Supplemental Digital Content is available in the text
Abstract
Current pegylated interferon-α (PEG-IFN) treatment for chronic hepatitis B (CHB) e-antigen (HBeAg)-positive patients are suboptimal, and effective ways of improving PEG-IFN treatment efficacy are needed.
This retrospective cohort study compared the efficacy of a PEG-IFN stepwise optimization treatment (PEG-IFN SOT) strategy with that of a 48-week PEG-IFN standard therapy (PEG-IFN ST) in HBeAg-positive CHB patients.
A total of 110 patients were included in our study. Of these, 70 received the PEG-IFN SOT and 40 received the PEG-IFN ST (control group). We based the decision whether to add adefovir and/or extend the PEG-IFN–based treatment to 96 weeks on the patients’ 12-week or 24-week early virological response (12W EVR, at least a 2 log10 reduction in HBV DNA copies/mL at week 12; 24W EVR, at least 1 log10 reduction in HBsAg IU/mL or HBsAg <1500 IU/mL at week 24) and their 48-week partial response (48W PR, 1.0 ≤HBeAg ≤10.0 S/CO or HBeAg >10.0 S/CO but HBsAg <1000 IU/mL).
The HBeAg seroconversion rate 24 weeks post-PEG-IFN treatment was significantly higher in the PEG-IFN SOT than the PEG-IFN ST group (50% vs 22.5%, P = 0.005). The HBsAg clearance rates in the PEG-IFN SOT and ST groups were 10% and 0% (P = 0.04), respectively. Receiving PEG-IFN SOT (OR = 0.26, P = 0.01), ALT × ULN at baseline (OR = 0.74, P = 0.003), and achieving 12 and 24W EVR (OR = 0.29, P = 0.03) were independent factors associated with HBeAg seroconversion.
PEG-IFN SOT is a promising strategy for achieving high rates of serological response in HBeAg-positive CHB patients.
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is a major public health problem affecting >350 million people worldwide. It is also a leading cause of chronic hepatitis B (CHB), cirrhosis, and hepatocellular carcinoma.1 China has a high prevalence of chronic HBV infection, with an HBV surface antigen (HBsAg) carrier rate of 7.18%.2 Although a well tolerated and effective vaccine has been available for many years, chronic HBV infection still causes significant morbidity and mortality.3
Recent studies have demonstrated that effective anti-HBV therapy arrests or delays CHB progression.4 However, the complete eradication of HBV is rarely achieved due to the persistence of covalently closed circular DNA in host hepatocytes.5 At present, antivirals for CHB patients include interferon (IFN) (both conventional IFN-α and pegylated IFN-α [PEG-IFN-α]) and nucleot(s)ide analogues (NAs).6 Although NAs therapy may effectively suppress HBV, the hepatitis B virus e antigen (HBeAg) seroconversion rate after 1 year of treatment is about 20% irrespective of the NAs type used.7–11 Furthermore, NA-induced HBeAg seroconversion may not durable after NAs discontinuation, and protracted NAs’ use is associated with increased drug resistance.6 Compared with NAs, the advantages of IFN treatment are limited duration, absence of resistance, and relatively higher rates of HBeAg seroconversion and HBsAg clearence. PEG-IFN has been shown to reduce the incidence of liver cirrhosis and hepatocellular carcinoma in patients who achieve HBeAg seroconversion.12 However, the HBeAg seroconversion and HBsAg clearence rates with a standard 48-week regimen of PEG-IFN in Chinese patients were unsatisfactory (approximately 30% and 1.8%, respectively).13
Therefore, much attention has focused on improving the antiviral efficacy of PEG-IFN treatment in CHB patients. It has been suggested that the optimal strategy for PEG-IFN treatment of HBeAg-positive patients includes treatment extension, a response-guided therapy strategy, and combining PEG-IFN with NAs.14–18 However, studies have indicated that there was no added benefit in terms of sustained suppression of viral replication from lamivudine (LAM) and PEG-IFN combination therapy compared with PEG-IFN alone.14,15 PEG-IFN plus telbivudine (LdT) exhibited a potent antiviral effect, but was prohibited due to a high risk of severe polyneuropathy.16 Neither entecavir (ETV) add-on nor ETV pretreatment demonstrated superiority to a 48-week PEG-IFN monotherapy.17 Other studies have indicated that the therapeutic effects of PEG-IFN plus adefovir (ADV) in HBeAg-positive patients were controversial.18–20 To date, no studies have investigated the ideal treatment regimen for combining PEG-IFN treatment extension, the addition of NAs, and/or the administration of PEG-IFN response-guided therapy.
Therefore, we aimed at evaluating the efficacy of a PEG-IFN stepwise optimization treatment (SOT) by assessing the 12- or 24-week and 48-week treatment responses to determine ADV add-on and/or PEG-IFN treatment extension in HBeAg-positive CHB patients. This strategy was compared with the current standard 48-week PEG-IFN monotherapy (ST).
METHODS
Patients
We evaluated 119 HBeAg-positive CHB patients who were treated with PEG-IFN-α-2a (PEG-IFN) at Department of the Infectious Diseases, Huashan Hospital, Fudan university, Shanghai, China, from January 2008 to December 2012. The inclusion criteria were pre-treatment HBV DNA levels of >5 log10 copies/mL, HBsAg-positive for >6 months, no exposure to NAs or interferon-α (including conventional or PEG-IFN-α) within the 6 months before PEG-IFN therapy, and having received at least 48 weeks of PEG-IFN-based therapy. Patients with a hepatitis C, D, E, or human immunodeficiency virus co-infection; pregnant women; transplant recipients; those receiving immunosuppressive therapy; those with a Child–Pugh score >5; and patients with immunologically mediated disorders, thyroid dysfunction, ophthalmological disorders, seizure disorders, or serious psychiatric illness were excluded. Patients were also excluded if they had evidence of anemia (hemoglobin levels <11.5 g/dL for women and <12.5 g/dL for men), a neutrophil count <1500 cells/mm3, or a platelet count <90 000 cells/mm3 at screening.
In total, 110 patients who met the inclusion criteria were enrolled. All patients were followed up for 120 weeks after PEG-IFN treatment initiation. In China, medical insurance only covers 48 weeks of PEG-IFN treatment for CHB patients, and PEG-IFN SOT may cause additional economic burden. Therefore, patients were assigned to the PEG-IFN SOT or PEG-IFN ST groups according to their own preferences. In our study, 70 patients received PEG-IFN SOT and 40 received 48-week PEG-IFN ST (Figure 1). In the PEG-IFN SOT group, 57 of 70 (81.4%) were treatment-naïve and 13 of 70 (18.6%) were treatment-experienced (9 had received NAs and 4 had received conventional IFN-α). In the PEG-IFN ST group, 32 of 40 (80%) were treatment-naïve and 8 of 40 (20%) were treatment-experienced (6 had received NAs and 2 had received conventional IFN-α). PEG-IFN was administered at 135 or 180 μg/week and ADV was administered at 10 mg once daily. All patients were identified through an electronic query of CHB HBeAg-positive records at our treatment center using International Classification of Diseases (ICD-9) diagnostic codes. Clinical and laboratory data were collected from the patient history record system.
FIGURE 1.
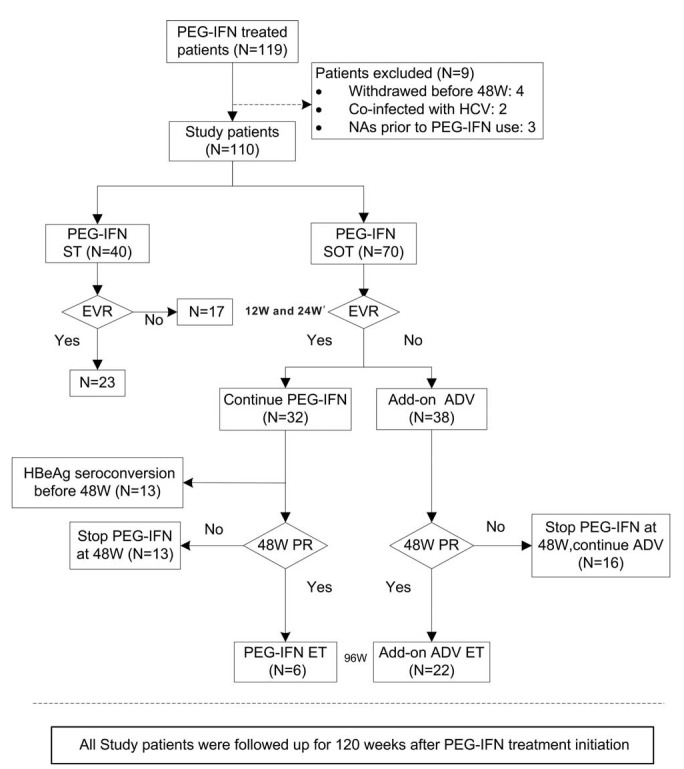
Flow chart of the study population and design. EVR, including 12-week EVR (12W EVR) and 24-week EVR (24W EVR) in our study at two time points. 12W EVR was defined as at least a 2 log10 reduction from baseline in HBV DNA copies/mL at week 12. Twenty-four-week EVR was defined as at least a 1 log10 reduction from baseline in HBsAg IU/mL or HBsAg < 1500 IU/mL at week 24. The 48W PR (48-week partial response) was defined as 1.0 ≤ HBeAg ≤ 10.0 S/CO or HBeAg >10.0 S/CO but HBsAg <1000 IU/mL at week 48. ADV = adefovir, ET = extended treatment, EVR = early virological response, HBeAg = hepatitis B virus e antigen, HCV = hepatitis C virus, NAs = nucleot(s)ide analogues, PEG-IFN SOT = pegylated interferon-α-2a stepwise optimization treatment, PEG-IFN ST = pegylated interferon-α-2a standard therapy, PR = partial response.
Our center's ethics committee reviewed and approved the study design, and all patients provided written informed consent before participation.
Study Design
The protocol for the PEG-IFN SOT is shown in Figure 1. Each patient started with PEG-IFN monotherapy. The first period proposed for monitoring was based on 12-week early virological response (12W EVR; at least a 2 log10 reduction in HBV DNA copies/mL at week 12) or 24-week early virological response (24W EVR; at least 1 log10 reduction in HBsAg IU/mL or HBsAg <1500 IU/mL at week 24). We made the decision to add ADV at week 12 or 24, based on 12W and 24W EVR, respectively. Patients who did not meet the 12W or 24W EVR required immediately ADV add-on at week 12 or 24 after PEG-IFN therapy. Subsequently, patients without HBeAg seroconversion were monitored again at week 48, based on their partial response (48W PR; 1.0 ≤ HBeAg ≤10.0 S/CO or HBeAg >10.0 S/CO but HBsAg <1000 IU/mL). For 12 and 24W EVR patients who achieved a 48W PR, PEG-IFN therapy was continued to 96 weeks. In contrast, those who did not achieve a 48W PR stopped PEG-IFN-α-2a therapy at week 48. For patients without 12W or 24W EVR who achieved a 48W PR, the PEG-IFN and ADV combination therapy was continued to 96 weeks. However, those who did not achieve a 48W PR stopped PEG-IFN administration at week 48, but continued oral ADV therapy.
Efficacy Analysis
The serological response was defined as HBeAg seroconversion or HBsAg clearance, and the virological response as HBV DNA levels <4 log10 copies/mL for patients on PEG-IFN monotherapy, but for patients receiving ADV add-on therapy, the virological response was defined as undetectable HBV DNA by a sensitive PCR assay.6 The primary efficacy parameter was HBeAg seroconversion determined 24 weeks post-PEG-IFN treatment. Secondary efficacy parameters included virological and serological response at week 48, HBsAg clearance and virological response 24 weeks post-PEG-IFN treatment, serological response at week 120 after PEG-IFN treatment initiation.
Safety
We assessed the frequency, nature, and severity of adverse events, as well as changes in clinical laboratory parameters and vital signs from baseline. Adverse events and concomitant medications were recorded every 4 weeks. We analyzed adverse events according to the World Health Organization recommendations for toxicity grading, as adapted for chronic liver disease.
Laboratory Assessments
At each visit, patients underwent analyses for HBV serological markers, HBV DNA quantification, HBsAg quantification, and alanine aminotransferase (ALT) levels. HBV serological markers were analyzed by Abbott chemiluminescence immunoassay (Abbott Laboratories, Abbott Park, IL). Serum HBV DNA concentration was assessed by real-time polymerase chain reaction with a lower detection limit of 1000 copies/mL (DAAN Diagnostics, Guangzhou, China). HBV genotype was detected by directly sequencing the HBV reverse-transcription domain. HBsAg was performed by ADICON Clinical Laboratories (Shanghai, China) using the Abbott ARCHITECT I2000 platform, based on a chemiluminescent microparticle immunoassay with a dynamic range of 0.05 to 250.0 IU/m. Sera with HBsAg above 250.0 IU/ml were subsequently 1:500 serially diluted and retested until they fell within the dynamic range.
Statistical Analysis
Descriptive statistics were used to summarize all variables. Categorical variables are expressed as proportions, and continuous variables are expressed as medians (ranges) or means and standard deviations. Pearson χ2 test or Fisher exact test was used to compare categorical variables, and Student t test or the Mann–Whitney U test was used to compare continuous variables. Independent predictors of HBeAg seroconversion 24 weeks post-PEG-IFN treatment were determined by univariate and multivariate logistic binary regression analyses including the following variables: age; male sex; baseline ALT levels; baseline albumin level; baseline bilirubin level; baseline platelet account; baseline creatinine level; baseline, 12-week, and 24-week HBV DNA concentrations (log10 copies/mL); HBsAg quantification (log10 IU/mL); HBeAg titers (S/CO); 12W and 24W EVR; and PEG-IFN-2α SOT, HBV DNA genotype, and PEG-IFN reductions during therapy. For all statistical tests, a 2-sided P value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS (version 17.0, SPSS Inc, Chicago, IL).
RESULTS
Patients
As shown in Figure 1, 40 study participants received 48 weeks of PEG-IFN ST, and 70 received PEG-IFN SOT. All patients in the PEG-IFN SOT group started with the PEG-IFN monotherapy for 12–24 weeks. A total of 32 patients (45.7%) who had 12W and 24W EVR received monotherapy, and the remaining 38 (54.3%) patients without 12W or 24W EVR (Non-EVR) needed ADV add-on at week 12 or 24. Among the patients who achieved 12W and 24W EVR, 13 achieved HBeAg seroconversion before week 48 and stopped PEG-IFN therapy at week 48, 6 achieved a 48W PR and were recommended to receive PEG-IFN extended monotherapy to 96 weeks, and 13 without a 48W PR stopped PEG-IFN treatment at week 48. At week 48, none of the non-EVR patients achieved HBeAg seroconversion, 22 patients with a PR received extended PEG-IFN and ADV combination therapy to 96 weeks, and 16 without a PR stopped PEG-IFN treatment at week 48, but continued ADV oral antiviral therapy. Patients in the PEG-IFN ST group received PEG-IFN monotherapy for 48 weeks, 23 (57.5%) had 12W and 24W EVR, and 17 (42.5%) had non-EVR. All patients were followed up for 120 weeks after PEG-IFN treatment initiation.
However, because of the retrospective cohort study design, 27 patients without HBeAg seroconversion 24 weeks post PEG-IFN monotherapy received antiviral therapy with NAs in both PEG-IFN SOT and PEG-IFN ST groups (This therapy was provided because of virological recurrence). In the PEG-IFN ST group, 20 of 31 non-HBeAg seroconversion patients received therapy with NAs (2 received LdT, 9 received ETV, and 9 received ADV) 24 weeks post PEG-IFN treatment. In the PEG-IFN SOT group, 7 of 11 non-HBeAg seroconversion patients with 12W and 24W EVR received therapy with NAs (1 received LdT, 4 received ETV, and 2 received ADV).
Patient Baseline Clinical Characteristics
The baseline clinical characteristics of patients in the PEG-IFN ST and SOT groups are shown in Table 1. There were no significant differences between 2 groups.
TABLE 1.
Baseline Clinical Characteristics of Patients

Treatment Efficacy in the Total Study Population
The HBeAg seroconversion rates at week 48 in the PEG-IFN SOT and ST groups were 18.6% (13/70) and 20% (8/40), respectively (P = 0.85, Figure 2A). At 24 weeks post-PEG-IFN treatment, the rates increased to 50% (35/70) and 22.5% (9/40), respectively (P = 0.005, Figure 2A). The HBsAg clearance rates at week 48 were 2.9 % (2/70) and 0% (0/40) in the PEG-IFN SOT and ST groups, respectively (P = 0.53, Figure 2B). At 24 weeks post-PEG-IFN treatment, the rate increased to 10% (7/70) in the PEG-IFN SOT group and remained at 0% (0/40) in the ST group (P = 0.04, Figure 2B). The HBeAg seroconversion rates at week 120 in the PEG-IFN SOT and ST groups were 50.0% (35/70) and 22.5% (9/40), respectively (P = 0.005, Figure 2A). The HBsAg clearance rates at week 120 in the PEG-IFN SOT and ST groups were 10.0% (7/70) and 0% (0/40), respectively (P = 0.04, Figure 2B).
FIGURE 2.
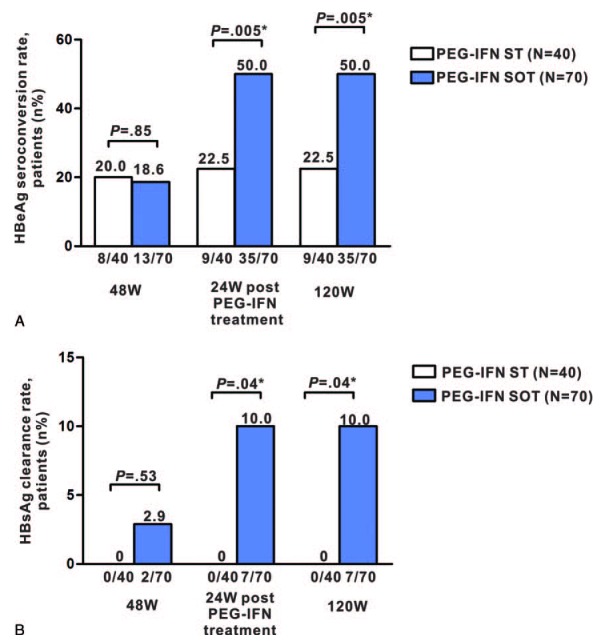
HBeAg seroconversion and HBsAg clearance rates. HBeAg seroconversion (A) and HBsAg clearance (B) rates are shown for the PEG-IFN ST and PEG-IFN SOT groups at week 48, 24 weeks post-PEG-IFN treatment, and 120 weeks after PEG-IFN therapy initiation. HBeAg = hepatitis B virus e antigen, HBsAg = hepatitis B virus surface antigen, PEG-IFN SOT = pegylated interferon-α-2a stepwise optimization treatment, PEG-IFN ST = pegylated interferon-α-2a standard therapy, W, week.
Treatment Efficacy by EVR Status
Among patients who achieved 12W and 24W EVR in the PEG-IFN SOT and ST groups, the HBeAg seroconversion rates at week 48 were 40.6% (13/32) and 30.4% (7/23), respectively (P = 0.53). At 24 weeks post-PEG-IFN treatment, the rates increased to 65.6% (21/32) and 34.8% (8/23), respectively (P = 0.03), and the HBsAg clearance rate increased from 3.1% (1/32) to 15.6% (5/32) in the PEG-IFN SOT group and remained at 0% (0/23) in the ST group (P = 0.05). The HBeAg seroconversion rates at week 120 were 65.6% (21/32) and 34.8% (8/23), respectively (P = 0.03), and the HBsAg clearance rates at week 120 were 15.6% (5/32) and 0% (0/23), respectively (P = 0.05) (Figure 3A and B).
FIGURE 3.
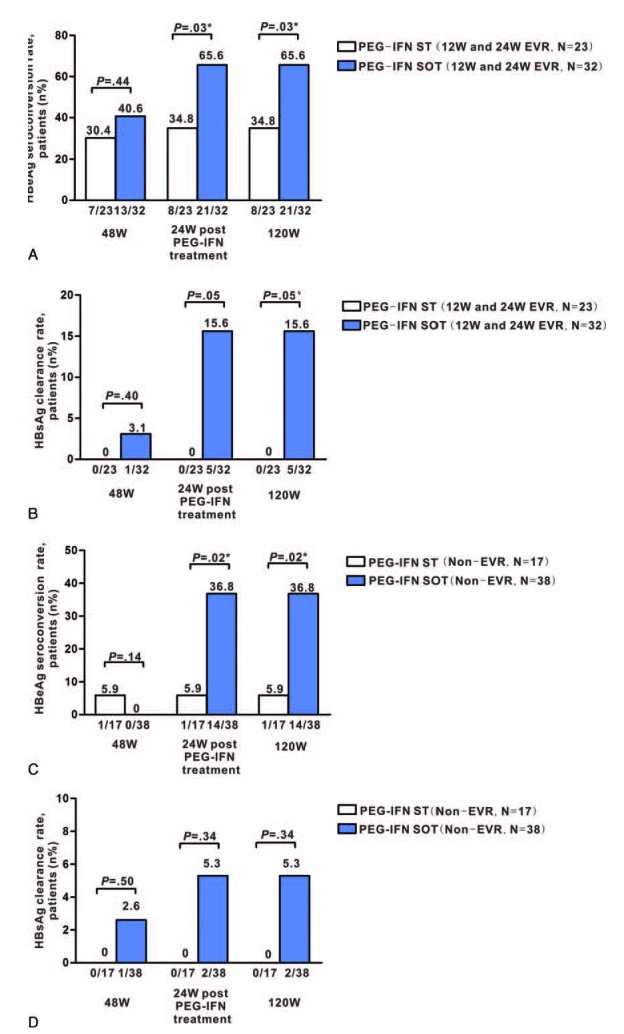
HBeAg and HBsAg clearance rates at week 48, 24 weeks post–PEG-IFN, and 120 weeks after PEG-IFN therapy initiation in patients with 12W and 24W EVR and non-EVR. HBeAg seroconversion and HBsAg clearance rates are shown in the PEG-IFN ST and PEG-IFN SOT groups at week 48 and 24 weeks post-PEG-IFN treatment, 120 weeks after PEG-IFN therapy initiation are shown for patients with EVR (A, B) and non-EVR patients (C, D). ADV = adefovir, EVR = early virological response, HBeAg = hepatitis B virus e antigen, HBsAg = hepatitis B virus surface antigen, PEG-IFN SOT = pegylated interferon-α-2a stepwise optimization treatment, PEG-IFN ST = pegylated interferon-α-2a standard therapy, W = week.
Among patients with non-EVR, the HBeAg seroconversion rates at week 48 were 0% (0/38) and 5.9% (1/17) in the PEG-IFN SOT and ST groups, respectively (P = 0.14). At 24 weeks post-PEG-IFN treatment, the rate increased to 36.8% (14/38) in the PEG-IFN SOT group and remained at 5.9% (1/17) in the PEG-IFN ST group (P = 0.02). The HBsAg clearance rate also increased from 2.6% (1/38) at week 48 to 5.3% (2/38) at 24 weeks post-PEG-IFN treatment in the PEG-IFN SOT group, whereas no patients in the PEG-IFN ST group achieved HBsAg clearance (P = 0.34). However, the HBeAg seroconversion and HBsAg clearance rates had not changed at week 120, as compared with 24 weeks post-PEG-IFN treatment (Figure 3C and D).
The virological response rates for non-EVR patients were 89.5% (34/38) and 23.5% (4/17) at week 48 in the PEG-IFN SOT and ST groups, respectively (P < 0.001). The rate increased to 94.7% (36/38) in the PEG-IFN SOT group 24 weeks post-PEG-IFN treatment and decreased to 17.6% (3/17) in the PEG-IFN ST group (P < 0.001, Table 2).
TABLE 2.
Response Rates at 48 Weeks and 24 Weeks Post-PEG-IFN-α-2a Treatment, 120 Weeks After PEG-IFN Initiation

The HBeAg seroconversion and HBsAg clearance rates of patients who did and did not achieve a 48W PR in the PEG-IFN SOT group are shown in Supplemental Figure 1, http://links.lww.com/MD/A246. HBsAg loss was achieved 24 weeks after the end of PEG-IFN extended therapy (96 weeks) in 3 of 6 patients who had EVR and PR without HBeAg seroconversion at week 48. All the 6 patients had HBeAg <10.0 S/CO, and the 3 patients with HBsAg loss had lower HBsAg levels (HBsAg < 1000 IU/mL) at week 48.
HBeAg seroconversion, HBsAg clearance, and virological response rates in patients with ADV add-on at week 12 or 24 are shown in Supplemental Figure 2, http://links.lww.com/MD/A246. In our study, 14 patients without 12W EVR received ADV add-on at week 12, and 24 patients without 24W EVR received ADV add-on at week 24 after PEG-IFN therapy initiation. The HBeAg seroconversion rates were 35.7% (5/14) and 37.5% (9/24) for patients receiving ADV add-on at week 12 and 24 (P = 0.914), respectively. The HBsAg loss rates were 7.1% (1/14) and 4.2% (1/24) for patients receiving ADV add-on at week 12 and 24 (P = 0.696), respectively. Further, the virological response rates were 92.9% (13/14) and 95.8% (23/24) for patients receiving ADV add-on at week 12 and 24 (P = 0.696), respectively. The serological response and virological response rates showed no significant differences between patients receiving ADV add-on at week 12 and 24.
Factors Associated With HBeAg Seroconversion at 24 Weeks Post-PEG-IFN Treatment
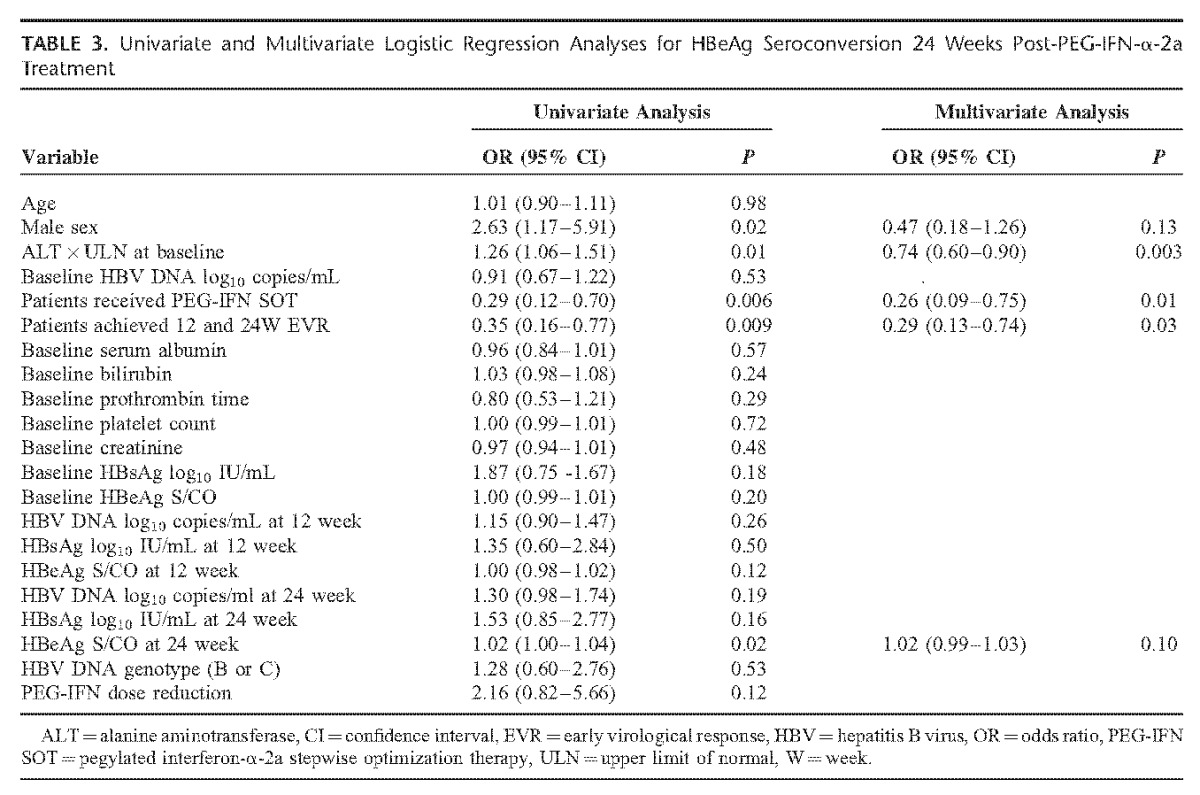
The results of univariate and multivariate analyses indicated that receiving PEG-IFN SOT (OR = 0.26, P = 0.01), ALT × ULN at baseline (OR = 0.74, P = 0.003), and achieving 12W and 24W EVR (OR = 0.29, P = 0.03) were significant independent predictors for HBeAg seroconversion 24 weeks post-PEG-IFN treatment in all study participants (Table 3).
TABLE 3.
Univariate and Multivariate Logistic Regression Analyses for HBeAg Seroconversion 24 Weeks Post-PEG-IFN-α-2a Treatment
Safety
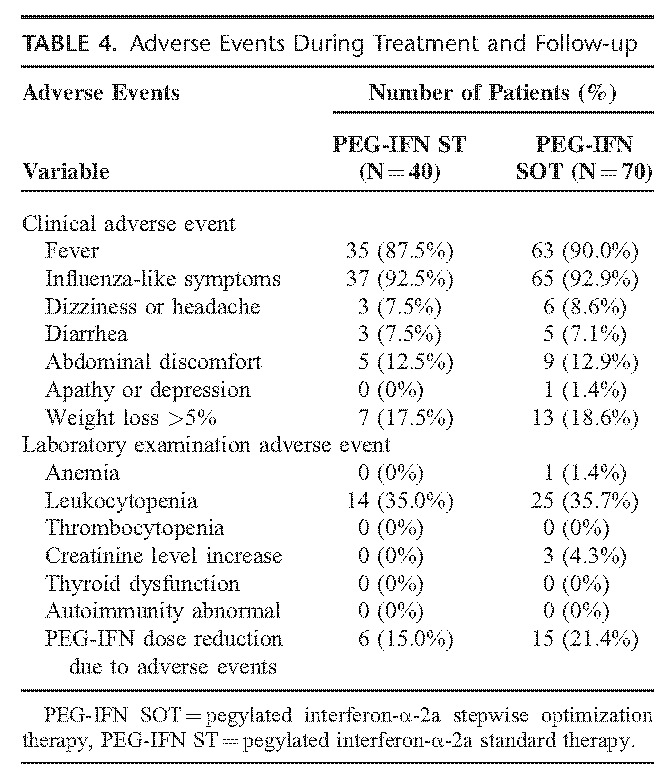
None of our study's patients had serious adverse effects during treatment or up to 24 weeks post-PEG-IFN treatment. The most common clinical adverse events in the PEG-IFN SOT and ST groups were influenza-like symptoms (92.3% and 92.5%, respectively), and the most common adverse event determined from laboratory examinations was leukocytopenia (35.7% and 35.0%, respectively). Twenty-one patients had PEG-IFN dose reductions from 180 to 135 μg because of neutropenia (a neutrophil count <750 cells/mm3 but >500 cells/mm3) of whom 6 belonged to the PEG-IFN ST group and 15 belonged to the PEG-IFN SOT group. During therapy, we also monitored whether patients developed elevated creatinine level (defined as over 2 times the baseline value or >133 μmol/L for the creatinine level). We found that 3 patients with ADV add-on developed a transient elevation of creatinine level over 2 times the baseline value, but <133 μmol/L (the upper limit of normal). Additionally, none of the patients in our study showed thyroid dysfunction or autoimmunity, either during treatment or at 24 weeks post PEG-IFN treatment (Table 4).
TABLE 4.
Adverse Events During Treatment and Follow-up
DISCUSSION
Currently, the recommended treatment regimens for HBeAg-positive CHB patients include long-term treatment with a potent NA or finite treatment with PEG-IFN. However, both treatments have limitations. The efficacy of PEG-IFN treatment extension or combination therapy of PEG-IFN and NA was still controversial,14–18 and an optimal strategy has not been established yet. The present study introduces a novel approach for the treatment of HBeAg-positive CHB patients based on PEG-IFN. We used 12W or 24W EVR and 48W PR to decide whether to add ADV and/or extend PEG-IFN–based treatment to 96 weeks. Our results showed higher serological response rates in patients receiving PEG-IFN SOT than those receiving PEG-IFN ST, suggesting that PEG-IFN SOT is an effective treatment for patients with HBeAg-positive chronic hepatitis B.
Several NAs, including LAM, LdT, ETV and ADV, have been tested in combination with PEG-IFN. The addition of LAM in HBeAg-positive CHB patients led to a greater decrease in serum HBV DNA levels during treatment but did not increase the HBeAg seroconversion rate or HBsAg loss.14,15 Neither ETV addition nor ETV pretreatment demonstrated superiority compared with PEG-IFN monotherapy for 48 weeks.17 Furthermore, a PEG-IFN and LdT combination therapy exerted a potent antiviral effect but conferred a high risk of severe polyneuropathy.16 Wursthorn et al demonstrated that the de-novo use of an ADV and PEG-IFN combination therapy led to a greater decline in intrahepatic covalently closed circular DNA levels; but the HBeAg seroconversion rate was only 33%.18 However, a recent clinical research observed the therapeutic efficacy and safety of an IFN plus ADV combination therapy, but reported inconsistent results.19,20
As the results of studies on IFN-α and NA combination antiviral therapy for CHB were unsatisfactory, other PEG-IFN-related strategies must be developed to improve treatment efficacy. Lampertico et al demonstrated that PEG-IFN treatment extension from 48 to 96 weeks in HBeAg-negative patients could improve clinical outcomes, significantly increase rates of viral suppression, and quicken HBsAg clearance.21 Although PEG-IFN treatment extension could improve efficacy in limited clinical practice reported, but additional side effects and high cost limited its use in all CHB patients receiving PEG-IFN therapy. Therefore, response-guided optimizing treatment at different time points has important clinical implications.
In NAs therapy for CHB patients, the roadmap strategy based on the 12- to 24-week virological response is a rational approach for improving long-term outcomes while minimizing the risk of drug resistance due to continued suboptimal monotherapy.22 Although recent PEG-IFN studies suggest that HBV DNA, HBsAg, and HBeAg levels at baseline and during treatment may allow clinicians to determine which patients are likely to achieve a sustained response,23 data from response-guided strategies for PEG-IFN in CHB patients are limited. The SOT study reported herein is a response-guided therapy based on both 12 to 24- and 48-week responses. Although the definition of EVR varies across PEG-IFN studies, the 2012 European Association for the Study of the Liver guidelines state that patients treated with PEG-IFN who achieve quick reductions in HBV DNA and/or HBsAg levels within 3 or 6 months of therapy have an increased probability of achieving a response to treatment.6 Piratvisuth demonstrated that 54.4% of patients with HBsAg levels <1500 IU/mL after 24 weeks of treatment achieved HBeAg seroconversion 6 months post-treatment. This is significantly higher than the seroconversion rates detected in patients with HBsAg levels of 1500 to 20 000 or >20 000 IU/mL.24 Chan et al found that 43% (9/21) of sustained responders versus 13% (9/71) of nonsustained responders experienced an HBsAg reduction of >1 log10 IU/mL at month 6 (P < 0.001).25 Based on these findings, we defined 12W EVR as having achieved a reduction in HBV DNA of 2 log10 copies/mL at least at week 12 and 24W EVR as having a reduction in HBsAg of 1 log10 at least or levels <1500 IU/mL at week 24. Moreover, there is no precise definition of a PR in PEG-IFN treatment till now. In the present study, we defined a PR as 1.0 ≤ HBeAg ≤ 10.0 S/CO or HBeAg >10.0 S/CO but HBsAg <1000 IU/mL at week 48.
Patients receiving the SOT exhibited more satisfactory results than those receiving ST in our study. The HBeAg seroconversion and HBsAg clearance rates 24 weeks post-PEG-IFN treatment in the PEG-IFN SOT group were significantly higher than that in the PEG-IFN ST group (HBeAg seroconversion, 50.0% vs 22.5%, P = 0.005; HBsAg clearance 10.0% vs 0%, P = 0.04). And the difference in HBeAg seroconversion and HBsAg clearance rate between 2 groups remained significantly at week 120 in our study. Previous data indicated that the HBeAg seroconversion rate in patients receiving a PEG-IFN-2α ST for 48 weeks was only 32%.14 Our subgroup analysis also showed that PEG-IFN SOT significantly increased the HBeAg seroconversion rates 24 weeks post-PEG-IFN treatment in patients who achieved 12W and 24W EVR.
Surprisingly, in PEG-IFN SOT group, the HBsAg clearance rate reached 50% (3/6) for 12 and 24W EVR and 48W PR patients who received PEG-IFN extended therapy to 96 weeks. However, all 6 of the patients had HBeAg <10.0 S/CO, and the 3 patients with HBsAg loss had lower HBsAg levels (HBsAg <1000 IU/mL) at week 48. In the OSST trial that has recently been reported by Qin Ning et al, switching to a finite course of PEG-IFN-α-2a significantly increased rates of HBsAg loss (to 22.2%) for ETV-treated patients who had HBeAg loss and HBsAg <1500 IU/mL at randomization.26 Hu et al have reported similar results from another clinical trial, in which the HBsAg loss rate of 31.2% was observed in patients who had HBeAg loss and HBsAg <1500 IU/mL at randomization.27 Because HBeAg seroconversion for HBeAg-positive CHB patients is a satisfactory endpoint that is associated with an improved prognosis,6 prolonged PEG-IFN treatment was not suggested for all the patients who achieved HBeAg seroconversion before week 48. It is rational to believe that extended PEG-IFN therapy may increase the HBsAg loss rate for patients with HBeAg seroconversion and lower HBsAg level after PEG-IFN treatment, irrespective of the time of HBeAg seroconversion. However, randomized controlled clinical trial is needed to support our view in the future.
In this study, for non-EVR patients, PEG-IFN SOT increased markedly the HBeAg seroconversion rate from 0% at week 48 to 36.8% at 24 weeks post-PEG-IFN treatment, which was significantly higher than the rate 5.9% in patients with the 48-week PEG-IFN ST at 24 weeks post-PEG-IFN treatment. Previous studies have indicated that ADV not only directly affected virus suppression and led to a greater decline in intrahepatic covalently closed circular DNA levels, but also had an indirect immunoregulatory capability in CHB patients.28–29 It might explain why patients with ADV add-on in the PEG-IFN SOT group were able to achieve a relatively high HBeAg seroconversion rate. As compared with the PEG-IFN ST group, patients with non-EVR in the PEG-IFN SOT group achieved a significantly higher rate of virological response at week 48 and at 24 weeks post-PEG-IFN treatment. This means that the addition of ADV effectively suppressed HBV replication in non-EVR patients.
The factors affecting HBeAg seroconversion 24 weeks post-PEG-IFN treatment were analyzed in our study. Multivariate analysis showed that receiving PEG-IFN SOT, achieving 12W to 24W EVR and ALT × ULN at baseline were independent predictors for HBeAg seroconversion at 24 weeks post-PEG-IFN treatment. These results indicate that PEG-IFN SOT is beneficial to achieve HBeAg seroconversion for HBeAg-positive CHB patients.
The adverse events observed in the present study are consistent with previous findings, suggesting that the PEG-IFN SOT used in our study does not result in more adverse events than the PEG-IFN ST. Our study has some limitations, including the single-center retrospective cohort study design, relatively sample size, and heterogeneous observation regimen. Despite its limitations, this SOT study is a promising strategy for achieving higher serological response rates in HBeAg-positive CHB patients.
In conclusion, our PEG-IFN-α-2a stepwise optimization treatment strategies, including the decision to add ADV and/or extent treatment, were based on patient responses to PEG-IFN at week 12, 24, and 48, and PEG-IFN SOT is a effective way of improving PEG-IFN treatment efficacy in HBeAg-positive CHB patients. Nevertheless, further studies are required to corroborate the present findings and investigate this promising strategy in further detail.
Acknowledgments
We thank Dr Xiaoqin Wang (Fudan University) for helpful suggestions.
Footnotes
Abbreviations: ADV = adefovir, ALT = alanine aminotransferase, CHB = chronic hepatitis B virus infection, ETV = entecavir, EVR = early virological response, HBeAg = hepatitis B virus e antigen, HBsAg = hepatitis B virus surface antigen, HBV = hepatitis B virus, ICD = International Classification of Diseases, IFN-α = interferon α, LAM = lamivudine, LdT = telbivudine, NA = nucleot(s)ide analogue, PEG = pegylated, PR = partial response, SOT = stepwise optimization treatment, ST = standard therapy, ULN = upper limit of normal.
PZ and FY contributed equally to this work.
This work was supported by Major Science and Technology Special Project of China (2012ZX10002007–001–002, 2013ZX10002001), the grants from Shanghai Science and Technology Committee (grant no. 14411966200) and the Shanghai Municipal Commission of Health and Family Planning Major Project (grant no. 201440042), and the Special Research Fund of Ministry of Health for Non-Profit Sector (grant no. 201302010).
The authors have no conflicts of interest to declare.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Website (www.md-journal.com).
REFERENCES
- 1.Custer B, Sullivan SD, Hazlet TK, et al. Global epidemiology of hepatitis B virus. J Clin Gastroenterol 2004; 38:S158–168. [DOI] [PubMed] [Google Scholar]
- 2.Liang X, Bi S, Yang W, et al. Epidemiological serosurvey of hepatitis B in China-declining HBV prevalence due to hepatitis B vaccination. Vaccine 2009; 27:6550–6557. [DOI] [PubMed] [Google Scholar]
- 3.Lok AS, McMahon BJ. Chronic hepatitis B. Hepatology 2007; 45:507–539. [DOI] [PubMed] [Google Scholar]
- 4.Feld JJ, Wong DK, Heathcote EJ. Endpoints of therapy in chronic hepatitis B. Hepatology 2009; 49:S96–102. [DOI] [PubMed] [Google Scholar]
- 5.Sung JJ, Wong ML, Bowden S, et al. Intrahepatic hepatitis B virus covalently closed circular DNA can be a predictor of sustained response to therapy. Gastroenterology 2005; 128:1890–1897. [DOI] [PubMed] [Google Scholar]
- 6.European Association for the Study of the Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012; 57:167–185. [DOI] [PubMed] [Google Scholar]
- 7.Lai CL, Chien RN, Leung NW, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med 1998; 339:61–68. [DOI] [PubMed] [Google Scholar]
- 8.Chang TT, Gish RG, de Man R, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med 2006; 354:1001–1010. [DOI] [PubMed] [Google Scholar]
- 9.Lai CL, Gane E, Liaw YF, et al. Telbivudine versus lamivudine in patients with chronic hepatitis B. N Engl J Med 2007; 357:2576–2588. [DOI] [PubMed] [Google Scholar]
- 10.Marcellin P, Chang TT, Lim SG, et al. Adefovir dipivoxil for the treatment of hepatitis B antigen-positive chronic hepatitis B. N Engl J Med 2003; 348:808–816. [DOI] [PubMed] [Google Scholar]
- 11.Marcellin P, Heathcote EJ, Buti M, et al. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med 2008; 359:2442–2455. [DOI] [PubMed] [Google Scholar]
- 12.Zonneveld M, Honkoop P, Hansen BE, et al. Long-term follow-up of alpha-interferon treatment of patients with chronic hepatitis B. Hepatology 2004; 39:804–810. [DOI] [PubMed] [Google Scholar]
- 13.Cheng J, Wang Y, Hou J, et al. Peginterferon alfa-2b in the treatment of Chinese patients with HBeAg-positive chronic hepatitis B: a randomized trial. J Clin Virol 2014; 61:509–516. [DOI] [PubMed] [Google Scholar]
- 14.Lau GK, Piratvisuth T, Luo KX, et al. Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. N Engl J Med 2005; 352:2682–2695. [DOI] [PubMed] [Google Scholar]
- 15.Janssen HL, Zonneveld M, Senturk H, et al. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet 2005; 365:123–129. [DOI] [PubMed] [Google Scholar]
- 16.Marcellin P, Wursthorn K, Wedemeyer H, et al. Telbivudine plus pegylated interferon alfa-2a in a randomized study in chronic hepatitis B was associated with unexpected high rate of peripheral neuropathy. J Hepatol 2014; 61:41–47. [DOI] [PubMed] [Google Scholar]
- 17.Xie Q, Zhou H, Bai X, et al. A randomized, open-label clinical study of combined pegylated interferon Alfa-2a (40KD) and entecavir treatment for hepatitis B “e” antigen-positive chronic hepatitis B. Clin Infect Dis 2014; 59:1714–1723. [DOI] [PubMed] [Google Scholar]
- 18.Wursthorn K, Lutgehetmann M, Dandri M, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology 2006; 44:675–684. [DOI] [PubMed] [Google Scholar]
- 19.Wang Y, Zhao C, Wang W, et al. Improved efficacy by individualized combination therapy with Peg IFN-a 2a and ADV in HBeAg positive chronic hepatitis B patients. Hepatogastroenterology 2012; 59:680–686. [DOI] [PubMed] [Google Scholar]
- 20.Cao Z, Ma L, Zhang H, et al. Extended treatment with peginterferon alpha-2a in combination with lamivudine or adefovir for 96 weeks yields high rates of HBeAg and HBsAg seroconversion. J Dig Dis 2013; 14:446–450. [DOI] [PubMed] [Google Scholar]
- 21.Lampertico P, Vigano M, Di Costanzo GG, et al. Randomised study comparing 48 and 96 weeks peginterferon (-2a therapy in genotype D HBeAg-negative chronic hepatitis B. Gut 2013; 62:290–298. [DOI] [PubMed] [Google Scholar]
- 22.Keeffe EB, Zeuzem S, Koff RS, et al. Report of an international workshop: Roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clin Gastroenterol Hepatol 2007; 5:890–897. [DOI] [PubMed] [Google Scholar]
- 23.Tang CM, Yau TO, Yu J. Management of chronic hepatitis B infection: current treatment guidelines, challenges, and new developments. World J Gastroenterol 2014; 20:6262–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piratvisuth T, Marcellin P, Popescu M, et al. Hepatitis B surface antigen: association with sustained response to peginterferon alfa-2a in hepatitis B e antigen-positive patients. Hepatol Int 2011; 7:429–436. [DOI] [PubMed] [Google Scholar]
- 25.Chan HL, Wong VS, Chim AM, et al. Serum HBsAg quantification to predict response to peginterferon therapy of e antigen positive chronic hepatitis B. Aliment Pharmacol Ther 2010; 32:1323–1331. [DOI] [PubMed] [Google Scholar]
- 26.Ning Q, Han M, Sun Y, et al. Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). J Hepatol 2014; 61:777–784. [DOI] [PubMed] [Google Scholar]
- 27.Hu P, Shang J, Zhang W, et al. Multi-center randomized study on the efficacy and safety of switching to peginterferon-α-2a (40KD) for 48 or 96 weeks in HBeAg positive CHB patients with a prior NUC history for 1 to 3 years: an interim analysis of NEW SWITCH study. LB-10 in Late-Breaking AASLD ABSTRACTS. Hepatology 2014; 60:1273A. [Google Scholar]
- 28.Jiang Y, Ma Z, Xin G, et al. Th1 and Th2 immune response in chronic hepatitis B patients during a long-term treatment with adefovir dipivoxil. Mediators Inflamm 2010; 2010:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang Y, Li W, Yu L, et al. Enhancing the antihepatitis B virus immune response by adefovir dipivoxil and entecavir therapies. Cell Mol Immunol 2010; 8:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]


