Abstract
The aim of this study was to evaluate the Parkinson disease (PD) prevalence of cognitive impairment in Taiwan.
The case–control study consisted of 6177 cognitive impairment patients and 24,708 noncognitive impairment as controls for the period of 2006 to 2010 and both of the groups aged ≥50 years. The multivariable logistic regression analyses were used to estimate the odds ratio (OR) for cognitive impairment, and the 95% confidence intervals (CIs) among patients with PD were compared with those of non-PD patients.
PD (adjusted odds ratio [aOR] is 3.07, 95% CI 2.76–3.41) is the one of the most contributed risk factors for cognitive impairment. Besides, we found a remarkable result of the diagnosed cognitive impairment of PD that was found highest in the first 6 months (aOR 11.98, 95% CI 8.51–16.86) and then decrease the incident year by year. The PD prevalence in a patient with cognitive impairment in our data present is 12.1% lower than those with truly dementia published previously and documented by western studies.
We found a remarkable result of the diagnosed cognitive impairment of PD that was found highest in the first 6 months and then decrease the incident year by year.
INTRODUCTION
Parkinson disease (PD) is a degenerative disorder of the central nervous system; it was originally described in 1817 by James Parkinson in the classic “Essay on the Shaking Palsy.”1 The motor symptoms appear early in the course of the disease, are the main parts of PD, and result from the death of dopamine-generating cells in the substantia nigra,2 a region of the midbrain3; although the cause of this cell death is not well known until now,4 connecting to an abnormal protein that accumulates inside neurons in the substantia nigra was found by Professor Lewy in 1900s and still bears the name of being a “Lewy body.”5 Except movement disorders, mild cognitive impairment noted in the early stage of PD is common and increases the risk for dementia.6–8 Besides, the cognitive impairments subtypes in PD are various, including dementia with Lewy bodies, Parkinson disease dementia (PDD), and dementia of Alzheimer type, which are not rare even, and it is difficult to separate the distinguishing.9–13 Clinically, the main difference between PDD and Lewy body dementia is a bit arbitrary; cognitive impairment characters in PD patients are different from Alzheimer dementia.14 Altogether, PD with cognitive impairment is multifactorial, in the involving subcortical parts, in the underline pathology,15,16 and in circuits of cortical connection with subcortical neurons.17 Traditionally, it had been thought to be linked to the late stage of PD, but recent evidences suggest that it may appear early in the evolution of PD.18–20 More evidences suggest that proposed risk for developing dementia in PD is 2 to 6 times greater than the prevalence rate in general population and it increases in relation to disease duration.11,17 To further evaluate the cognitive impairment and diagnosed dementia prevalence of PD in Taiwan, we conducted the population-based case–control studies for retrospectively assessment whether cognitive impairment developed in evolution of PD and risk factors of comorbidity. In our data limitation and study design, we cannot further separate the cognitive impairment subtype. To the best of our knowledge, there are no population-based case–control studies that outline the possible relationship between PD and cognitive impairment in Taiwan. The objective of this study was to investigate the slope trend and risk factors of diagnosed dementia among patients with PD in Taiwan.
METHODS
Data Sources and Study Population
Since 1995, the Taiwanese government implemented the NHI program, which provides general health insurance coverage to almost the entire Taiwanese population. Until the end of 2009, the insurance program had established contracts with 97% of clinics and hospitals. We performed a case–control study of medical information from the nationwide population-based data released by the National Health Research Institute (NHRI) for the period of 1996 to 2010. With approval from the NHRI, we used the scrambled patient identification numbers to interlink files including outpatient, inpatient claims and the registry of beneficiaries. Available sociodemographic information for study subjects included sex, birth date, occupation, and place of residence. Diagnoses were coded according to the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). The NHRI encrypts the patients’ personal information for privacy protection and provides researchers with anonymous identification numbers associated with the relevant claim information, which includes the patient's sex, date of birth, registry of medical services, and medication prescriptions. Patient consent is not required for accessing the National Health Insurance Research Database (NHIRD) or Longitudinal Health Insurance Database (LHID). This study was exempted by the Institutional Review Board of China Medical University in central Taiwan (CMU-REC-101–012).
We used the claims data set of the LHID, which consists of 1,000,000 people randomly selected from all those insured, with claims data abstracted from 1996 to 2010. The NHRI reported no substantial differences in age and sex between the LHID and all insured (http://nhird.nhri.org.tw/en/index.htm). Information on these databases has been presented in the previous articles. Other studies have also demonstrated the accuracy and high validity of diagnoses in the NHIRD.
Study Subjects
According to case–control study design, we identified patients aged ≥50 years with a newly diagnosed cognitive impairment, cording as dementia (ICD-9 codes 290, 294.1 and 331.0, and 331.82), during the period of 2006 to 2010 as the case group. The index date was defined as the date of cognitive impairment diagnosis. The control group was aged ≥50 years and randomly selected from subjects in the database without cognitive impairment during the same time period and frequency matched with the age (every 5 years) and sex, at a ratio of 1:2. The index date of the control subjects was randomly assigned a month and a day with the same year as the matched cases. Patients with missing information on sex and age were excluded from the study. The interesting risk factor was the history of PD (ICD-9 code 332). Histories of diabetes (ICD-9 code 250), coronary artery disease (CAD) (ICD-9 codes 410–414), hypertension (ICD-9 codes 401–405), hyperlipidemia (ICD-9 code 272), stroke (ICD-9 codes 430–438), and head injury (ICD-9 codes 850–854, 959.01) were identified based on the diagnoses of hospital admissions before the index date.
Besides the age and sex, we also considered the urbanization of the residence and the occupation as demographic factors. The occupation was classified as 3 groups: white collar: the longer indoor work, like institution worker, business worker, and others; blue collar: longer outdoor worker such as those for farmers, industrial laborers, and others; and others: retired, low income, and so on. According to the Liu report,21 we categorized the subjects registered for insurance into 4 levels of urbanization based on the index of incorporating variables indicating population density (people/km2), and population ratio of different educational levels, population ratio of elderly, population ratio of people of agriculture workers, and the number of physicians per 100,000 people. The level 1 of the urbanization presented the highest urbanization level and the level 4+ meant lowest levels.
Diagnosed Dementia Coded
For those clinical consultation patients who have symptoms of lapses in memory and decline in the ability to talk, read, and write, problems with finding the right words or change mood, change personality, etc, the brain images assist to the evaluation of intracranial insults, and degenerative and vascular events. The serum and blood chemistries examinations served to exclude the metabolic or systemic conditions. Minimental statement examination and Clinical Dementia Rating was used to evaluate and follow up the cognitive function. After complete evaluation, the disease could be coded as the correct conditions as possible, 290, 331, 294, or others according to generalized collected data evidences that fulfill the Diagnostic and Statistical Manual of Mental Disorders IV or Movement Disorder Society different dementia diagnosis criteria. However, from the database, we can see the disease coded but not the disease severity, which may be ranged from mild cognitive impairment, mild dementia to severe dementia. Therefore, the statement we conduct cognitive impairment in this study include different types and severities of dementia.
Statistical Analysis
Demographic and clinical characteristic of cognitive impairment patients and control subjects were presented using the total number (percentage) for categorical variables. Differences were examined using the χ2 test for categorical variables. The sex-specific, age-specific, and comorbidity-specific odds ratios (ORs) of cognitive impairment were determined using logistic regression.
Logistic regression analyses were used to estimate the OR for cognitive impairment, and the 95% confidence intervals (CIs) among patients with PD were compared with those of non-PD patients. The models were adjustment by age, sex, CAD, diabetes, hypertension, stroke, hyperlipidemia, and head injury. Both crude ORs and multivariable adjusted ORs (aORs) were used to measure the risk for dementia. We used the SAS statistical package (version 9.1; SAS Institute Inc., Cary, NC) for statistical analyses. Statistical significance was defined as 2 sided, P < 0.05.
RESULTS
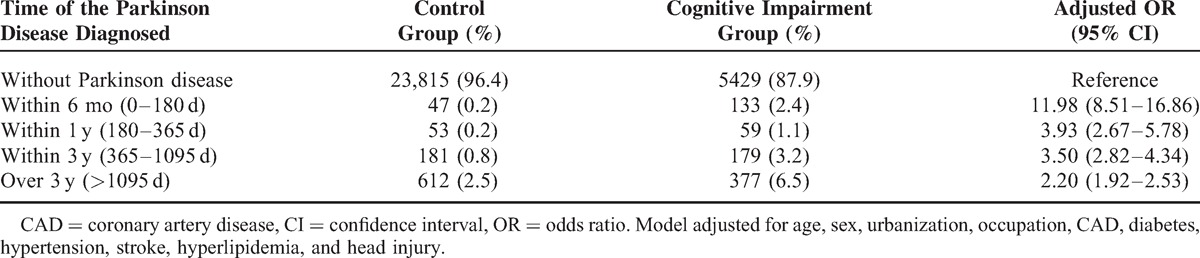
The case–control study consisted of 6177 cognitive impairment patients and 24,708 noncognitive impairment patients as controls for the period of 2006 to 2010. The distribution of age and sex between cases and controls were similar (Table 1). Approximately 46.4% of the overall participants were male. Most subjects were >70 years old (about 79.7%). Urbanization level did not have significant difference. Cases and controls had a quiet different distribution of overall baseline comorbidities (P < 0.0001), such as occupation, hypertension, diabetes, CAD, stroke, hyperlipidemia, and head injury. The blue-collar level had higher prevalence than white-collar group (P < 0.0001).
TABLE 1.
Demographic Status and Comorbidity Compared Between Control Group and Cognitive Impairment Group
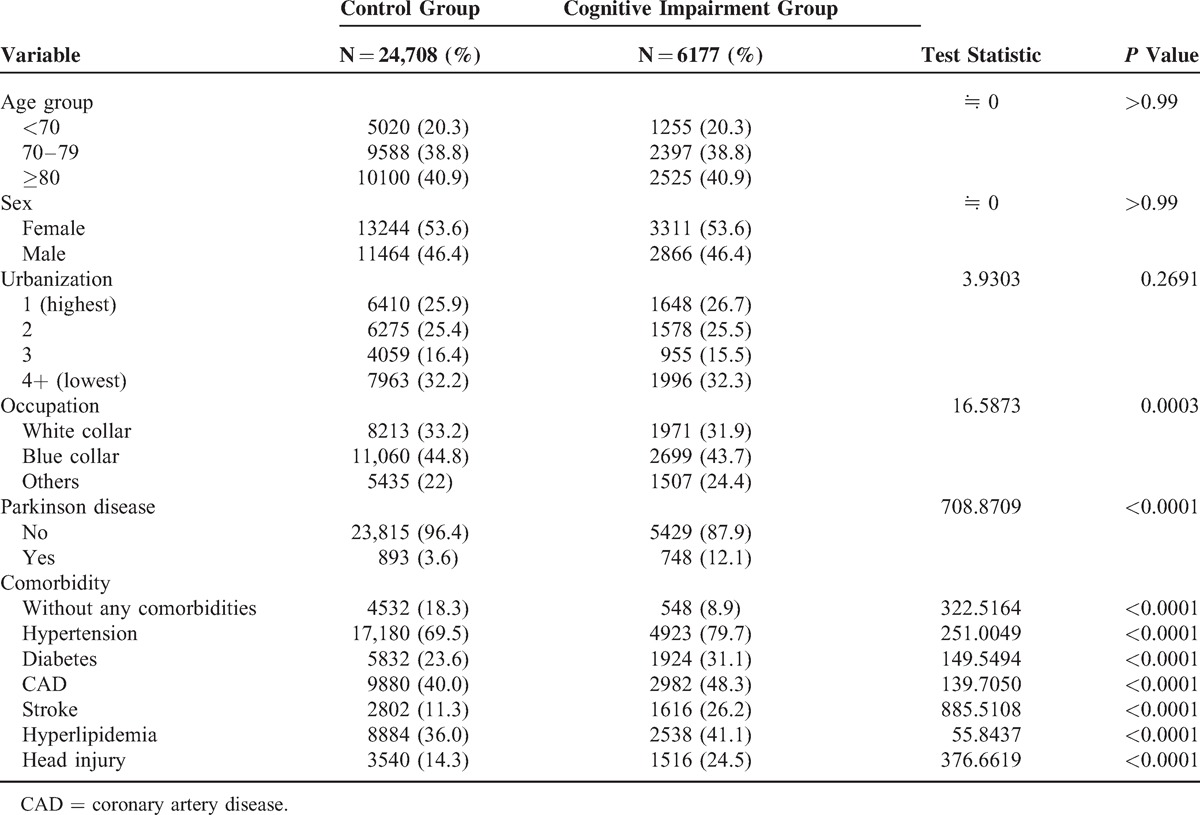
Table 2 presents the OR and aOR of cognitive impairment between patients with PD and without PD according to demographic factors and comorbidity. The crude OR was 3.67 (95% CI 3.32–4.07) risk for cognitive impairment. When we adjusted for age, sex, and other comorbidities, aOR showed that the individual with PD had 3.07 (95% CI 2.76–3.41) risk for dementia, relative to the individual without PD.
TABLE 2.
Odds Ratios for Cognitive Impairment in Individual With Parkinson Disease Relative to Without Parkinson Disease
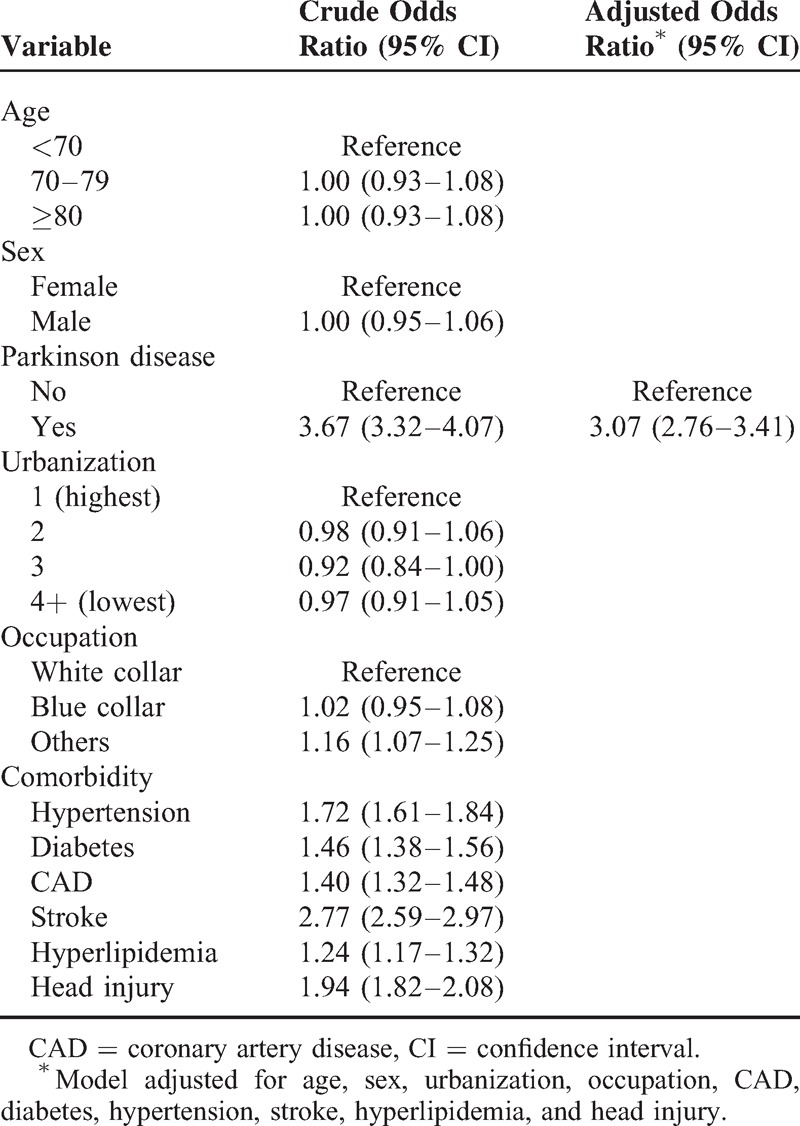
Table 3 pointed out the comorbidities interaction and estimated OR for diagnosed dementia among different existing morbidities. The estimated OR was adjusted for age, sex, urbanization, and occupation. Whether hypertension existed or not, OR for cognitive impairment among PD patients showed the significant high risk for diagnosed cognitive impairment that were 3.26 (95% CI 2.92–3.65) to 5.38 (95% CI 4.15–6.99) when compared to the non-PD group. Among diabetes patients, OR for diagnosed cognitive impairment also showed significant high risk from 2.83 (95% CI 2.36–3.40) to 4.05 (95% CI 3.58–4.58). In the CAD morbidity, OR for diagnosed cognitive impairment was present as significant high risk from 2.86 (95% CI 2.50–3.28) to 4.70 (95% CI 4.03–5.48). In stroke, OR for diagnosed cognitive impairment was presence as significant high risk from 1.71 (95% CI 1.41–2.08) to 4.27 (95% CI 3.78–4.82). In head injury, OR for diagnosed cognitive impairment was also present as significant high risk from 2.94 (95% CI 2.40–3.60) to 3.74 (95% CI 3.32–4.21). In the hyperlipidemia, OR for cognitive impairment was about 3.48 to 3.74-fold risk, despite without statistical significance.
TABLE 3.
Effect of Parkinson Disease to the Cognitive Impairment Under Different Comorbidity Status
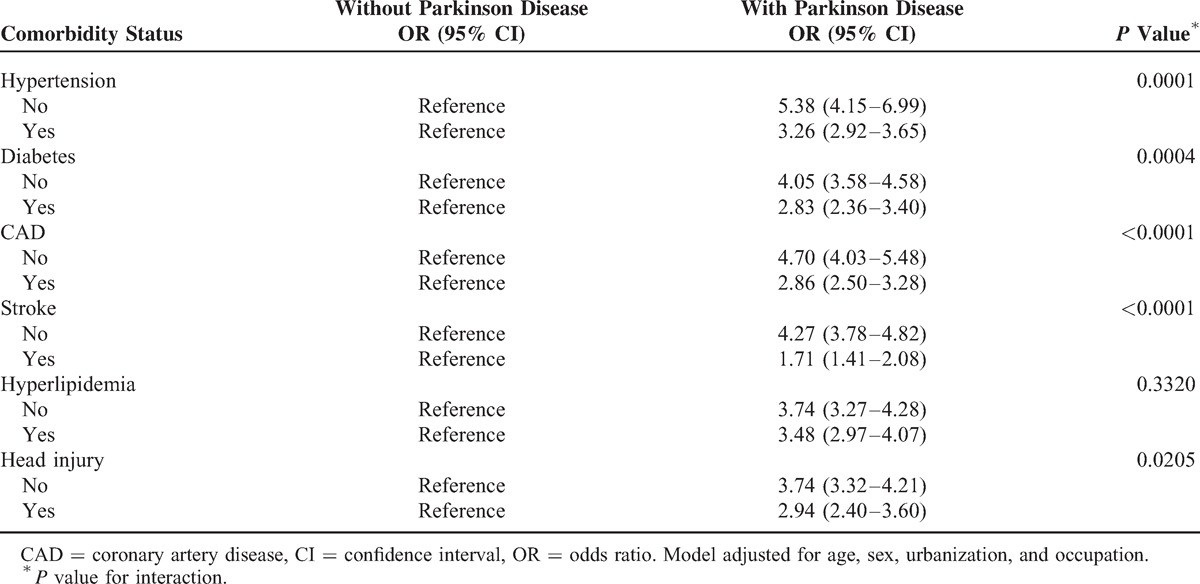
Analyzing the association of time of the PD diagnosed showed that subjects with short diagnosed period within 6 months (0–180 days) had a highest risk for diagnosed cognitive impairment (aOR 11.98, 95% CI 8.51–16.86), the risk for cognitive impairment among participants without PD as reference. Within 1 year, the aOR for diagnosed cognitive impairment (aOR 3.93, 95% CI 2.67–5.78), and within 3 years (365–1095 days), the aOR for diagnosed cognitive impairment (aOR 3.50, 95% CI 2.82–4.34), also demonstrated higher risk for diagnosed cognitive impairment. When the diagnosed time was >3 years, the aOR for diagnosed cognitive impairment was reduced to 2.20-fold (95% CI 1.92–2.53). The aOR for diagnosed cognitive impairment between with and without PD group among the different diagnosed time of PD showed a reverse gradient.
DISCUSSION
We analyzed a population-based case–control study of PD enrolment in the National Health Insurance Database 2006 to 2010, living in Taiwan. We found that a remarkable result of the diagnosed cognitive impairment of PD were found highest in the first 6 months (aOR 11.98, 95% CI 8.51–16.86) and then decrease the incident year by year; the first year of aOR is 3.93 (95% CI 2.67–5.78), within 3 years is 3.50 (95% CI 2.82–4.34), and >3 years aOR is 2.20 (95% CI 1.92–2.53) (Table 4). The education plays an important role in our study; Blue-collar patients (43.7%) have higher prevalence than white collar (31.9%) (P < 0.0001), although the lower education was associated with a greater risk for dementia in many but not all studies.22 From the record, we can understand that early neuropsychological abnormalities are many but easy to unheed in PD previously due to lack of routine clinical test using neurophysiologic markers with cognitive assessment in early disease stages. Therefore, it may be low estimate cognitive impairment in the past studies. The previous systemic review study emphasizes that the exact prevalence of dementia in PD is not clearly known,10 but the current data show that it occurs frequently and has more impact on reduction in quality of live.17,23 However, the diagnosed cognitive impairment in our database cannot clarify the etiologies or subtypes. We can expect to add routine test in early-stage PD of combining neurophysiologic markers with cognitive assessment in PD that can substantially improve dementia risk profiling in PD, providing potential benefits for clinical care as well as for the future development of therapeutic strategies.24–27
TABLE 4.
Association Between Diagnosed Timing of the Parkinson Disease and Cognitive Impairment Risk
Furthermore, we compare chronic diseases, head injury, and stroke; our studies demonstrate that the most contributed risk factor for diagnosed cognitive impairment is PD per se (OR is 3.67, 95% CI 2.76–3.41), followed by stroke (OR is 2.77, 95% CI 2.59–2.97), hypertension (OR is 1.72, 95% CI 1.61–1.84), diabetes mellitus (OR is 1.46, 95% CI 1.38–1.56), CAD (OR is 1.40, 95% CI 1.32–1.48), and hyperlipidemia (OR is 1.24, 95% CI 1.17–1.32) (Table 2.). The age and sex are not different in our studies.
Though, we are interested in whether or not the ethnic and genetic contribute to cognitive impairment either, we did not get the information from our database and it need to be explored in future studies.
The prevalence rate of PD with diagnosed cognitive impairment in our data present is 12.1% lower than those with truly dementia published previously and documented by western studies.11,25,28 We consider that it may have underestimation in our database, for estimates are influenced even more by case-finding strategies. Record-based studies and studies done in clinical settings do not include patients who have not met the criteria of clinical diagnosis of dementia, but is often difficult to make meaningful conclusion in PD patients, and thus underestimate the prevalence or incidence of diagnosed cognitive impairment in PD patients.
In addition, the study was subject to some limitations, which must be mentioned. First, the NHIRD does not provide detailed information on patients such as their alcohol consumption, body mass index, physical activity, and socioeconomic status. All of these are possible confounding factors in this study. Second, the evidence derived from a case–control study is generally of a lower methodological quality than that from randomized trials because an observation study design is subject to many biases related to adjustment for confounds. Despite our meticulous study design with adequate control of confounding factors, a key limitation was that bias could still remain because of possible unmeasured or unknown confounders. Third, the registries in NHI claims primarily serve the purpose of administrative billing and do not undergo verification for scientific purposes. We were unable to contact the patients directly to obtain more information because of the anonymity assured by the identification numbers. However, the data that we obtained on the diagnoses of PD and diagnosed dementia were highly reliable. Finally, additional large population-based unbiased randomized trials are required, and it would be essential to confirm our current findings before drawing any firm conclusions.
Footnotes
Abbreviations: aOR = adjusted odds ratio, CI = confidence interval, ICD-9-CM = International Classification of Diseases, Ninth Revision, Clinical Modification, LHID = Longitudinal Health Insurance Database, NHIRD = National Health Insurance Research Database, NHRI = National Health Research Institute, PD = Parkinson disease, PDD = Parkinson disease dementia.
The authors’ individual contributions were as follows. Conception and design: Y-CH, C-HK. Administrative support: C-HK. Collection and assembly of data: All authors. Data analysis and interpretation: All authors. Manuscript writing: All authors. Final approval of manuscript: All authors.
This work was supported by the Health and Welfare Surcharge of Tobacco Products, China Medical University Hospital Cancer Research Center of Excellence (MOHW104-TD-B-111-03, Taiwan), Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW104-TDU-B-212-113002), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM104010092), NRPB Stroke Clinical Trial Consortium (MOST 103-2325-B-039 -006), Tseng-Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding received for this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Pearce JM. Aspects of the history of Parkinson's disease. J Neurol Neurosurg Psychiatry 1989; 52 suppl:6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hornykiewicz O. The discovery of dopamine deficiency in the parkinsonian brain. J Neural Transm Suppl 2006; (70):9–15. [DOI] [PubMed] [Google Scholar]
- 3.Obeso JA, Rodriguez-Oroz MC, Goetz CG, et al. Missing pieces in the Parkinson's disease puzzle. Nat Med 2010; 16:653–661. [DOI] [PubMed] [Google Scholar]
- 4.Andrew J, Lees JH, Tamas Revesz. Parkinson's disease. Lancet 2009; 373:2055–2066. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues e Silva AM, Geldsetzer F, Holdorff B, et al. Who was the man who discovered the “Lewy bodies”? Mov Disord 2010; 25:1765–1773. [DOI] [PubMed] [Google Scholar]
- 6.Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology 2010; 75:1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erro R, Santangelo G, Barone P, et al. Do subjective memory complaints herald the onset of mild cognitive impairment in Parkinson disease? J Geriatr Psychiatry Neurol 2014; 27:276–281. [DOI] [PubMed] [Google Scholar]
- 8.Pedersen KF, Larsen JP, Tysnes OB, et al. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol 2013; 70:580–586. [DOI] [PubMed] [Google Scholar]
- 9.Aarsland D, Andersen K, Larsen JP, et al. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol 2003; 60:387–392. [DOI] [PubMed] [Google Scholar]
- 10.Dag Aarsland M, Julia Zaccai, Carol Brayne. A systematic review of prevalence studies of dementia in Parkinson's disease. Mov Disord 2005; 20:1255–1263. [DOI] [PubMed] [Google Scholar]
- 11.Aarsland D, Kurz MW. The epidemiology of dementia associated with Parkinson disease. J Neurol Sci 2010; 289:18–22. [DOI] [PubMed] [Google Scholar]
- 12.Savica R, Grossardt BR, Bower JH, et al. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol 2013; 70:1396–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jellinger KA. Mild cognitive impairment in Parkinson disease: heterogenous mechanisms. J Neural Transm 2013; 120:157–167. [DOI] [PubMed] [Google Scholar]
- 14.Svenningsson P, Westman E, Ballard C, et al. Cognitive impairment in patients with Parkinson's disease: diagnosis, biomarkers, and treatment. Lancet Neurol 2012; 11:697–707. [DOI] [PubMed] [Google Scholar]
- 15.Compta Y1, Parkkinen L, O'Sullivan SS, et al. Lewy- and Alzheimer-type pathologies in Parkinson's disease dementia: which is more important. Brain 2011; 134:1493–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yarnall AJ, Breen DP, Duncan GW, et al. Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology 2014; 82:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caballol N, Marti MJ, Tolosa E. Cognitive dysfunction and dementia in Parkinson disease. Mov Disord 2007; 22 suppl 17:S358–S366. [DOI] [PubMed] [Google Scholar]
- 18.Aarsland D, Bronnick K, Larsen JP, et al. Norwegian ParkWest Study Group. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology 2009; 72:1121–1126. [DOI] [PubMed] [Google Scholar]
- 19.Benito-Leon J, Louis ED, Posada IJ, et al. Population-based case-control study of cognitive function in early Parkinson's disease (NEDICES). J Neurol Sci 2011; 310:176–182. [DOI] [PubMed] [Google Scholar]
- 20.Sanchez-Ferro A, Benito-Leon J, Louis ED, et al. Rate of cognitive decline in premotor Parkinson's disease: a prospective study (NEDICES). Mov Disord 2013; 28:161–168. [DOI] [PubMed] [Google Scholar]
- 21.Liu C, Hung Y, Chuang Y, et al. Incorporating development stratification of Taiwan townships into sampling design of large scale health interview survey. J Health Manage 2006; 4:1–22. [Google Scholar]
- 22.Sharp ES, Margaret Gatz. The relationship between education and dementia: an updated systematic review. Alzheimer Dis Assoc Disord 2011; 25:286–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Germain S, Adam S, Olivier C, et al. Does cognitive impairment influence burden in caregivers of patients with Alzheimer's disease? J Alzheimer Dis 2009; 17:105–114. [DOI] [PubMed] [Google Scholar]
- 24.Olde Dubbelink KT, Hillebrand A, Twisk JW, et al. Predicting dementia in Parkinson disease by combining neurophysiologic and cognitive markers. Neurology 2014; 82:263–270. [DOI] [PubMed] [Google Scholar]
- 25.Wang HF, Yu JT, Tang SW, et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson's disease, Parkinson's disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J Neurol Neurosurg Psychiatry 2015; 86:135–143. [DOI] [PubMed] [Google Scholar]
- 26.Emre M, Tsolaki M, Bonuccelli U, et al. Memantine for patients with Parkinson's disease dementia or dementia with Lewy bodies: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010; 9:969–977. [DOI] [PubMed] [Google Scholar]
- 27.Lonneke ML, de Lau MMBB. Epidemiology of Parkinson's disease. Lancet Neurol 2006; 5:525–535. [DOI] [PubMed] [Google Scholar]
- 28.Lin RT, Lai CL, Tai CT, et al. Prevalence and subtypes of dementia in southern Taiwan: impact of age, sex, education, and urbanization. J Neurol Sci 1998; 160:67–75. [DOI] [PubMed] [Google Scholar]


