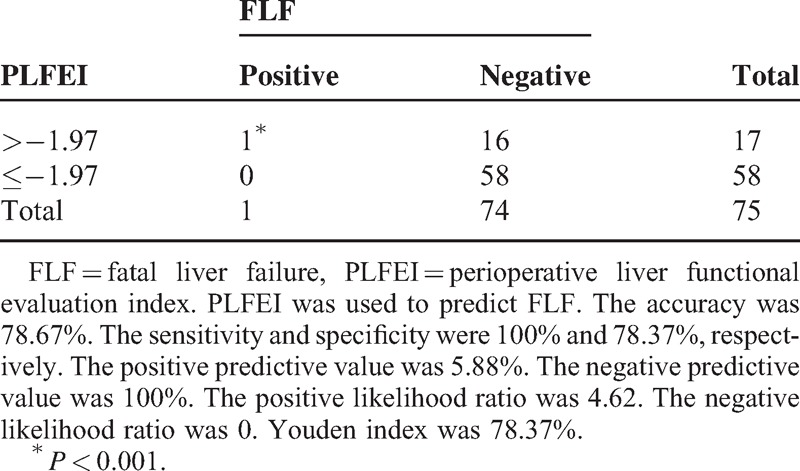
The sensitivity and the negative predictive value were 100% and the accuracy and the specificity were 80% among the validating samples for predicting FLF (Table 5).
TABLE 5.
Evaluation of PLFEI in Predicting FLF
DISCUSSION
Main Influencing Factors for POLD
Many perioperative factors existing in preoperative, intraoperative, and postoperative phases are involved in POLD. First, some factors in preoperative phase affected postoperative liver function of patients with liver resection. Our data showed that preoperative ICGR15 among patients without POLD was lower than with POLD, which is similar to the result described by Okabe et al.13 Preoperative ICG clearance tests like ICGR15 and ICGK are clinically used to assess LRF, and many reports confirmed that they are safe, sensitive, and accurate quantitative methods evaluating LRF before liver resection; avoiding “high ICGR15” can prevent postoperative patients from liver dysfunction.5,24 We also found that preoperative INR among patients without POLD was less than with POLD, which resembles to the previous reports.3,25 PT was used as an independent prognostic factor in patients after liver resection when PT activity is >80%.25 Preoperative PT is >14 seconds; the death risk in postoperative patients will increase.3 Second, intraoperative factors had some effects on POLD. OBV in the group without POLD was less than with POLD. Bleeding in liver resection process is difficult to avoid. Excessive operative bleeding not only causes liver tissue hypoperfusion, which subsequently leads to liver tissue hypoxia/ischemia injury, but also affects postoperative hypoalbuminemia and aggravates the burden of liver. A large number of studies have shown that excessive operative bleeding significantly increases postoperative morbidity and mortality, especially in the patients with cirrhosis.9–12 Operating time in the group without POLD was shorter than with POLD. The duration of surgery is directly proportional to the postoperative recovery of patients. The prolonged surgery affects the liver function recovery and causes higher morbidity in patients after liver resection.13,14 Finally, some postoperative factors influenced POLD. The SRLV in the group without POLD was larger than with POLD. Liver volume is closely related to LRF.26 When RLV is below a certain threshold, the patient is prone to liver dysfunction, even FLF, and high mortality rate.13 SRLV is used as an even more individualized approach to evaluate residual LRF of patients.
NAFLD, abnormal BMI, and diabetes are considered as risk factors of complications after liver resection.27–31 In western countries, NAFLD is a prevalent problem.32 But in China, >90% of liver cancer patients infected with hepatitis B or hepatitis C,33 and most of them had liver cirrhosis. In agreement with this report, there were >90% patients with viral hepatitis and only 3 patients with NAFLD were observed in our study, therefore, viral hepatitis was the major whereas NAFLD was the minor factor leading to cirrhosis that affected LRF. BMI had a significant difference between patients without and with POLD, P = 0.018. But, there was no significant difference between patients without and with POLD in BMI by grade, P = 0.158, and no significant difference among four subgroups of BMI by multiple comparisons, similar to some study.34–36 This study showed that diabetes had no significant differences between patients without and with liver dysfunction, similar to the literature.37
In conclusion, we found that 3 preoperative determinants such as higher BMI, higher ICGR15, and larger INR affected POLD in patients with liver resection; 3 risk factors during operation like massive OBV, too much blood transfusion, and longer operation time played key roles in postoperative liver function insufficiency; 1 postoperative factor like undersized SRLV caused by excessive excision of liver tissue also influenced postoperative liver function.
Validity and Reliability of PLFEI in Predicting the Status of Postoperative Liver Function for Patients With Liver Cancer Resection
We proposed the PLFEI and tried to include intraoperative influencing factor into the postoperative liver function evaluation system. When PLFEI is >−2.16, the sensitivity was 90.3% and the specificity was 73.5% for evaluating POLD. When PLFEI is >−1.97, the sensitivity was 100% and the specificity was 68.8% for evaluating FLF. Many evaluation methods of liver function only rely on the detection of preoperative LRF, and some methods pay close attention to the postoperative RLV. Although a variety of studies suggested that some intraoperative factors significantly affect the postoperative liver function,9–14 there was not a single evaluation method that took intraoperative factors into considerations. PLFEI includes intraoperative influencing factor and it is more comprehensive and objective evaluation method for perioperative liver function and for prediction of postoperative liver function. It is known that operation time and OBV may vary owing to different surgeons, the patient's postoperative outcome is likely to be different. Therefore, when preoperative liver function evaluation method was used to predict the maximum excision volume of liver, intraoperative influencing factors should not be ignored.
Clinical Application of the PLFEI
PLFEI might be evaluated even before surgery. Every patient could have a preoperative ICG test to obtain the ICGR15 value, TLV and LRV could be estimated through a CT scan before surgery, and then SRLV could be figured out. Rough OBV could be estimated according to imaging data and doctors’ experience. If there is a less estimated OBV and a higher estimated PLFEI value, this patient should be dealt with more carefully. Especially when the estimated PLFEI is >−1.97, the surgery should be carefully designed to ensure the safety of patients, even giving up the surgery. During the surgery, bleeding should be reduced as far as possible, and some effective hemostatic measures and new surgical devices should be taken.22
We can calculate and find out the PLFEI immediately after the surgery. Before surgery, the preoperative ICGR15 value could be determined, the TLV can be estimated through a CT scan, the LRV can be obtained during surgery, OBV can be obtained immediately after the surgery, and then the PLFEI will be figured out. Postoperative treatment, especially protecting liver therapy, should be enhanced in the patient with a higher PLFEI value, in order to reduce POLD.
Therefore, we conclude that PLFEI will be a more comprehensive, sensitive, and accurate index assessing perioperative LRF in liver cancer patients who receive liver resection, and keeping PLFEI <−1.97 is a safety margin for preventing FLF in PLC patients after liver resection.
Disadvantages of This Study
With all the mentioned advantages of PLFEI in mind, however, some disadvantages need to be considered. First, the number of cases is relatively small. Second, there are only 3 patients with NAFLD involved in this study and no patient with severe cirrhosis. Finally, OBV is difficult to be estimated accurately before surgery. Therefore, the related comprehensive evaluation methods need further study.
CONCLUSIONS
PLFEI will be a more comprehensive, sensitive, and accurate index assessing perioperative LRF in liver cancer patients who receive liver resection. Also keeping PLFEI <−1.97 is a safety margin for preventing FLF in PLC patients after liver resection.
Footnotes
Abbreviations: BMI = body mass index, BSA = body surface area, CT = computed tomography, FLF = fatal liver failure, ICG = indocyanine green, ICGK = indocyanine green elimination rate, ICGR15 = indocyanine green retention rate at 15 minute, INR = international normalized ratio, LRF = liver reserve function, NAFLD = nonalcoholic fatty liver disease, OBV = operative bleeding volume, PLC = primary liver cancer, PLFEI = preoperative liver functional evaluation index, POLD = postoperative liver dysfunction, PT = prothrombin time, ROC = receiver-operating characteristic, SRLV = standard remnant liver volume, TLV = total liver volume.
JL and BL contributed equally to this work.
This work was supported in part by the National Natural Science Foundation of China (Nos. 31370917 and 81430014), the Natural Science Foundation of Guangxi Province (No. 2014GXNSFDA118019), the Science and Technology Planning Project of Guangxi Province (1298003-2-1 and 1140003B-79), the Lijiang Scholarship Foundation and the Science and Technology Planning Project of Guilin City (20110119-1-8), the Key Project of Educational Commission, Guangxi Province (201102ZD024), the Key Project of Medical Research Foundation, Department of Public Health, Guangxi Province (2011006), the Guangxi Distinguished Experts Special Fund, the Guangxi Health and Family Planning Commission “139” leading talents training plan, the Project supported by the Guangxi culture of new century academic and technical leader of special funds, and Scientific Research Innovation Team in Colleges and Universities of Guangxi.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Rahbari NN, Mehrabi A, Mollberg NM, et al. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg 2011; 253:453–469. [DOI] [PubMed] [Google Scholar]
- 2.Cescon M, Vetrone G, Grazi GL, et al. Trends in perioperative outcome after hepatic resection: analysis of 1500 consecutive unselected cases over 20 years. Ann Surg 2009; 249:995–1002. [DOI] [PubMed] [Google Scholar]
- 3.Yang T, Zhang J, Lu JH, et al. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg 2011; 35:2073–2082. [DOI] [PubMed] [Google Scholar]
- 4.Li H, Li B, Wei Y. Potential factors dedicated to postoperative liver dysfunction in patients with normal preoperative ICG-15 clearance rate. Dig Dis Sci 2012; 58:1163–1164. [DOI] [PubMed] [Google Scholar]
- 5.Greco E, Nanji S, Bromberg IL, et al. Predictors of peri-opertative morbidity and liver dysfunction after hepatic resection in patients with chronic liver disease. HPB (Oxford) 2011; 13:559–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levesque E, Hoti E, Azoulay D, et al. Non-invasive ICG-clearance: a useful tool for the management of hepatic artery thrombosis following liver transplantation. Clin Transplant 2011; 25:297–301. [DOI] [PubMed] [Google Scholar]
- 7.Tralhao JG, Hoti E, Oliveiros B, et al. Study of perioperative liver function by dynamic monitoring of ICG-clearance. Hepatogastroenterology 2012; 59:1179–1183. [DOI] [PubMed] [Google Scholar]
- 8.Du Z, Li B, Wei Y, et al. A new scoring system for assessment of liver function after successful hepatectomy in patients with hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int 2011; 10:265–269. [DOI] [PubMed] [Google Scholar]
- 9.Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002; 236:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smyrniotis V, Farantos C, Kostopanagiotou G, et al. Vascular control during hepatectomy: review of methods and results. World J Surg 2005; 29:1384–1396. [DOI] [PubMed] [Google Scholar]
- 11.Rahbari NN, Koch M, Mehrabi A, et al. Portal triad clamping versus vascular exclusion for vascular control during hepatic resection: a systematic review and meta-analysis. J Gastrointest Surg 2009; 13:558–568. [DOI] [PubMed] [Google Scholar]
- 12.Sitzmann JV, Greene PS. Perioperative predictors of morbidity following hepatic resection for neoplasm. A multivariate analysis of a single surgeon experience with 105 patients. Ann Surg 1994; 219:13–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okabe H, Beppu T, Chikamoto A, et al. Remnant liver volume-based predictors of postoperative liver dysfunction after hepatectomy: analysis of 625 consecutive patients from a single institution. Int J Clin Oncol 2014; 19:614–621. [DOI] [PubMed] [Google Scholar]
- 14.Sadamori H, Yagi T, Shinoura S, et al. Risk factors for major morbidity after liver resection for hepatocellular carcinoma. Br J Surg 2013; 100:122–129. [DOI] [PubMed] [Google Scholar]
- 15.Paugam-Burtz C, Janny S, Delefosse D, et al. Prospective validation of the “fifty-fifty” criteria as an early and accurate predictor of death after liver resection in intensive care unit patients. Ann Surg 2009; 249:124–128. [DOI] [PubMed] [Google Scholar]
- 16.Brunt EM, Kleiner DE, Wilson LA, et al. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology 2011; 53:810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mathur AK, Ghaferi AA, Osborne NH, et al. Body mass index and adverse perioperative outcomes following hepatic resection. J Gastrointest Surg 2010; 14:1285–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition 1989; 5:303–311; discussion 312–313. [PubMed] [Google Scholar]
- 19.Pugh RN, Murray-Lyon IM, Dawson JL, et al. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 1973; 60:646–649. [DOI] [PubMed] [Google Scholar]
- 20.Farrell GC, Chitturi S, Lau GK, et al. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol 2007; 22:775–777. [DOI] [PubMed] [Google Scholar]
- 21.Chitturi S, Farrell GC, Hashimoto E, et al. Non-alcoholic fatty liver disease in the Asia-Pacific region: definitions and overview of proposed guidelines. J Gastroenterol Hepatol 2007; 22:778–787. [DOI] [PubMed] [Google Scholar]
- 22.Aramaki O, Takayama T, Higaki T, et al. Decreased blood loss reduces postoperative complications in resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci 2014; 21:585–591. [DOI] [PubMed] [Google Scholar]
- 23.Kim YK, Chin JH, Kang SJ, et al. Association between central venous pressure and blood loss during hepatic resection in 984 living donors. Acta Anaesthesiol Scand 2009; 53:601–606. [DOI] [PubMed] [Google Scholar]
- 24.Ren Z, Xu Y, Zhu S. Indocyanine green retention test avoiding liver failure after hepatectomy for hepatolithiasis. Hepatogastroenterology 2012; 59:782–784. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi M, Ikeda K, Kawamura Y, et al. High serum des-gamma-carboxy prothrombin level predicts poor prognosis after radiofrequency ablation of hepatocellular carcinoma. Cancer 2009; 115:571–580. [DOI] [PubMed] [Google Scholar]
- 26.Gruttadauria S, Vasta F, Minervini MI, et al. Significance of the effective remnant liver volume in major hepatectomies. Am Surg 2005; 71:235–240. [PubMed] [Google Scholar]
- 27.Ting CT, Chen RC, Chen CC, et al. Diabetes worsens the surgical outcomes in cirrhotic patients with hepatocellular carcinoma. Tohoku J Exp Med 2012; 227:73–81. [DOI] [PubMed] [Google Scholar]
- 28.Komura T, Mizukoshi E, Kita Y, et al. Impact of diabetes on recurrence of hepatocellular carcinoma after surgical treatment in patients with viral hepatitis. Am J Gastroenterol 2007; 102:1939–1946. [DOI] [PubMed] [Google Scholar]
- 29.Kooby DA, Fong Y, Suriawinata A, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg 2003; 7:1034–1044. [DOI] [PubMed] [Google Scholar]
- 30.McCormack L, Petrowsky H, Jochum W, et al. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg 2007; 245:923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walsh MJ. Systematic review and meta-analysis of steatosis as a risk factor in major hepatic resection. Br J Surg 2010; 97:1331–1339. [DOI] [PubMed] [Google Scholar]
- 32.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology 2010; 51:1820–1832. [DOI] [PubMed] [Google Scholar]
- 33.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer 2006; 118:3030–3044. [DOI] [PubMed] [Google Scholar]
- 34.Cucchetti A, Cescon M, Ercolani G, et al. Safety of hepatic resection in overweight and obese patients with cirrhosis. Br J Surg 2011; 98:1147–1154. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka S, Iimuro Y, Hirano T, et al. Safety of hepatic resection for hepatocellular carcinoma in obese patients with cirrhosis. Surg Today 2013; 43:1290–1297. [DOI] [PubMed] [Google Scholar]
- 36.Wiggans MG, Lordan JT, Shahtahmassebi G, et al. The interaction between diabetes, body mass index, hepatic steatosis, and risk of liver resection: insulin dependent diabetes is the greatest risk for major complications. HPB Surg 2014; 2014:586159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang YY, Huang S, Zhong JH, et al. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma after curative hepatectomy. PLoS One 2014; 9:e113858. [DOI] [PMC free article] [PubMed] [Google Scholar]


